Nanocomposite Multilayer Binary Nitride Coatings Based on Transition and Refractory Metals: Structure and Properties
Abstract
:1. Introduction
2. General Features of Multilayer Coatings
3. Microstructure, Physical-Mechanical and Tribological Properties of Multilayer Coatings
4. Structure, Phase Composition, Mechanical and Tribological Properties of Some Multilayer Coatings
5. Conclusions
Funding
Conflicts of Interest
References
- Koltunowicz, T.N.; Zhukowski, P.; Fedotov, A.K.; Larkin, A.V.; Patryn, A.; Andryevskyy, B.; Saad, A.; Fedotova, J.A.; Fedotova, V.V. Influence of Matrix Type on Negative Capacitance Effect in Nanogranular Composite Films FeCoZr-Insulator. Elektron. IR Elektrotechnika 2013, 19, 37–40. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Jain, A.; Rajam, K.S. Structure, Hardness and Thermal Stability of Nanolayered TiN/CrN Multilayer Coatings. Vacuum 2003, 72, 241–248. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A.; Ronkainen, H. Coatings Tribology—Contact Mechanisms and Surface Design. Tribol. Int. 1998, 31, 107–120. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Single Layer and Multilayer Wear Resistant Coatings of (Ti,Al)N: A Review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Jehn, H.A. Multicomponent and Multiphase Hard Coatings for Tribological Applications. Surf. Coat. Technol. 2000, 131, 433–440. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Skorb, E.V. Chapter 13—Multi-Layer Smart Coatings for Corrosion Protection of Aluminium Alloys and Steel. In Handbook of Smart Coatings for Materials Protection; Makhlouf, A.S.H., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 307–327. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H. Chapter 6—Protective Coatings for Automotive, Aerospace and Military Applications: Current Prospects and Future Trends. In Handbook of Smart Coatings for Materials Protection; Makhlouf, A.S.H., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 121–131. [Google Scholar] [CrossRef]
- Beresnev, V.M.; Bondar, O.V.; Postolnyi, B.O.; Lisovenko, M.O.; Abadias, G.; Chartier, P.; Kolesnikov, D.A.; Borisyuk, V.N.; Mukushev, B.A.; Zhollybekov, B.R.; et al. Comparison of Tribological Characteristics of Nanostructured TiN, MoN and TiN/MoN Arc-PVD Coatings. J. Frict. Wear 2014, 35, 374–382. [Google Scholar] [CrossRef]
- Khadem, M.; Penkov, O.V.; Yang, H.-K.; Kim, D.-E. Tribology of Multilayer Coatings for Wear Reduction: A Review. Friction 2017, 5, 248–262. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Zhang, G.; Jiang, X.; Wang, L.; Xue, Q. Microstructures and Properties of Zr/CrN Multilayer Coatings Fabricated by Multi-Arc Ion Plating. Tribol. Int. 2017, 106, 78–87. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Browne, T.; Heckerman, B.; Bowman, C.; Gorokhovsky, V.; Meletis, E.I. Mechanical and Tribological Properties of TiN/Ti Multilayer Coating. Surf. Coat. Technol. 2010, 205, 146–151. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Structural, Mechanical and Tribocorrosion Behaviour in Artificial Seawater of CrN/AlN Nano-Multilayer Coatings on F690 Steel Substrates. Appl. Surf. Sci. 2018, 428, 404–414. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Yakushchenko, I.V.; Beresnev, V.M. The Structure and Properties of High-Entropy Alloys and Nitride Coatings Based on Them. Russ. Chem. Rev. 2014, 83, 1027. [Google Scholar] [CrossRef]
- Pogrebnyak, A.D.; Shpak, A.P.; Azarenkov, N.A.; Beresnev, V.M. Structures and Properties of Hard and Superhard Nanocomposite Coatings. Phys.-Uspekhi 2009, 52, 29. [Google Scholar] [CrossRef]
- Vereschaka, A.; Tabakov, V.; Grigoriev, S.; Sitnikov, N.; Oganyan, G.; Andreev, N.; Milovich, F. Investigation of Wear Dynamics for Cutting Tools with Multilayer Composite Nanostructured Coatings in Turning Constructional Steel. Wear 2019, 420–421, 17–37. [Google Scholar] [CrossRef]
- Kindlund, H.; Sangiovanni, D.G.; Martínez-de-Olcoz, L.; Lu, J.; Jensen, J.; Birch, J.; Petrov, I.; Greene, J.E.; Chirita, V.; Hultman, L. Toughness Enhancement in Hard Ceramic Thin Films by Alloy Design. APL Mater. 2013, 1, 042104. [Google Scholar] [CrossRef]
- Boing, D.; de Oliveira, A.J.; Schroeter, R.B. Limiting Conditions for Application of PVD (TiAlN) and CVD (TiCN/Al2O3/TiN) Coated Cemented Carbide Grades in the Turning of Hardened Steels. Wear 2018, 416–417, 54–61. [Google Scholar] [CrossRef]
