Electrochemical Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Specimen Preparation
2.2. Test Methods
3. Results and Discussion
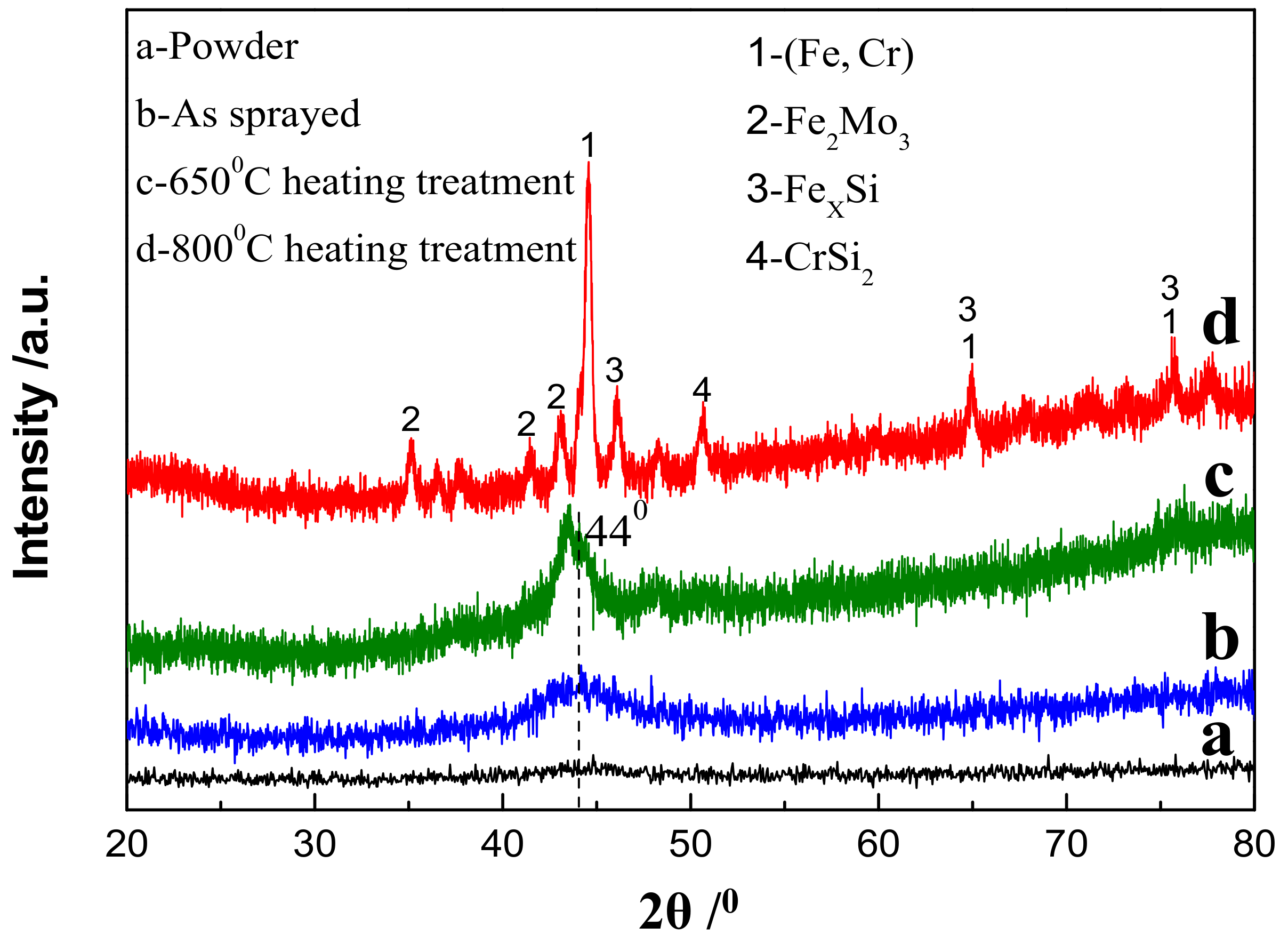
3.1. Phase Structure of Fe-Based Amorphous/Nanocrystalline Coating (AC)
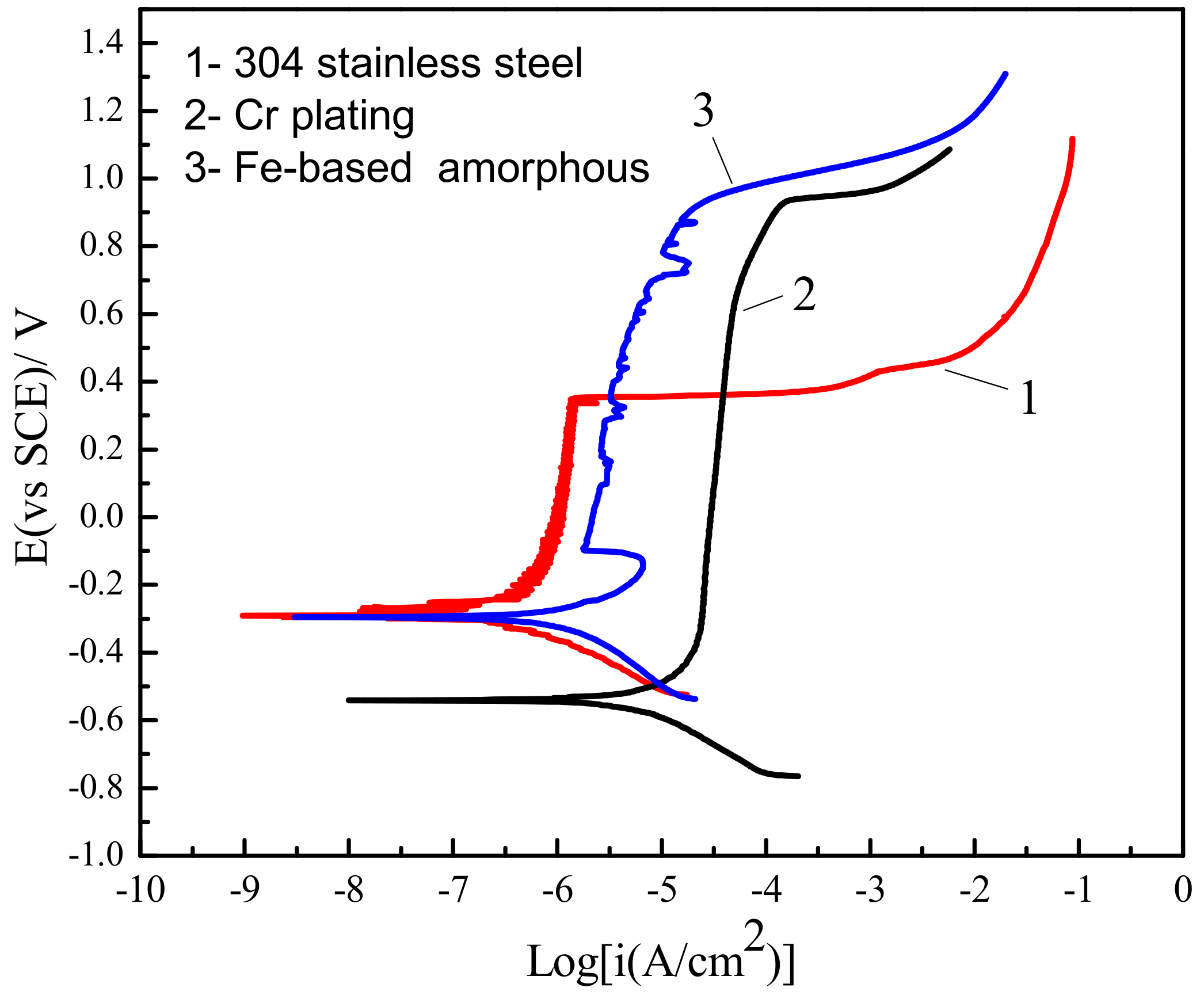
3.2. Corrosion Resistance of Fe-Based Amorphous/Nanocrystalline Coating (AC) vs. Conventional Materials
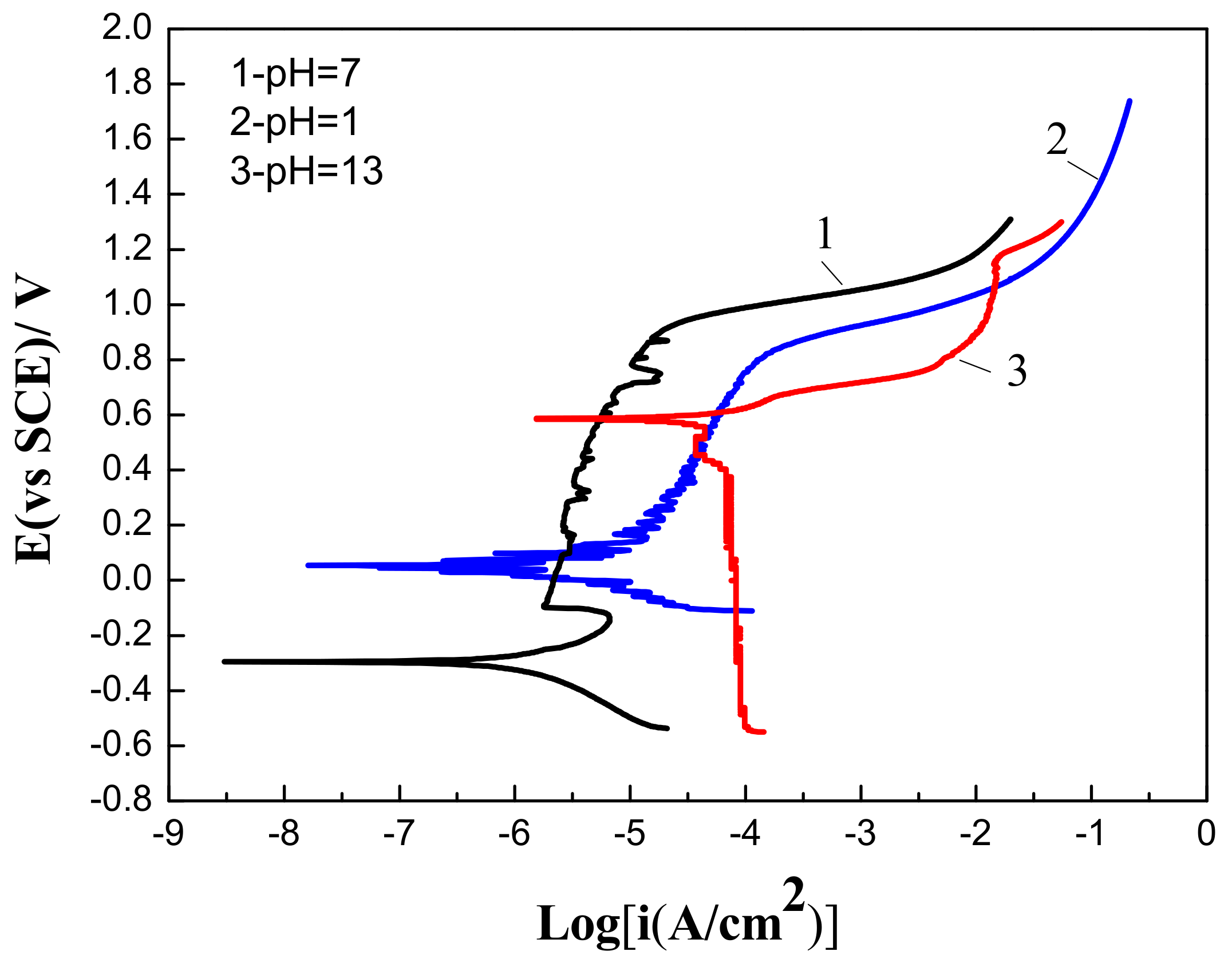
3.3. The Effects of pH Values on Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating
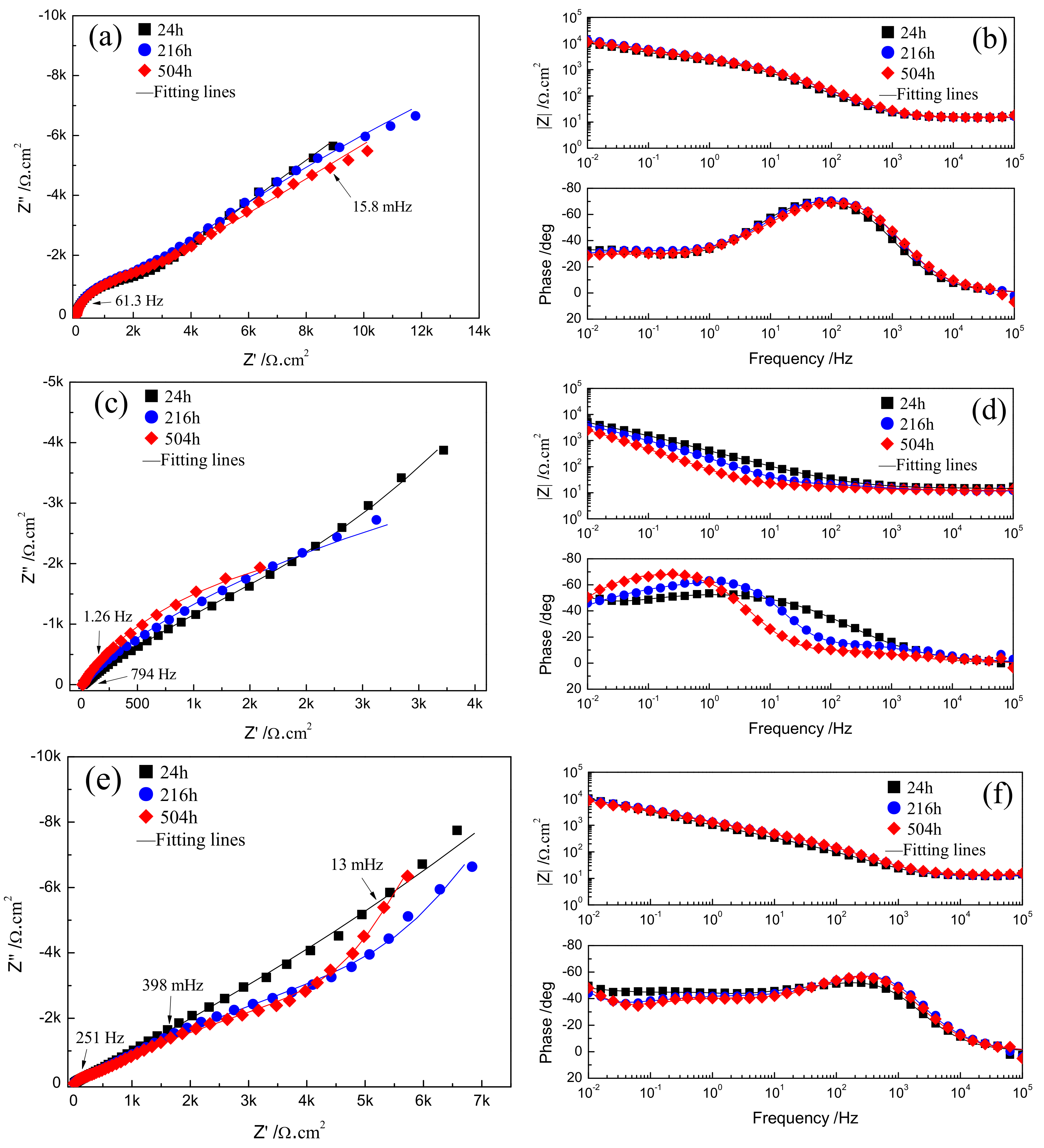
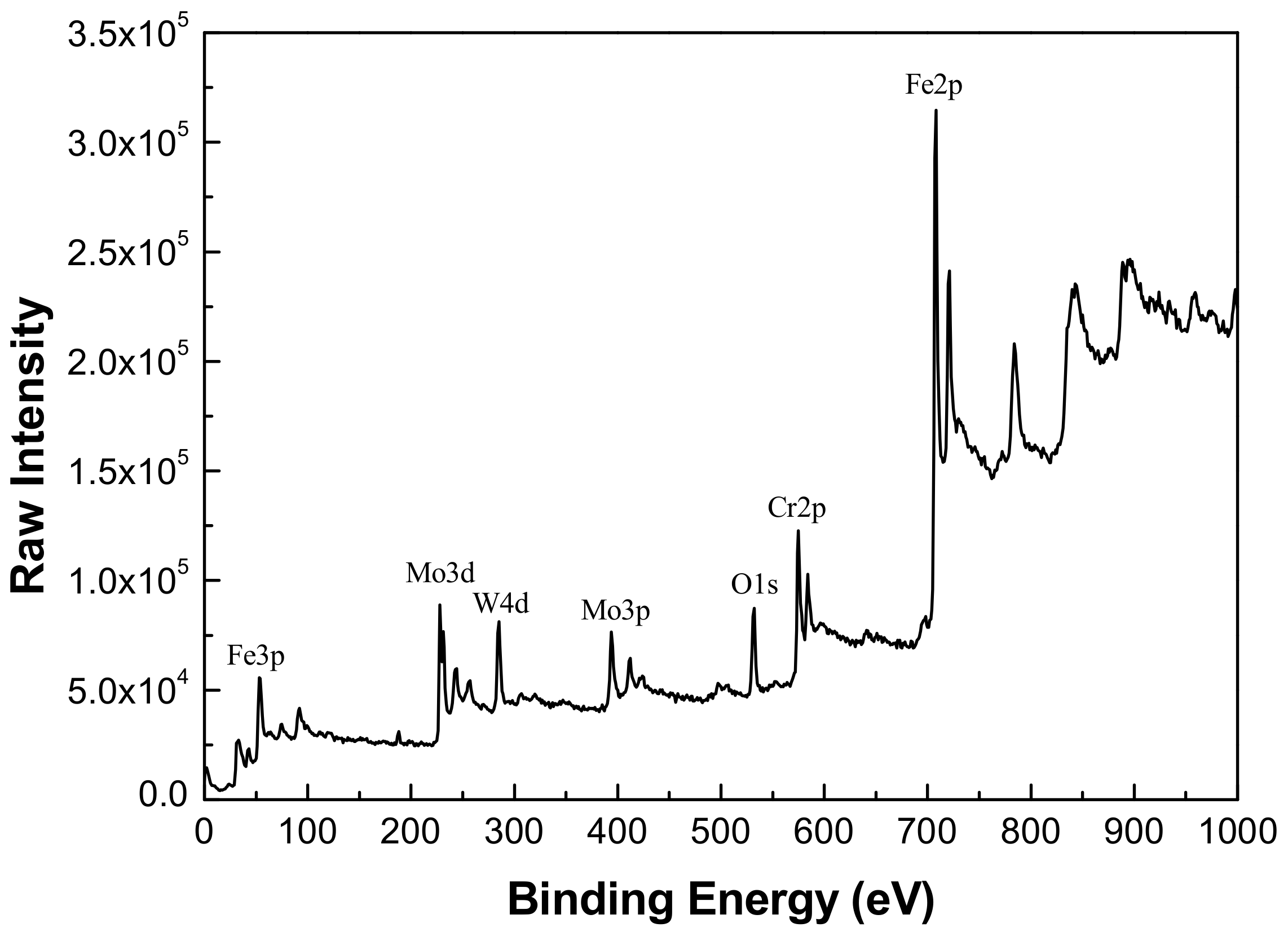
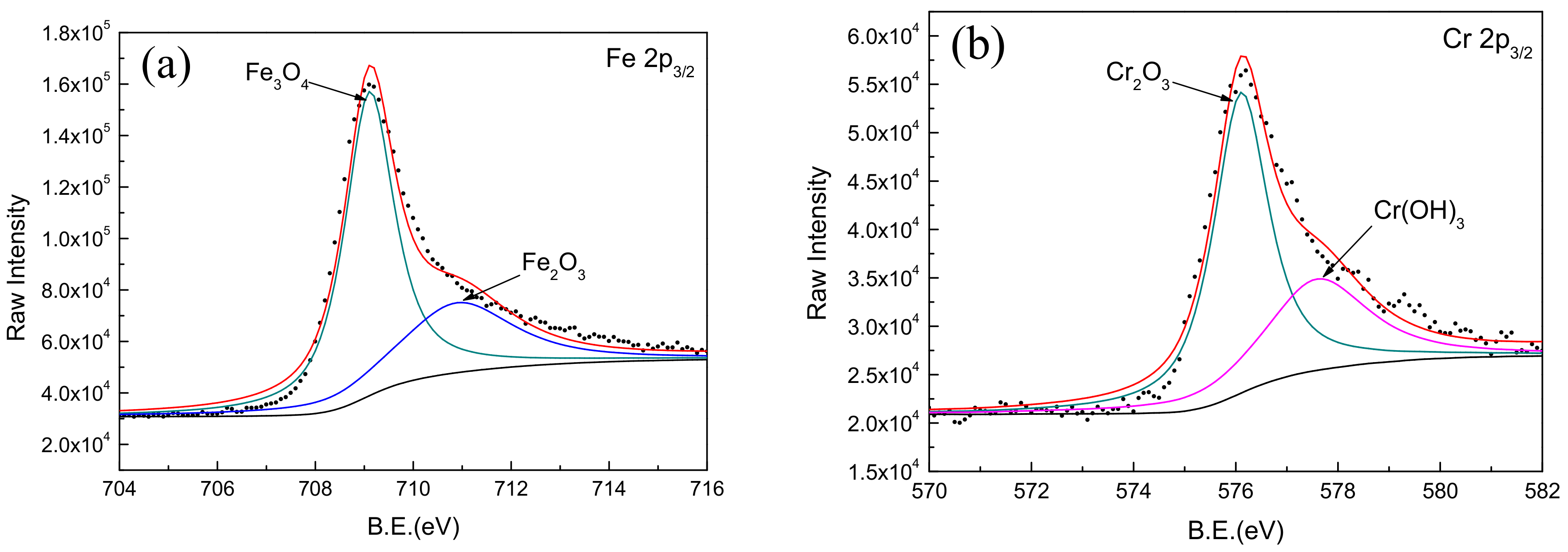
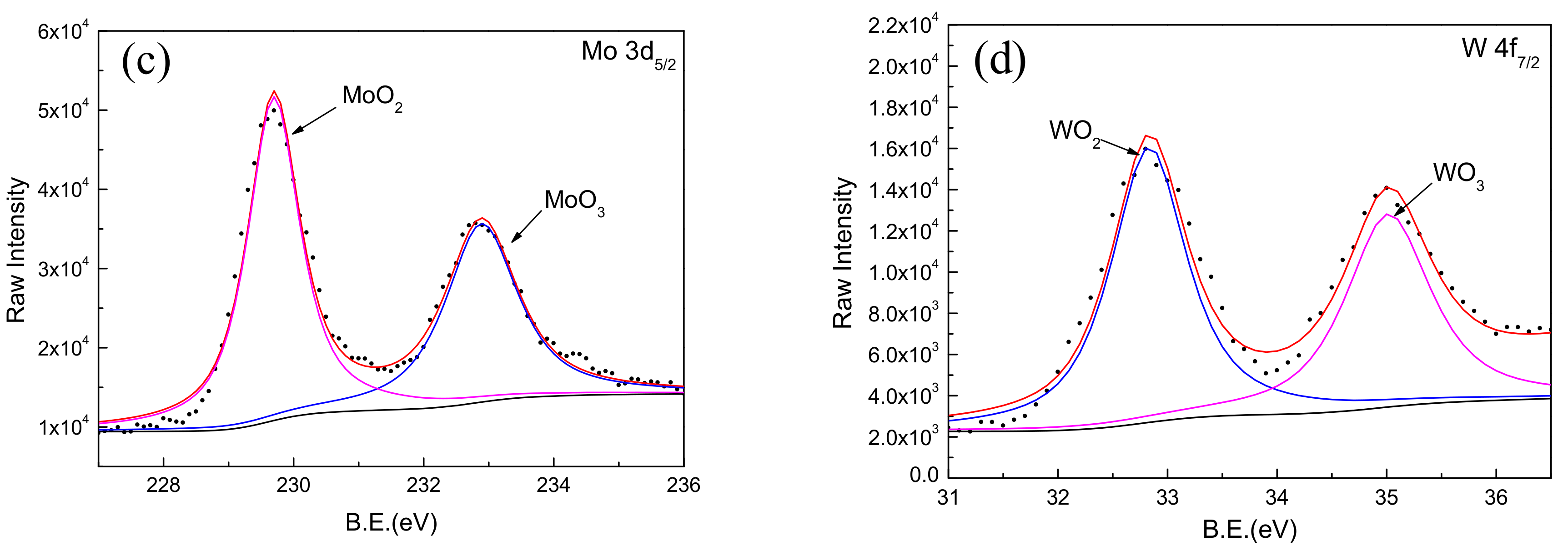
3.4. Composition Analysis of Passive Film Formed on Fe-Based Amorphous/Nanocrystalline Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsutsumi, Y.; Nishikata, A.; Tsuru, T. Pitting corrosion mechanism of Type 304 stainless steel under a droplet of chloride solutions. Corros. Sci. 2007, 49, 1394–1407. