Effect of Urea-Formaldehyde-Coated Epoxy Microcapsule Modification on Gloss, Toughness and Chromatic Distortion of Acrylic Copolymers Waterborne Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Preparation of Microcapsules
2.3. Preparation of Coatings
2.4. Performance Test
3. Results and Discussion
3.1. Effect of Microcapsule on the Properties of Waterborne Coatings
3.1.1. Analysis of Gloss of Coating
3.1.2. Color Difference Analysis of Coating
3.1.3. Flexibility Analysis of Coating
3.2. Effect of Different Coating Processes
3.2.1. Analysis of Gloss of Coating
3.2.2. Color Difference Analysis of Coating
3.2.3. Flexibility Analysis of Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, J.; Jiang, Y.; Zhang, T.; Dai, Y.; Yang, D.; Qiu, F.; Yu, Z.P.; Yang, P. Synthesis of UV-curing waterborne polyurethane-acrylate coating and its photopolymerization kinetics using FT-IR and photo-DSC methods. Prog. Org. Coat. 2018, 122, 10–18. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Lu, R.; Miyakoshi, T. Synergistic effect of addition of fillers on properties of interior waterborne UV-curing wood coatings. Coatings 2017, 8, 9. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, F.; Wang, Y.; Yang, D.; Wu, W.; Chen, Z.; Zhu, J. Preparation, mechanical properties of waterborne polyurethane and crosslinked polyurethane-acrylate composite. J. Appl. Polym. Sci. 2012, 124, 958–968. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Qian, J. Synthesis and properties of a novel UV-curable waterborne hyperbranched polyurethane. J. Coat. Tech. Res. 2014, 11, 319–328. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Zhou, D.; Zhang, Y.; Wang, X.; Yang, R. Biodegradable polyvinyl alcohol nanocomposites made from rice straw fibrils: Mechanical and thermal properties. J. Compos. Mater. 2013, 47, 1449–1459. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, T.; Yang, M.; Zhang, Q. Preparation and characterization of novel melamine modified poly(urea-formaldehyde) self-repairing microcapsules. Coll. Surf. A 2010, 371, 91–97. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Z.; Zhang, J. Compressive creep and recovery behaviors of seat cushions in upholstered. Wood Fiber Sci. 2015, 47, 431–444. [Google Scholar]