- Ahmad, Z. Chapter 1—Introduction to Corrosion. In Principles of Corrosion Engineering and Corrosion Control; Ahmad, Z., Ed.; Butterworth-Heinemann: Oxford, UK, 2006; pp. 1–8. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Konarski, P.; Misnik, M.; Ivashchenko, V.I.; Kempiński, M.; Mediukh, N.R.; Pogrebnjak, A.D.; Beresnev, V.M.; et al. A New Type of (TiZrNbTaHf)N/MoN Nanocomposite Coating: Microstructure and Properties Depending on Energy of Incident Ions. Compos. Part B Eng. 2018, 146, 132–144. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Deepthi, B.; Rajam, K.S. Chapter 10—Transition Metal Nitride-Based Nanolayered Multilayer Coatings and Nanocomposite Coatings as Novel Superhard Materials. In Nanostructured Thin Films and Coatings: Mechanical Properties; CRC Press: Boca Raton, FL, USA, 2010; pp. 427–480. [Google Scholar]
- Gaffney, J.S.; Marley, N.A. Chapter 3—Chemical Bonding—The Formation of Materials. In General Chemistry for Engineers; Gaffney, J.S., Marley, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–116. [Google Scholar] [CrossRef]
- Needham, P. The Source of Chemical Bonding. Stud. Hist. Philos. Sci. Part A 2014, 45, 1–13. [Google Scholar] [CrossRef]
- Nilsson, A.; Pettersson, L.G.M. Chapter 2—Adsorbate Electronic Structure and Bonding on Metal Surfaces. In Chemical Bonding at Surfaces and Interfaces; Nilsson, A., Pettersson, L.G.M., Nørskov, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 57–142. [Google Scholar] [CrossRef]
- Stephanos, J.J.; Addison, A.W. Chapter 3—Chemical Bonding. In Electrons, Atoms and Molecules in Inorganic Chemistry; Stephanos, J.J., Addison, A.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 147–226. [Google Scholar] [CrossRef]
- Weber, M.; Iatsunskyi, I.; Coy, E.; Miele, P.; Cornu, D.; Bechelany, M. Novel and Facile Route for the Synthesis of Tunable Boron Nitride Nanotubes Combining Atomic Layer Deposition and Annealing Processes for Water Purification. Adv. Mater. Interfaces 2018, 5, 1800056. [Google Scholar] [CrossRef]
- Weber, M.; Coy, E.; Iatsunskyi, I.; Yate, L.; Miele, P.; Bechelany, M. Mechanical Properties of Boron Nitride Thin Films Prepared by Atomic Layer Deposition. CrystEngComm 2017, 19, 6089–6094. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing and Applications; Noyes publications: Westwood, NJ, USA, 1996. [Google Scholar]
- Mayrhofer, P.H.; Mitterer, C.; Clemens, H. Self-Organized Nanostructures in Hard Ceramic Coatings. Adv. Eng. Mater. 2005, 7, 1071–1082. [Google Scholar] [CrossRef]
- Holleck, H.; Schier, V. Multilayer PVD Coatings for Wear Protection. Surf. Coat. Technol. 1995, 76–77, 328–336. [Google Scholar] [CrossRef]
- Wai-Kee, L.; Gong-Du, Z.; Thomas, S.W.M. Advanced Structural Inorganic Chemistry; Oxford University Press Inc.: New York, NY, USA, 2008. [Google Scholar]
- Rasaki, S.A.; Zhang, B.; Anbalgam, K.; Thomas, T.; Yang, M. Synthesis and Application of Nano-Structured Metal Nitrides and Carbides: A Review. Prog. Solid State Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Othmer, K. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Dong, S.; Chen, X.; Zhang, X.; Cui, G. Nanostructured Transition Metal Nitrides for Energy Storage and Fuel Cells. Coord. Chem. Rev. 2013, 257, 1946–1956. [Google Scholar] [CrossRef]
- Salamat, A.; Hector, A.L.; Kroll, P.; McMillan, P.F. Nitrogen-Rich Transition Metal Nitrides. Coord. Chem. Rev. 2013, 257, 2063–2072. [Google Scholar] [CrossRef]
- Samsonolf, G.V.; Pryadko, I.F.; Pryadko, L.F. A Configurational Model of Matter, 1st ed.; Tybulewicz, A., Translator; Consultants Bureau: New York, NY, USA, 1973. [Google Scholar]
- Dong, H. Tribological Properties of Titanium-Based Alloys. In Surface Engineering of Light Alloys: Aluminium, Magnesium and Titanium Alloys; Woodhead Publishing Limited: Cambridge, MA, USA, 2010; pp. 58–82. [Google Scholar]
- Voznyi, A.; Kosyak, V.; Opanasyuk, A.; Tirkusova, N.; Grase, L.; Medvids, A.; Mezinskis, G. Structural and Electrical Properties of SnS2 Thin Films. Mater. Chem. Phys. 2016, 173, 52–61. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Pshyk, A.; Dyadyura, K. Adaptive Multicomponent Nanocomposite Coatings in Surface Engineering. Phys.-Uspekhi 2017, 60, 586. [Google Scholar] [CrossRef]
- Kurbatov, D.; Opanasyuk, A.; Khlyap, H. Substrate-Temperature Effect on the Microstructural and Optical Properties of ZnS Thin Films Obtained by Close-Spaced Vacuum Sublimation. Phys. Status Solidi A 2009, 206, 1549–1557. [Google Scholar] [CrossRef]
- Żukowski, P.; Koltunowicz, T.N.; Bondariev, V.; Fedotov, A.K.; Fedotova, J.A. Determining the Percolation Threshold for (FeCoZr)x(CaF2)(100−x) Nanocomposites Produced by Pure Argon Ion-Beam Sputtering. J. Alloy. Compd. 2016, 683, 62–66. [Google Scholar] [CrossRef]
