Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (Dosidicus Gigas)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Muscle Protein Extraction
2.3. Film Elaboration
2.4. Light Transmittance and Transparency
2.5. Tensile Strength and Elongation Percentage
2.6. Water Solubility
2.7. Water Vapor Transmission Rate (WVTR)
2.8. Thermogravimetric Analysis (TGA)
2.9. Fourier Transform Infrared Spectroscopy (FT-IR)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Films Obtained
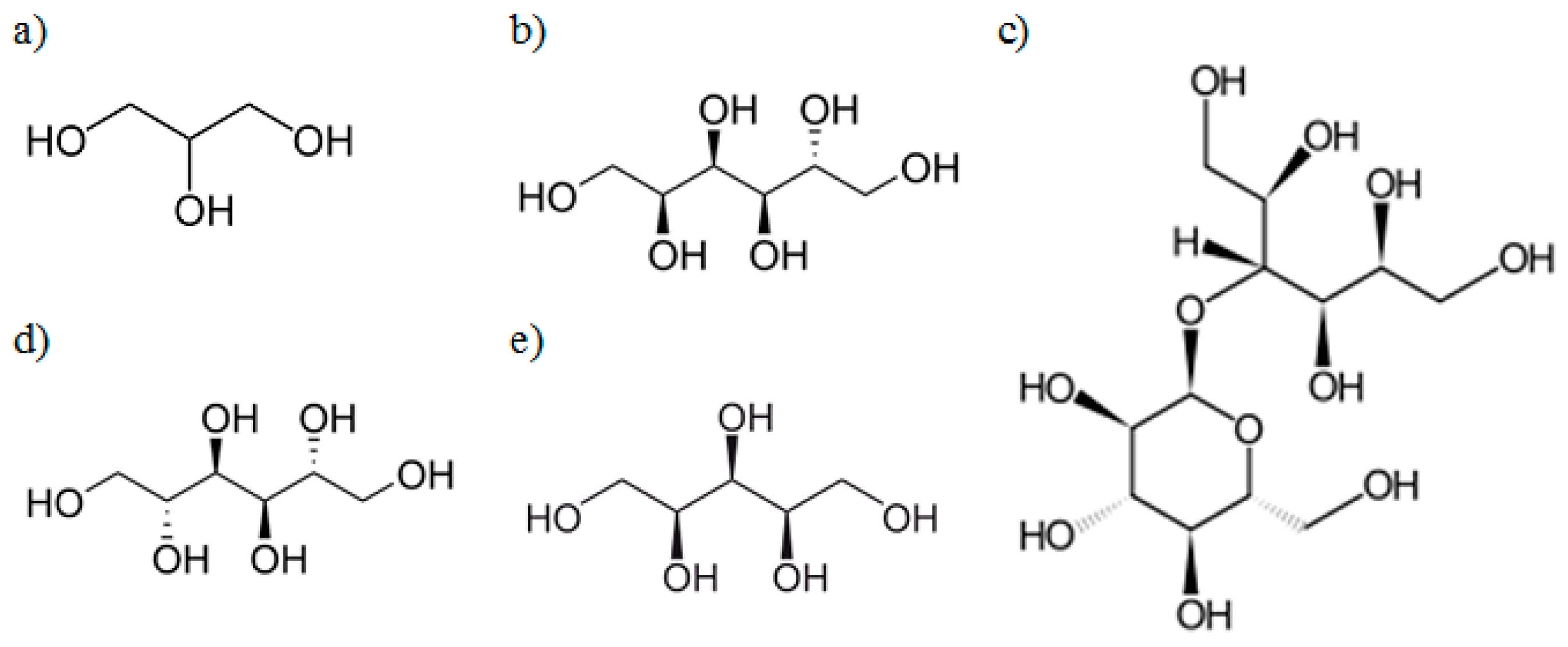
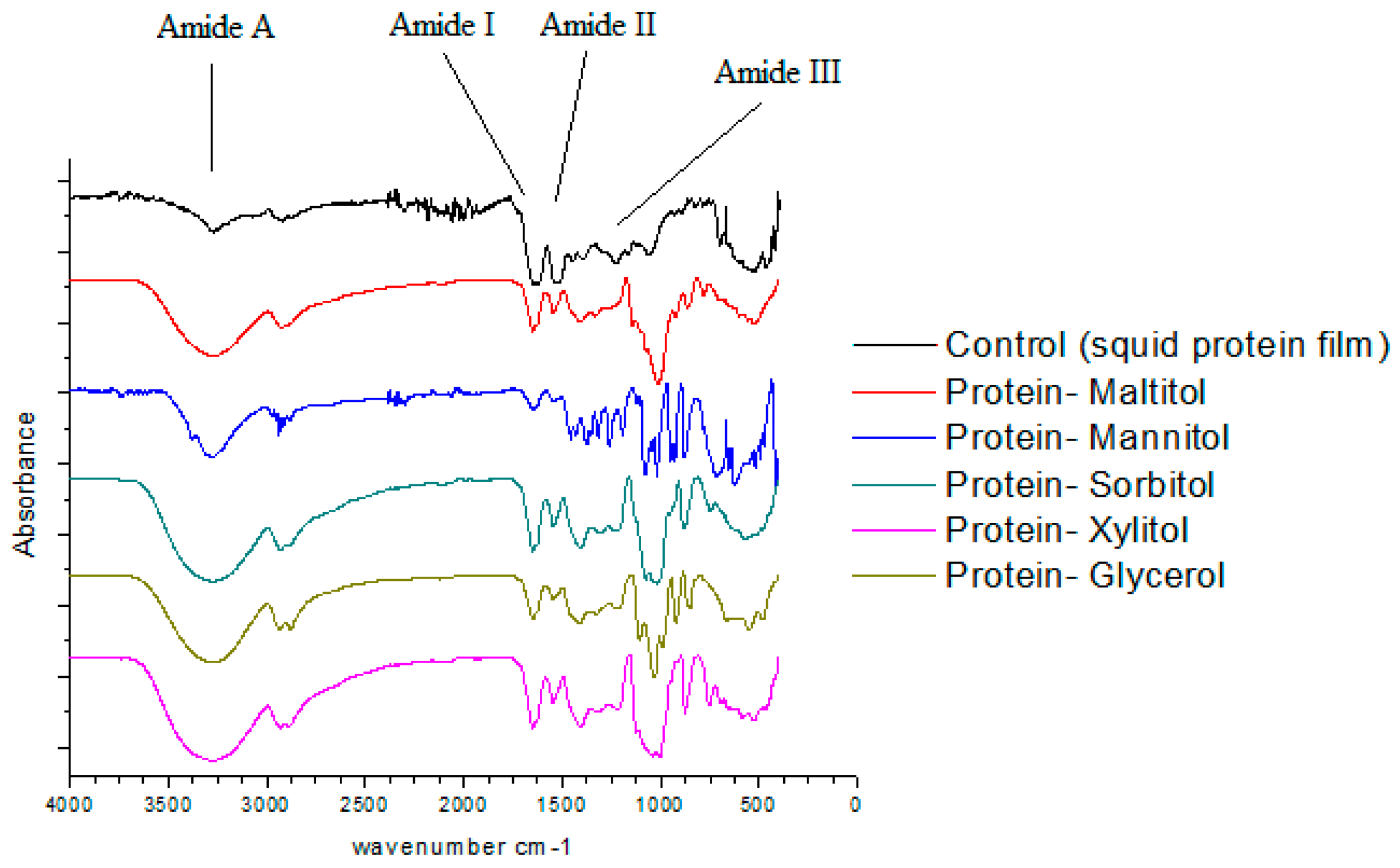
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
