Correlation Between Stoichiometry of NbxNy Coatings Produced by DC Magnetron Sputtering with Electrical Conductivity and the Hall Coefficient
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
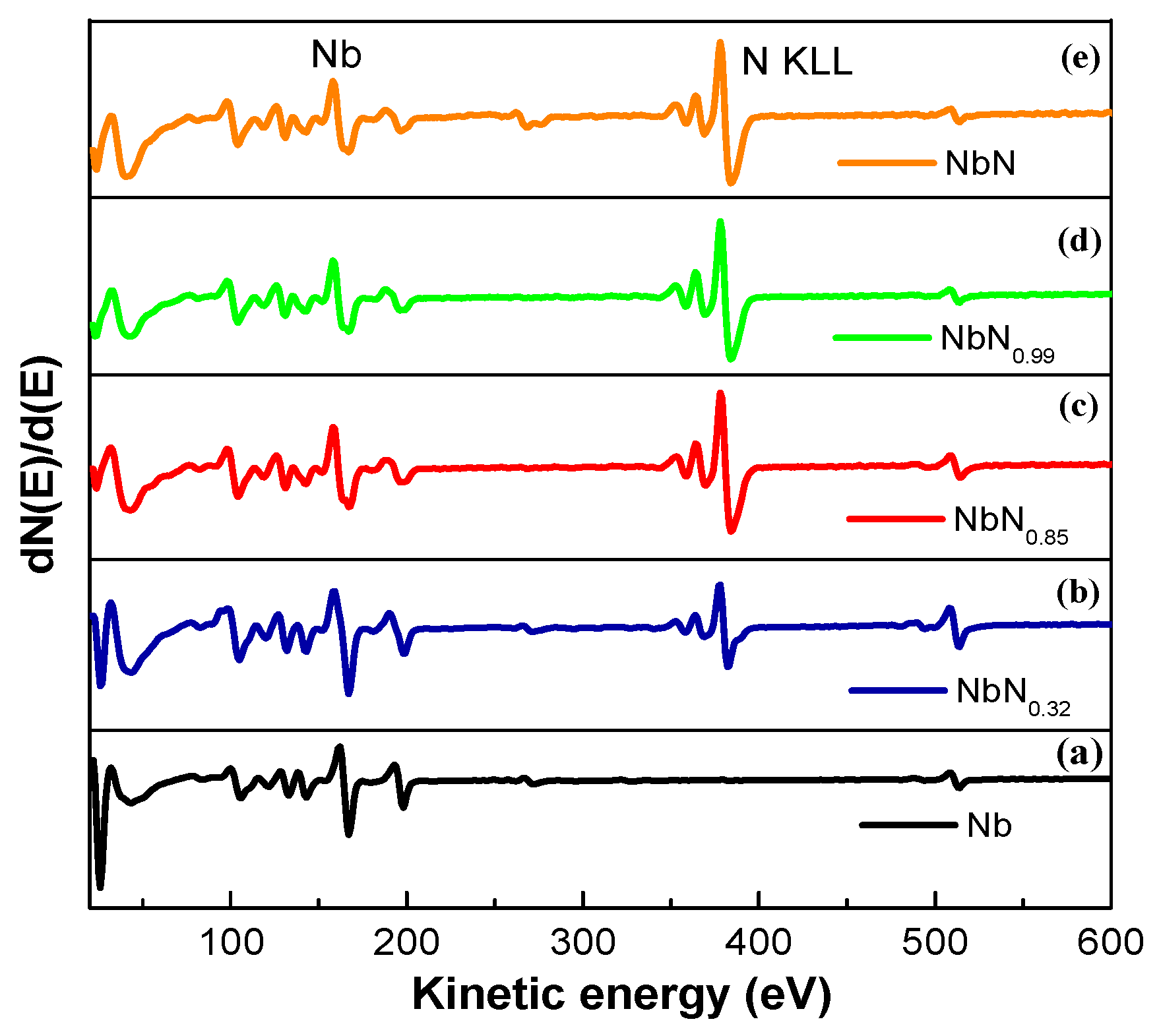
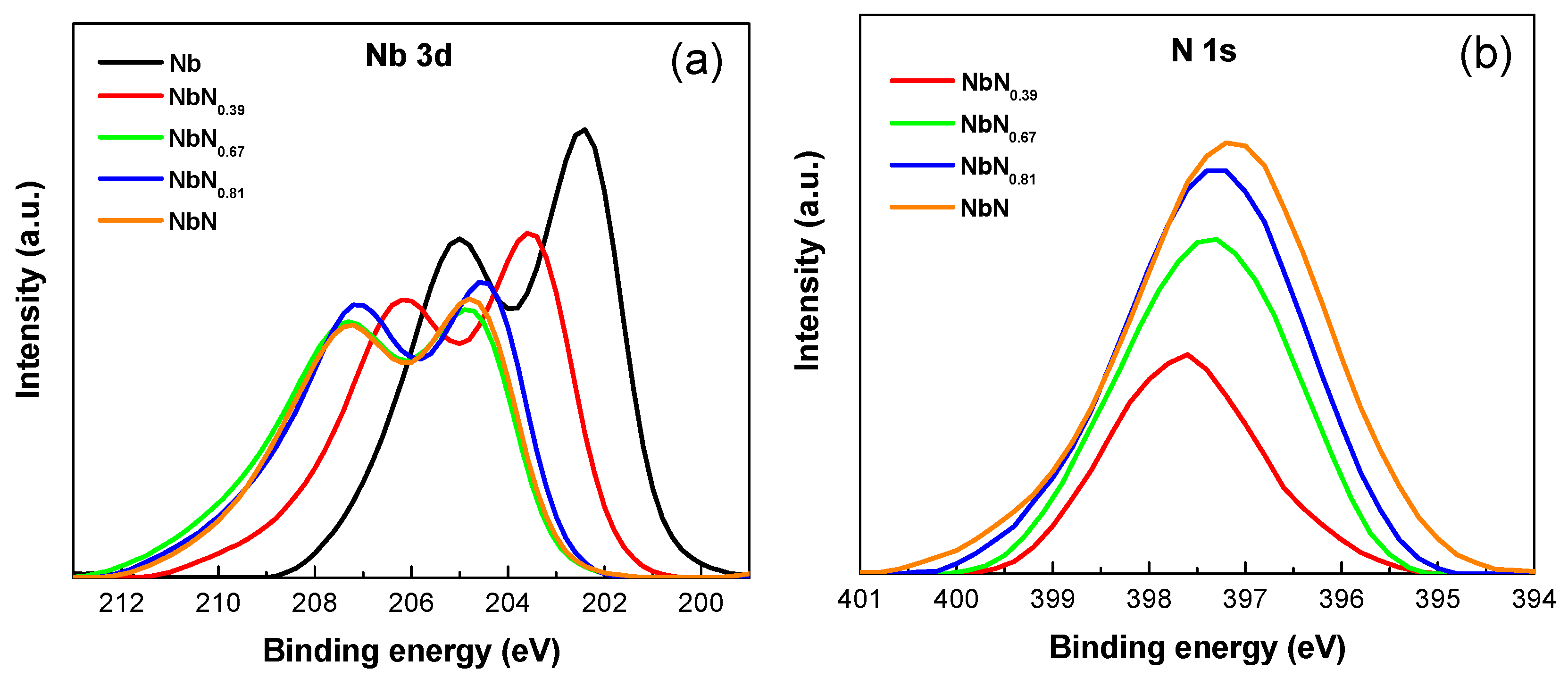
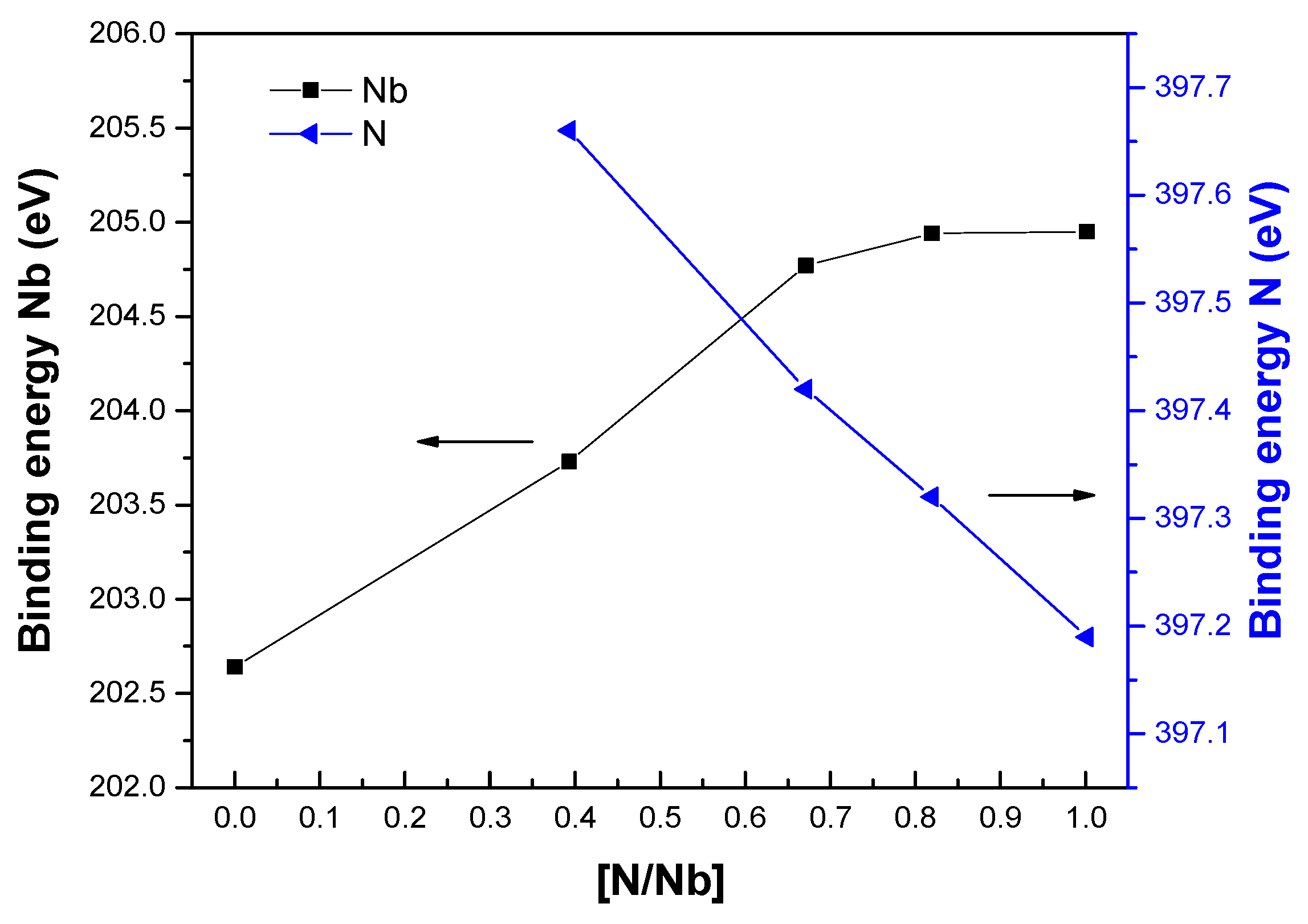
3.1. AES and XPS Characterization
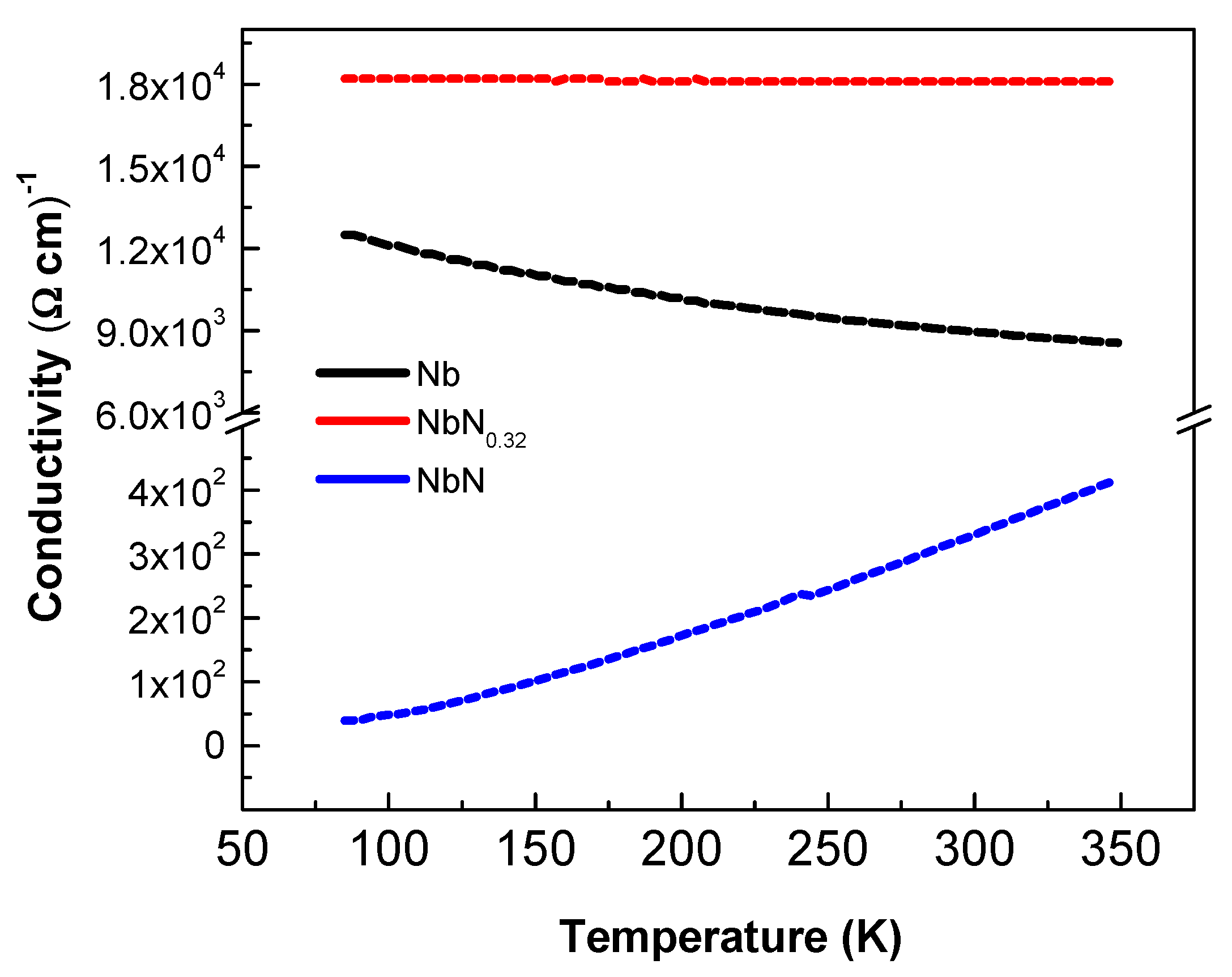
3.2. Electrical Conductivity of Coatings
3.3. Hall Effect Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugimoto, N.; Motohiro, T. Anisotropic I–V characteristics of spontaneously emerged periodic stripes of superconducting NbN thin films on Si trench sidewall by RF magnetron sputtering. Vacuum 2013, 93, 13–21. [Google Scholar] [CrossRef]
- Bhattacharya, C. Effect of shock on transition metal carbides and nitrides {MC/N (M = Zr, Nb, Ta, Ti)}. Comput. Mater. Sci. 2017, 127, 85–95. [Google Scholar] [CrossRef]
- Han, K.; Lin, G.; Dong, C.; Tai, K.; Jiang, X. Experimental study on atomic-scale strengthening mechanism of the IVB transition-metal nitrides. J. Alloy. Compd. 2017, 696, 572–579. [Google Scholar] [CrossRef]
- Jouve, G.; Séverac, C.; Cantacuzène, S. XPS study of NbN and (NbTi)N superconducting coatings. Thin Solid Films 1996, 287, 146–153. [Google Scholar] [CrossRef]
- Mercier, F.; Coindeau, S.; Lay, S.; Crisci, A.; Benz, M.; Encinas, T.; Boichot, R.; Mantoux, A.; Jimenez, C.; Weiss, F.; et al. Niobium nitride thin films deposited by high temperature chemical vapor deposition. Surf. Coat. Technol. 2014, 260, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Klug, J.A.; Becker, N.G.; Groll, N.R.; Cao, C.; Weimer, M.S.; Pellin, M.J.; Zasadzinski, J.F.; Proslier, T. Heteroepitaxy of group IV-VI nitrides by atomic layer deposition. Appl. Phys. Lett. 2013, 103, 12–16. [Google Scholar] [CrossRef]
