Feasibility and Surface Evaluation of the Pigment from Scytalidium cuboideum for Inkjet Printing on Textiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pigment Production
2.2. Crystallization
2.3. Textiles
2.4. Immersion
2.5. Dripping
2.6. Inkjet Printing
2.6.1. CTAB-Based Ink
2.6.2. Ethanol-Based Ink
2.6.3. Acetone-Based Ink
2.7. Inkjet Printing
2.8. Image Scanning
2.9. Scanning Electron Microscopy (SEM)
3. Results
3.1. Textile Controls
3.2. Dripping and Immersion
3.2.1. Cotton
3.2.2. Polyester
3.3. Printing: CTAB, EtOH and Acetone
3.3.1. Controls
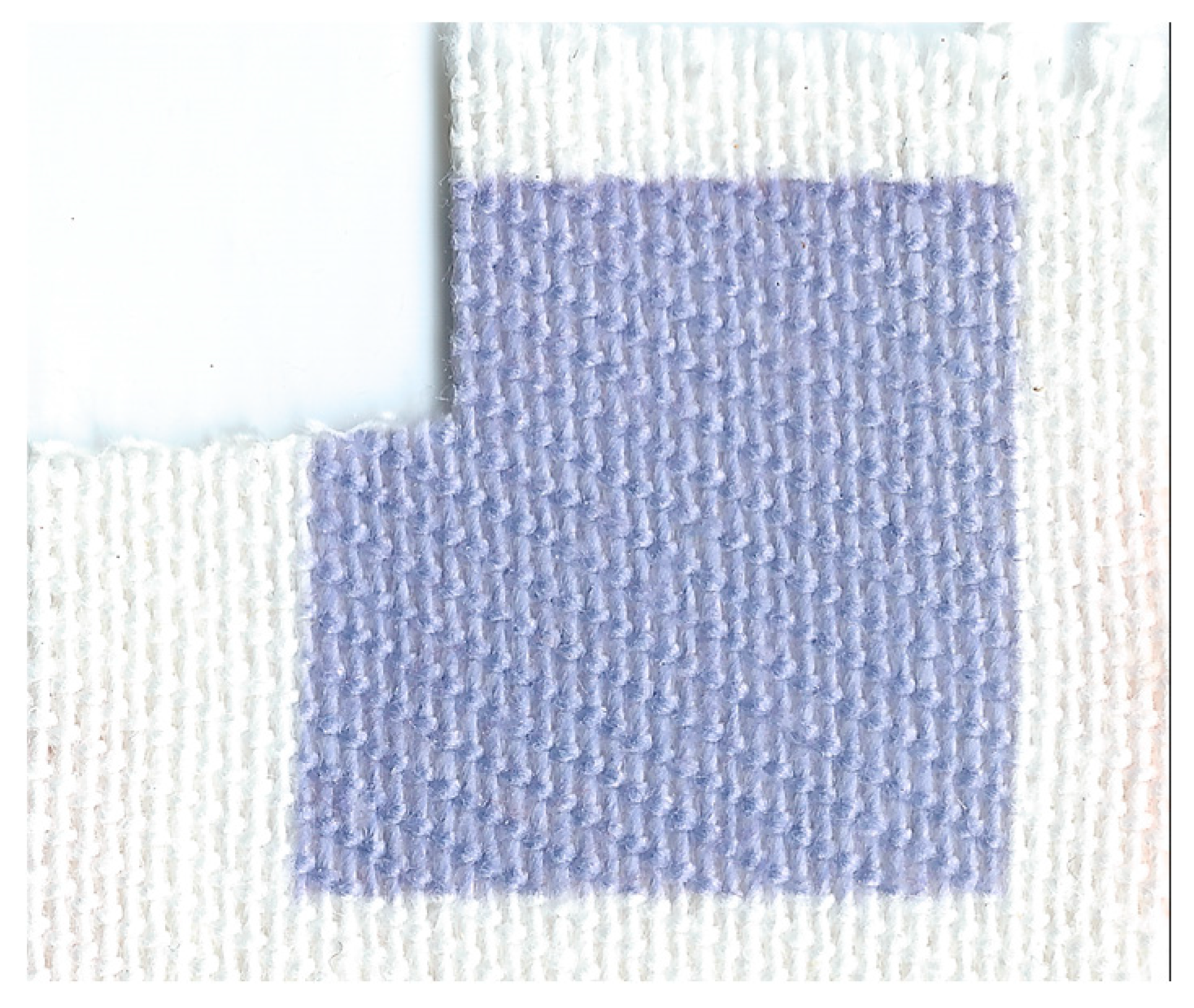
3.3.2. Cotton and Polyester
3.4. Printing: Acetone
4. Discussion
4.1. Dripping and Immersion
4.2. Printing: CTAB, Ethanol, and Acetone
4.3. Fungal Pigments—Breaking the Rules
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schoeser, M. World Textiles, a Concise History, 1st ed.; Thames & Hudson Ltd: New York, NY, USA, 2003; p. 224. [Google Scholar]
- Ferreira, E.S.; Hulme, A.N.; McNab, H.; Quye, A. The natural constituents of historical textile dyes. Chem. Soc. Rev. 2004, 33, 329–336. [Google Scholar] [CrossRef]
- Robinson, S. A History of Dyed Textiles, 1st ed.; Studio Vista Limited: London, UK, 1969; p. 112. [Google Scholar]
- Miles, L.W.C. Textile Priting, 1st ed.; Merrow Publishing Co. Ltd.: London, UK, 1971; p. 46. [Google Scholar]
- Pointing, S. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar] [PubMed]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Nilsson, I.; Möller, A.; Mattiasson, B.; Rubindamayugi, M.; Welander, U. Decolorization of synthetic and real textile wastewater by the use of white-rot fungi. Enzym. Microb. Technol. 2006, 38, 94–100. [Google Scholar] [CrossRef]
- Zaffalon, V. Climate change, carbon mitigation and textiles. Text. World 2010, 160, 34–35. [Google Scholar]
- Mussak, R.A.M.; Bechtold, T. Natural colorants in textile dyeing. In Handbook of Natural Colorants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 315–337. [Google Scholar]
- Poorniammal, R.; Parthiban, M.; Gunasekaran, S.; Murugesan, R.; Thilagavathi, G. Natural dye production from Thermomyces sp. Fungi for textile application. Indian J. Fibre Text. Res. 2013, 38, 276–279. [Google Scholar]
- Samanta, A.K.; Agarwal, P. Application of natural dyes on textiles. Indian J. Fibre Text. Res. 2009, 34, 384–399. [Google Scholar]
- Samanta, A.K.; Konar, A. Dyeing of textiles with natural dyes. In Natural Dyes, 1st ed.; Kumbasar, D.E.A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 29–56. [Google Scholar]
- Gupta, S. Inkjet printing-a revolutionary ecofriendly technique for textile printing. Indian J. Fibre Text. Res. 2001, 26, 156–161. [Google Scholar]
- Kulube, C. Colour generation in textile ink-jet printing. S. Afr. J. Sci. 1998, 94, 469–472. [Google Scholar]
- Ujiie, H.X. Digital Printing of Textiles; Woodhead Publishing: Sawston, UK, 2006. [Google Scholar]
- Cie, C. Ink-Jet Textile Printing, 1st ed.; Woodhead Publishing Limited: Sawston, UK, 2015; p. 183. [Google Scholar]