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting corrosion behaviour of austenitic stainless steels-combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Pang, S.J.; Zhang, T.; Asami, K.; Inoue, A. Bulk glassy Fe-Cr-Mo-C-B alloys with high corrosion resistance. Corros. Sci. 2002, 44, 1847–1856. [Google Scholar] [CrossRef]
- Souza, C.A.C.; May, J.E.; Carlos, I.A.; de Oliveira, M.F.; Kuri, S.E.; Kiminami, C.S. Influence of the corrosion on the saturation magnetic density of amorphous and nanocrystalline Fe73Nb3Si15.5B7.5Cu1 and Fe80Zr3.5Nb3.5B12Cu1 alloys. J. Non-Cryst. Solids 2002, 304, 210–216. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Chen, Y.X.; Wang, Z.H.; Xu, B.S. High-temperature erosion resistance of FeBSiNb amorphous coatings deposited by arc spraying for boiler applications. J. Therm. Spray Technol. 2013, 22, 820–827. [Google Scholar] [CrossRef]
- Guo, R.Q.; Zhang, C.; Yang, Y.; Peng, Y.; Liu, L. Corrosion and wear resistance of a Fe-based amorphous coating in underground environment. Intermetallics 2012, 30, 94–99. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Wang, W.; Yasir, M.; Liu, L. Microstructure and mechanical properties of Fe-based amorphous composite coatings reinforced by stainless steel powders. J. Mater. Sci. Technol. 