- Olaya, J.J.; Rodil, S.E.; Muhl, S. Comparative study of niobium nitride coatings deposited by unbalanced and balanced magnetron sputtering. Thin Solid Films 2008, 516, 8319–8326. [Google Scholar] [CrossRef]
- Olaya, J.J.; Huerta, L.; Rodil, S.E.; Escamilla, R. Superconducting niobium nitride films deposited by unbalanced magnetron sputtering. Thin Solid Films 2008, 516, 8768–8773. [Google Scholar] [CrossRef]
- Reinhard, C.; Ehiasarian, A.P.; Hovsepian, P.E. CrN/NbN superlattice structured coatings with enhanced corrosion resistance achieved by high power impulse magnetron sputtering interface pre-treatment. Thin Solid Films 2007, 515, 3685–3692. [Google Scholar] [CrossRef]
- Araujo, J.A.; Giorjao, R.A.R.; Bettini, J.; Souza, R.M.; Tschiptschin, A.P. Modeling intrinsic residual stresses built-up during growth of nanostructured multilayer NbN/CrN coatings. Surf. Coat. Technol. 2016, 308, 264–272. [Google Scholar] [CrossRef]
- Koscielska, B. Electrical conductivity of NbN-SiO2 films obtained by ammonolysis of Nb2O5-SiO2 sol-gel derived coatings. J. Non. Cryst. Solids 2008, 354, 1549–1552. [Google Scholar] [CrossRef]
- Park, W.W.; Kim, E.K.; Jeon, J.H.; Choi, J.Y.; Moon, S.W.; Lim, S.H.; Han, S.H. Wear of UHMWPE against nitrogen-ion-implanted and NbN-coated Co-Cr-Mo alloy formed by plasma immersion ion implantation and deposition for artificial joints. Appl. Surf. Sci. 2012, 258, 8228–8233. [Google Scholar] [CrossRef]
