The Dielectric Properties Improvement of Cable Insulation Layer by Different Morphology Nanoparticles Doping into LDPE
Abstract
:1. Introduction
2. Experiment Materials and Nanoparticles Modification
2.1. Raw Materials and Equipments
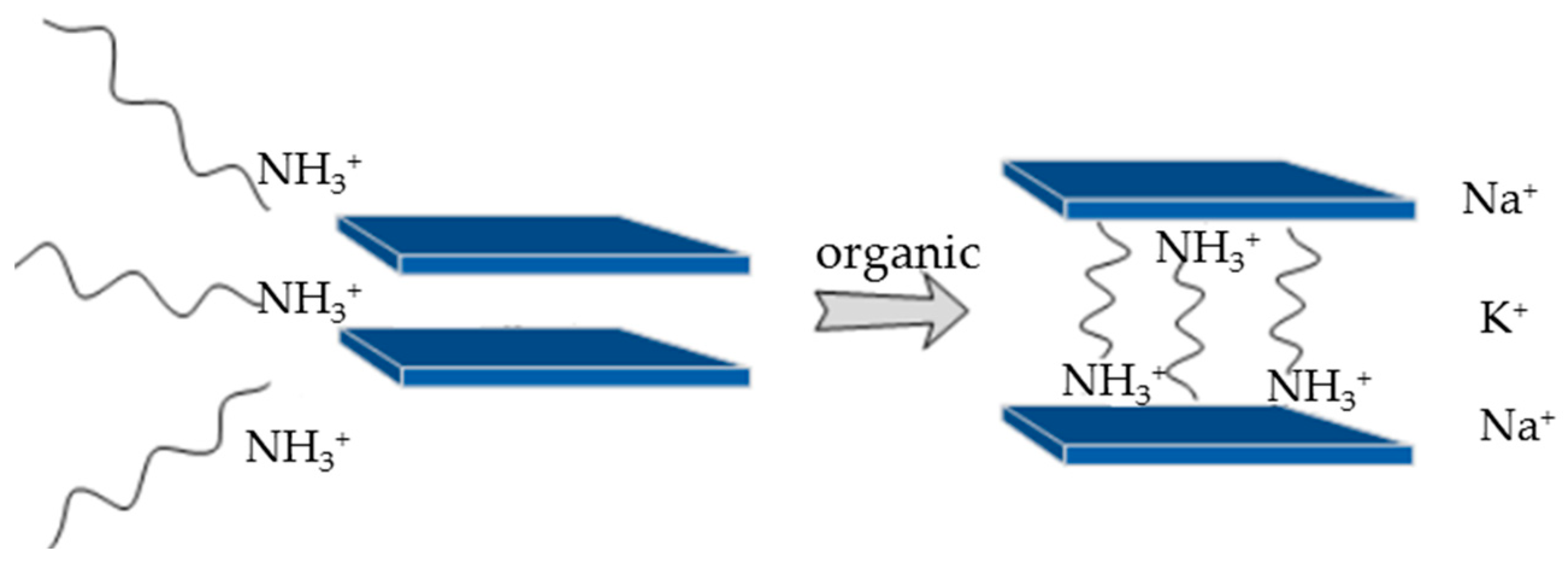
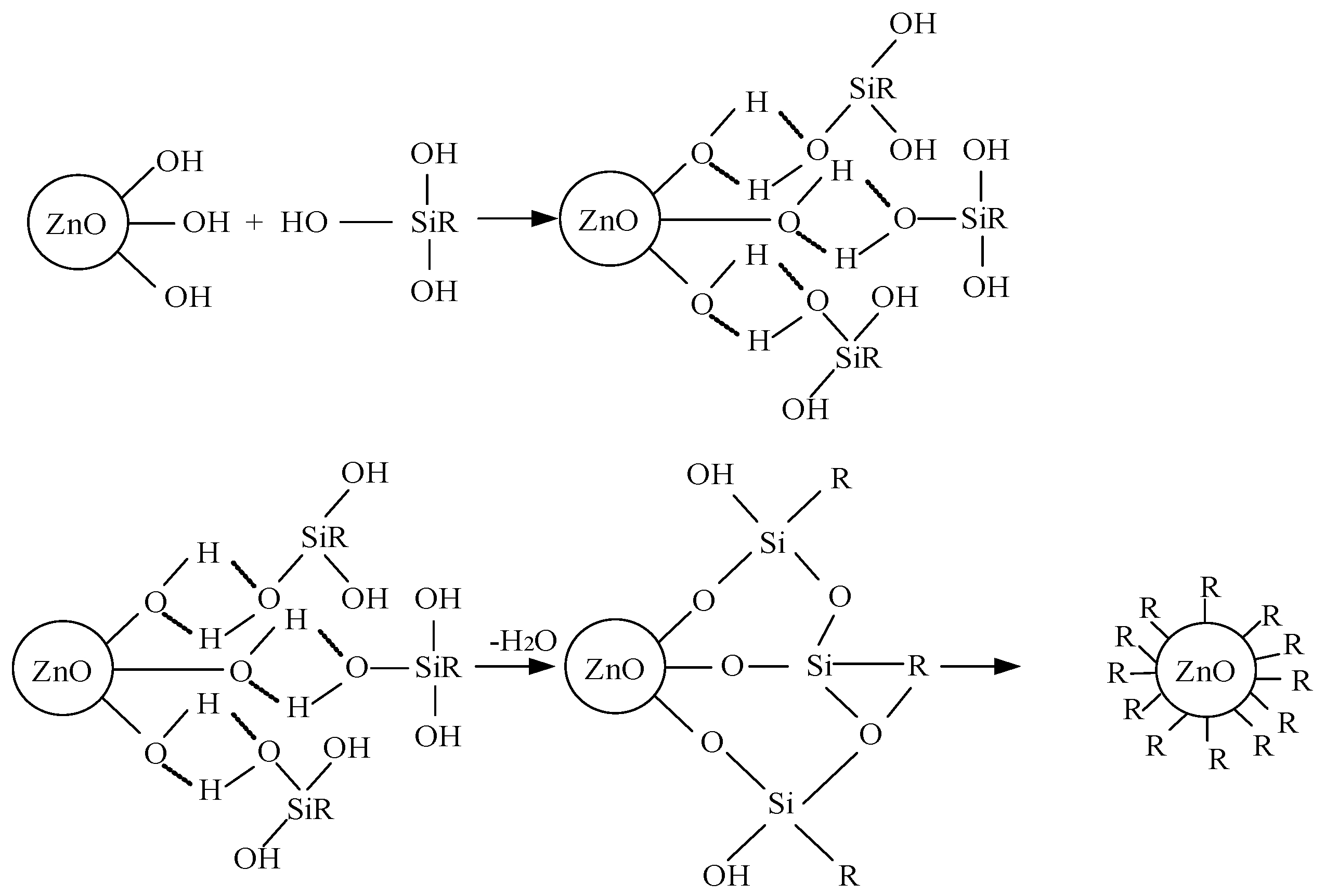
2.2. Surface Modification of Different Nanoparticles
2.3. Nanocomposites Preparation
3. Results and Discussion
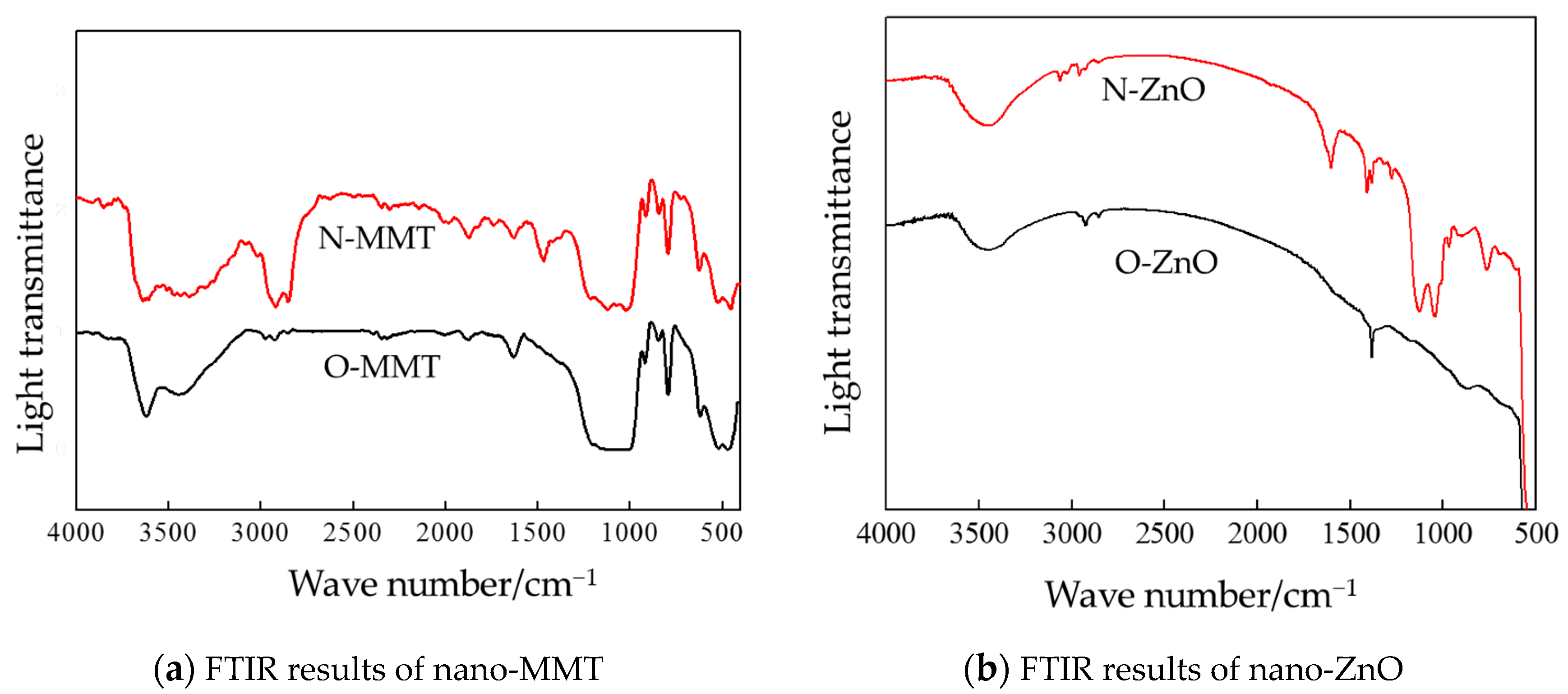