- Kan, C.W.; Yuen, C.W.M. Digital ink-jet printing on textiles. Res. J. Text. Appar. 2012, 16, 1–24. [Google Scholar] [CrossRef]
- Fryberg, M. Dyes for ink–jet printing. Rev. Prog. Coloration Relat. Top. 2005, 35, 1–30. [Google Scholar] [CrossRef]
- Weber, G.; Chen, H.-L.; Hinsch, E.; Freitas, S.; Robinson, S. Pigments extracted from the wood-staining fungi Chlorociboria aeruginosa, Scytalidium cuboideum, and S. ganodermophthorum show potential for use as textile dyes. Coloration Technol. 2014, 130, 445–452. [Google Scholar] [CrossRef]
- Hinsch, E.M.; Weber, G.; Chen, H.-L.; Robinson, S.C. Colorfastness of extracted wood-staining fungal pigments on fabrics: A new potential for textile dyes. J. Text. Appar. Technol. Manag. 2015, 9. [Google Scholar]
- Palomino Agurto, M.E.; Vega Gutierrez, S.M.; Chen, H.-L.; Robinson, S.C. Wood-rotting fungal pigments as colorant coatings on oil-based textile dyes. Coatings 2017, 7, 152. [Google Scholar] [CrossRef]
- Edwards, R.L.; Kale, N. The structure of xylindein. Tetrahedron 1965, 21, 2095–2107. [Google Scholar] [CrossRef]
- Saikawa, Y.; Watanabe, T.; Hashimoto, K.; Nakata, A. Absolute configuration and tautomeric structure of xylindein, a blue-green pigment of Chlorociboria species. Phytochemistry 2000, 55, 237–240. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.M.; Hazell, K.K.; Simonsen, J.; Robinson, S.C. Description of a naphthoquinonic crystal produced by the fungus Scytalidium cuboideum. Molecules 2018, 23, 1905. [Google Scholar] [CrossRef]
- Otterstedt, A. Investigating green marquetry on bowed-string instruments. The leaves be Greene. Galpin Soc. J. 2001, 54, 330–338. [Google Scholar] [CrossRef]
- Michaelsen, H.; Unger, A.; Fischer, C.-H. Blaugrüne färbung an intarsienhölzern des 16. Bis 18. Jahrhunderts. Restauro 1992, 98, 17–25. [Google Scholar]
- Blanchette, R.A.; Wilmering, A.M.; Baumeister, M. The use of green-stained wood caused by the fungus Chlorociboria in intarsia masterpieces from the 15th century. Holzforschung 1992, 46, 225–232. [Google Scholar] [CrossRef]
- Blackburn, G.M.; Ekong, D.E.; Nielson, A.H.; Todd, L. Xylindein. Chimia 1965, 19, 208–212. [Google Scholar]
- Giles, R.G.F.; Reuben, M.K.; Roos, G.H.P. A quinonoid napthtopyranone as a model for the synthesis of the pigment xylindeine. Photochemical formation of the lactone ring. S. Afr. J. Chem. 1979, 32, 127–129. [Google Scholar]
- Giles, R.G.F.; Green, I.R.; Hugo, V.I. Model studies towards xylindein precursors. S. Afr. J. Chem. 1990, 48, 28–33. [Google Scholar]
- Maeda, M.; Yamauchi, T.; Oshima, K.; Shimomura, M.; Miyauchi, S.; Mukae, K.; Sakaki, T.; Shibata, M.; Wakamatsu, K. Extraction of xylindein from Chlorociboria aeruginosa complex and its biological characteristics. Bull. Nagaoka Univ. Technol. 2003, 25, 105–111. [Google Scholar]
- Beck, H.G.; Freitas, S.; Weber, G.; Robinson, S.C.; Morrell, J.J. Resistance of Fungal Derived Pigments to Ultraviolet Light Exposure; IRG/WP, Ed.; International Research Group in Wood Protection: St George, UT, USA, 2014. [Google Scholar]