- Zhitomirsky, V.N. Structure and properties of cathodic vacuum arc deposited NbN and NbN-based multi-component and multi-layer coatings. Surf. Coat. Technol. 2007, 201, 6122–6130. [Google Scholar] [CrossRef]
- Huang, W. Thermodynamic assessment of the Nb–N system. Metall. Mater. Trans. A 1996, 27, 3591–3600. [Google Scholar] [CrossRef]
- Chu, X. Deposition and properties of polycrystalline TiN/NbN superlattice coatings. J. Vac. Sci. Technol. A Vacuum Surf. Film 1992, 10, 1604. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.J.; Kinder, T.J.; Preston, E.W. The deposition of NbN and NbC thin films by filtered vacuum cathodic arc deposition. Surf. Coat. Technol. 2003, 163–164, 347–352. [Google Scholar] [CrossRef]
- Chu, X.; Barnett, S.A.; Wong, M.S.; Sproul, W.D. Reactive unbalanced magnetron sputter deposition of polycrystalline TiN/NbN superlattice coatings. Surf. Coat. Technol. 1993, 57, 13–18. [Google Scholar] [CrossRef]
- Singh, K.; Bidaye, A.C.; Suri, A.K. Magnetron sputtered NbN films with electroplated Cr interlayer. Vacuum 2011, 86, 267–274. [Google Scholar] [CrossRef]
- Du, X.K.; Wang, T.M.; Wang, C.; Chen, B.L.; Zhou, L. Microstructure and optical characterization of magnetron sputtered NbN thin films. Chin. J. Aeronaut. 2007, 20, 140–144. [Google Scholar] [CrossRef]
- Singh, K.; Krishnamurthy, N.; Suri, A.K. Adhesion and wear studies of magnetron sputtered NbN films. Tribol. Int. 2012, 50, 16–25. [Google Scholar] [CrossRef]
- Menon, C.S.; Pankajakshan, V.S. Electrical conductivity and transition temperature of NbN thin films. Bull. Mater. Sci. 1987, 9, 187–191. [Google Scholar] [CrossRef]