3.1. Chemical Structure Test and Analysis
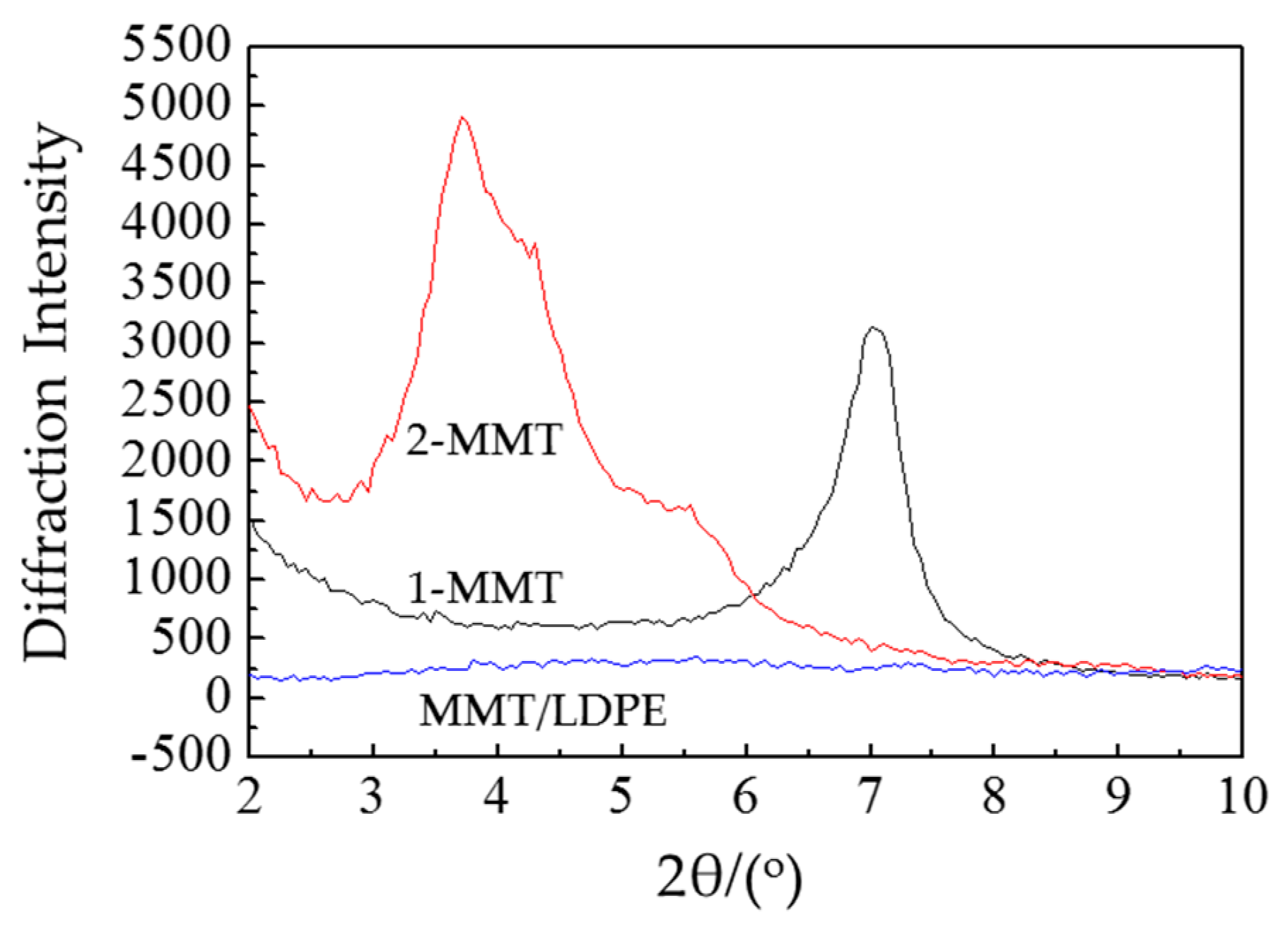
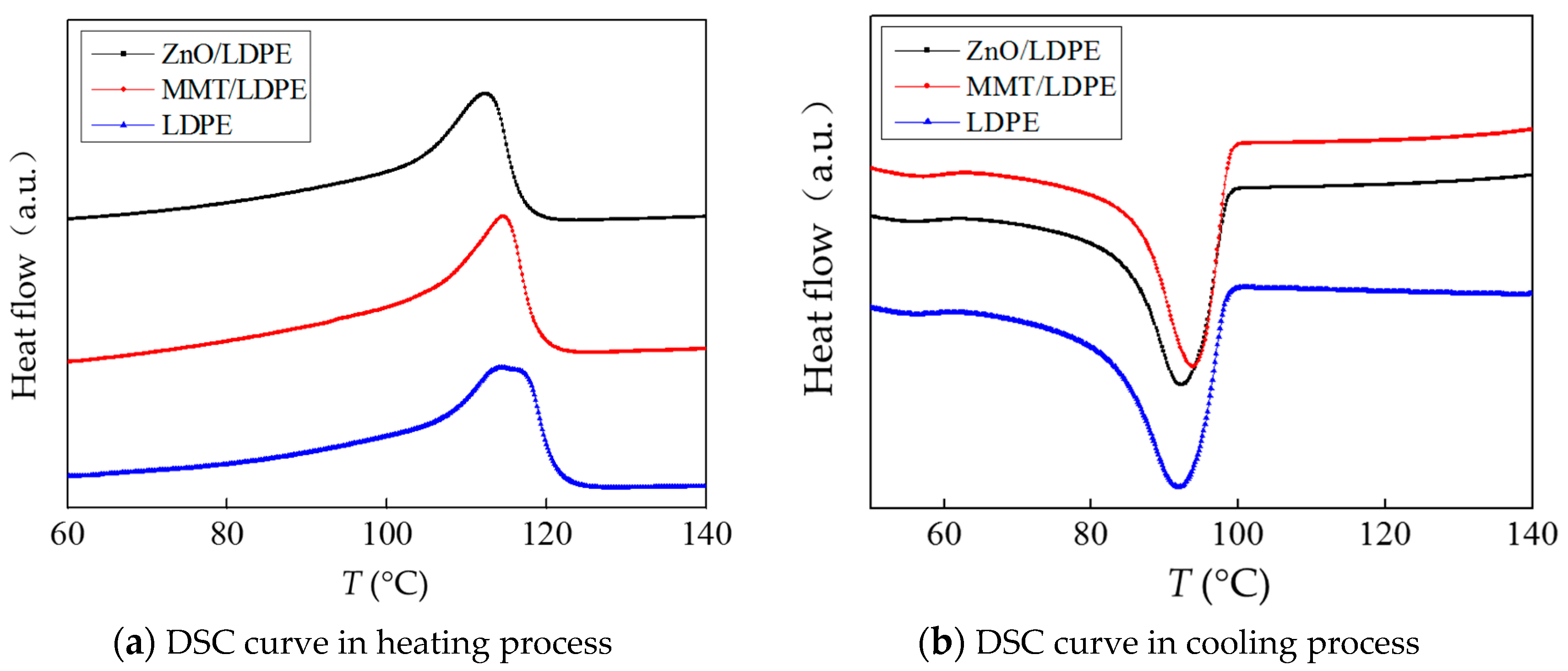
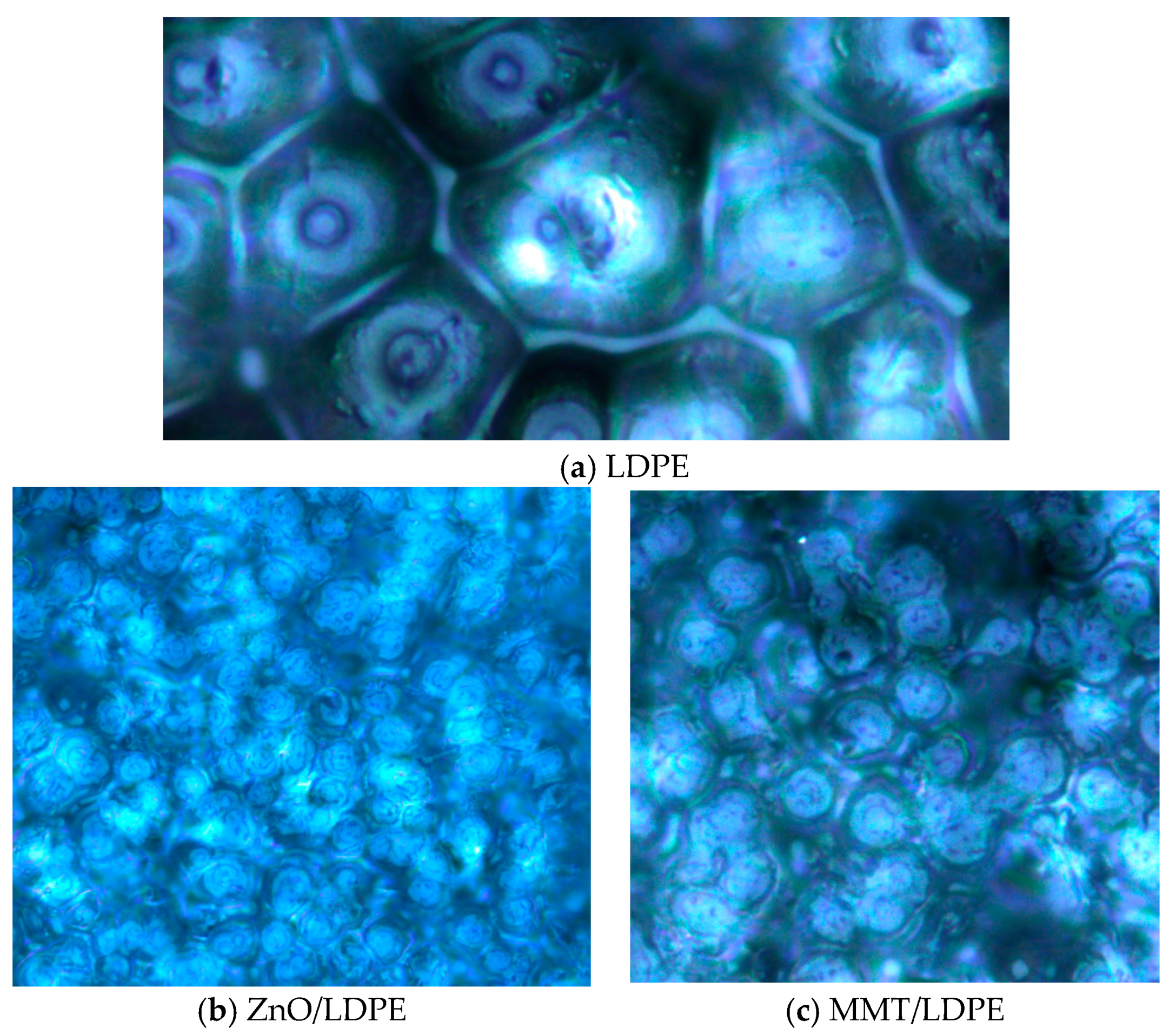
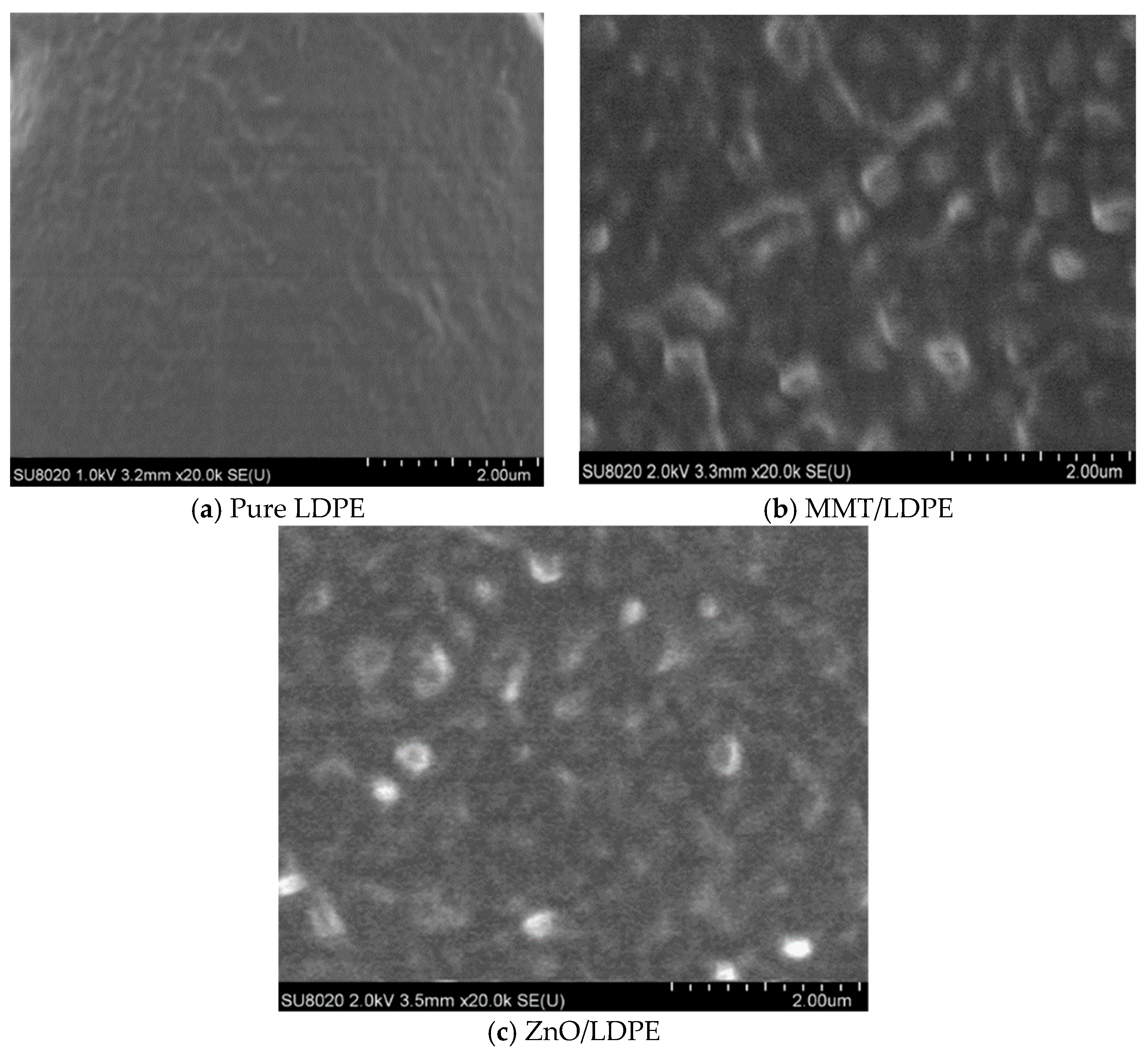