2015, 31, 43–47. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Ma, H.; Jiang, K.; Chang, C.; Wang, J.-Q.; Song, Z.; Wang, X.; Li, R.-W. Improved corrosion resistance of novel Fe-based amorphous alloys. Mater. Des. 2016, 95, 225–230. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C. Fe-based amorphous coatings: Structures and properties. Thin Solid Films 2014, 561, 70–86. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Z.; Xiao, P.; Zhu, X. Spraying of Fe-based amorphous coating with high corrosionresistance by HVAF. J. Manuf. Process. 2016, 22, 34–38. [Google Scholar] [CrossRef]
- Wu, H.; Lan, X.; Liu, Y.; Li, F.; Zhang, W.; Chen, Z.; Zai, X.; Zeng, H. Fabrication, tribological and corrosion behaviors of detonation gun sprayed Fe-based metallic glass coating. Trans. Nonferr. Met. Soc. China 2016, 26, 1629–1637. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Sun, W.H.; Wang, J.Q. Erosion-corrosion of HVOF-sprayed Fe-based amorphous metallic coating under impingement by a sand-containing NaCl solution. Corros. Sci. 2013, 76, 337–347. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Hou, G.; An, Y.; Liu, G. The effect of high-velocity oxy-fuel spraying parameters on microstructure, corrosion and wear resistance of Fe-based metallic glass coatings. J. Non-Cryst. Solids 2014, 406, 37–44. [Google Scholar] [CrossRef]
- Ni, H.S.; Liu, X.H.; Chang, X.C.; Hou, W.L.; Liu, W.; Wang, J.Q. High performance amorphous steel coating prepared by HVOF thermal spraying. J. Alloy Compd. 2009, 467, 163–167. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Guo, S.F.; Pan, F.S.; Zhang, H.J.; Zhang, D.F.; Wang, J.F.; Miao, J.; Su, C.; Zhang, C. Fe-based amorphous coating for corrosion protection of magnesiumalloy. Mater. Des. 2016, 108, 624–631. [Google Scholar] [CrossRef]
- Wua, J.; Zhang, S.D.; Sun, W.H.; Gao, Y.; Wang, J.Q. Enhanced corrosion resistance in Fe-based amorphous coatings through eliminating Cr-depleted zones. Corros. Sci. 2018, 136, 161–173. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Peng, Y.; Yu, Y.; Liu, L. Effects of crystallization on the corrosion resistance of Fe-based amorphous coatings. Corros. Sci. 2012, 59, 10–19. [Google Scholar] [CrossRef]