- Gu, Y.; Wu, Z.; Zhang, J. Load-deflection behavior of rattan chair seats. Wood Fiber Sci. 2016, 48, 13–24. [Google Scholar]
- Verma, G.; Dhoke, S.K.; Khanna, A.S. Polyester based-siloxane modified waterborne anticorrosive hydrophobic coating on copper. Surf. Coat. Tech. 2015, 221, 1229–1235. [Google Scholar] [CrossRef]
- Jeong, J.; Han, Y.; Yang, J.; Kwak, D.S.; Jeong, H.M. Waterborne polyurethane modified with poly(ethylene glycol) macromer for waterproof breathable coating. Prog. Org. Coat. 2017, 103, 69–75. [Google Scholar] [CrossRef]
- Nikolic, M.; Barsberg, S.; Larsen, F.H.; Lof, D.; Mortensen, K.; Sanadi, A.R. Mechanical characteristics of alkyd binder reinforced by surface modified colloidal nano silica. Prog. Org. Coat. 2016, 90, 147–153. [Google Scholar] [CrossRef]
- Guo, W.; Jia, Y.; Tian, K.; Xu, Z.; Jiao, J.; Li, R.; Wu, Y.; Cao, L.; Wang, H. UV-Triggered self-healing of a single robust SiO2 microcapsule based on cationic polymerization for potential application in aerospace coatings. ACS Appl. Mater. Inter. 2016, 8, 21046–21054. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, W.; Xin, Y.; Wang, H.; Zhao, Y.; Li, W. Preparation and characterization of microcapsule containing epoxy resin and its self-healing performance of anticorrosion covering material. Chin. Sci. Bull. 2011, 56, 439–443. [Google Scholar] [CrossRef]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Zhou, Q. Preparation and characterization of microcapsules based self-healing coatings containing epoxy ester as healing agent. Prog. Org. Coat. 2018, 125, 403–410. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Dutil, I.; Gauquelin, L.; Yan, N.; Farnood, R.R. Preparation of self-healing acrylic latex coatings using novel oil-filled ethyl cellulose microcapsules. Prog. Org. Coat. 2015, 85, 168–177. [Google Scholar] [CrossRef]