- Hashmi, S. Comprehensive Materials Processing, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Toth, L.E. Transition Metal Carbides and Nitrides, 1st ed.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Goldschmidt, H.J. Interstitial Alloys, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1967. [Google Scholar]
- Pogrebnjak, A.D.; Beresnev, V.M.; Smyrnova, K.V.; Kravchenko, Y.O.; Zukowski, P.V.; Bondarenko, G.G. The Influence of Nitrogen Pressure on the Fabrication of the Two-Phase Superhard Nanocomposite (TiZrNbAlYCr)N Coatings. Mater. Lett. 2018, 211, 316–318. [Google Scholar] [CrossRef]
- Holleck, H. Material Selection for Hard Coatings. J. Vac. Sci. Technol. A 1986, 4, 2661–2669. [Google Scholar] [CrossRef]
- Goltsman, G.; Korneev, A.; Izbenko, V.; Smirnov, K.; Kouminov, P.; Voronov, B.; Kaurova, N.; Verevkin, A.; Zhang, J.; Pearlman, A.; et al. Nano-Structured Superconducting Single-Photon Detectors. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2004, 520, 527–529. [Google Scholar] [CrossRef]
- Kerman, A.J.; Dauler, E.A.; Keicher, W.E.; Yang, J.K.W.; Berggren, K.K.; Gol’tsman, G.; Voronov, B. Kinetic-Inductance-Limited Reset Time of Superconducting Nanowire Photon Counters. Appl. Phys. Lett. 2006, 88, 111116. [Google Scholar] [CrossRef]
- Bitauld, D.; Marsili, F.; Fiore, A.; Gaggero, A.; Mattioli, F.; Leoni, R.; Benkahoul, M.; Lévy, F. NbN Nanowire Superconducting Single Photon Detectors Fabricated on MgO Substrates. J. Mod. Opt. 2009, 56, 395–400. [Google Scholar] [CrossRef]
- Villégier, J.-C.; Delaet, B.; Feautrier, P.; Frey, L.; Delacour, C.; Bouchiat, V. Fabrication of High-Speed Single Photon Detectors in NbN for Quantum Information Processing. J. Phys. Conf. Ser. 2006, 43, 1373. [Google Scholar] [CrossRef]
- Semenov, A. Hot-Electrons in Superconducting Nanostructures for Detection of Terahertz Radiation. Cryogenics 2009, 49, 656–659. [Google Scholar] [CrossRef]
- Gol’tsman, G.N.; Gershenzon, E.M. Phonon-Cooled Hot-Electron Bolometric Mixer: Overview of Recent Results. Appl. Supercond. 1999, 6, 649–655. [Google Scholar] [CrossRef]
- Żukowski, P.; Kołtunowicz, T.; Partyka, J.; Fedotova, Y.A.; Larkin, A.V. Electrical Properties of Nanostructures (CoFeZr)x+(Al2O3)1−x with Use of Alternating Current. Vacuum 2009, 83, S275–S279. [Google Scholar] [CrossRef]
- Stueber, M.; Holleck, H.; Leiste, H.; Seemann, K.; Ulrich, S.; Ziebert, C. Concepts for the Design of Advanced Nanoscale PVD Multilayer Protective Thin Films. J. Alloy. Compd. 2009, 483, 321–333. [Google Scholar] [CrossRef]
- Bull, S.J.; Jones, A.M. Multilayer Coatings for Improved Performance. Surf. Coat. Technol. 1996, 78, 173–184. [Google Scholar] [CrossRef]
- Sakakima, H.; Osano, K.; Ihara, K.; Satomi, M. Multilayered Films of Nitride Alloys as Magnetic Head Materials. J. Magn. Magn. Mater. 1991, 93, 349–355. [Google Scholar] [CrossRef]
- Wadsworth, I.; Smith, I.J.; Donohue, L.A.; Münz, W.-D. Thermal Stability and Oxidation Resistance of TiAlN/CrN Multilayer Coatings. Surf. Coat. Technol. 1997, 94–95, 315–321. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhang, J.J.; Yang, J.; Wang, L.Q.; Li, D.J. Influence of Ar/N2 Flow Ratio on Structure and Properties of Nanoscale ZrN/WN Multilayered Coatings. Surf. Coat. Technol. 2007, 201, 5472–5476. [Google Scholar] [CrossRef]
- Sadki, E.S.; Barber, Z.H.; Lloyd, S.J.; Blamire, M.G.; Campbell, A.M. Effects of Interlayer Coupling on the Irreversibility Lines of NbN/AlN Superconducting Multilayers. Phys. Rev. Lett. 2000, 85, 4168–4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celis, J.-P.; Prakash, B. Surface Modification of Materials by Plasma Immersion Ion Implantation. In Materials Surface Processing by Directed Energy Techniques; Pauleau, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Vieira, T.; Castanho, J.; Louro, C. Hard Coatings Based on Metal Nitrides, Metal Carbides and Nanocomposite Materials: PVD Process and Properties. In Materials Surface Processing by Directed Energy Techniques; Pauleau, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Falco, C.M.; Slaughter, J.M. Characterization of Metallic Multilayers for X-Ray Optics. J. Magn. Magn. Mater. 1993, 126, 3–7. [Google Scholar] [CrossRef]
- Gilewicz, A.; Warcholinski, B. Deposition and Characterisation of Mo2N/CrN Multilayer Coatings Prepared by Cathodic Arc Evaporation. Surf. Coat. Technol. 2015, 279, 126–133. [Google Scholar] [CrossRef]
- Hultman, L. Thermal Stability of Nitride Thin Films. Vacuum 2000, 57, 1–30. [Google Scholar] [CrossRef]
