Nanocrystalline Cermet Coatings for Erosion–Corrosion Protection
Abstract
:1. Introduction
2. Cr3C2-NiCr and WC-Based Cermet Coatings
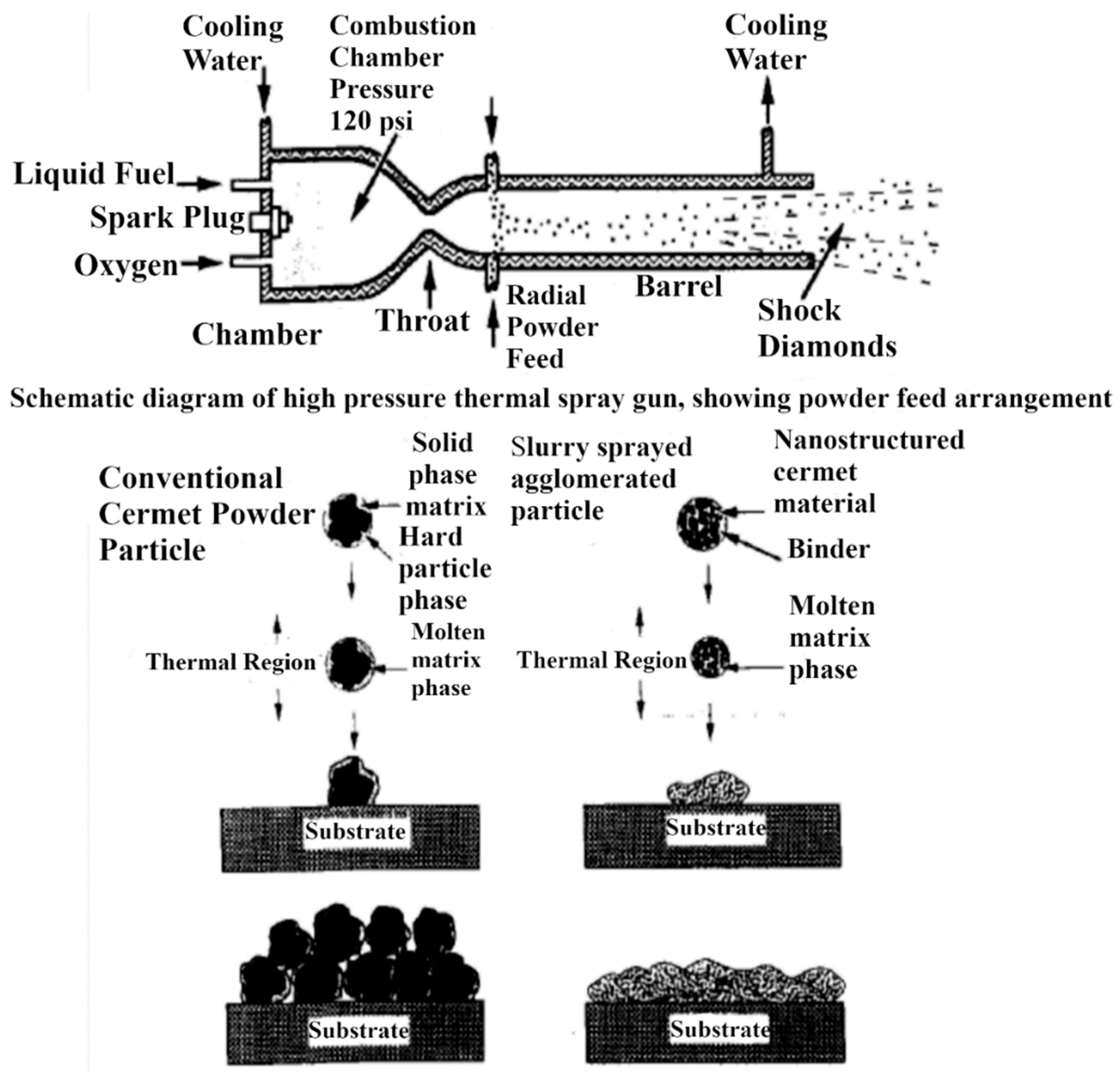
2.1. Thermal Spray Techniques for Synthesis of Cr3C2-NiCr and WC-Based Cermet Coatings
2.2. Chracterisation of Cr3C2-NiCr and WC-Based Cermet Coatings
2.3. Nanostructured Cermet Coatings
2.3.1. Synthesis of Nanocrystalline Feedstock Powders
- (a)
- Chemical processing: In general, for industrial application, chromium carbide cermet powders are synthesized by crushing, grinding, blending, and consolidation of the constituent powders. The limitation of this method is that the scale of the morphological and compositional variation is determined by size of the phases in the blended powder; also, during processing, it is prone to include impurities, whereas the advanced thermochemical method uses precursor compounds in which respective elements A, B, and C are mixed at molecular level [59]. A, B, and C compounds are made using aqueous solution and transferred into nanostructured AC/B followed by thermochemical conversion. The first step involved spray drying of aqueous solution containing appropriate quantities of Cr, Ni, and C to synthesize Cr3C2/Ni cermet as the end product [59]. Spray-dried precursor powder is heated at >1100 °C in a tube furnace for 5 h in an inert gas environment [59]. This heat treatment allows solid-state chemical reactions resulting in conversion of spray-dried powder to nanostructured form [59]. Peter et al. [60] patented aqueous solution reaction (ASR) and organic solution reaction (OSR) methods for producing nanostructured Cr-C/NiCr and WC/Co powders in the form of loose agglomerates of variable size and morphology. Using these procedures, powders can be ultrasonically dispersed in an aqueous or organic liquid medium with a polymer or paraffin binder and spray dried to form uniform-sized spherical agglomerates of 5–25 μm diameter. Moreover, during thermal spraying, the nano-composite powders experience partial melting and undergo splat quenching when they impact the substrate surface [60].
- (b)
- Mechanical alloying/milling: This method is used to produce a large quantity of nanostructured material. This method can also be employed to produce a variety of compositions. There are four types of mills available to carry out milling: (1) Attritor mill, (2) uniball mill, (3) vibratory mill, and (4) planetary mill. Among them, attritor mill has the highest charge capacity. It utilizes a stainless-steel container with several horizontal impellers joined to a rotating (around 180 rpm) vertical shaft driven by external motor [25]. It consists of a ball-to-powder-mass ratio of 20:1 [25,61]. Rotating impellers energize the moving balls, which further causes grain size refinement of powder by impact under a controlled milling environment. A controlled environment is necessary to decrease contamination of powder and control powder temperature. The milling environment can be gaseous, liquid, or solid. In some cases, using a liquid environment like liquid nitrogen, methanol, acetone, etc., can affect powder particle size and morphology [25]. Xu [62] reported that the embedded sub-particle or plurality of embedded sub-particles may include various metal, carbon, metal oxide, metal nitride, metal carbide, intermetallic compounds, or cermet particles, or a combination thereof by any suitable method, including, for example, by ball milling or cryo-milling hard particles together with the particle core material.