- Nigro, A.; Nobile, G.; Rubino, M.G.; Vaglio, R. Electrical resistivity of polycrystalline niobium nitride films. Phys. Rev. B 1988, 37, 3970–3972. [Google Scholar] [CrossRef]
- Polakovic, T.; Lendinez, S.; Pearson, J.E.; Hoffmann, A.; Yefremenko, V.; Chang, C.L.; Armstrong, W.; Hafidi, K.; Karapetrov, G.; Novosad, V. Room temperature deposition of superconducting niobium nitride films by ion beam assisted sputtering. Cit. APL Mater. 2018, 6, 076107. [Google Scholar] [CrossRef]
- Cabanel, R.; Chaussy, J.; Mazuer, J.; Villegier, J.; Villegier, J.C. From localization to superconductivity in granular niobium nitride thin films. J. Phys. 1988, 49, 795–802. [Google Scholar] [CrossRef]
- Bourgeois, O.; André, E.; Macovei, C.; Chaussy, J. Liquid nitrogen to room-temperature thermometry using niobium nitride thin films. Rev. Sci. Instrum. 2006, 77, 126108. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectros. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Benia, H.; Guemmaz, M.; Schmerber, G.; Mosser, A.; Parlebas, J.-C. Investigations on non-stoichiometric zirconium nitrides. Appl. Surf. Sci. 2002, 200, 231–238. [Google Scholar] [CrossRef]
- Ponon, N.K.; Appleby, D.J.R.; Arac, E.; King, P.J.; Ganti, S.; Kwa, K.S.K.; O’Neill, A. Effect of deposition conditions and post deposition anneal on reactively sputtered titanium nitride thin films. Thin Solid Films 2015, 578, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, R.Y. Amorphous molybdenum nitride thin films prepared by reactive sputter deposition. Mater. Sci. Eng. B 2004, 112, 42–49. [Google Scholar] [CrossRef]