- Ataei, S.; Khorasani, S.N.; Torkaman, R.; Neisiany, R.E.; Koochaki, M.S. Self-healing performance of an epoxy coating containing microencapsulated alkyd resin based on coconut oil. Prog. Org. Coat. 2015, 120, 160–166. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Pedersen, L.T.; Kiil, S. Microcapsule-based self-healing anticorrosive coatings: Capsule size, coating formulation, and exposure testing. Prog. Org. Coat. 2012, 75, 309–318. [Google Scholar] [CrossRef]
- Wang, H.; Hu, S.; Cai, S.; Yu, F. Preparation and properties of bisphenol a epoxy resin microcapsules coated with melamine-formaldehyde resin. Polyn. Bull. 2014, 71, 2407–2419. [Google Scholar] [CrossRef]
- Safaei, F.; Khorasani, S.N.; Rahnama, H.; Neisiany, R.E.; Koochaki, M.S. Single microcapsules containing epoxy healing agent used for development in the fabrication of cost efficients self-healing epoxy coating. Pro. Org. Coat. 2018, 114, 40–46. [Google Scholar] [CrossRef]
- Sadrabadi, T.E.; Allahkaram, S.R.; Staab, T.; Towhidi, N. Preparation and characterization of durable micro/nanocapsules for use in self-healing anticorrosive coatings. Polym. Sci. Ser. B 2017, 59, 281–291. [Google Scholar] [CrossRef]
- Es-haghi, H.; Mirabedini, S.M.; Imani, M.; Farnood, R.R. Mechanical and self-healing properties of a water-based acrylic latex containing linseed oil filled microcapsules: Effect of pre-silanization of microcapsules’ shell compound. Compos. Part B Eng. 2015, 85, 305–314. [Google Scholar]
- Lu, R.; Wan, Y.; Honda, T.; Ishimura, T.; Kamiya, Y.; Miyakoshi, T. Design and characterization of modified urethane lacquer coating. Prog. Org. Coat. 2006, 57, 215–222. [Google Scholar] [CrossRef]
- Perfetti, G.; Arfsten, J.; Kwade, A.; Wildeboer, W.J.; Meesters, G.M.H. Repeated impacts tests and nanoindentation as complementary tools for mechanical characterization of polymer-coated particles. J. Appl. Polym. Sci. 2010, 118, 790–804. [Google Scholar]
- Ghazali, H.; Ye, L.; Zhang, M. Mode II interlaminar fracture toughness of CF/EP composite containing microencapsulated healing resins. Compos. Sci. Technol. 2017, 142, 275–285. [Google Scholar] [CrossRef]