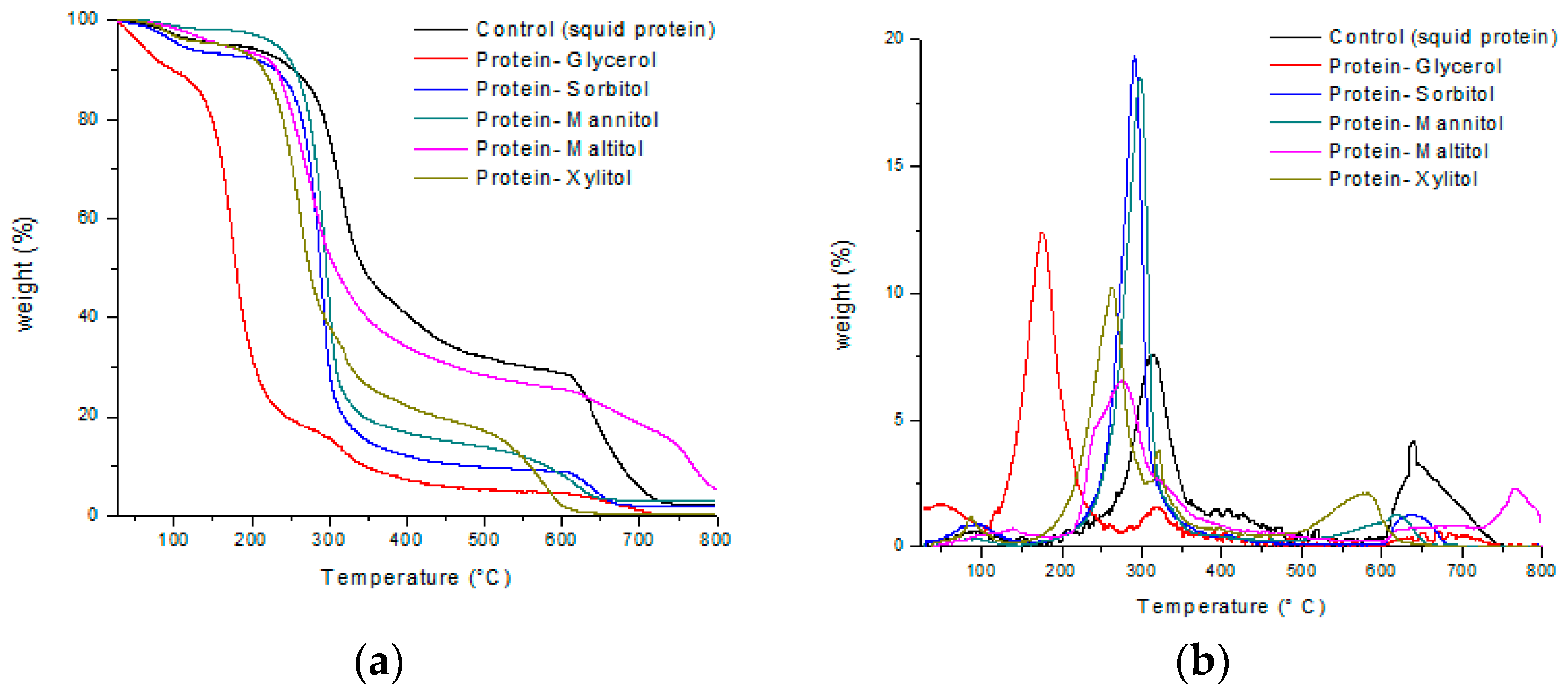
3.3. Thermogravimetric Analysis (TGA)
3.4. Light Transmittance and Transparency
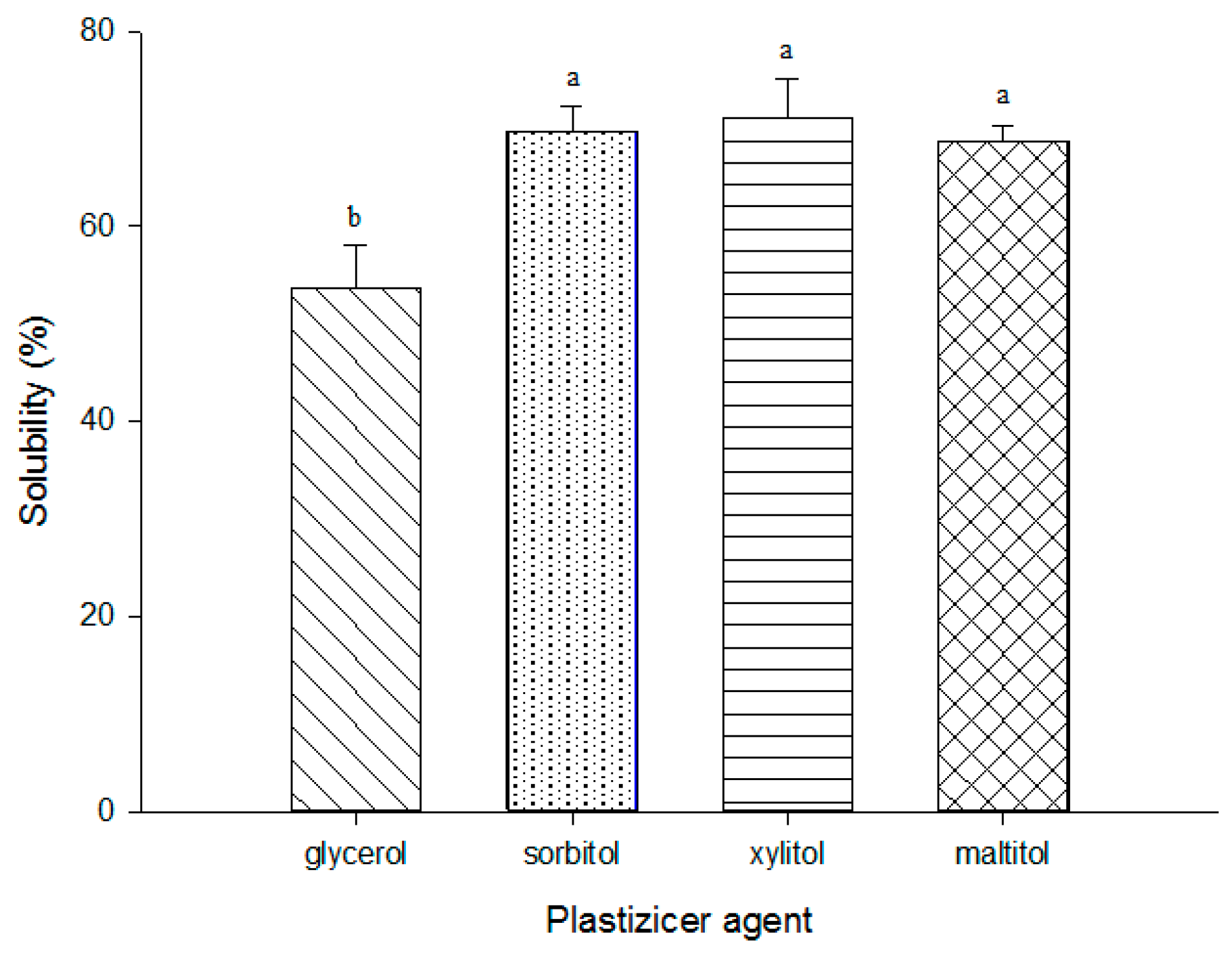
3.5. Film Solubility in Water
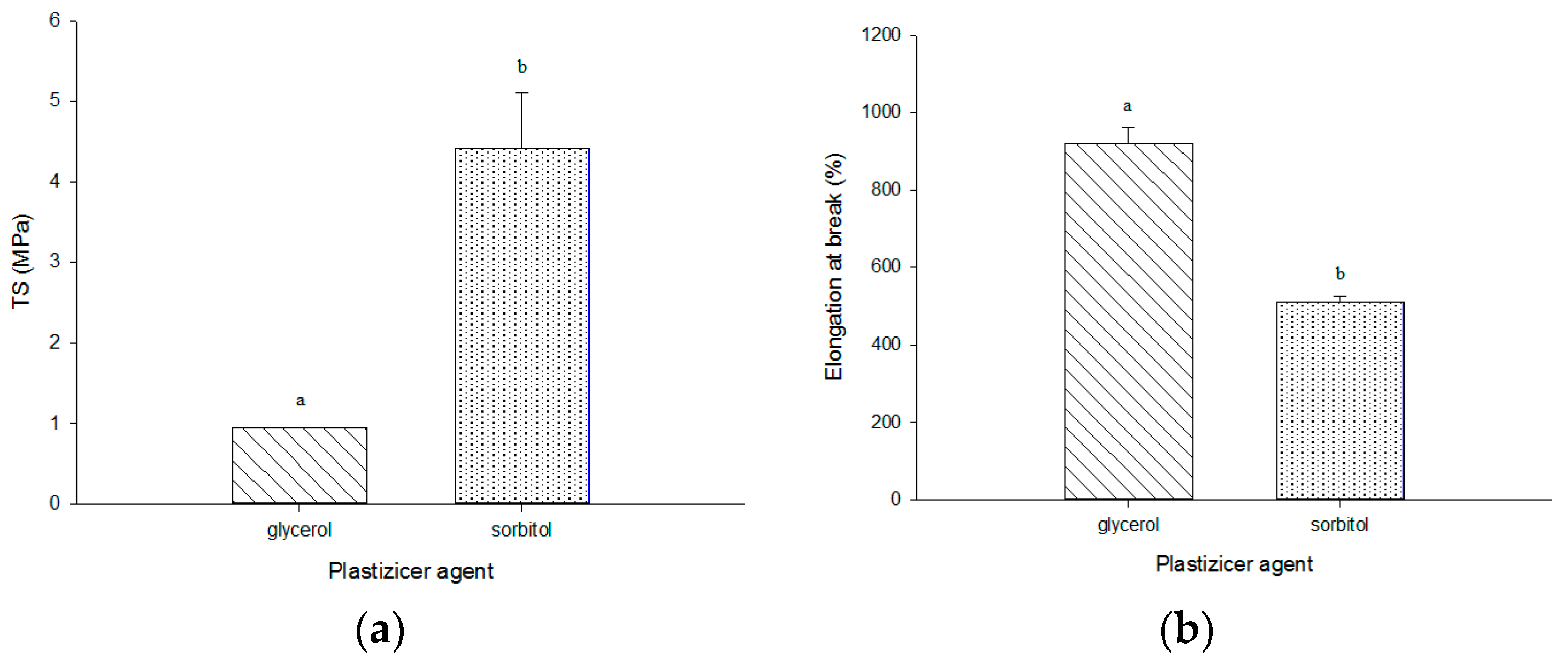
3.6. Mechanical Properties
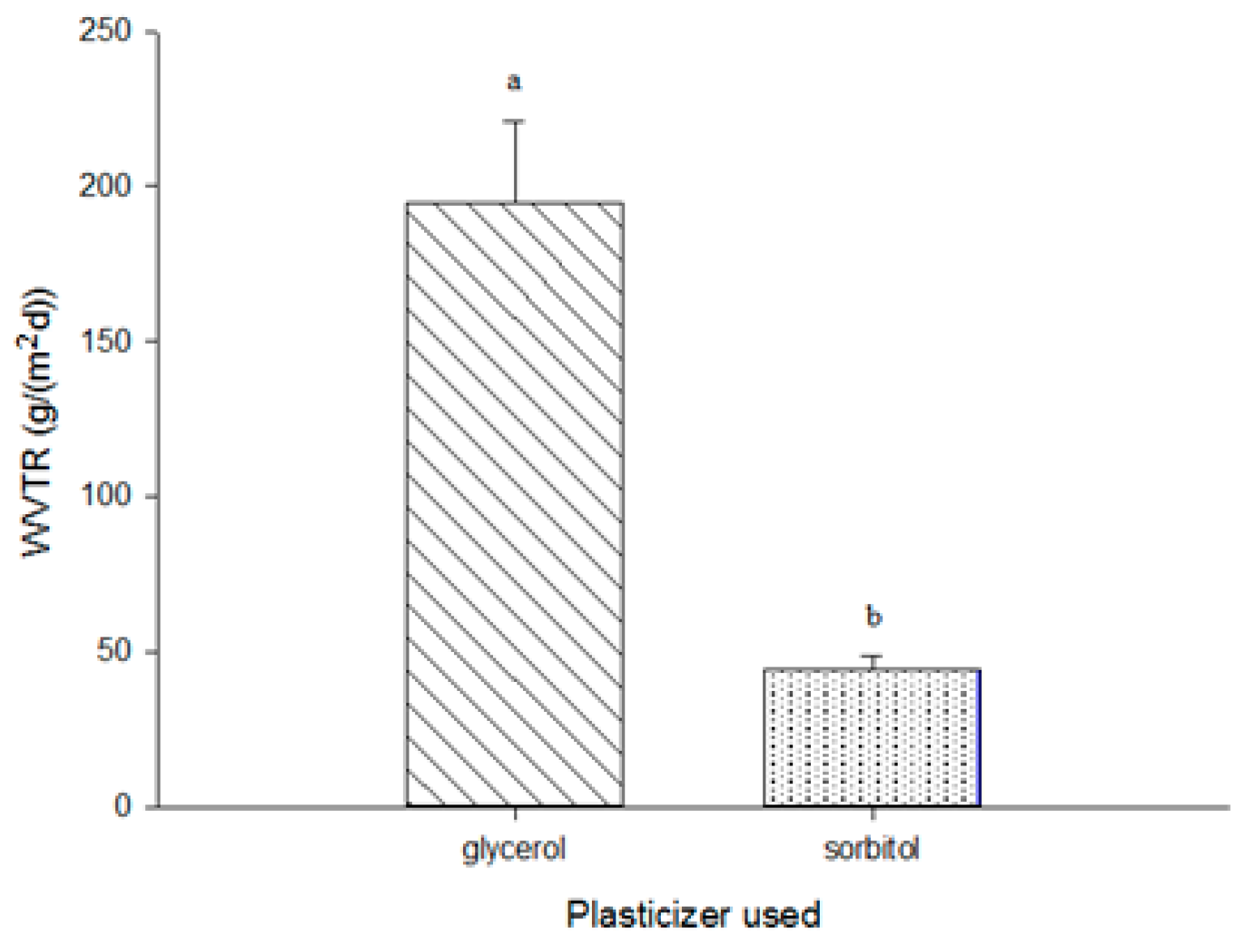
3.7. Water-Vapor Transmission Rate (WVTR)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez-Ríos, E. Edible protain films: Sources and behavior. Packag. Technol. Sci. 2017, 31, 113–122. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Rawdkuen, S. Effect of protein concentrations on the properties of fish myofibrilar protein based film compared with PVC film. J. Food Sci. Technol. 2016, 53, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The potential of proteins for producing food packaging materials: A review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Mekpiroon, A.; Lerdrattranataywere, W.; Jutidamrongphan, W. Prespective of waste utilization in seafood industry. Int. J. Manag. Appl. Sci. 2016, 2, 96–99. [Google Scholar]
- FAO. FAO Global Capture Production Database Updated to 2016. Available online: http://www.fao.org/3/I9540ES/i9540es.pdf (accessed on 7 December 2018).