- Medeiros, H.S.; Pessoa, R.S.; Sagás, J.C.; Fraga, M.A.; Santos, L.V.; Maciel, H.S.; Massi, M.; da Silva Sobrinho, A.S.; Maia da Costa, M.E.H.M. Effect of nitrogen content in amorphous SiCxNyOz thin films deposited by low temperature reactive magnetron co-sputtering technique. Surf. Coat. Technol. 2011, 206, 1787–1795. [Google Scholar] [CrossRef]
- Yau, B.-S.; Huang, J.-L.; Lii, D.-F. Effects of nitrogen flow rates on reactively sputtered nanocrystal-(Ti,Al) xN1−x/amorphous-SiyN1−y nanolaminate films. J. Mater. Res. 2004, 19, 2322–2329. [Google Scholar] [CrossRef]
- Willmott, P. An Introduction to Synchrotron Radiation: Techniques and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Prieto, P.; Kirby, R.E. X-ray photoelectron spectroscopy study of the difference between reactively evaporated and direct sputter-deposited TiN films and their oxidation properties. J. Vac. Sci. Technol. A Vac. Surf. Film 1995, 13, 2819–2826. [Google Scholar] [CrossRef]
- Cannelli, G.; Cannelli, G.B. Thermal properties of Nb from acoustic and electrical resistivity measurements in the temperature range 60–340 K. J. Appl. Phys. 1976, 47, 17–21. [Google Scholar] [CrossRef]
- Chand, M.; Mishra, A.; Xiong, Y.M.; Kamlapure, A.; Chockalingam, S.P.; Jesudasan, J.; Bagwe, V.; Mondal, M.; Adams, P.W.; Tripathi, V.; et al. Temperature dependence of resistivity and Hall coefficient in strongly disordered NbN thin films. Phys. Rev. B 2009, 80, 134514. [Google Scholar] [CrossRef]
- Hurd, C.M. The Hall Effect in Metals and Alloys; Springer: Boston, MA, USA, 1972. [Google Scholar]
- Zaytseva, I.; Abal’oshev, O.; Dłużewski, P.; Paszkowicz, W.; Zhu, L.Y.; Chien, C.L.; Kończykowski, M.; Cieplak, M.Z. Negative Hall coefficient of ultrathin niobium in Si/Nb/Si trilayers. Phys. Rev. B 2014, 90, 060505. [Google Scholar] [CrossRef] [Green Version]