3.2. Crystalline Structure Test and Analysis
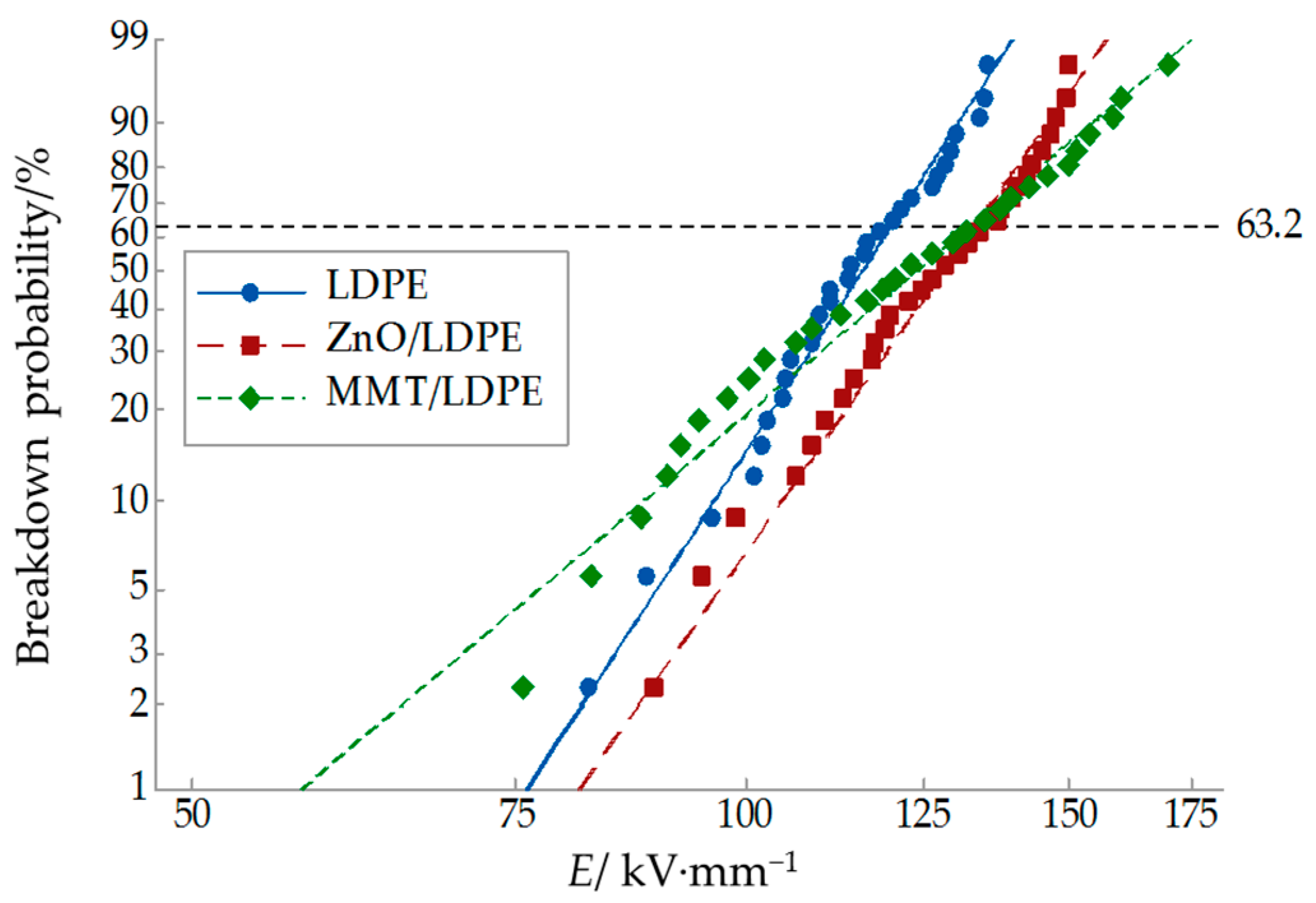
3.3. Breakdown Characteristic Test and Analysis
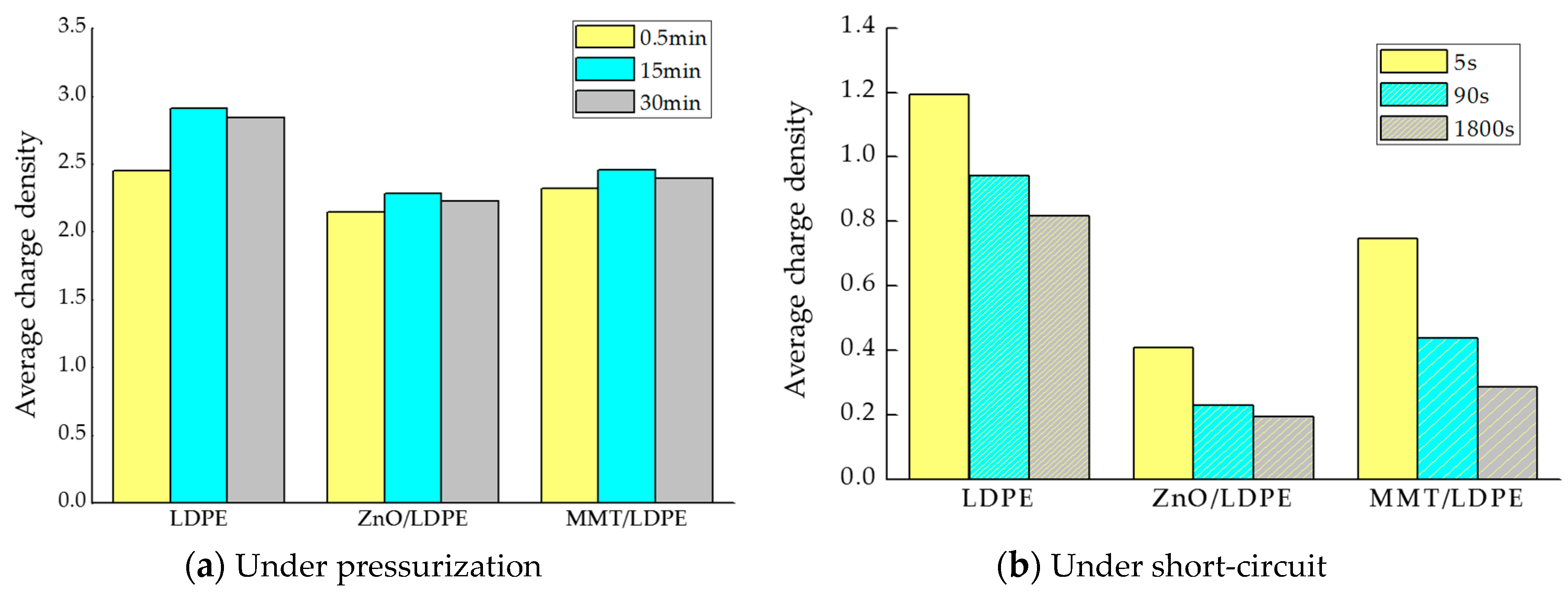
3.4. Space Charge Characteristic Test and Analysis
4. Conclusions
- The compatibility of modified nanoparticles with LDPE was excellent. The quantity of crystal cell in nano-MMT/LDPE and nano-ZnO/LDPE increased, the crystal size decreased, and the distribution of crystal cells was close. Being compared with pure LDPE, the crystallization rate of nanocomposite was faster and the crystallinity was higher. Besides, the amorphous regions in nanocomposite decreased, the migration path of carriers was tortuous, and the free path got shorter.