- Blanco-Pascual, N.; Fernández-Martín, F.; Montero, M.P. Effect of diferent protein extracts from Dosidicus gigas muscle co-products on edible films development. Food Hydrocoll. 2013, 33, 118–131. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Fernández-Martín, F.; Montero, M.P. Jumbo squid (Dosidicus gigas) myofibrillar protein concentrate for edible packaging films and storage stability. LWT Food Sci. Technol. 2014, 55, 543–550. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Properties of biodegradable blend films based on fish myofibrillar protein and polyvinyl alcohol as influenced by blend composition and pH level. J. Food Eng. 2010, 100, 85–92. [Google Scholar] [CrossRef]
- Adeodato-Vieira, M.G.; Altenhofen da Silva, M.; Oliveira dos Santos, L.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef]
- Tong, Q.; Xiao, Q.; Lim, L.-T. Effects of glycerol, sorbitol, xylitol and fructose plasticisers on mechanical and moisture barrier properties of pullulan-alginate-carboxymethylcellulose blend films. Int. J. Food Sci. Technol. 2013, 48, 870–878. [Google Scholar] [CrossRef]
- Cortés-Ruiz, J.A.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Carvallo-Ruiz, M.G.; García-Sánchez, G. Production and functional evaluation of a protein concentrate from giant squid (Dosidicus gigas) by acid dissolution and isoelectric precipitation. Food Chem. 2008, 110, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Shiku, Y.; Hamaguchi, P.Y.; Tanaka, M. Effect of pH on the preparation of edible films based on fish myofibrillar proteins. Fish. Sci. 2003, 69, 1026–1032. [Google Scholar] [CrossRef]
- D882-91 Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 1996.
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Osako, K. Development and characterisaton of blend films based on fish skin. Food Hydrocoll. 2014, 39, 58–67. [Google Scholar] [CrossRef]
- E96/E96M-05 Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2005.
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Sockalingam, K.; Abdullah, H.Z. Extraction and characterization of gelatin biopolymer from black tilapia (Oreochromis mossambicus) scales. AIP Conf. Proc. 2015, 1, 020053. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Dandurand, J.; Samouillan, V.; Lacoste-Ferré, M.H.; Lacabanne, C.; Bochicchio, B.; Pepe, A. Conformational and termal characterization of a synthetic peptidic fragment inspired from human tropoelastin: Signature of the amyloid fibers. Pathol. Biol. 2014, 62, 100–107. [Google Scholar] [CrossRef]
- Rocha, M.; Cardozo, M.A.; Raffi, J.E.; El Halal, S.L.M.; Souza, M.M.; Prentice, C. Functional, thermal, and physicochemical properties of proteins from Argentine croaker (Umbrina canosai) recovered by solubilization/precipitation or a washing process. Int. Food Res. J. 2017, 24, 579. [Google Scholar]
- Spectral Database for Organic Compounds, SDBS. Available online: http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 7 December 2018).
- Schimid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Oujifard, A.; Benjakul, S.; Prodpran, T.; Seyfabadi, J. Properties of red tilapia (Oreochromis niloticus) protein based film as affected by cryoprotectants. Food Hydrocoll. 2013, 32, 245–251. [Google Scholar] [CrossRef]
- Zavareze, E.D.A.; El Halal, E.L.M.; Marques e Silva, R.; Días, A.R.G.; Prentice, C.H. Mechanical, barrier and morphological properties of biodegradable films based on muscle and waste proteins from the whitemouth croaker (Micropogonias furnieri). J. Food Process. Preserv. 2014, 38, 1973–1981. [Google Scholar] [CrossRef]
- Mahadevaiah; Shivakumara, L.R.; Demappa, T.; Singh, V. Mechanical and barrier properties of hydroxy propyl methyl cellulose edible polymer films with plasticizer combinations. J. Food Process. Preserv. 2016, 41, e13020. [Google Scholar] [CrossRef]