- Gubin, A.I.; Il’in, K.S.; Vitusevich, S.A.; Siegel, M.; Klein, N. Dependence of magnetic penetration depth on the thickness of superconducting Nb thin films. Phys. Rev. B 2005, 72, 064503. [Google Scholar] [CrossRef] [Green Version]
- Lemberger, T.R.; Hetel, I.; Knepper, J.W.; Yang, F.Y. Penetration depth study of very thin superconducting Nb films. Phys. Rev. B 2007, 76, 094515. [Google Scholar] [CrossRef]
- Semenov, A.; Engel, A.; Il’in, K.; Gol’tsman, G.; Siegel, M.; Hübers, H.-W. Ultimate performance of a superconducting quantum detector. Eur. Phys. J. Appl. Phys. 2003, 21, 171–178. [Google Scholar] [CrossRef]
- Demchenko, I.N.; Lisowski, W.; Syryanyy, Y.; Melikhov, Y.; Zaytseva, I.; Konstantynov, P.; Chernyshova, M.; Cieplak, M.Z. Use of XPS to clarify the Hall coefficient sign variation in thin niobium layers buried in silicon. Appl. Surf. Sci. 2017, 399, 32–40. [Google Scholar] [CrossRef]
- Clark, A.H. A review of band structure and transport mechanisms in elemental amorphous semiconductors. J. Non. Cryst. Solids 1970, 2, 52–65. [Google Scholar] [CrossRef]
- Clark, A.H. Electrical and optical properties of amorphous germanium. Phys. Rev. 1967, 154, 750–757. [Google Scholar] [CrossRef]
- Gubanov, A.I. Band theory for the three-dimensional model of a liquid [1]. In Quantum Electron Theory of Amorphous Conductors; Springer: Boston, MA, USA, 1965; pp. 111–130. [Google Scholar]
| N2/Ar Ratio | N2 Flow (sccm) | Nb (%) | N (%) | NbxNy |
|---|---|---|---|---|
| 1/10 | 1.0 | 75.5 ± 1.4 | 24.5 ± 0.9 | NbN0.32 |