- Robinson, S.C.; Tudor, D.; Zhang, W.R.; Ng, S.; Cooper, P.A. Ability of three yellow pigment producing fungi to colour wood under controlled conditions. Int. Wood Prod. J. 2014, 5, 103–107. [Google Scholar] [CrossRef]
- Kang, H.; Sigler, L.; Lee, J.; Gibas, C.; Yun, S.; Lee, Y. Xylogone ganodermophthora sp. nov., an ascomycetous pathogen causing yellow rot on cultivated mushroom Ganoderma lucidum in Korea. Mycologia 2010, 102, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Utilizing pigment-producing fungi to add commercial value to american beech (Fagus grandifolia). Appl. Microbiol. Biotechnol. 2012, 93, 1041–1048. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.; Van Court, R.C.; Stone, D.; Konkler, M.; Groth, E.; Robinson, S. Relationship between molarity and color in the crystal (‘dramada’) produced by Scytalidium cuboideum, in two solvents. Molecules 2018, 23, 2581. [Google Scholar] [CrossRef]
- Hinsch, E.M. A Comparative Analysis of Extracted Fungal Pigments and Commercially Available Dyes for Colorizing Textiles. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2015. [Google Scholar]
- Hinsch, E.M.; Robinson, S.C. Mechanical color reading of wood-staining fungal pigment textile dyes: An alternative method for determining colorfastness. Coatings 2016, 6, 25. [Google Scholar] [CrossRef]
- Pittis, L.; Rodrigues de Oliveira, D.; Vega Gutierrez, S.M.; Robinson, S.C. Alternative carrier solvents for pigments extracted from spalting fungi. Materials 2018, 11, 897. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Tudor, D.; Snider, H.; Cooper, P.A. Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems. AMB Express 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Robinson, S.C.; Weber, G.; Hinsch, E.; Vega Gutierrez, S.M.; Pittis, L.; Freitas, S. Utilizing extracted fungal pigments for wood spalting: A comparison of induced fungal pigmentation to fungal dyeing. J. Coat. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.M.; Robinson, S.C. Microscopic analysis of pigments extracted from spalting fungi. J. Fungi 2017, 3, 15. [Google Scholar] [CrossRef]
- Young, J.A. Dichloromethane. J. Chem. Educ. 2004, 81, 1415. [Google Scholar] [CrossRef]
- Almoughni, H.; Gong, H. Capillary flow of liquid water through yarns: A theoretical model. Text. Res. J. 2015, 85, 722–732. [Google Scholar] [CrossRef]
- Palomino Agurto, M.E. Wood-Rotting Fungal Pigments as Colorant Coatings on Oil-Based Textile Dyes: A Detailed View of The Interaction Between Fungal Pigments and Some Commercial Fabrics. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2018. [Google Scholar]
- Leelajariyakul, S.; Noguchi, H.; Kiatkamjornwong, S. Surface-modified and micro-encapsulated pigmented inks for ink jet printing on textile fabrics. Prog. Org. Coat. 2008, 62, 145–161. [Google Scholar] [CrossRef]
- Xue, C.-H.; Shi, M.-M.; Chen, H.-Z.; Wu, G.; Wang, M. Preparation and application of nanoscale microemulsion as binder for fabric inkjet printing. Colloids Surf. A Physicochem. Eng. Asp. 2006, 287, 147–152. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Arafa, A.A.; Zahran, M.K.; Abdou, L.A.W. Characterisation and application of pigmented uv-curable inkjet inks. Pigment Resin Technol. 2018, 47, 164–172. [Google Scholar] [CrossRef]