- Pascall, M.A.; Lin, S.J. The application of edible polymeric films and coatings in the food industry. J. Food Process. Technol. 2013, 4, e116. [Google Scholar] [CrossRef]
- Hamaguchi, P.Y.; Yin, W.W.; Tanaka, M. Effect of pH on the formation of edible film made from the muscle proteins of blue marlin (Makaira mazara). Food Chem. 2007, 100, 914–920. [Google Scholar] [CrossRef]
- Othman, S.H.; Edwal, S.A.M.; Risyon, N.P.; Basha, R.K.; Talib, R.A. Water sorption and water permeability properties of edible film made from potato peel waste. Food Sci. Technol. 2017, 37, 63–70. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vähä-Nissi, M.; Harlin, A.; Salomäki, M.; Areva, S.; Korhonen, J.T.; Karppinen, M. Enhanced water vapor barrier properties for biopolymer films by polyelectrolyte multilayer and atomic layer deposited Al2O3 double-coating. Appl. Surf. Sci. 2011, 257, 9451–9454. [Google Scholar] [CrossRef]
- Schmid, M. Properties of cast films made from different ratios od whey protein isolate, hydrolysed whey protein isolate and glycerol. Materials 2013, 6, 3254–3269. [Google Scholar] [CrossRef]
- Graciano-Verdugo, A.Z.; Peralta, E.; Soto-Valdez, H. Permeabilidad y vida útil de los alimentos. AlimenPack 2006, 2, 15–19. [Google Scholar]

| Film | Light Transmittance (%) | Transparency | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 250 | 350 | 400 | 500 | 600 | 700 | 800 | ||
| Gly | 0.0 ± 0.0 b | 0.3 ± 0.2 ab | 51.9 ± 3.3 a | 61.8 ± 3.7 a | 66.1 ± 2.9 a | 71.0 ± 3.9 a | 71.9 ± 3.9 a | 72.4 ± 4.0 a | 0.5 c |
| Sor | 0.0 ± 0.0 b | 1.0 ± 0.7 a | 22.7 ± 2.4 b | 26.8 ± 3.4 b | 29.6 ± 3.7 b | 30.9 ± 3.9 b | 31.8 ± 4.0 b | 32.3 ± 4.1b | 0.7 b |
| Mal | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 44.5 ± 16.1a | 52.4 ± 17.4 a | 57.5 ± 18.1 a | 59.2 ± 18.3 a | 59.6 ± 18.5 a | 60.1 ± 18.5 a | 0.5 c |
| Man | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.8 ± 0.2 c | 0.9 ± 0.2 c | 1.2 ± 0.3 c | 1.4 ± 0.4 c | 1.6 ± 0.5 c | 1.7 ± 0.6 c | 9.8 a |
| Xyl | 0.3 ± 0.1 a | 0.6 ± 0.2 ab | 36.6 ± 5.0 ab | 44.0 ± 6.3 ab | 49.9 ± 6.9 ab | 52.3 ± 7.1 ab | 54.0 ± 7.1 ab | 55.0 ± 7.0 ab | 0.1d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murrieta-Martínez, C.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Ramírez-Wong, B.; Santacruz-Ortega, H.; Santos-Sauceda, I.; Olibarría-Rodríguez, G.; Márquez-Ríos, E. Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (Dosidicus Gigas). Coatings 2019, 9, 77. https://doi.org/10.3390/coatings9020077
Murrieta-Martínez C, Soto-Valdez H, Pacheco-Aguilar R, Torres-Arreola W, Rodríguez-Felix F, Ramírez-Wong B, Santacruz-Ortega H, Santos-Sauceda I, Olibarría-Rodríguez G, Márquez-Ríos E. Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (Dosidicus Gigas). Coatings. 2019; 9(2):77. https://doi.org/10.3390/coatings9020077
Chicago/Turabian StyleMurrieta-Martínez, Claudia, Herlinda Soto-Valdez, Ramón Pacheco-Aguilar, Wilfrido Torres-Arreola, Francisco Rodríguez-Felix, Benjamín Ramírez-Wong, Hisila Santacruz-Ortega, Irela Santos-Sauceda, Guillermo Olibarría-Rodríguez, and Enrique Márquez-Ríos. 2019. "Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (Dosidicus Gigas)" Coatings 9, no. 2: 77. https://doi.org/10.3390/coatings9020077
APA StyleMurrieta-Martínez, C., Soto-Valdez, H., Pacheco-Aguilar, R., Torres-Arreola, W., Rodríguez-Felix, F., Ramírez-Wong, B., Santacruz-Ortega, H., Santos-Sauceda, I., Olibarría-Rodríguez, G., & Márquez-Ríos, E. (2019). Effect of Different Polyalcohols as Plasticizers on the Functional Properties of Squid Protein Film (Dosidicus Gigas). Coatings, 9(2), 77. https://doi.org/10.3390/coatings9020077