| 1.5/10 | 1.5 | 54.0 ± 1.5 | 46.0 ± 0.8 | NbN0.85 |
| 2/10 | 2.0 | 50.3 ± 1.8 | 49.7 ± 0.9 | NbN0.99 |
| 2.5/10 | 2.5 | 50.0 ± 1.5 | 50.0 ± 0.9 | NbN |
| N2/Ar Ratio | N2 Flow (sccm) | Nb (%) | N (%) | NbxNy |
|---|---|---|---|---|
| 1/10 | 1.0 | 71.8 ± 1.1 | 28.2 ± 2.1 | NbN0.39 |
| 1.5/10 | 1.5 | 59.8 ± 1.1 | 40.2 ± 1.9 | NbN0.67 |
| 2/10 | 2.0 | 54.9 ± 1.3 | 45.0 ± 1.9 | NbN0.81 |
| 2.5/10 | 2.5 | 49.9 ± 1.1 | 50.0 ± 1.3 | NbN |
| Sample | N2 Flow (sccm) | Conductivity (Ω cm)−1 | Hall Coefficient (cm3/C) |
|---|---|---|---|
| Nb | 0.0 | 8.90 × 103 | −2.18 × 10−4 |
| NbN0.32 | 1.0 | 1.81 × 104 | 4.00 × 10−5 |
| NbN | 2.0 | 3.33 × 102 | −2.14 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzon-Fontecha, A.; Castillo, H.A.; Escobar-Rincón, D.; Restrepo-Parra, E.; de la Cruz, W. Correlation Between Stoichiometry of NbxNy Coatings Produced by DC Magnetron Sputtering with Electrical Conductivity and the Hall Coefficient. Coatings 2019, 9, 196. https://doi.org/10.3390/coatings9030196
Garzon-Fontecha A, Castillo HA, Escobar-Rincón D, Restrepo-Parra E, de la Cruz W. Correlation Between Stoichiometry of NbxNy Coatings Produced by DC Magnetron Sputtering with Electrical Conductivity and the Hall Coefficient. Coatings. 2019; 9(3):196. https://doi.org/10.3390/coatings9030196
Chicago/Turabian StyleGarzon-Fontecha, Angélica, Harvi A. Castillo, Daniel Escobar-Rincón, Elisabeth Restrepo-Parra, and Wencel de la Cruz. 2019. "Correlation Between Stoichiometry of NbxNy Coatings Produced by DC Magnetron Sputtering with Electrical Conductivity and the Hall Coefficient" Coatings 9, no. 3: 196. https://doi.org/10.3390/coatings9030196