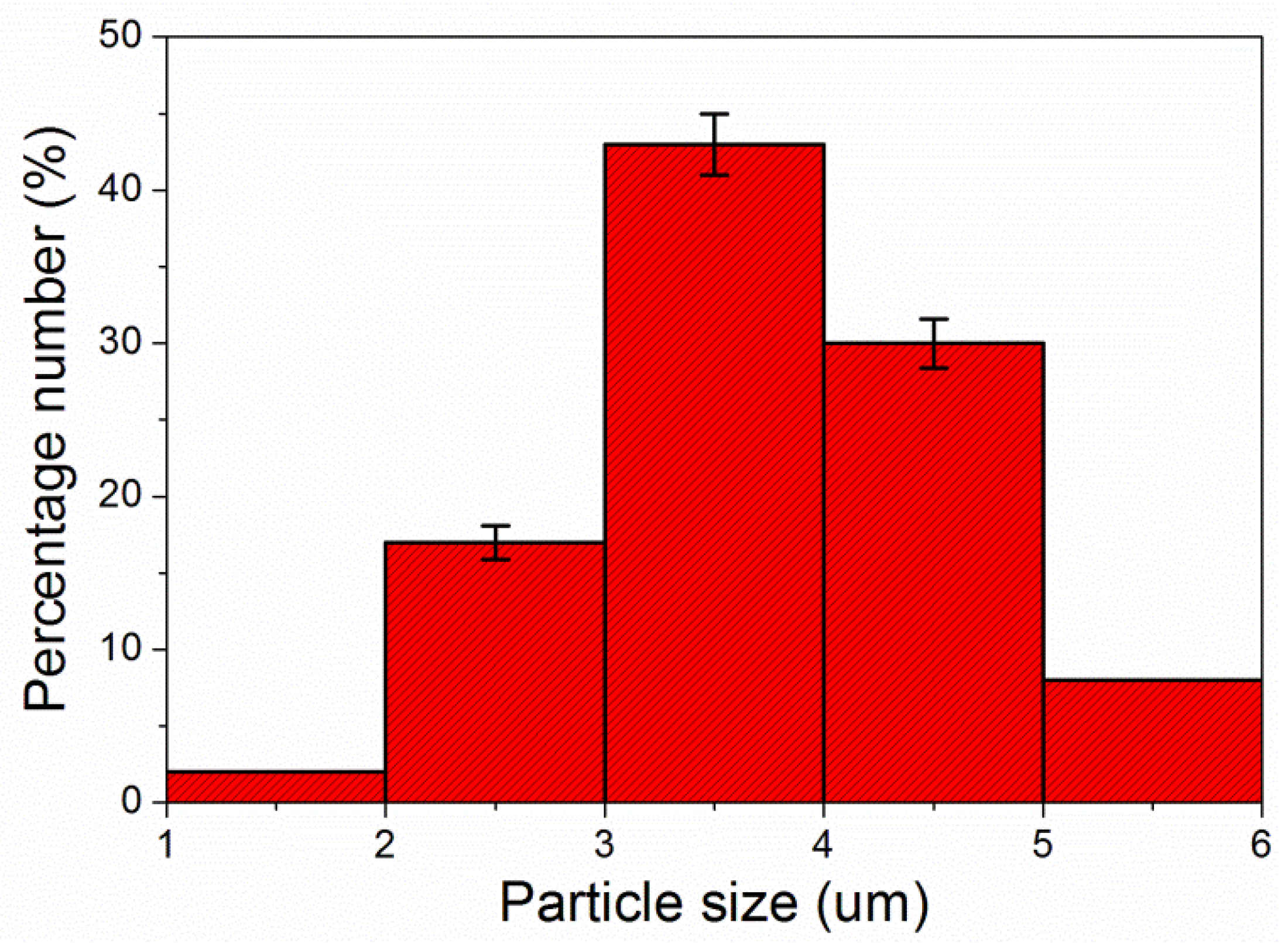
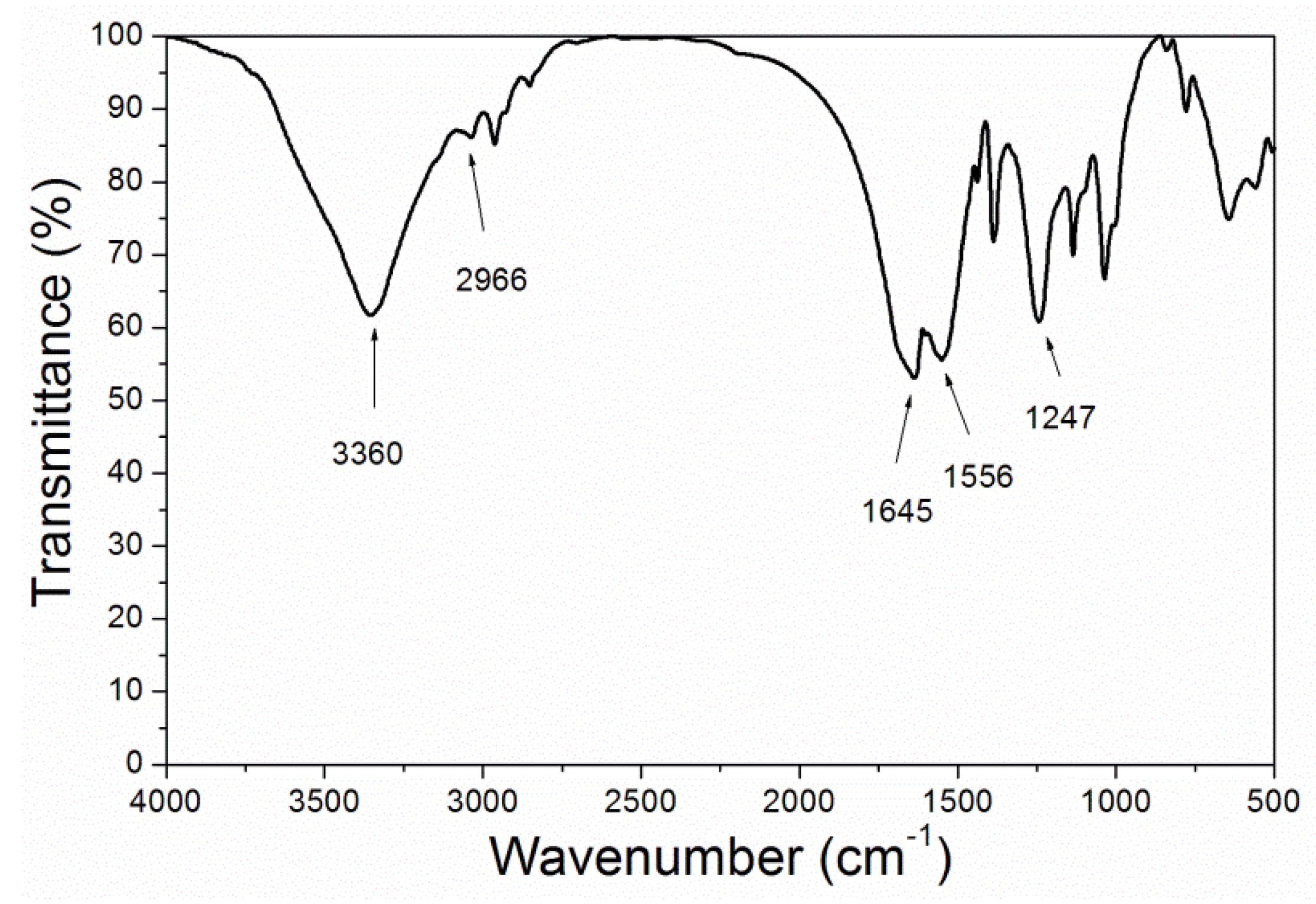









| Samples | Layers of Primer | Layers of Topcoat |
|---|---|---|
| 1 | 2 | 2 |
| 2 | 2 | 3 |
| 3 | 3 | 2 |
| 4 | 3 | 3 |
| Microcapsule Content/% | L | a* | b* | L’ | a*’ | b*’ | ΔL | Δa* | Δb* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 59.80 ± 0 | 13.00 ± 0.02 | 21.20 ± 0.02 | 59.3 ± 0.01 | 11.8 ± 0.01 | 21.20 ± 0.22 | 0.50 ± 0.01 | 1.20 ± 0.02 | 0 ± 0.21 | 1.3 ± 0.05 |
| 1.0 | 60.60 ± 0 | 12.40 ± 0 | 20.30 ± 0.07 | 60.20 ± 0.02 | 10.90 ± 0.01 | 21.10 ± 0.01 | 0.40 ± 0.02 | 1.50 ± 0.01 | −0.80 ± 0.07 | 1.70 ± 0.05 |
| 3.0 | 61.50 ± 0 | 11.80 ± 0.08 | 20.20 ± 0.01 | 61.30 ± 0.02 | 10.10 ± 0.07 | 19.00 ± 0.01 | 0.20 ± 0.01 | 1.70 ± 0.04 | 1.20 ± 0.01 | 2.10 ± 0.05 |
| 5.0 | 62.40 ± 0.02 | 11.00 ± 0.22 | 15.80 ± 0.06 | 62.40 ± 0 | 9.00 ± 0.23 | 18.00 ± 0.14 | 0 ± 0.02 | 2.00 ± 0.04 | −2.20 ± 0.09 | 3.00 ± 0.05 |
| 8.0 | 62.4 0 ± 0.02 | 11.00 ± 0.09 | 22.60 ± 0.02 | 61.90 ± 0.01 | 10.30 ± 0.08 | 15.90 ± 0.02 | 0.50 ± 0.02 | 0.70 ± 0.01 | 6.70 ± 0.04 | 6.80 ± 0.06 |
| 10.0 | 65.20 ± 0.09 | 8.00 ± 0.01 | 15.60 ± 0.04 | 62.60 ± 0.02 | 9.20 ± 0.04 | 14.40 ± 0.02 | 2.60 ± 0.07 | −1.20 ± 0.05 | 1.20 ± 0.04 | 3.10 ± 0.05 |
| 12.0 | 62.30 ± 0.09 | 9.30 ± 0.04 | 15.20 ± 0.08 | 62.50 ± 0.09 | 10.80 ± 0.02 | 13.90 ± 0.08 | −0.20 ± 0.01 | −1.50 ± 0.04 | 1.30 ± 0.08 | 2.0 ± 0.05 |
| Coating Process | L | a* | b* | L’ | a*’ | b*’ | ΔL | Δa* | Δb* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| Two-layer primer and two-layer topcoat | 58.90 ± 0.02 | 10.40 ± 0.02 | 15.60 ± 0.04 | 58.90 ± 0.02 | 9.60 ± 0.02 | 17.90 ± 0.08 | 0 ± 0.01 | 0.80 ± 0.01 | −2.30 ± 0.07 | 2.40 ± 0.05 |
| Two-layer primer and three-layer topcoat | 60.40 ± 0.02 | 9.50 ± 0.02 | 17.90 ± 0.09 | 60.40 ± 0.02 | 10.50 ± 0.07 | 17.40±0.08 | 0 ± 0.01 | −1.00 ± 0.06 | 0.50 ± 0.01 | 1.10 ± 0.05 |
| Three-layer primer and two-layer topcoat | 63.90 ± 0.05 | 6.50 ± 0.02 | 13.70 ± 0.02 | 63.20 ± 0.02 | 6.00 ± 0.01 | 13.40 ± 0.02 | 0.70 ± 0.03 | 0.50 ± 0.01 | 0.30 ± 0.01 | 0.90 ± 0.05 |