- Molina-Aldareguia, J.M.; Lloyd, S.J. Chapter 3—Multilayered Materials: A Palette for the Materials Artist. In Advances in Nanoengineering: Electronics, Materials and Assembly; Davies, A.G., Thompson, G.M.T., Eds.; World Scientific Publishing Co.: Singapore, 2007; Chapter 3. [Google Scholar]
- Smallman, R.E.; Ngan, A.H.W. Chapter 10—Surfaces, Grain Boundaries and Interfaces. In Modern Physical Metallurgy (Eighth Edition); Smallman, R.E., Ngan, A.H.W., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 415–442. [Google Scholar] [CrossRef]
- Hogmark, S.; Jacobson, S.; Larsson, M. Design and Evaluation of Tribological Coatings. Wear 2000, 246, 20–33. [Google Scholar] [CrossRef]
- Holleck, H.; Schulz, H. Preparation and Behaviour of Wear-Resistant TiC/TiB2, TiN/TiB2 and TiC/TiN Coatings with High Amounts of Phase Boundaries. Surf. Coat. Technol. 1988, 36, 707–714. [Google Scholar] [CrossRef]
- Koehler, J.S. Attempt to Design a Strong Solid. Phys. Rev. B 1970, 2, 547–551. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, D.; Fu, Y.; Du, H. Toughening of Hard Nanostructural Thin Films: A Critical Review. Surf. Coat. Technol. 2005, 198, 2–8. [Google Scholar] [CrossRef]
- Tjong, S.C.; Chen, H. Nanocrystalline Materials and Coatings. Mater. Sci. Eng. R. Rep. 2004, 45, 1–88. [Google Scholar] [CrossRef]
- Inspektor, A.; Salvador, P.A. Architecture of PVD Coatings for Metalcutting Applications: A Review. Surf. Coat. Technol. 2014, 257, 138–153. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Kempiński, M.; Pogrebnjak, A.D.; Beresnev, V.M.; Jurga, S. Structural and Mechanical Characterization of (TiZrNbHfTa)N/WN Multilayered Nitride Coatings. Mater. Lett. 2018, 229, 364–367. [Google Scholar] [CrossRef]
- Chauhan, A.; Vaish, R. Hard Coating Material Selection Using Multi-Criteria Decision Making. Mater. Des. 2013, 44, 240–245. [Google Scholar] [CrossRef]
- Kravchenko, Y.O.; Coy, L.E.; Peplińska, B.; Iatsunskyi, I.; Załęski, K.; Kempiǹski, M.; Beresnev, V.M.; Konarski, P.; Jurga, S.; Pogrebnjak, A.D. Nano-Multilayered Coatings of (TiAlSiY)N/MeN (Me=Mo, Cr and Zr): Influence of Composition of the Alternating Layer on Their Structural and Mechanical Properties. J. Alloy. Compd. 2018, 767, 483–495. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bondar, O.V.; Abadias, G.; Ivashchenko, V.; Sobol, O.V.; Jurga, S.; Coy, E. Structural and Mechanical Properties of NbN and Nb-Si-N Films: Experiment and Molecular Dynamics Simulations. Ceram. Int. 2016, 42, 11743–11756. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Beresnev, V.M.; Nyemchenko, U.S.; Ivashchenko, V.I.; Kravchenko, Y.O.; Shaimardanov, Z.K.; Plotnikov, S.V.; Maksakova, O. The Effects of Cr and Si Additions and Deposition Conditions on the Structure and Properties of the (Zr-Ti-Nb)N Coatings. Ceram. Int. 2017, 43, 771–782. [Google Scholar] [CrossRef]
- Macías, H.A.; Yate, L.; Coy, L.E.; Olaya, J.J.; Aperador, W. Effect of Nitrogen Flow Ratio on Microstructure, Mechanical and Tribological Properties of TiWSiNx Thin Film Deposited by Magnetron Co-Sputtering. Appl. Surf. Sci. 2018, 456, 445–456. [Google Scholar] [CrossRef]
- Zhou, Y.; Asaki, R.; Soe, W.-H.; Yamamoto, R.; Chen, R.; Iwabuchi, A. Hardness Anomaly, Plastic Deformation Work and Fretting Wear Properties of Polycrystalline TiN/CrN Multilayers. Wear 1999, 236, 159–164. [Google Scholar] [CrossRef]
- Yashar, P.C.; Sproul, W.D. Nanometer Scale Multilayered Hard Coatings. Vacuum 1999, 55, 179–190. [Google Scholar] [CrossRef]
- Vieira, M.T.; Ramos, A.S. The Influence of Ductile Interlayers on the Mechanical Performance of Tungsten Nitride Coatings. J. Mater. Process. Technol. 1999, 92–93, 156–161. [Google Scholar] [CrossRef]
- Berger, M.; Wiklund, U.; Eriksson, M.; Engqvist, H.; Jacobson, S. The Multilayer Effect in Abrasion—Optimising the Combination of Hard and Tough Phases. Surf. Coat. Technol. 1999, 116–119, 1138–1144. [Google Scholar] [CrossRef]
- Maksakova, O.V.; Simoẽs, S.; Pogrebnjak, A.D.; Bondar, O.V.; Kravchenko, Y.O.; Koltunowicz, T.N.; Shaimardanov, Z.K. Multilayered ZrN/CrN Coatings with Enhanced Thermal and Mechanical Properties. J. Alloy. Compd. 2019, 776, 679–690. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Singh, J.; Wolfe, D.E. Nanostructured Component Fabrication by Electron Beam-Physical Vapor Deposition. J. Mater. Eng. Perform. 2005, 14, 448–459. [Google Scholar] [CrossRef]
- Schintlmeister, W.; Wallgram, W.; Kanz, J.; Gigl, K. Cutting Tool Materials Coated by Chemical Vapour Deposition. Wear 1984, 100, 153–169. [Google Scholar] [CrossRef]