- Raicheff, R.; Zaprianova, V.; Gattef, E. Effect of structural relaxation on electrochemical corrosion behaviour of amorphous alloys. J. Mater. Sci. Lett. 1997, 16, 1701–1704. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar]
- Long, Z.L.; Chang, C.T.; Ding, Y.H.; Shao, Y.; Zhang, P.; Shen, B.L.; Inoue, A. Corrosion behavior of Fe-based ferromagnetic (Fe, Ni)-B-Si-Nb bulk glassy alloys in aqueous electrolytes. J. Non-Cryst. Solids 2008, 354, 4609–4613. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhang, W.L.; Wang, S.G.; Gu, X.J.; Wang, J.Q. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Bakare, M.S.; Voisey, K.T.; Chokethawai, K.; McCartney, D.G. Corrosion behaviour of crystalline and amorphous forms of the glass forming alloy Fe43Cr16Mo16C15B10. J. Alloys Compd. 2012, 527, 210–218. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Song, J.; Deng, C.; Deng, C. Microstructure and corrosion behavior of Fe-based amorphous coating prepared by HVOF. J. Alloys Compd. 2017, 721, 506–511. [Google Scholar] [CrossRef]
- Zhang, C.; Chan, K.C.; Wu, Y.; Liu, L. Pitting initiation in Fe-based amorphous coatings. Acta Mater. 2012, 60, 4152–4159. [Google Scholar] [CrossRef]