2.3.2. Thermal Spraying of Nanocrystalline Coatings
2.3.3. Characterization of Nano-Crystalline Powder Synthesized by Mechanical Milling
3. Wear Resistance of Cr3C2-NiCr and WC-Based Nanostructured Coatings
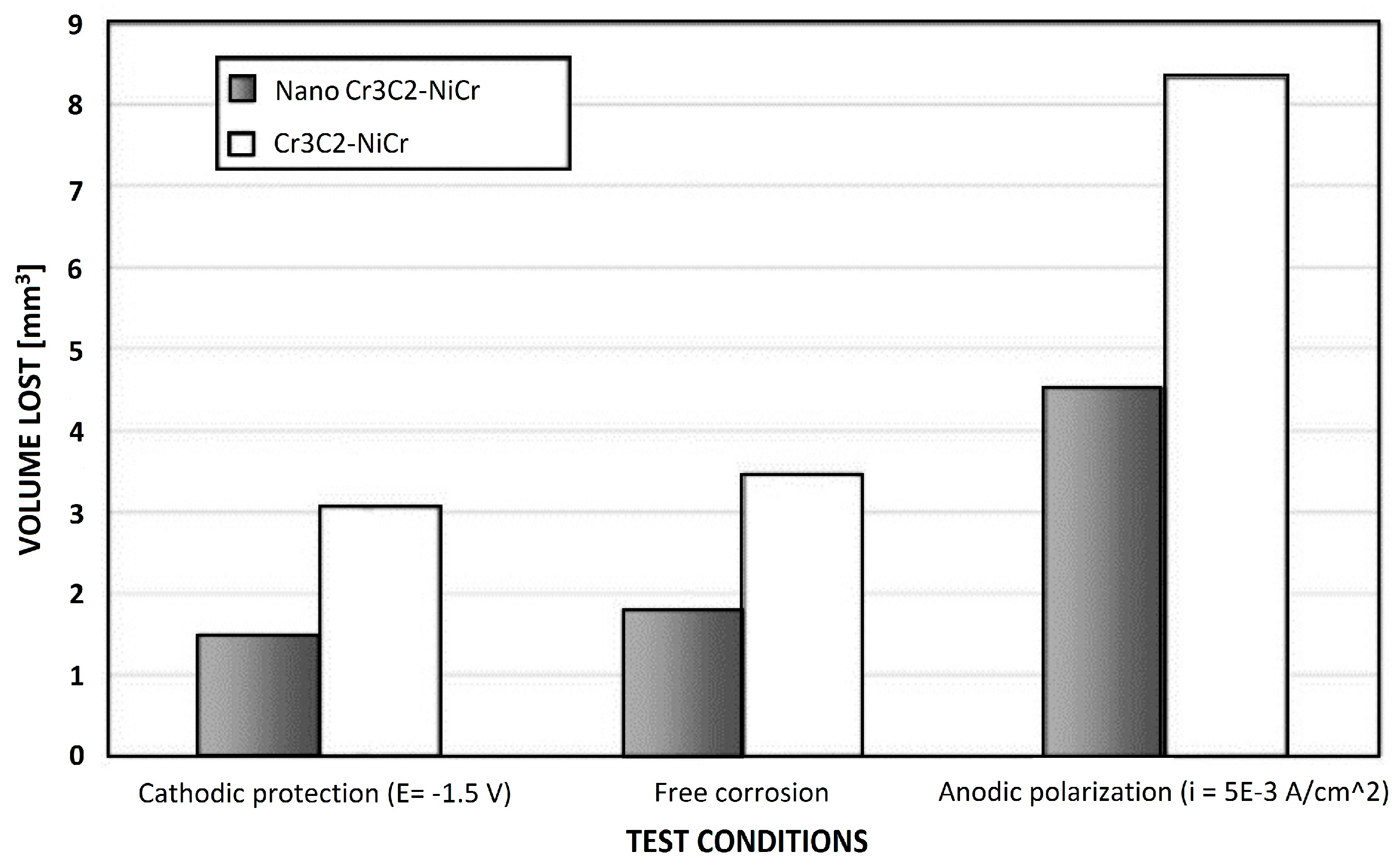
4. Erosion–Corrosion Resistance of Cr3C2-NiCr and WC-Based Nanostructured Coatings
- Wear-corrosion test at open circuit potential (0.6 wt. % NaCl solution) using 200 rpm rotation/5 N applied load;
- Corrosion test at open circuit potential in the same electrolyte without any applied load;
- Lubricated wear test was obtained in the same electrolyte using 200 rpm rotation speed and 5 N applied load.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, H.; Chittosiya, C.; Shukla, V.N. HVOF Sprayed WC Based Cermet Coating for Mitigation of Cavitation, Erosion & Abrasion in Hydro Turbine Blade. Mater. Today Proc. 2018, 5, 6413–6420. [Google Scholar] [CrossRef]
- Rayes, M.M.E.; Abdo, H.S.; Khalil, K.A. Erosion—Corrosion of Cermet Coating. Int. J. Electrochem. Sci. 2013, 8, 1117–1137. [Google Scholar]
- Prasanna, N.D.; Siddaraju, C.; Shetty, G.; Ramesh, M.R.; Reddy, M. Studies on the role of HVOF coatings to combat erosion in turbine alloys. Mater. Today Proc. 2018, 5, 3130–3136. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Tiwari, A. Graphene: The Thinnest Known Coating for Corrosion Protection. JOM 2014, 66, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Dumée, L.F.; He, L.; Wang, Z.; Sheath, P.; Xiong, J.; Feng, C.; Tan, M.Y.; She, F.; Duke, M.; Gray, S.; et al. Growth of nano-textured graphene coatings across highly porous stainless steel supports towards corrosion resistant coatings. Carbon 2015, 87, 395–408. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Rossi, S.; Cristel, R.; Bonora, P.L. Corrosion and wear behaviour of HVOF cermet coatings used to replace hard chromium. Electrochim. Acta 2004, 49, 2803–2814. [Google Scholar] [CrossRef]
- Mann, B.S.; Arya, V. Abrasive and erosive wear characteristics of plasma nitriding and HVOF coatings: Their application in hydro turbines. Wear 2001, 249, 354–360. [Google Scholar] [CrossRef]
- Hazra, S.; Sabiruddin, K.; Bandyopadhyay, P.P. Plasma and HVOF sprayed WC–Co coatings as hard chrome replacement solution. Surf. Eng. 2012, 28, 37–43. [Google Scholar] [CrossRef]
- Shukla, V.; Jayaganthan, R.; Tewari, V. Degradation behaviour and microstructural characterisation of HVOF-sprayed Cr3C2-NiCr cermet coatings in molten salt environment. Int. J. Mater. Prod. Technol. 2016, 53, 15–27. [Google Scholar] [CrossRef]
- Kamal, S.; Sharma, K.V.; Jayaganthan, R.; Prakash, S. Hot corrosion behavior of thermal spray coatings on superalloy in coal-fired boiler environment. J. Mater. Res. 2015, 30, 2829–2843. [Google Scholar] [CrossRef]
- Rana, N.; Mahapatra, M.M.; Jayaganthan, R.; Prakash, S. Deposition of nanocrystalline coatings by modified LVOF thermal spray method. J. Alloys Compd. 2014, 615, 779–783. [Google Scholar] [CrossRef]
- Chen, H.; Si, Y.Q.; McCartney, D.G. An analytical approach to the β-phase coarsening behaviour in a thermally sprayed CoNiCrAlY bond coat alloy. J. Alloys Compd. 2017, 704, 359–365. [Google Scholar] [CrossRef]
- Rana, N.; Mahapatra, M.M.; Jayaganthan, R.; Prakash, S. High-Temperature Oxidation and Hot Corrosion Studies on NiCrAlY Coatings Deposited by Flame-Spray Technique. J. Therm. Spray Technol. 2015, 24, 769–777. [Google Scholar] [CrossRef]
- Rana, N.; Jayaganthan, R.; Prakash, S. Microstructural Features and Oxidation Behaviour of NiCrAlY Coatings Obtained by HVOF Process. Adv. Mater. Res. 2012, 585, 507–511. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Gao, W.; Zhang, J.; Zheng, Y.; Zheng, Y. Slurry erosion-corrosion resistance and microbial corrosion electrochemical characteristics of HVOF sprayed WC-10Co-4Cr coating for offshore hydraulic machinery. Int. J. Refract. Met. Hard Mater. 2018, 74, 7–13. [Google Scholar] [CrossRef]
- Parida, M.; Nanda, S.P.; Bhuyan, S.K.; Mishra, S.C. Sea water Corrosion of Nickel based Plasma Spray Coating. Iop Conf. Ser. Mater. Sci. Eng. 2018, 338, 012051. [Google Scholar] [CrossRef] [Green Version]
- Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T.; Steeger, M. Impact wear of an HVOF-sprayed Cr3C2-NiCr coating. Int. J. Refract. Met. Hard Mater. 2018, 70, 191–196. [Google Scholar] [CrossRef]
- Guilemany, J.; Dosta, S.; Nin, J.; Miguel, J. Study of the properties of WC-Co nanostructured coatings sprayed by high-velocity oxyfuel. J. Therm. Spray Technol. 2005, 14, 405–413. [Google Scholar]