- Stephenson, D.A.; Agapiou, J.S. Metal Cutting Theory and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Yang, S.; Li, X.; Cooke, K.E.; Teer, D.G. A Study of TiMoN Nano-Multilayer Coatings Deposited by CFUBMSIP Using DC and HIPIMS Power. Appl. Surf. Sci. 2012, 258, 2062–2067. [Google Scholar] [CrossRef]
- Chen, J.; Ji, R.; Khan, R.H.U.; Li, X.; Beake, B.D.; Dong, H. Effects of Mechanical Properties and Layer Structure on the Cyclic Dynamic Loading of TiN-Based Coatings. Surf. Coat. Technol. 2011, 206, 522–529. [Google Scholar] [CrossRef]
- Mendibide, C.; Fontaine, J.; Steyer, P.; Esnouf, C. Dry Sliding Wear Model of Nanometer Scale Multilayered TiN/CrN PVD Hard Coatings. Tribol. Lett. 2004, 17, 779–789. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Grigoriev, S.N. Study of Cracking Mechanisms in Multi-Layered Composite Nano-Structured Coatings. Wear 2017, 378–379, 43–57. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Grigoriev, S.N.; Sitnikov, N.N.; Batako, A.D. Delamination and Longitudinal Cracking in Multi-Layered Composite Nano-Structured Coatings and Their Influence on Cutting Tool Life. Wear 2017, 390–391, 209–219. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, M.X.; Yang, J.; Liu, Q.X.; Li, D.J. Enhancing Mechanical and Tribological Performance of Multilayered CrN/ZrN Coatings. Surf. Coat. Technol. 2007, 201, 5186–5189. [Google Scholar] [CrossRef]
- Paulitsch, J.; Schenkel, M.; Schintlmeister, A.; Hutter, H.; Mayrhofer, P.H. Low Friction CrN/TiN Multilayer Coatings Prepared by a Hybrid High Power Impulse Magnetron Sputtering/DC Magnetron Sputtering Deposition Technique. Thin Solid Films 2010, 518, 5553–5557. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Rajam, K.S. Structure and Properties of Reactive DC Magnetron Sputtered TiN/NbN Hard Superlattices. Surf. Coat. Technol. 2004, 183, 174–183. [Google Scholar] [CrossRef]
- Helmersson, U.; Todorova, S.; Barnett, S.A.; Sundgren, J.-E.; Markert, L.C.; Greene, J.E. Growth of Single-crystal TiN/VN Strained-layer Superlattices with Extremely High Mechanical Hardness. J. Appl. Phys. 1987, 62, 481–484. [Google Scholar] [CrossRef]
- Nordin, M.; Larsson, M.; Hogmark, S. Mechanical and Tribological Properties of Multilayered PVD TiN/CrN, TiN/MoN, TiN/NbN and TiN/TaN Coatings on Cemented Carbide. Surf. Coat. Technol. 1998, 106, 234–241. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Lewis, D.B.; Münz, W.-D. Recent Progress in Large Scale Manufacturing of Multilayer/Superlattice Hard Coatings. Surf. Coat. Technol. 2000, 133–134, 166–175. [Google Scholar] [CrossRef]
- Ou, Y.X.; Lin, J.; Che, H.L.; Moore, J.J.; Sproul, W.D.; Lei, M.K. Mechanical and Tribological Properties of CrN/TiN Superlattice Coatings Deposited by a Combination of Arc-Free Deep Oscillation Magnetron Sputtering with Pulsed Dc Magnetron Sputtering. Thin Solid Films 2015, 594, 147–155. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Ivashchenko, V.; Bondar, O.; Beresnev, V.; Sobol, O.; Załęski, K.; Jurga, S.; Coy, E.; Konarski, P.; Postolnyi, B. Multilayered Vacuum-Arc Nanocomposite TiN/ZrN Coatings before and after Annealing: Structure, Properties, First-Principles Calculations. Mater. Charact. 2017, 134, 55–63. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M.; Bondar, O.V.; Postolnyi, B.O.; Zaleski, K.; Coy, E.; Jurga, S.; Lisovenko, M.O.; Konarski, P.; Rebouta, L.; et al. Superhard CrN/MoN Coatings with Multilayer Architecture. Mater. Des. 2018, 153, 47–59. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Eyidi, D.; Abadias, G.; Bondar, O.V.; Beresnev, V.M.; Sobol, O.V. Structure and Properties of Arc Evaporated Nanoscale TiN/MoN Multilayered Systems. Int. J. Refract. Met. Hard Mater. 2015, 48, 222–228. [Google Scholar] [CrossRef]
- Chu, X.; Wong, M.S.; Sproul, W.D.; Rohde, S.L.; Barnett, S.A. Deposition and Properties of Polycrystalline TiN/NbN Superlattice Coatings. J. Vac. Sci. Technol. Vac. Surf. Films 1992, 10, 1604–1609. [Google Scholar] [CrossRef]
- Sproul, W.D. High-Rate Reactive DC Magnetron Sputtering of Oxide and Nitride Superlattice Coatings. Vacuum 1998, 51, 641–646. [Google Scholar] [CrossRef]
- Chu, X.; Barnett, S.A.; Wong, M.S.; Sproul, W.D. Reactive Unbalanced Magnetron Sputter Deposition of Polycrystalline TiN/NbN Superlattice Coatings. Surf. Coat. Technol. 1993, 57, 13–18. [Google Scholar] [CrossRef]
- Luo, Q.; Zhou, Z.; Rainforth, W.M.; Hovsepian, P.E. TEM-EELS Study of Low-Friction Superlattice TiAlN/VN Coating: The Wear Mechanisms. Tribol. Lett. 2006, 24, 171–178. [Google Scholar] [CrossRef]