- Naka, M.; Hashimoto, K.; Masumoto, T. High corrosion resistance of amorphous Fe-Mo and Fe-W alloys in HCl. J. Non-Cryst. Solids 1978, 29, 61–65. [Google Scholar] [CrossRef]
- Aghuy, A.A.; Zakeri, M.; Moayed, M.H.; Mazinani, M. Effect of grain size on pitting corrosion of 304L austenitic stainless steel. Corros. Sci. 2015, 94, 368–376. [Google Scholar] [CrossRef]
- Cao, C. On electrochemical techniques for interface inhibitor research. Corros. Sci. 1996, 38, 2073–2082. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Yan, C.; Du, K.; Wang, F. Corrosion behaviors of Zn/Al-Mn alloy composite coatings deposited on magnesium alloy AZ31B (Mg-Al-Zn). Electrochim. Acta 2009, 55, 560–571. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting corrosion of metals: A review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Malik, A.U.; Kutty, P.C.M.; Siddiqi, N.A.; Andijani, I.N.; Ahmed, S. The influence of pH and chloride concentration on the corrosion behaviour of AISI 316 steel in aqueous solutions. Corros. Sci. 1992, 33, 1809–1827. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Chen, W. Surface enriched molybdenum enhancing the corrosion resistance of 316L stainless steel. Mater. Lett. 2016, 171, 38–41. [Google Scholar] [CrossRef]
- Huang, H.-H. Effect of chemical composition on the corrosion behavior of Ni-Cr-Mo dental casting alloys. J. Biomed. Mater. Res. Banner 2002, 60, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Stolica, N. Pitting corrosion on Fe-Cr and Fe-Cr-Ni alloys. Corros. Sci. 1969, 9, 455–470. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Wu, W.; Qiao, Q.; Li, Y.; Li, X. Effect of pH and chloride on the micro-mechanism of pitting corrosionfor high strength pipeline steel in aerated NaCl solutions. Appl. Surf. Sci. 2015, 349, 746–756. [Google Scholar] [CrossRef]
- Roy, A.K.; Fleming, D.L.; Gordon, S.R. Effect of chloride concentration and pH on pitting corrosion of waste package container materials. In Proceedings of the 190th Meeting of The Electrochemical Society, San Antonio, TX, USA, 6–11 October 1996. [Google Scholar]
- Search for the binding energy of an element and line. Available online: http://srdata.nist.gov/xps/ElmSpectralSrch.aspx?selEnergy=PE (accessed on 26 March 2019).