- Reyes-Mojena, M.Á.; Sánchez-Orozco, M.; Carvajal-Fals, H.; Sagaró-Zamora, R.; Camello-Lima, C.R. A comparative study on slurry erosion behavior of HVOF sprayed coatings. DYNA 2017, 84, 239–246. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, H.; Singh, N. Fire side erosion–corrosion protection of boiler tubes by nanostructured coatings. Mater. Corros. 2015, 66, 695–709. [Google Scholar] [CrossRef]
- Liu, Y.; Hang, Z.; Chen, H.; Ceng, S.; Gou, G.; Wang, X.; Tu, M.; Wu, X. Erosion–Corrosion Property of CeO2-Modified HVOF WC-Co Coating. J. Therm. Spray Technol. 2016, 25, 815–822. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Howse, H.; Wade, S.A.; Berndt, C.C. Manufacturing of nickel based cermet coatings by the HVOF process. Surf. Eng. 2016, 32, 713–724. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. Characterisation and Corrosion-Erosion Behaviour of Carbide based Thermal Spray Coatings. J. Miner. Mater. Charact. Eng. 2012, 11, 569–586. [Google Scholar] [CrossRef]
- Khan, T.I.; Saha, G.; Glenesk, L.B. Nanostructured composite coatings for oil sand’s applications. Surf. Eng. 2010, 26, 540–545. [Google Scholar] [CrossRef]
- He, J.; Schoenung, J.M. Nanostructured coatings. Mater. Sci. Eng. A 2002, 336, 274–319. [Google Scholar] [CrossRef]
- Amin, S.; Panchal, H. A Review on Thermal Spray Coating Processes. Int. J. Curr. Trends Eng. Res. 2016, 2, 556–563. [Google Scholar]
- Stein, K.J.; Schorr, B.S.; Marder, A.R. Erosion of thermal spray MCr–Cr3C2 cermet coatings. Wear 1999, 224, 153–159. [Google Scholar] [CrossRef]
- Jacobs, L.; Hyland, M.M.; De Bonte, M. Study of the influence of microstructural properties on the sliding-wear behavior of HVOF and HVAF sprayed WC-cermet coatings. J. Therm. Spray Technol. 1999, 8, 125–132. [Google Scholar] [CrossRef]
- Hawthorne, H.M.; Arsenault, B.; Immarigeon, J.P.; Legoux, J.G.; Parameswaran, V.R. Comparison of slurry and dry erosion behaviour of some HVOF thermal sprayed coatings. Wear 1999, 225–229, 825–834. [Google Scholar] [CrossRef]
- Browning, J. Viewing the future of high-velocity oxyfuel (HVOF) and high-velocity air fuel (HVAF) thermal spraying. J. Therm. Spray Technol. 1999, 8, 351. [Google Scholar]
- Morales, A.T. Coating for Superplastic and Quick Plastic Forming Tool and Process of Using. U.S. Patent US6655181B2, 2 December 2003. [Google Scholar]
- Li, C.J.; Wang, Y.Y.; Yang, G.J.; Ohmori, A.; Khor, K.A. Effect of solid carbide particle size on deposition behaviour, microstructure and wear performance of HVOF cermet coatings. Mater. Sci. Technol. 2004, 20, 1087–1096. [Google Scholar] [CrossRef]
- Ji, G.-C.; Li, C.-J.; Wang, Y.-Y.; Li, W.-Y. Microstructural characterization and abrasive wear performance of HVOF sprayed Cr3C2–NiCr coating. Surf. Coat. Technol. 2006, 200, 6749–6757. [Google Scholar] [CrossRef]
- Ahmed, R.; Ali, O.; Faisal, N.H.; Al-Anazi, N.M.; Al-Mutairi, S.; Toma, F.L.; Berger, L.M.; Potthoff, A.; Goosen, M.F.A. Sliding wear investigation of suspension sprayed WC–Co nanocomposite coatings. Wear 2015, 322–323, 133–150. [Google Scholar] [CrossRef]
- Pawlowski, L. Suspension and solution thermal spray coatings. Surf. Coat. Technol. 2009, 203, 2807–2829. [Google Scholar] [CrossRef]
- Killinger, A.; Kuhn, M.; Gadow, R. High-Velocity Suspension Flame Spraying (HVSFS), a new approach for spraying nanoparticles with hypersonic speed. Surf. Coat. Technol. 2006, 201, 1922–1929. [Google Scholar] [CrossRef]
- Fauchais, P.; Montavon, G.; Lima, R.S.; Marple, B.R. Engineering a new class of thermal spray nano-based microstructures from agglomerated nanostructured particles, suspensions and solutions: An invited review. J. Phys. D Appl. Phys. 2011, 44, 093001. [Google Scholar] [CrossRef]
- Berghaus, J.O.; Marple, B.; Moreau, C. Suspension plasma spraying of nanostructured WC-12Co coatings. J. Therm. Spray Technol. 2006, 15, 676–681. [Google Scholar] [CrossRef]
- Korpiola, K. High Temperature Oxidation of Metal, Alloy and Cermet Powders in HVOF Spraying Process; Helsinki University of Technology: Espoo, Finland, 2004. [Google Scholar]
- Ahmed, R.; Faisal, N.H.; Al-Anazi, N.M.; Al-Mutairi, S.; Toma, F.-L.; Berger, L.-M.; Potthoff, A.; Polychroniadis, E.K.; Sall, M.; Chaliampalias, D.; et al. Structure Property Relationship of Suspension Thermally Sprayed WC-Co Nanocomposite Coatings. J. Therm. Spray Technol. 2015, 24, 357–377. [Google Scholar] [CrossRef]
- Vashishtha, N.; Khatirkar, R.K.; Sapate, S.G. Tribological behaviour of HVOF sprayed WC-12Co, WC-10Co-4Cr and Cr3C2–25NiCr coatings. Tribol. Int. 2017, 105, 55–68. [Google Scholar] [CrossRef]