- Zhou, Z.; Calvert, C.C.; Rainforth, W.M.; Luo, Q.; Chen, L.; Hovsepian, P.E. Investigating Worn Surfaces of Nanoscale TiAlN/VN Multilayer Coating Using FIB and TEM. J. Phys. Conf. Ser. 2006, 26, 95. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Luo, Q.; Robinson, G.; Pittman, M.; Howarth, M.; Doerwald, D.; Tietema, R.; Sim, W.M.; Deeming, A.; Zeus, T. TiAlN/VN Superlattice Structured PVD Coatings: A New Alternative in Machining of Aluminium Alloys for Aerospace and Automotive Components. Surf. Coat. Technol. 2006, 201, 265–272. [Google Scholar] [CrossRef]
- Tytko, D.; Choi, P.-P.; Raabe, D. Oxidation Behavior of AlN/CrN Multilayered Hard Coatings. Nano Converg. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-Y.; Yang, S.-J.; Wang, D.-Y. Characterization of TiCr(C,N)/Amorphous Carbon Coatings Synthesized by a Cathodic Arc Deposition Process. Thin Solid Films 2007, 515, 4722–4726. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Wong, K.C.; Mitchell, K.A.R.; Namavar, F.; Tobin, E.; Mihut, D.M.; Rohde, S.L. Characterization of Titanium Chromium Nitride Nanocomposite Protective Coatings. Appl. Surf. Sci. 2004, 229, 387–394. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.J.; Mishra, B.; Pinkas, M.; Sproul, W.D. Nano-Structured CrN/AlN Multilayer Coatings Synthesized by Pulsed Closed Field Unbalanced Magnetron Sputtering. Surf. Coat. Technol. 2009, 204, 936–940. [Google Scholar] [CrossRef]
- Rizzo, A.; Signore, M.A.; Valerini, D. PVD Protective Multilayer Coatings for Tribological Applications. Energ. Ambient Innov. 2012, 3, 102–108. [Google Scholar]
- Chu, X.; Barnett, S.A. Model of Superlattice Yield Stress and Hardness Enhancements. J. Appl. Phys. 1995, 77, 4403–4411. [Google Scholar] [CrossRef]
- Anderson, P.M.; Li, C. Hall-Petch Relations for Multilayered Materials. Nanostruct. Mater. 1995, 5, 349–362. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Amaya, C.; Yate, L.; Gómez, M.E.; Zambrano, G.; Alvarado-Rivera, J.; Muñoz-Saldaña, J.; Prieto, P. TiCN/TiNbCN Multilayer Coatings with Enhanced Mechanical Properties. Appl. Surf. Sci. 2010, 256, 5898–5904. [Google Scholar] [CrossRef]
- Kato, M.; Mori, T.; Schwartz, L.H. Hardening by Spinodal Modulated Structure. Acta Metall. 1980, 28, 285–290. [Google Scholar] [CrossRef]
- Cammarata, R.C. The Supermodulus Effect in Compositionally Modulated Thin Films. Scr. Metall. 1986, 20, 479–486. [Google Scholar] [CrossRef]
- Bondar, O.V.; Stolbovoy, V.A.; Kylyshkanov, M.K.; Plotnikov, S.V.; Erdybaeva, N.K.; Piotrowska, K.; Czarnacka, K.; Karwat, C. Dependence of Mechanical and Tribotechnical Properties of Multilayered TiN/ZrN Coatings on Deposition. Przeglad Elektrotechniczny 2015, 91, 233–236. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Rapaud, O.; Allain, N.; Mercs, D.; Baraket, M.; Dong, C.; Coddet, C. Microstructures and Tribological Properties of CrN/ZrN Nanoscale Multilayer Coatings. Appl. Surf. Sci. 2009, 255, 4020–4026. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bondar, O.V.; Abadias, G.; Eyidi, D.; Beresnev, V.M.; Sobol, O.V.; Postolnyi, B.O.; Zukowski, P. Investigation of Nanoscale TiN/MoN Multilayered Systems, Fabricated Using Arc Evaporation. Acta Phys. Pol. A 2015, 128, 836–840. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.G.J.; Karvankova, P.; Prochazka, J. Different Approaches to Superhard Coatings and Nanocomposites. Thin Solid Films 2005, 476, 1–29. [Google Scholar] [CrossRef]
- Beresnev, V.M.; Klimenko, S.A.; Sobol’, O.V.; Grankin, S.S.; Stolbovoi, V.A.; Turbin, P.V.; Novikov, V.Y.; Meilekhov, A.A.; Litovchenko, S.V.; Malikova, L.V. Effect of the Deposition Parameters on the Phase–Structure State, Hardness and Tribological Characteristics of Mo2N/CrN Vacuum–Arc Multilayer Coatings. J. Superhard Mater. 2016, 38, 114–122. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bondar, O.V.; Erdybaeva, N.K.; Plotnikov, S.V.; Turbin, P.V.; Grankin, S.S.; Stolbovoy, V.A.; Sobol, O.V.; Kolesnikov, D.A.; Kozak, C. Influence of Thermal Annealing and Deposition Conditions on Structure and Physical-Mechanical Properties of Multilayered Nanosized TiN/ZrN Coatings. Przeglad Elektrotechniczny 2015, 91, 228–232. [Google Scholar]
- Musil, J. Hard Nanocomposite Coatings: Thermal Stability, Oxidation Resistance and Toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M.; Bondar, O.V.; Abadias, G.; Chartier, P.; Postol’nyi, B.A.; Andreev, A.A.; Sobol’, O.V. The Effect of Nanolayer Thickness on the Structure and Properties of Multilayer TiN/MoN Coatings. Tech. Phys. Lett. 2014, 40, 215–218. [Google Scholar] [CrossRef]
- Postolnyi, B.O.; Beresnev, V.M.; Abadias, G.; Bondar, O.V.; Rebouta, L.; Araujo, J.P.; Pogrebnjak, A.D. Multilayer Design of CrN/MoN Protective Coatings for Enhanced Hardness and Toughness. J. Alloy. Compd. 2017, 725, 1188–1198. [Google Scholar] [CrossRef]