- Thierry, D.; Zou, F. Localized electrochemical impedance spectroscopy for studying pitting corrosion on stainless steels. J. Electrochem. Soc. 1997, 144, 1208–1215. [Google Scholar]
- Loable, C.; Viçosa, I.N.; Mesquita, T.J.; Mantel, M.; Nogueira, R.P.; Berthom, G.; Chauveau, E.; Roche, V. Synergy between molybdenum and nitrogen on the pitting corrosion and passive film resistance of austenitic stainless steels as a pH-dependent effect. Mater. Chem. Phys. 2017, 186, 237–245. [Google Scholar] [CrossRef]
- Sunseri, C.; Piazza, S.; Quarto, F.D. Photocurrent spectroscopic investigations of passive films on chromium. J. Electrochem. Soc. 1990, 137, 2411–2417. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W. Flatband potentials and donor densities of polycrystalline α-Fe2O3 determined from Mott-Schottky plots. J. Electrochem. Soc. 1978, 125, 723–726. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Burstein, G.T. The role of alloyed molybdenum in the inhibition of pitting corrosion in stainless steels. Corros. Sci. 2001, 43, 485–516. [Google Scholar] [CrossRef]
- Long, Z.L.; Shao, Y.; Deng, X.H.; Zhang, Z.C.; Jiang, Y.; Zhang, P.; Shen, B.L.; Inoue, A. Cr effects on magnetic and corrosion properties of Fe-Co-Si-B-Nb-Cr bulk glassy alloys with high glass-forming ability. Intermetallics 2007, 15, 1453–1458. [Google Scholar] [CrossRef]
- Streicher, M.A. Development of pitting resistant Fe-Cr-Mo alloys. Corrosion 1974, 30, 77–91. [Google Scholar] [CrossRef]
- Otsubo, F.; Kishitake, K. Corrosion resistance of Fe-16%Cr-30%Mo-(C,B,P) amorphous coatings sprayed by HVOF and APS processes. Mater. Trans. 2005, 46, 80–83. [Google Scholar] [CrossRef]


| Parameters | Optimized Value |
|---|---|
| Oxygen (O2) (L/min) | 900 |
| Kerosene (Ke) (L/h) | 22 |
| O2/Ke burning ratio(λ) | 1.18 |
| Combustion pressure (Bar) | 8.5 |
| Nozzle | 150/11 |
| Carrier gas (N2) (L/min) | 9 |
| Powder feed rate (g/min) | 100 |
| Spray distance (mm) | 380 |
| Transverse speed (mm/s) | 1000 |
| Deposition efficiency (%) | 30 –45 |
| Samples | Epit (mVSCE) | Ecorr (mVSCE) | Epit − Ecorr (mV) | Ip (A/cm2) |
|---|---|---|---|---|
| 304 stainless steel | 388 ± 11 | –277 ± 11 | 665 ± 20 | (0.73–1.3) × 10−6 |
| Cr plating | 918 ± 20 | –543 ± 12 | 1461 ± 34 | (0.20–1.5) × 10−4 |
| Fe-based AC | 929 ± 18 | –294 ± 17 | 1223 ± 34 | (0.22–1.1) × 10−5 |
| Samples | Rsol (cm2) | Rpore (cm2) | Rp (cm2) | CPEfilm | CPEdl | ||
|---|---|---|---|---|---|---|---|
| Y0 (cm−2·s−n) | n | Y0 (cm−2·s−n) | n | ||||
| pH = 7, 24 h | 14.91 | 1561 | 1.7 × 105 | 2.77 × 10−5 | 0.89 | 3.39 × 10−4 | 0.44 |
| pH = 7, 216 h | 14.96 | 1060 | 3.7 × 1011 | 1.84 × 10−5 | 0.91 | 2.24 × 10−4 | 0.38 |
| pH = 7, 504 h | 15.31 | 831.4 | 1.7 × 106 | 1.82 × 10−5 | 0.90 | 2.36 × 10−4 | 0.35 |
| pH = 1, 24 h | 14.55 | 10530 | 4.6 × 104 | 7.8 × 10−4 | 0.62 | 13.7 × 10−4 | 1 |
| pH = 1, 216 h | 11.45 | 18 | 1.5 × 104 | 10.3 × 10−4 | 0.59 | 3.5 × 10−4 | 0.96 |
| pH = 1, 504 h | 12.17 | 9 | 6.6 × 103 | 9.4 × 10−4 | 0.68 | 21.6 × 10−4 | 0.87 |
| Samples | Rsol (cm2) | Rpore (cm2) | Rp (cm2) | Rdiff (cm2) | CPEfilm | CPEdl | Zw−Y0 (cm2·s−1/2) | ||
|---|---|---|---|---|---|---|---|---|---|
| Y0 (cm−2·s−n) | n | Y0 (cm−2·s−n) | n | ||||||
| pH = 13, 24 h | 12.44 | 6 | 8.7 × 1014 | 1118 | 6.50 × 10−6 | 0.99 | 2.61 × 10−4 | 0.52 | 1.4 × 10−4 |
| pH = 13, 216 h | 12.73 | 20380 | 1.4 × 1013 | 205.8 | 1.89 × 10−5 | 0.88 | 8.10 × 10−4 | 1.00 | 2.7 × 10−4 |
| pH = 13, 504 h | 13.89 | 14560 | 8.3 × 1013 | 242.5 | 2.00 × 10−5 | 0.88 | 9.24 × 10−4 | 1.00 | 2.9 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Deng, C.; Song, J.; Deng, C.; Liu, M.; Dai, M. Electrochemical Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed. Coatings 2019, 9, 226. https://doi.org/10.3390/coatings9040226
Zhang J, Deng C, Song J, Deng C, Liu M, Dai M. Electrochemical Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed. Coatings. 2019; 9(4):226. https://doi.org/10.3390/coatings9040226
Chicago/Turabian StyleZhang, Jifu, Chunming Deng, Jinbing Song, Changguang Deng, Min Liu, and Mingjiang Dai. 2019. "Electrochemical Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed" Coatings 9, no. 4: 226. https://doi.org/10.3390/coatings9040226