- Kear, B.H.; Strutt, P.R. Chemical processing and applications for nanostructured materials. Nanostructured Mater. 1995, 6, 227–236. [Google Scholar] [CrossRef]
- He, J.; Ice, M.; Lavernia, E.J. Synthesis and characterization of nanostructured Cr3C2 NiCr. Nanostruct. Mater. 1998, 10, 1271–1283. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, H.; Wang, Y.; Mao, S.; Niu, J.; Chen, Y.; Shang, M. Synthesis of chromium carbide (Cr3C2) nanopowders by the carbonization of the precursor. Int. J. Refract. Met. Hard Mater. 2011, 29, 614–617. [Google Scholar] [CrossRef]
- Anand, K.; Subramanian, P.R.; Gray, D.M.; Sampath, S.; Huang, S.-C.; Nelson, W.A.; Hasz, W.C. Nano-structured Coating Systems. WIPO Patent Application WO2005056879, 23 June 2005. [Google Scholar]
- Tjong, S.C.; Chen, H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R: Rep. 2004, 45, 1–88. [Google Scholar] [CrossRef]
- Bolelli, G.; Berger, L.M.; Börner, T.; Koivuluoto, H.; Matikainen, V.; Lusvarghi, L.; Lyphout, C.; Markocsan, N.; Nylén, P.; Sassatelli, P.; et al. Sliding and abrasive wear behaviour of HVOF- and HVAF-sprayed Cr3C2–NiCr hardmetal coatings. Wear 2016, 358–359, 32–50. [Google Scholar] [CrossRef]
- Hodgkiess, T.; Neville, A.; Shrestha, S. Electrochemical and mechanical interactions during erosion–corrosion of a high-velocity oxy-fuel coating and a stainless steel. Wear 1999, 233–235, 623–634. [Google Scholar] [CrossRef]
- Stack, M.M.; Abd El Badia, T.M. Mapping erosion–corrosion of WC/Co–Cr based composite coatings: Particle velocity and applied potential effects. Surf. Coat. Technol. 2006, 201, 1335–1347. [Google Scholar] [CrossRef]
- Alegría-Ortega, J.A.; Ocampo-Carmona, L.M.; Suárez-Bustamante, F.A.; Olaya-Flórez, J.J. Erosion–corrosion wear of Cr/CrN multi-layer coating deposited on AISI-304 stainless steel using the unbalanced magnetron (UBM) sputtering system. Wear 2012, 290–291, 149–153. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.M.; Wei, R. Effect of ion bombardment on structural, mechanical, erosion and corrosion properties of Ti–Si–C–N nanocomposite coatings. Surf. Coat. Technol. 2014, 258, 320–328. [Google Scholar] [CrossRef]
- Mishra, S.B.; Chandra, K.; Prakash, S. Studies on erosion-corrosion behaviour of plasma sprayed Ni3Al coating in a coal-fired thermal power plant environment at 540 °C. Anti-Corros. Methods Mater. 2017, 64, 540–549. [Google Scholar] [CrossRef]
- Venkatesh, L.; Pitchuka, S.B.; Sivakumar, G.; Gundakaram, R.C.; Joshi, S.V.; Samajdar, I. Microstructural response of various chromium carbide based coatings to erosion and nano impact testing. Wear 2017, 386–387, 72–79. [Google Scholar] [CrossRef]
- Hemmati, A.R.; Soltanieh, S.M.; Masoudpanah, S.M. On the Interaction Between Erosion and Corrosion in Chromium Carbide Coating. J. Bio-Tribo-Corros. 2018, 4, 10. [Google Scholar] [CrossRef]
- Liang, X.B.; Cheng, J.B.; Bai, J.Y.; Xu, B.S. Erosion properties of Fe based amorphous/nanocrystalline coatings prepared by wire arc spraying process. Surf. Eng. 2013, 26, 209–215. [Google Scholar] [CrossRef]
- Roy, M.; Pauschitz, A.; Polak, R.; Franek, F. Comparative evaluation of ambient temperature friction behaviour of thermal sprayed Cr3C2–25(Ni20Cr) coatings with conventional and nano-crystalline grains. Tribol. Int. 2006, 39, 29–38. [Google Scholar] [CrossRef]
- Pawlowski, L. Finely grained nanometric and submicrometric coatings by thermal spraying: A review. Surf. Coat. Technol. 2008, 202, 4318–4328. [Google Scholar] [CrossRef]
- Yanpin, L.; Junpeng, M.; Weifeng, Y. Aplication of nanotechnology on hydraulic turbine abrasion and erosion. In Proceedings of the 2010 International Conference on Power System Technology, Hangzhou, China, 24–28 October 2010; pp. 1–3. [Google Scholar]
- Luo, P.; Strutt, P.R. Thermal chemical synthesis of nanostructured chromium carbide cermets. Mater. Sci. Eng. A 1995, 204, 181–185. [Google Scholar] [CrossRef]
- Strutt, P.R.; Kear, B.H.; Boland, R.F. Thermal Spray Method for the Formation of Nanostructured Coatings. U.S. Patent US6277448B2, 21 August 2001. [Google Scholar]
- Tkachivskyi, D.; Juhani, K.; Surženkov, A.; Kulu, P.; Viljus, M.; Traksmaa, R.; Jankauskas, V.; Leišys, R. Production of Thermal Spray Cr3C2-Ni Powders by Mechanically Activated Synthesis. Key Eng. Mater. 2019, 799, 31–36. [Google Scholar] [CrossRef]
- Xu, Z. Nanostructured Powder Metal Compact. European Patent EP2750819A4, 20 January 2016. [Google Scholar]