- Yuen, C.W.M.; Ku, S.K.A.; Kan, C.W.; Choi, P.S.R. Enhancing textile ink-jet printing with chitosan. Coloration Technol. 2007, 123, 267–270. [Google Scholar] [CrossRef]
- El-Hennawi, H.M.; Shahin, A.A.; Rekaby, M.; Ragheb, A.A. Ink-jet printing of bio-treated linen, polyester fabrics and their blend. Carbohydr. Polym. 2015, 118, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Soleimani-Gorgani, A.; Karami, Z. The effect of biodegradable organic acids on the improvement of cotton ink-jet printing and antibacterial activity. Fibers Polym. 2016, 17, 512–520. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Chen, K.; Yin, Y. Improvement of ink-jet printing performances using β-cyclodextrin forming inclusion complex on cotton fabric. Fibers Polym. 2017, 18, 619–624. [Google Scholar] [CrossRef]
- Leva, M.; Gherasimescu, C.; Butnaru, R. Study regarding the ink-jet printing with reactive dyes of natural silk. The influence of pretreatment conditions on the intensity of the prints. Ind. Text. 2011, 62, 151–154. [Google Scholar]
- Wataoka, I. Ink-jet printing using natural dyes. Sen-I Gakkaishi 2012, 68, 176–179. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S. Cotton fabric inkjet printing with acid dyes. Text. Res. J. 2003, 73, 809–814. [Google Scholar] [CrossRef]
- Karanikas, E.K.; Nikolaidis, N.F.; Tsatsaroni, E.G. Synthesis, characterization, and application of hetarylazo disperse colorants: Preparation and properties of ink-jet inks with active agents for polyester printing. J. Appl. Polym. Sci. 2012, 125, 3396–3403. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, X. Influencing factors of carmine ink jet printing effect on silk fabric. Text. Aux. 2017, 34, 46–49. (In Chinese) [Google Scholar]
- Savvidis, G.; Karanikas, E.; Nikolaidis, N.; Eleftheriadis, I.; Tsatsaroni, E. Ink-jet printing of cotton with natural dyes. Coloration Technol. 2014, 130, 200–204. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega Gutierrez, S.M.; He, Y.; Cao, Y.; Stone, D.; Walsh, Z.; Malhotra, R.; Chen, H.-L.; Chang, C.-H.; Robinson, S.C. Feasibility and Surface Evaluation of the Pigment from Scytalidium cuboideum for Inkjet Printing on Textiles. Coatings 2019, 9, 266. https://doi.org/10.3390/coatings9040266
Vega Gutierrez SM, He Y, Cao Y, Stone D, Walsh Z, Malhotra R, Chen H-L, Chang C-H, Robinson SC. Feasibility and Surface Evaluation of the Pigment from Scytalidium cuboideum for Inkjet Printing on Textiles. Coatings. 2019; 9(4):266. https://doi.org/10.3390/coatings9040266
Chicago/Turabian StyleVega Gutierrez, Sarath M., Yujuan He, Yu Cao, Derek Stone, Zielle Walsh, Rajiv Malhotra, Hsiou-Lien Chen, Chih-Hung Chang, and Seri C. Robinson. 2019. "Feasibility and Surface Evaluation of the Pigment from Scytalidium cuboideum for Inkjet Printing on Textiles" Coatings 9, no. 4: 266. https://doi.org/10.3390/coatings9040266