- Bondar, O.V.; Postol’nyi, B.A.; Beresnev, V.M.; Abadias, G.; Chartier, P.; Sobol, O.V.; Kolesnikov, D.A.; Komarov, F.F.; Lisovenko, M.O.; Andreev, A.A. Composition, Structure and Tribotechnical Properties of TiN, MoN Single-Layer and TiN/MoN Multilayer Coatings. J. Superhard Mater. 2015, 37, 27–38. [Google Scholar] [CrossRef]
- Postolnyi, B.; Bondar, O.; Opielak, M.; Rogalski, P.; Araújo, J.P. Structural Analysis of Multilayer Metal Nitride Films CrN/MoN Using Electron Backscatter Diffraction (EBSD). In SPIE—Advanced Topics in Optoelectronics, Microelectronics and Nanotechnologies VIII; SPIE: Bellingham, WA, USA, 2016; Volume 10010, p. 100100E. [Google Scholar] [CrossRef]
- Pogrebnyak, A.D.; Bondar, O.V.; Zhollybekov, B.; Konstantinov, S.; Konarski, P.; Beresnev, V.M.; Kupchishin, A.I. Influence of the Bilayer Thickness of Nanostructured Multilayer MoN/CrN Coating on Its Microstructure, Hardness and Elemental Composition. Phys. Solid State 2017, 59, 1798–1802. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.-Y. Review: Mechanical Behavior of Metal/Ceramic Interfaces in Nanolayered Composites—Experiments and Modeling. J. Mater. Sci. 2018, 53, 5562–5583. [Google Scholar] [CrossRef]
- Liu, Y.; Bufford, D.; Wang, H.; Sun, C.; Zhang, X. Mechanical Properties of Highly Textured Cu/Ni Multilayers. Acta Mater. 2011, 59, 1924–1933. [Google Scholar] [CrossRef]
- Khomenko, A.V.; Prodanov, N.V. Molecular Dynamics of Cleavage and Flake Formation during the Interaction of a Graphite Surface with a Rigid Nanoasperity. Carbon 2010, 48, 1234–1243. [Google Scholar] [CrossRef]
- Rogström, L.; Johnson, L.J.S.; Johansson, M.P.; Ahlgren, M.; Hultman, L.; Odén, M. Thermal Stability and Mechanical Properties of Arc Evaporated ZrN/ZrAlN Multilayers. Thin Solid Films 2010, 519, 694–699. [Google Scholar] [CrossRef]
- Kato, K.; Adachi, K. Wear Mechanisms. In Modern Tribology Handbook, Two Volume Set; Bhushan, B., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 273–299. [Google Scholar]
- Ge, F.; Zhu, P.; Meng, F.; Huang, F. Enhancing the Wear Resistance of Magnetron Sputtered VN Coating by Si Addition. Wear 2016, 354–355, 32–40. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Abadias, G.; Bondar, O.V.; Postolnyi, P.O.; Lisovenko, M.O.; Kyrychenko, O.V.; Andreev, A.A.; Beresnev, V.M.; Kolesnikov, D.A.; Opielak, M. Structure and Properties of Multilayer Nanostructured Coatings TiN/MoN Depending on Deposition Conditions. Acta Phys. Pol. A 2014, 125, 1280–2183. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.J.; Kinder, T.J.; Preston, E.W. The Deposition of NbN and NbC Thin Films by Filtered Vacuum Cathodic Arc Deposition. Surf. Coat. Technol. 2003, 163–164, 347–352. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M. Nanocoatings Nanosystems Nanotechnologies, 1st ed.; Bentham Science: Emirate of Sharjah, UAE, 2012. [Google Scholar]
- Beake, B.D.; Fox-Rabinovich, G.S. High-Temperature Coatings. In Multifunctional Materials for Tribological Applications; Wood, R.J.K., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 161–203. [Google Scholar]
- Guo, J.; Wang, H.; Meng, F.; Liu, X.; Huang, F. Tuning the H/E* Ratio and E* of AlN Coatings by Copper Addition. Surf. Coat. Technol. 2013, 228, 68–75. [Google Scholar] [CrossRef]
- Khomenko, A.V.; Yushchenko, O.V. Solid-Liquid Transition of Ultrathin Lubricant Film. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2003, 68, 036110. [Google Scholar] [CrossRef] [PubMed]
- Pogrebnjak, A.D.; Kravchenko, Y.O.; Bondar, O.V.; Zhollybekov, B.; Kupchishin, A.I. Structural Features and Tribological Properties of Multilayer Coatings Based on Refractory Metals. Prot. Met. Phys. Chem. Surf. 2018, 54, 240–258. [Google Scholar] [CrossRef]
- Ou, Y.X.; Lin, J.; Che, H.L.; Sproul, W.D.; Moore, J.J.; Lei, M.K. Mechanical and Tribological Properties of CrN/TiN Multilayer Coatings Deposited by Pulsed Dc Magnetron Sputtering. Surf. Coat. Technol. 2015, 276, 152–159. [Google Scholar] [CrossRef]
- Ahmad, Z. Chapter 7—Coatings. In Principles of Corrosion Engineering and Corrosion Control; Ahmad, Z., Ed.; Butterworth-Heinemann: Oxford, UK, 2006; pp. 382–437. [Google Scholar] [CrossRef]
- De Sánchez, N.A.; Suárez, H.E.J.; Vivas, Z. Fracture Resistant and Wear Corrosion Performance of CrN/ZrN Bilayers Deposited onto AISI 420 Stainless Steel. Adv. Mater. Res. 2008, 38, 63–75. [Google Scholar] [CrossRef]