- Lavernia, E.J. Method for Thermal Spraying of Nanocrystalline Coatings and Materials for the Same. U.S. Patent 5939146, 17 August 1999. [Google Scholar]
- Lima, R.S.; Marple, B.R. Thermal Spray Coatings Engineered from Nanostructured Ceramic Agglomerated Powders for Structural, Thermal Barrier and Biomedical Applications: A Review. J. Therm. Spray Technol. 2007, 16, 40–63. [Google Scholar] [CrossRef] [Green Version]
- Tao, K.; Zhou, X.-L.; Cui, H.; Zhang, J.-S. Oxidation and hot corrosion behaviors of HVAF-sprayed conventional and nanostructured NiCrC coatings. Trans. Nonferrous Met. Soc. China 2009, 19, 1151–1160. [Google Scholar] [CrossRef]
- He, J.; Lavernia, E.J. Precipitation phenomenon in nanostructured Cr3C2–NiCr coatings. Mater. Sci. Eng. A 2001, 301, 69–79. [Google Scholar] [CrossRef]
- Gomari, S.; Sharafi, S. Microstructural characterization of nanocrystalline chromium carbides synthesized by high energy ball milling. J. Alloys Compd. 2010, 490, 26–30. [Google Scholar] [CrossRef]
- Bonache, V.; Salvador, M.D.; Fernández, A.; Borrell, A. Fabrication of full density near-nanostructured cemented carbides by combination of VC/Cr3C2 addition and consolidation by SPS and HIP technologies. Int. J. Refract. Met. Hard Mater. 2011, 29, 202–208. [Google Scholar] [CrossRef]
- Lampke, T.; Wielage, B.; Pokhmurska, H.; Rupprecht, C.; Schuberth, S.; Drehmann, R.; Schreiber, F. Development of particle-reinforced nanostructured iron-based composite alloys for thermal spraying. Surf. Coat. Technol. 2011, 205, 3671–3676. [Google Scholar] [CrossRef]
- Sharafi, S.; Gomari, S. Effects of milling and subsequent consolidation treatment on the microstructural properties and hardness of the nanocrystalline chromium carbide powders. Int. J. Refract. Met. Hard Mater. 2012, 30, 57–63. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, H.; Zhang, S.; Song, W.; Mao, S.; Chen, Y. Effect of reaction time on phase composition and microstructure of chromium carbide nanopowders. Int. J. Refract. Met. Hard Mater. 2013, 41, 558–562. [Google Scholar] [CrossRef]
- Eigen, N.; Gärtner, F.; Klassen, T.; Aust, E.; Bormann, R.; Kreye, H. Microstructures and properties of nanostructured thermal sprayed coatings using high-energy milled cermet powders. Surf. Coat. Technol. 2005, 195, 344–357. [Google Scholar] [CrossRef]
- Shukla, V.N.; Jayaganthan, R.; Tewari, V.K. Degradation Behavior of HVOF-Sprayed Cr3C2-25%NiCr Cermet Coatings Exposed to High Temperature Environment. Mater. Today Proc. 2015, 2, 1805–1813. [Google Scholar] [CrossRef]
- He, J.; Ice, M.; Lavernia, E.J. Synthesis and Characterization of Nanocomposite Coatings. In Nanostructured Films and Coatings; Chow, G.-M., Ovid’ko, I.A., Tsakalakos, T., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 131–148. [Google Scholar]
- He, J.; Ice, M.; Schoenung, J.M.; Lavernia, E.J.; Shin, D.H. Thermal stability of nanostructured Cr3C2-NiCr coatings. J. Therm. Spray Technol. 2001, 10, 293–300. [Google Scholar] [CrossRef]
- Jellad, A.; Labdi, S.; Malibert, C.; Renou, G. Nanomechanical and nanowear properties of Cr3C2 thin films deposited by rf sputtering. Wear 2008, 264, 893–898. [Google Scholar] [CrossRef]
- Jellad, A.; Labdi, S.; Benameur, T. On the hardness and the inherent ductility of chromium carbide nanostructured coatings prepared by RF sputtering. J. Alloys Compd. 2009, 483, 464–467. [Google Scholar] [CrossRef]
- Roy, M.; Pauschitz, A.; Wernisch, J.; Franek, F. The influence of temperature on the wear of Cr3C2–25(Ni20Cr) coating—Comparison between nanocrystalline grains and conventional grains. Wear 2004, 257, 799–811. [Google Scholar] [CrossRef]
- Shmyreva, T.P.; Knapp, J.; Kleyman, A.S. Amorphous-Nanocrystalline-Microcrystalline Coatings and Methods of Production Thereof. U.S. Patent US8465602B2, 18 June 2013. [Google Scholar]
- Wang, B.Q. Effect of alkali chlorides on erosion-corrosion of cooled mild steel and Cr3C2-NiCr coating. Wear 1996, 199, 268–274. [Google Scholar] [CrossRef]
- Peat, T.; Galloway, A.; Toumpis, A.; Harvey, D.; Yang, W.-H. Performance evaluation of HVOF deposited cermet coatings under dry and slurry erosion. Surf. Coat. Technol. 2016, 300, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Sidhu, B.S. Characterization and High-Temperature Erosion Behaviour of HVOF Thermal Spray Cermet Coatings. J. Mater. Eng. Perform. 2016, 25, 250–258. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, K.; Li, Y.; Deng, C.; Zeng, K. High temperature wear performance of HVOF-sprayed Cr3C2-WC-NiCoCrMo and Cr3C2-NiCr hardmetal coatings. Appl. Surf. Sci. 2017, 416, 33–44. [Google Scholar] [CrossRef]