- According to the breakdown field strength test at different temperatures, the breakdown field strength of three samples increased initially, and then decreased with increasing temperature. When the testing temperature was lower than 60 °C, the breakdown field strength of nanocomposite was higher than that of pure LDPE. Under room temperature (25 °C), the breakdown field strength of nano-MMT/LDPE and nano-ZnO/LDPE increased by 10.3% and 11.1% respectively, compared to that of pure LDPE.
- According to the result of TSC, the trap density of nanocomposites was great, and many deep traps existed in materials. The carriers were captured effectively. The electric field strength decreased and the dielectric properties of the polymer improved. According to the space charge distribution test, the space charge accumulation in LDPE was restrained by nanoparticles doping. Among them, the space charges in nano-ZnO/LDPE was the least, and the release rate of charges in nano-MMT/LDPE was the fastest under short-circuit.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iyer, G.; Gorur, R.S.; Krivda, A. Corona resistance of epoxy nanocomposites: Experimental results and modeling. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 118–125. [Google Scholar] [CrossRef]
- Castellon, J.; Nguyen, H.N.; Agnel, S.; Toureille, A.; Frechette, M.; Savoie, S.; Krivda, A.; Schmidt, L.E. Electrical properties analysis of micro and nano composite epoxy resin materials. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 651–658. [Google Scholar] [CrossRef]
- Fukano, Y.; Sugawara, Y.; Morita, S. Nanometer resolution measurement of dielectric breakdown of silicon dioxide films with AFM/STM. In Proceedings of the 1992 International Conference on Solid State Devices and Materials, Tsukuba, Japan, 26–28 August 1992; Volume 41, pp. 17–19. [Google Scholar]
- Zha, J.W.; Dang, Z.M.; Li, W.K.; Zhu, Y.H.; Chen, G. Effect of micro-Si3N4–nano-Al2O3 co-filled particles on thermal conductivity, dielectric and mechanical properties of silicone rubber composites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1989–1996. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tohyama, K.; Kozako, M.; Kawano, S.; Ito, K.; Miyake, H.; Ohki, Y.; Tanaka, Y.; Hirose, Y. Charge behavior of epoxy/silica nanocomposites under AC high fields. In Proceedings of the IEEE International Conference on Solid Dielectrics, Bologna, Italy, 30 June–4 July 2013; pp. 892–895. [Google Scholar]
- Chen, X.; Wang, X.; Wu, K.; Peng, Z.R.; Cheng, Y.H.; Tu, D.M. Space charge measurement in LPDE films under temperature gradient and DC stress. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1796–1805. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Guo, N.; Wang, R.; Zhang, T. Dielectric propeties of nano ZnO/low density polyethylene composites. Acta Mater. Compos. Sin. 2015, 32, 1351–1360. [Google Scholar]
- Langdon, T.J. The significance of grain boundaries in the flow of polycrystalline materials. Mater. Sci. Forum 1995, 189–190, 31–42. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, L.-J.; Li, X.-L.; Wu, G.-N. AC breakdown characteristics of nanometer insulating oil. High Volt. Appar. 2012, 48, 94–99. [Google Scholar]
- Guo, J.; Chen, Y.; Jia, Z.; Tanaka, T.; Wu, J.; Cheng, Y. Study on electrical properties of micro-nano structured epoxy composites. In Proceedings of the International Symposium on Electrical Insulating Materials, Niigata, Japan, 1–5 June 2014; pp. 441–444. [Google Scholar]
- Dong, M.; Li, Y.; Dai, J.; Wang, J.; Li, J. Research status and progress on liquid nanocomposite dielectrics. Insul. Mater. 2016, 12, 1–7. [Google Scholar]
- Zhang, L.; Zhou, Y. Effect of nanparticle surface modificantion on breakdown and space charge behavior of XLPE/SiO2 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1554–1564. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, X.; Wu, K.; Chen, X.; Cheng, Y.; Dissado, L.A. Dependence of charge accumulation on sample thickness in Nano-SiO2 doped IDPE. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 337–345. [Google Scholar]
- Wang, W.; Min, D.; Li, S. Understanding the conduction and breakdown properties of polyethylene nanodielectrics: Effect of deep traps. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 564–572. [Google Scholar] [CrossRef]
- Ju, S.; Chen, M.; Zhang, H.; Zhang, Z. Dielectric properties of nanosilica/low-density polyethylene composites: The surface chemistry of nanoparticles and deep traps induced by nanoparticles. Express Polym. Lett. 2014, 8, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.; Wang, X.; Fan, Y. A new method of auto-separating thermally stimulated current. J. Appl. Phys. 1992, 72, 4254–4257. [Google Scholar] [CrossRef]