| Three-layer primer and three-layer topcoat | 65.20± 0.13 | 7.40 ± 0.02 | 14.80 ± 0.01 | 61.80 ± 0.06 | 7.70 ± 0.02 | 16.20 ± 0.02 | 3.40 ± 0.07 | −0.30 ± 0.01 | 1.40 ± 0.02 | 3.70 ± 0.08 |
| Coating Process | L | a* | b* | L’ | a*’ | b*’ | ΔL | Δa* | Δb* | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|
| Two-layer primer and two-layer topcoat | 65.90± 0.02 | 5.60 ± 0.02 | 14.30 ± 0.02 | 64.10 ± 0.02 | 6.30 ± 0.02 | 11.90 ± 0.02 | 1.80 ± 0.01 | −0.70 ± 0.01 | 2.40 ± 0.03 | 3.10 ± 0.05 |
| Two-layer primer and three-layer topcoat | 64.00± 0.29 | 9.30 ± 0.02 | 14.10 ± 0.01 | 62.90 ± 0.27 | 9.10 ± 0.02 | 14.20 ± 0.01 | 1.10 ± 0.02 | 0.20 ± 0.01 | −0.10 ± 0 | 1.10 ± 0.05 |
| Three-layer primer and two-layer topcoat | 61.80± 0.09 | 8.10 ± 0 | 15.10 ± 0.01 | 62.90 ± 0.05 | 8.00 ± 0 | 14.90 ± 0.01 | −1.10 ± 0.04 | 0.10 ± 0 | 0.20 ± 0 | 1.10 ± 0.05 |
| Three-layer primer and three-layer topcoat | 64.80 ± 0 | 7.90 ± 0.09 | 14.70 ± 0.02 | 64.70 ± 0.02 | 6.70 ± 0 | 13.80 ± 0.02 | 0.10 ± 0.02 | 1.20 ± 0.06 | 0.90 ± 0.01 | 1.50 ± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Wang, L.; Qian, X. Effect of Urea-Formaldehyde-Coated Epoxy Microcapsule Modification on Gloss, Toughness and Chromatic Distortion of Acrylic Copolymers Waterborne Coating. Coatings 2019, 9, 239. https://doi.org/10.3390/coatings9040239
Yan X, Wang L, Qian X. Effect of Urea-Formaldehyde-Coated Epoxy Microcapsule Modification on Gloss, Toughness and Chromatic Distortion of Acrylic Copolymers Waterborne Coating. Coatings. 2019; 9(4):239. https://doi.org/10.3390/coatings9040239
Chicago/Turabian StyleYan, Xiaoxing, Lin Wang, and Xingyu Qian. 2019. "Effect of Urea-Formaldehyde-Coated Epoxy Microcapsule Modification on Gloss, Toughness and Chromatic Distortion of Acrylic Copolymers Waterborne Coating" Coatings 9, no. 4: 239. https://doi.org/10.3390/coatings9040239
APA StyleYan, X., Wang, L., & Qian, X. (2019). Effect of Urea-Formaldehyde-Coated Epoxy Microcapsule Modification on Gloss, Toughness and Chromatic Distortion of Acrylic Copolymers Waterborne Coating. Coatings, 9(4), 239. https://doi.org/10.3390/coatings9040239