- Kumar, R.K.; Kamaraj, M.; Seetharamu, S.; Anand Kumar, S. A pragmatic approach and quantitative assessment of silt erosion characteristics of HVOF and HVAF processed WC-CoCr coatings and 16Cr5Ni steel for hydro turbine applications. Mater. Des. 2017, 132, 79–95. [Google Scholar] [CrossRef]
- Chakradhar, R.P.S.; Prasad, G.; Venkateswarlu, K.; Srivastava, M. An Investigation on the Wear and Corrosion Behavior of HVOF-Sprayed WC-12Co-Al2O3 Cermet Coating. J. Mater. Eng. Perform. 2018, 27, 1241–1248. [Google Scholar] [CrossRef]
- Bhatia, R.; Singh, H.; Sidhu, B.S. Characterisation of 80% Cr3C2-20% (Ni-20cr) Coating and Erosion Behaviour. Asian J. Eng. Appl. Technol. 2012, 1, 5–12. [Google Scholar]
- Espallargas, N.; Berget, J.; Guilemany, J.M.; Benedetti, A.V.; Suegama, P.H. Cr3C2–NiCr and WC–Ni thermal spray coatings as alternatives to hard chromium for erosion–corrosion resistance. Surf. Coat. Technol. 2008, 202, 1405–1417. [Google Scholar] [CrossRef]
- Staia, M.H.; Valente, T.; Bartuli, C.; Lewis, D.B.; Constable, C.P.; Roman, A.; Lesage, J.; Chicot, D.; Mesmacque, G. Part II: Tribological performance of Cr3C2-25% NiCr reactive plasma sprayed coatings deposited at different pressures. Surf. Coat. Technol. 2001, 146–147, 563–570. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Valentinelli, L.; Rossi, S.; Segna, S. Tribocorrosion behaviour of HVOF cermet coatings. Corros. Sci. 2007, 49, 2781–2799. [Google Scholar] [CrossRef]
- Gariboldi, E.; Rovatti, L.; Lecis, N.; Mondora, L.; Mondora, G.A. Tribological and mechanical behaviour of Cr3C2–NiCr thermally sprayed coatings after prolonged aging. Surf. Coat. Technol. 2016, 305, 83–92. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Lupescu, S.; Benchea, M.; Vizureanu, P. Preliminary Microstructural and Microscratch Results of Ni-Cr-Fe and Cr3C2-NiCr Coatings on Magnesium Substrate. Iop Conf. Ser. Mater. Sci. Eng. 2017, 209, 012024. [Google Scholar] [CrossRef]
- Skandan, G.; Yao, R.; Kear, B.H.; Qiao, Y.; Liu, L.; Fischer, T.E. Multimodal powders: A new class of feedstock material for thermal spraying of hard coatings. Scr. Mater. 2001, 44, 1699–1702. [Google Scholar] [CrossRef]
- He, J.; Ice, M.; Lavernia, E.J.; Dallek, S. Synthesis of nanostructured WC-12 pct Co coating using mechanical milling and high velocity oxygen fuel thermal spraying. Metall. Mater. Trans. A 2000, 31, 541–553. [Google Scholar] [CrossRef]
- He, J.; Lavernia, E.J.; Liu, Y.; Qiao, Y.; Fischer, T.E. Near-nanostructured WC-18 pct Co coatings with low amounts of non-WC carbide phase: Part I. Synthesis and characterization. Metall. Mater. Trans. A 2002, 33, 145–157. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The Effect of Heat Treatment on the Oxidation Mechanism of Blended Powder Cr3C2-NiCr Coatings. J. Therm. Spray Technol. 2010, 19, 119–127. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The role of microstructure in the mechanism of high velocity erosion of Cr3C2–NiCr thermal spray coatings: Part 2—Heat treated coatings. Surf. Coat. Technol. 2009, 203, 1094–1100. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The role of microstructure in the mechanism of high velocity erosion of Cr3C2–NiCr thermal spray coatings: Part 1—As-sprayed coatings. Surf. Coat. Technol. 2009, 203, 1086–1093. [Google Scholar] [CrossRef]
- Matthews, S.; Hyland, M.; James, B. Microhardness variation in relation to carbide development in heat treated Cr3C2–NiCr thermal spray coatings. Acta Mater. 2003, 51, 4267–4277. [Google Scholar] [CrossRef]
- Matthews, S.J.; James, B.J.; Hyland, M.M. Microstructural influence on erosion behaviour of thermal spray coatings. Mater. Charact. 2007, 58, 59–64. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. High temperature erosion of Cr3C2-NiCr thermal spray coatings—The role of phase microstructure. Surf. Coat. Technol. 2009, 203, 1144–1153. [Google Scholar] [CrossRef]
- Wang, B.Q. Dependence of erosion–corrosion on carbide/metal matrix proportion for HVOF Cr3C2–NiCr coatings. Surf. Eng. 1998, 14, 165–169. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Espallargas, N.; Suegama, P.H.; Benedetti, A.V. Comparative study of Cr3C2–NiCr coatings obtained by HVOF and hard chromium coatings. Corros. Sci. 2006, 48, 2998–3013. [Google Scholar] [CrossRef]
- Akhtari Zavareh, M.; Sarhan, A.A.D.M.; Razak, B.B.; Basirun, W.J. The tribological and electrochemical behavior of HVOF-sprayed Cr3C2–NiCr ceramic coating on carbon steel. Ceram. Int. 2015, 41, 5387–5396. [Google Scholar] [CrossRef]