- Fu, M.; Dissado, L.A.; Chen, G.; Fothergill, J.C. Space charge formation and its modified electric field under applied voltage reversal and temperature gradient in XLPE cable. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Komameni, S.; Roy, D.M. Multi-phase ceramic composites made by sol-gel technique. Mater. Res. Soc. Symp. Proc. 1984, 32, 347–359. [Google Scholar] [CrossRef]
- Zha, J.W.; Zhu, Y.H.; Li, W.K.; Bai, J.; Dang, Z.M. Low dielectric permittivity and high thermal conductivity silicone rubber composites with micro-nano-sized particles. Appl. Phys. Lett. 2012, 101, 062905. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Krasilnikov, N.A.; Raab, G.I. Grain boundary effects in nanocrystalline copper. Mater. Sci. Forum 1999, 294–296, 701–706. [Google Scholar] [CrossRef]
- Aht-Ong, D.; Benjapornthavee, H. Preparation of poly(ethylene terephthalate)/montmorillonite nanocomposites films by solution technique. In Proceedings of the International Conference on Nanoscience and Nanotechnology, Melbourne, Australia, 25–29 February 2008; pp. 25–29. [Google Scholar]
- Cui, X.; Chen, T. Preparation and surface modification of nanometer zinc oxide are reviewed. Rubber Technol. Mark. 2010, 13, 9–14. [Google Scholar]
- Cheng, Y.; Guo, N.; Wang, R.; Zhang, X. Effects of nano-ZnO and montmorillonte on dielectric propeties of low density polyethylene. Acta Mater. Compos. Sin. 2015, 32, 94–100. [Google Scholar]
- Zhou, P.; Qi, H.; Zhu, Z.; Qin, H.; Li, H.; Chu, C.; Yan, M. Development of SiC/PVB composite powders for selective laser sintering additive manufacturing of SiC. Materials 2018, 11, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J. Nanometer plastics and its application in engineering plastics modification. Eng. Plast. Appl. 2002, 30, 47. [Google Scholar]
- Zhu, C. The Polymer Structure Analysis; Science Press: Beijing, China, 2002; pp. 182–184. [Google Scholar]
- Luo, Y.; Wu, G.; Peng, J. The research progress of Polymer nanocomposite dielectrics-properties of interface performance. High Volt. Technol. 2012, 9, 2455–2464. [Google Scholar]
- Nelson, J.K.; Hu, Y. Nanocomposite dielectrics-properties and implications. J. Phys. D Appl. Phys. 2005, 38, 213–222. [Google Scholar] [CrossRef]
- Bjellheim, P.; Helgee, B. Aromatic polyimides: Synthesis, characterization, and evaluation of electric strength. J. Appl. Polym. Sci. 2003, 48, 1587–1596. [Google Scholar] [CrossRef]
- Takeda, T.; Suzuki, H.; Okamoto, T. Correlation between space charge distribution under DC voltage and dielectric breakdown properties in XLPE under impulse voltage superposed onto DC voltage. In Proceedings of the International Symposium on Electrical Insulating Materials, Himeji, Japan, 22 November 2001; pp. 493–496. [Google Scholar]
- Ieda, M.; Mizutani, T.; Suzuoki, Y. TSC and TL studies of carrier trapping in insulating polymers. Mem. Fac. Eng. Nagoya Univ. Jpn. 1980, 32, 173–219. [Google Scholar]
- Takada, T.; Hayase, Y.; Tanaka, Y. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Ieda, M. Electrical conduction and carrier traps in polymeric materials. IEEE Trans. Electr. Insul. 1984, 19, 162–178. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, S.Q.; Wang, X. In the process of polyethylene electrical aging, the influence of Nanometer ZnO for space charge and the breakdown characteristics. Insul. Mater. 2008, 41, 44–48. [Google Scholar]
- Tanaka, Y.; Chen, G.; Zhao, Y.; Davies, A.E.; Vaughan, A.S.; Takada, T. Effect of additives on morphology and space charge accumulation in low density polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 148–154. [Google Scholar] [CrossRef]


| Sample | LDPE (wt.%) | Nanoparticles (wt.%) | Preparation Method |
|---|---|---|---|
| LDPE | 100 | 0 | Melt blending |
| MMT/LDPE | 97 | 3 (MMT) | Melt intercalation |
| ZnO/LDPE | 97 | 3 (ZnO) | Melt blending |
| Sample | Tm/°C | Tc/°C | ΔTc/°C | Xc/% |
|---|---|---|---|---|
| LDPE | 117.7 | 92.3 | 17.5 | 36.71 |
| MMT/LDPE | 115.2 | 95.0 | 13.1 | 38.72 |
| ZnO/LDPE | 114.5 | 96.8 | 13.2 | 39.20 |
| Sample | E/(kV·mm−1) | N | β |
|---|---|---|---|
| LDPE | 120.0 | 30 | 10.06 |
| MMT/LDPE | 132.4 | 30 | 5.48 |
| ZnO/LDPE | 133.3 | 30 | 9.24 |
| Name | PE | LDPE/MMT | LDPE/ZnO |
|---|---|---|---|
| Peak temperature/°C | 52 | 72 | 58 |
| Trap depth/V | 0.51 | 0.64 | 0.56 |
| Released of charges/pC | 25,000 | 31,000 | 530,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Cheng, Y.; Zhang, X. The Dielectric Properties Improvement of Cable Insulation Layer by Different Morphology Nanoparticles Doping into LDPE. Coatings 2019, 9, 204. https://doi.org/10.3390/coatings9030204
Yu G, Cheng Y, Zhang X. The Dielectric Properties Improvement of Cable Insulation Layer by Different Morphology Nanoparticles Doping into LDPE. Coatings. 2019; 9(3):204. https://doi.org/10.3390/coatings9030204
Chicago/Turabian StyleYu, Guang, Yujia Cheng, and Xiaohong Zhang. 2019. "The Dielectric Properties Improvement of Cable Insulation Layer by Different Morphology Nanoparticles Doping into LDPE" Coatings 9, no. 3: 204. https://doi.org/10.3390/coatings9030204