- Khaled, K.F.; Al-Mhyawi, S.R. Electrochemical and Density Function Theory Investigations of L- Arginine as Corrosion Inhibitor for Steel in 3.5% NaCl. Int. J. Electrochem. Sci. 2014, 8, 4055–4072. [Google Scholar]
- Maksakova, O.V.; Pogrebnjak, A.D.; Beresnev, V.M. Features of Investigations of Multilayer Nitride Coatings Based on Cr and Zr. Usp Fiz Met 2018, 19, 25–48. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Corrosion, Passivation and Wear Behaviors of Electrodeposited Ni–Al2O3–SiC Nano-Composite Coatings. Surf. Coat. Technol. 2016, 304, 502–511. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Dong, D.; Yang, Q.; Hu, M. Tribological Performance of SiC Coating for Carbon/Carbon Composites at Elevated Temperatures. Ceram. Int. 2018, 44, 11233–11238. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Shi, Y.; Zhou, W. LPCVD-Based SiO2/SiC Multi-Layers Coating on Graphite for Improved Anti-Oxidation at Wide-Ranged Temperatures. Compos. Part B Eng. 2018, 146, 155–164. [Google Scholar] [CrossRef]
- Kot, M.; Major, L.; Lackner, J.; Rakowski, W. Carbon Based Coatings with Improved Fracture and Wear Resistance. In Innovations in Biomedical Engineering; Gzik, M., Tkacz, E., Paszenda, Z., Piętka, E., Eds.; Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2017; pp. 40–48. [Google Scholar]
- Pogrebnjak, A.D.; Ivashchenko, V.I.; Skrynskyy, P.L.; Bondar, O.V.; Konarski, P.; Załęski, K.; Jurga, S.; Coy, E. Experimental and Theoretical Studies of the Physicochemical and Mechanical Properties of Multi-Layered TiN/SiC Films: Temperature Effects on the Nanocomposite Structure. Compos. Part B Eng. 2018, 142, 85–94. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Daviau, K.; Lee, K.K.M. Zinc-Blende to Rocksalt Transition in SiC in a Laser-Heated Diamond-Anvil Cell. Phys. Rev. B 2017, 95, 134108. [Google Scholar] [CrossRef]
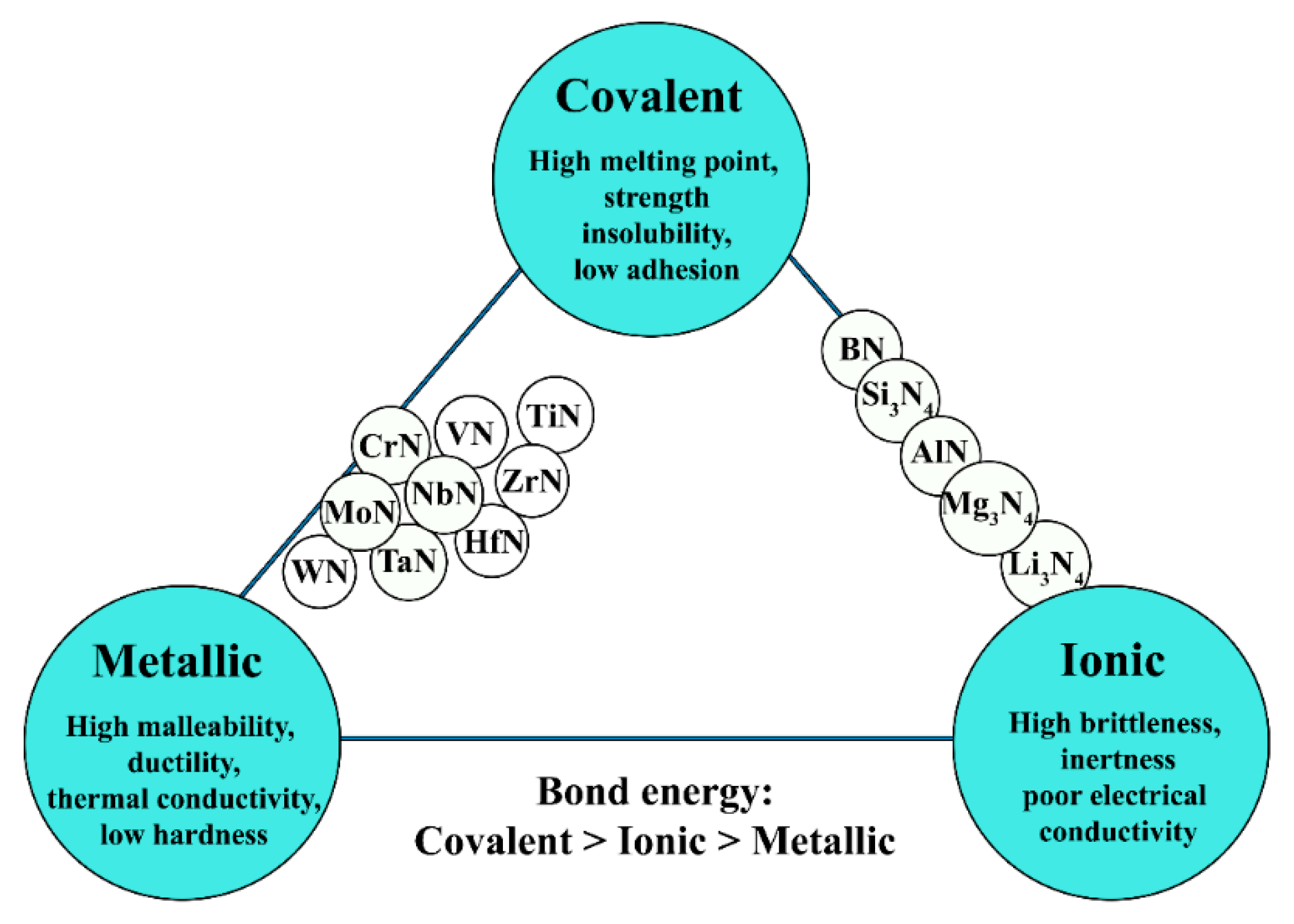
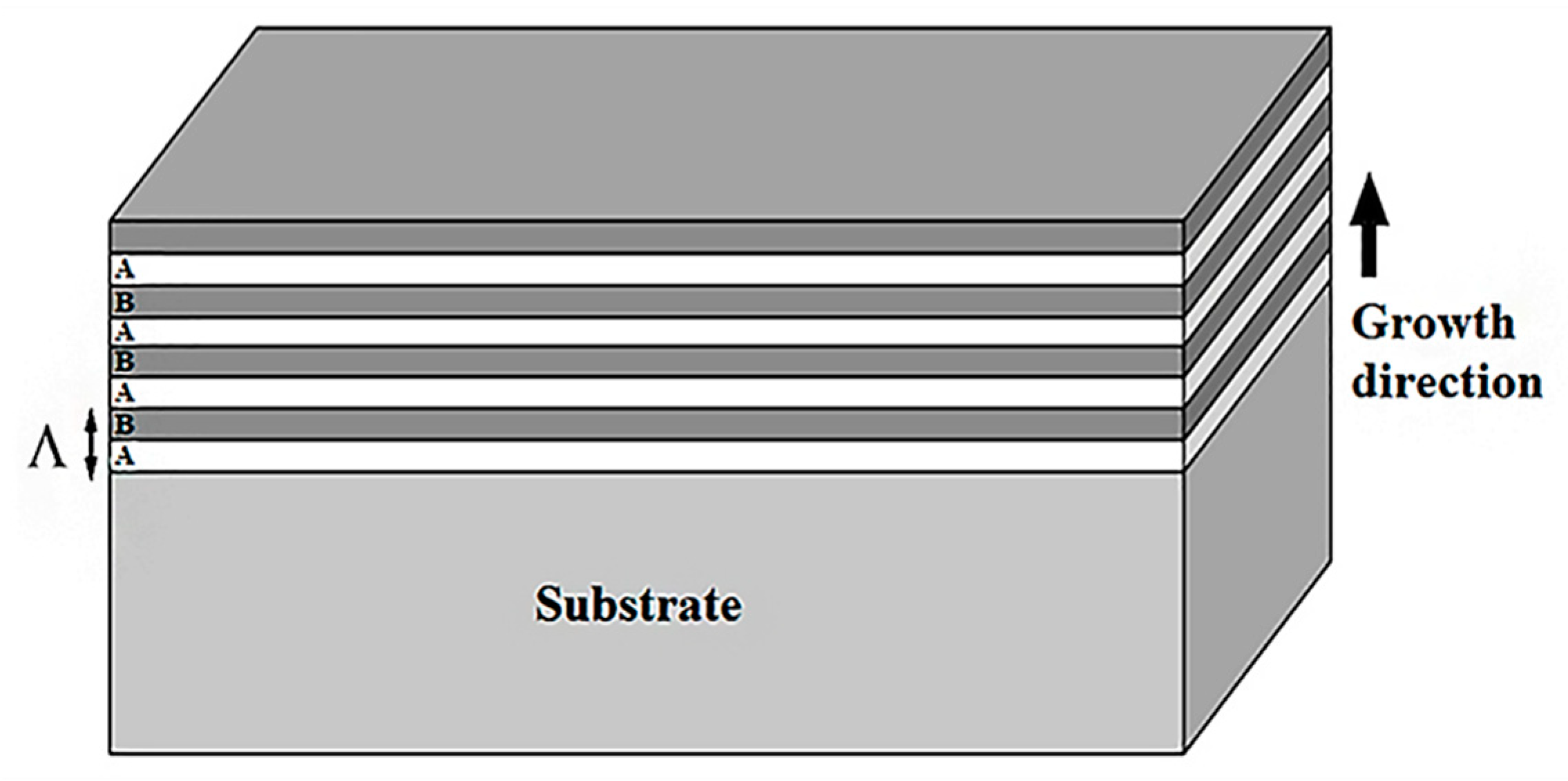















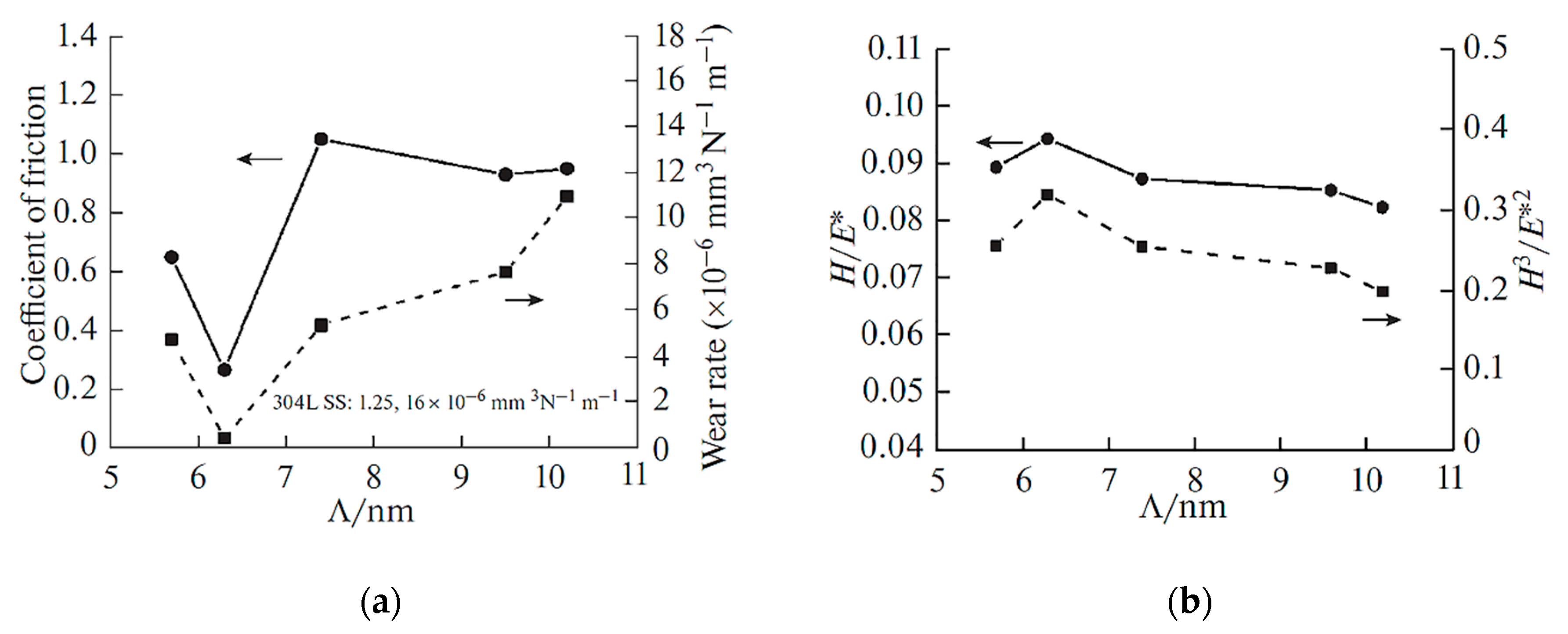




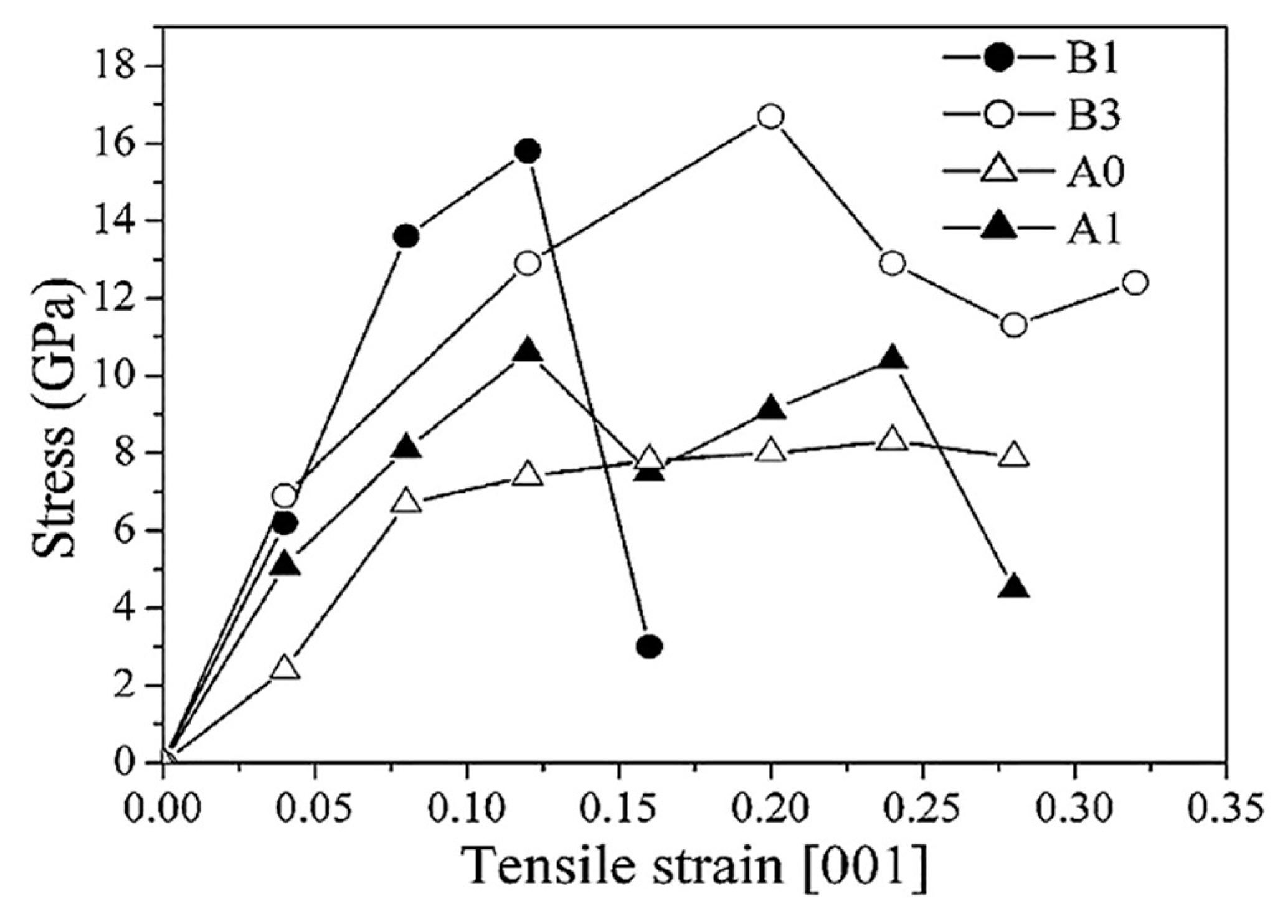
| Metal/Nitride | TM (°C) |
|---|---|
| Ti/TiN | 1668/3290 |
| Zr/ZrN | 1855/2960 |
| Hf/HfN | 2233/3305 |
| V/VN | 1910/2050 |
| Ta/TaN | 3015/3090 |
| Cr/CrN | 1907/1770 |
| Mo/MoN | 2010/1750 |
| Coating | Deposition Technique | Substrate Bias Voltage (V) | Total Thicknes (μm) | Bilayer Thickness (nm) | Hardness (GPa) | Young’s Modulus (GPa) | Ref. |
|---|---|---|---|---|---|---|---|
| ZrN/WN | Magnetron sputtering | −40 | 0.8 | 30.4 | 34 | 423.8 | [57] |
| CrN/ZrN | Unbalanced reactive magnetron sputtering | −200 | 1.0 ÷ 1.2 | 1.5 | 32 | 307.8 | [92] |
| CrN/TiN | High-power impulse magnetron sputtering | −1000 V + constant sputtering potential of −180 V applied to the Cr facing Ti target | 2.0 | 6.4 | 26 | 375 | [93] |
| TiN/NbN | DC planar magnetron sputtering | −200 | 2.0 | 4.8 | 39.2 | – | [94] |
| TiN/VN | Magnetron sputtering | −420 | 2.5 | 4.8 | 44 ± 10 | – | [95] |
| TiN/MoN | Ion plating of TiN and magnetron sputtering of MoN | −110 | 3.5 ± 0.1 | 9.0 | 29.0 ± 2.5 | – | [96] |
| TiN/TaN | Ion plating of TiN and magnetron sputtering of TaN | −110 | 3.5 ± 0.1 | 11 | 34.9 ± 2.4 | – | [96] |
| CrN/NbN (N/Me = 1) | Combined arc/unbalanced magnetron sputtering | −75 | 3.0 ÷ 5.0 | 3.54 | 42 | – | [97] |
| CrN/TiN | Arc-free deep oscillation magnetron sputtering + pulsed DC magnetron sputtering | −500 | 2.0 ÷ 3.0 | 6.3 | 36 | 360 ± 4 | [98] |
| TiN/ZrN | Vacuum-arc evaporation | −200 | 19.0 | 39 | 42 | 347.39 | [99] |
| CrN/MoN | Vacuum-arc evaporation | −20 | 15.6 | 44 | 42.3 | – | [100] |
| TiN/MoN | Vacuum-arc evaporation | −40 | 6.8 ÷ 8.2 | 50 | 30 | 420 | [101] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogrebnjak, A.; Smyrnova, K.; Bondar, O. Nanocomposite Multilayer Binary Nitride Coatings Based on Transition and Refractory Metals: Structure and Properties. Coatings 2019, 9, 155. https://doi.org/10.3390/coatings9030155
Pogrebnjak A, Smyrnova K, Bondar O. Nanocomposite Multilayer Binary Nitride Coatings Based on Transition and Refractory Metals: Structure and Properties. Coatings. 2019; 9(3):155. https://doi.org/10.3390/coatings9030155
Chicago/Turabian StylePogrebnjak, Alexander, Kateryna Smyrnova, and Oleksandr Bondar. 2019. "Nanocomposite Multilayer Binary Nitride Coatings Based on Transition and Refractory Metals: Structure and Properties" Coatings 9, no. 3: 155. https://doi.org/10.3390/coatings9030155
APA StylePogrebnjak, A., Smyrnova, K., & Bondar, O. (2019). Nanocomposite Multilayer Binary Nitride Coatings Based on Transition and Refractory Metals: Structure and Properties. Coatings, 9(3), 155. https://doi.org/10.3390/coatings9030155