- Pileggi, R.; Tului, M.; Stocchi, D.; Lionetti, S. Tribo-corrosion behaviour of chromium carbide based coatings deposited by HVOF. Surf. Coat. Technol. 2015, 268, 247–251. [Google Scholar] [CrossRef]
- Akhtari Zavareh, M.; Mohammed Sarhan, A.A.D.; Akhtari Zavareh, P.; Basirun, W.J. Electrochemical corrosion behavior of carbon steel pipes coated with a protective ceramic layer using plasma and HVOF thermal spray techniques for oil and gas. Ceram. Int. 2016, 42, 3397–3406. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Chen, S. Solid particle erosion-wear behaviour of Cr3C2–NiCr coating on Ni-based superalloy. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Kumarasamy, M.; Natarajan, S. Selection and characterisation of HVOF cermet coatings for volutes of high capacity pumps of opencast lignite mines. Surf. Eng. 2016, 32, 229–237. [Google Scholar] [CrossRef]
- De Souza, V.A.; Neville, A. Corrosion and erosion damage mechanisms during erosion–corrosion of WC–Co–Cr cermet coatings. Wear 2003, 255, 146–156. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Zhang, J.; Zheng, Y.; Qin, Y.; Gao, W.; Li, G. Cavitation Erosion Behavior and Mechanism of HVOF Sprayed WC-10Co-4Cr Coating in 3.5 wt% NaCl Solution. Trans. Indian Inst. Met. 2015, 68, 151–159. [Google Scholar] [CrossRef]
- Wang, B.Q.; Luer, K. The erosion-oxidation behavior of HVOF Cr3C2-NiCr cermet coating. Wear 1994, 174, 177–185. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Marginean, G. Wear and corrosion behaviour of thermally sprayed cermet coatings. Surf. Coat. Technol. 2001, 138, 149–158. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Z.Z.; Luo, Q.; Rahman, A.; Jiao, J.; Qu, S.J.; Zheng, Y.G.; Shen, J. Corrosion and erosion–corrosion behaviour of activated combustion high-velocity air fuel sprayed Fe-based amorphous coatings in chloride-containing solutions. Corros. Sci. 2015, 98, 339–353. [Google Scholar] [CrossRef]
- Amarendra, H.J.; Prathap, M.S.; Karthik, S.; Darshan, B.M.; Devaraj; Girish, P.C.; Runa, V.T. Combined Slurry and Cavitation Erosion Resistance of Hvof Thermal Spray Coated Stainless Steel. Mater. Today Proc. 2017, 4, 465–470. [Google Scholar] [CrossRef]
- Saha, G.C.; Khan, T.I.; Zhang, G.A. Erosion–corrosion resistance of microcrystalline and near-nanocrystalline WC–17Co high velocity oxy-fuel thermal spray coatings. Corros. Sci. 2011, 53, 2106–2114. [Google Scholar] [CrossRef]
- Cheng, D.; Tellkamp, V.L.; Lavernia, C.J.; Lavernia, E.J. Corrosion Properties of Nanocrystalline Co–Cr Coatings. Ann. Biomed. Eng. 2001, 29, 803–809. [Google Scholar] [CrossRef]
- Wang, F.; Geng, S. High Temperature Oxidation and Corrosion Resistant Nanocrystalline Coatings. Surf. Eng. 2013, 19, 32–36. [Google Scholar] [CrossRef]
- Stack, M.M.; Abd El-Badia, T.M. Some comments on mapping the combined effects of slurry concentration, impact velocity and electrochemical potential on the erosion–corrosion of WC/Co–Cr coatings. Wear 2008, 264, 826–837. [Google Scholar] [CrossRef]
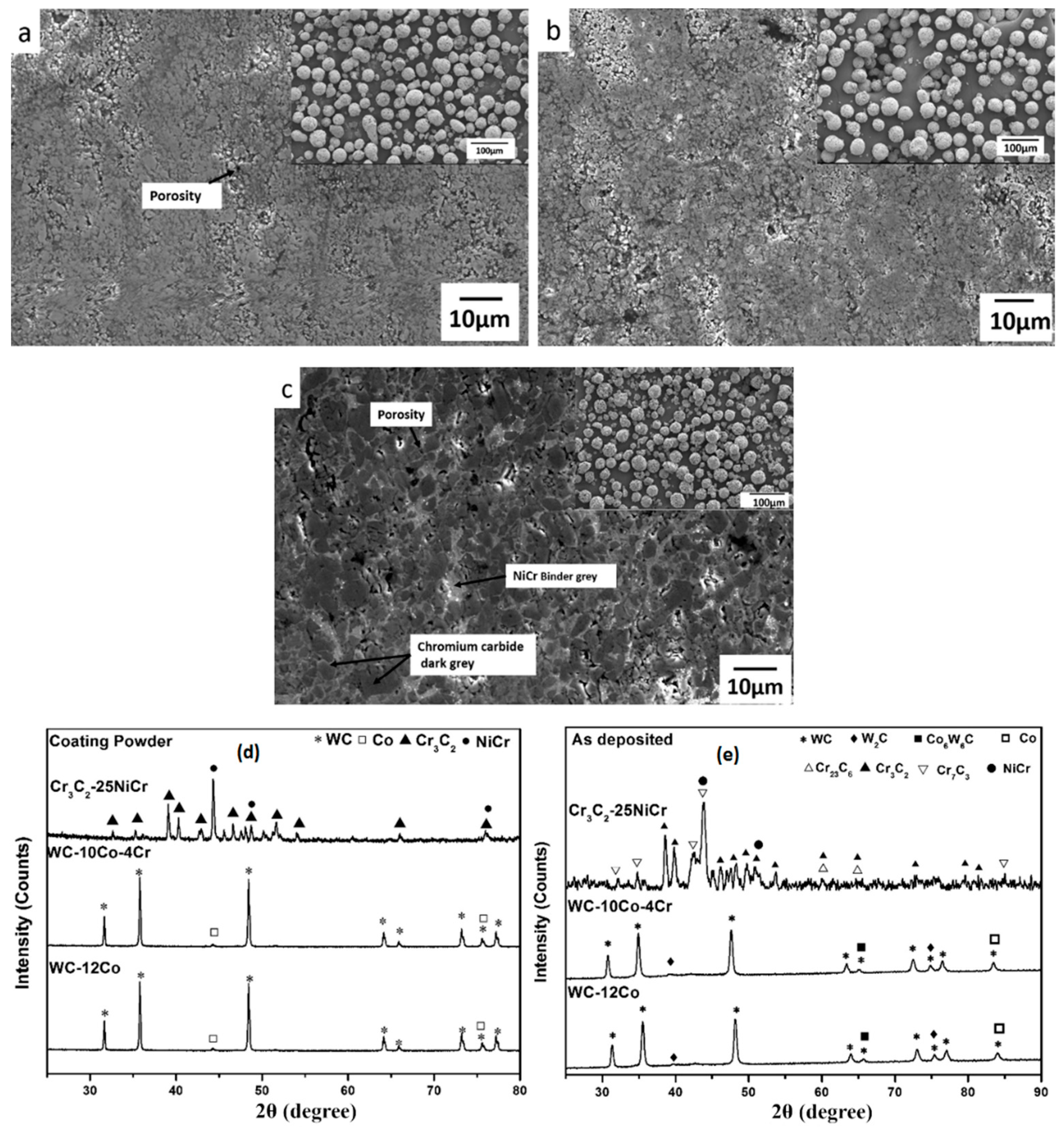



| Process | Coating | Particle Size (µm) | Porosity (%) | Thickness (µm) | Micro-hardness | Roughness (Ra) | Fracture Toughness (MPa m1/2) | Elastic Modulus (GPa) | Specific Wear Coefficient k, (mm3/Nm) | Friction Coefficient | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HVOF | Cr3C2–20(80Ni20Cr) WC–Ni | −45 + 10 −45 + 15 | - | 221 ± 16 118 ± 8 | 982 ± 35 (HV0.1) 977 ± 82 (HV0.1) | 2.74 ± 0.31 4.71 ± 0.44 | 4.40 ± 0.31 3.06 ± 0.86 | - | - | - | [87] |
| HVOF | Cr3C2–25NiCr | - | 1–2 | 500–590 | 614–810 (HV0.3) | - | - | - | - | - | [80] |
| CAPS | Cr3C2–25NiCr | - | - | 84.1–155.2 | 290.7–609.1 (HV0.3) | 6.75–10.83 | - | - | 5.72 × 10−7–2.29 × 10−7 | 0.48–0.718 | [88] |
| HVOF | WC–17Co WC–12Co WC–10Co4Cr Cr3C2–40NiCr Cr3C2–25NiCr | 20–40 | - | 100–200 | 1024 (HV0.3) 1038 1154 855 918 | 3.7–4.9 | 2.6 - 2.4 3.7 3.5 | - | - | - | [89] |
| HVOF | Conventional WC–12Co Nanostructured WC–12Co CeO2-modified WC–12Co | 15–45 5–45 15–45 | 0.60 ± 0.04 0.52 ± 0.04 0.35 ± 0.03 | - | 10.6 ± 0.3 (GPa) 12.2 ± 0.4 12.2 ± 0.4 | - | 4.0 ± 0.3 5.5 ± 0.4 5.2 ± 0.4 | 119.6 ± 9.3 226.9 ± 18.7 204.1 ± 17.1 | - | - | [21] |
| HVOF | WC–CoCr Cr3C2–NiCr Al2O3 | −45 + 15 −45 + 15 −22 + 5 | 0.240 1.306 1.347 | 163 176 294 | 1364 (HV) 1006 1164 | 4.119 1.855 1.063 | - | - | - | - | [81] |
| HVOF | Cr3C2–25NiCr | −45 + 15 | - | 250 | 780 ± 116 (HV) | <0.1 | 380 | 2 × 10−7 (100 h at 400 °C) | 0.65–0.55 | [90] | |
| HVAF HVOF | Cr3C2–25NiCr | −38/+10 −45/+15 | 4–6 | 300–400 | 1021 ± 110 (HV0.1) 919 ± 161 | - | 3.62 ± 0.35 4.13 ± 0.33 | 185.5 ± 1.5 170.7 ± 1.9 | 5 × 10−6 (at 400 °C) 1 × 10−5 (RT) | 0.70–0.75(RT) 0.05 (at 400 °C) | [47] |
| HVOF | Cr3C2–25NiCr WC-10Co–4Cr | −45/+15 −45/+15 | 1.56 2 | 225 221 | ~800 (HV) ~1000 | 3.35 6.51 | - | - | - | - | [82] |
| HVOF | Cr3C2–25NiCr Cr3C2–WC–NiCoCrMo | −45/+15 | 1.4 ± 0.1 1.1 ± 0.1 | - | 1029 ± 94 (HV0.3) 1153 ± 42 | - | - | - | 33.3 ± 1.6–37.2 ± 2.1 × 10−6 (450–650 °C) 6.3 ± 0.5–11.5 ± 0.3 × 10−6 | (450–650 °C) 0.474 ± 0.034–0.427 ± 0.032 0.574 ± 0.038–0.481 ± 0.041 | [83] |
| HVOF | WC−12Co WC−10Co−4Cr Cr3C2–25NiCr | −45/+15 | 1.2 1.1 1.5 | 350 | 1270 ± 100 (HV0.3) 1148 ± 100 825 ± 80 | 5.23 5.28 6.18 | 4.5 ± 1.0 5.1 ± 0.7 3.7 ± 0.9 | - | - | - | [41] |
| HVAF HVOF | WC–10Cr–4Co | −45/+16 −30/+5 | 0.52 ± 0.13 0.98 ± 0.3 | 380–420 | 1473 ± 40 (HV0.3) 1180 ± 70 | 2.28–4.48 | 5.60 ± 0.15 3.86 ± 0.7 | - | - | - | [84] |
| APS | Cr3C2–NiCr Ni–Cr–Fe | 10–90 | - | 200 150 | 0.239 (GPa) 0.217 | - | - | 22.795 23.375 | - | 0.515 1.298 | [91] |
| HVOF | WC–12Co WC–12Co–10Al2O3 WC–12Co–15Al2O3 | WC-12Co: 24 ± 5 Al2O3: 10 ± 5 | - | 255 ± 5 | 950 ± 50 (HK) 1200 ± 50 1300 ± 50 | 5.93 5.41 5.44 | - | - | 2.99 × 10−5 5.26 × 10−6 3.19 × 10−6 | 0.8 0.5 0.7 | [85] |
| HVOF | Cr3C2–25NiCr | −45/+15 | <1 | 260–280 | 949 ± 83 (HV0.1) | 0.70 | - | 205 ± 17 | - | - | [17] |
| HVOF | Cr3C2–20NiCr | - | <1.8 | 325 | 902 (HV) | 5.206 | - | - | - | - | [86] |
| HVOF | WC/Co coating: Multimodal M1 Multimodal M2 Conventional coarse grained Ultrafine grained Nanocrystalline | WC/12Co: (WC/Co: 2–3, WC: ~30 nm) WC/10Co: (WC/Co: 2–3, WC: ~30 nm) - 0.2–0.3 0.03–0.05 | - | - | 1100 820 1080 1150 1150 | - | - | - | 5.3 × 10−6 6.7 × 10−6 4.5 × 10−6 3.7 × 10−6 3.7 × 10−6 | - | [92] |
| HVOF | Commercial WC–12Co Nanostructured WC–12Co | −45/+5.5 Agglomerated: 40 Carbide particle: 6 ± 3 (nm) | - | - | 1129 ± 50 (HV0.3) 1135 ± 50 | - | - | - | - | - | [93] |
| HVOF | WC–18Co | 20 32 38 | - | 100 | 1004 (HV0.1) 825 735 | - | - | - | 0.28 × 10−6 0.32 × 10−6 0.30 × 10−6 | - | [94] |
| Process | Coating | Substrate | Test method | Main findings | Ref. |
|---|---|---|---|---|---|
| HVOF Arc spray Plasma spray Flame spray Flame spray Plasma spray | Cr3C2–25NiCr FeCrSiB Ni base Cr3C2–6SiO–4Al2O3 Cr3C2–12SiO–2Al2O3–4MgO WC–NiCrCo | 1018 steel | Erosion test | HVOF Cr3C2–NiCr coating offers better erosion–corrosion resistance compared to those of 1018 steel and of other cermet and ceramic coatings due to its compactness, high density, fine grain structure. and a homogeneous distribution of the skeletal network of hard carbide/oxide within a ductile and corrosion-resistant metallic binder. | [110] |
| HVOF | Ni–Cr–Si–B–C | Austenitic stainless steel (UNS S31603) | Weight loss test Potentiodynamic anodic polarization test | The cermet coating was found to be better than the stainless steel in material loss during solid–liquid conditions. During solid-free impingement, greater material loss was observed, and this was attributed to the relative influences of erosion and corrosion. | [48] |
| HVOF Flame spray Plasma spray | WC–Cr3C2–NiCr Cr3C2–NiCr WC–Co WC–Co–Cr Cr3C2–Al2O3–TiO2 Cr3O2 | High alloyed 1.4571 steel | Erosion corrosion test (0.1 M NaOH and 0.1 M H2SO4) Electrochemical polarization measurements and salt spray test | WC–Co coating showed the least resistant among the other coatings. WC–based coating exhibits the improved erosion and corrosion resistance by the addition of 4 wt. % Cr. HVOF-sprayed Cr3C2–NiCr coating is considered to be an excellent replacement for the thermal sprayed Cr3C2 coatings due to its low erosion and corrosion rate. | [111] |
| Super D-Gun thermal Spray | WC–Co–Cr | Stainless steels (UNS S31603 and UNS S32760) | Erosion–corrosion impingement test Electrochemical analysis DC anodic polarization test | Compared with stainless-steel WC–Co–Cr thermal-sprayed coatings offered good protection against wear and corrosion in liquid–solid impingement. Coating damage is controlled by erosion processes and is more important at the lower solid levels. Corrosion is affected by erosion processes at lower solid levels. | [108] |
| HVOF | WC/Co–Cr | Mild steel | Erosion–corrosion impingement test Electrochemical analysis | Erosion–corrosion regimes for the substrate and the coating are affected by the impact velocity and applied voltage. Interaction of mechanical and electrochemical processes are responsible for degradation of the coating. | [49] |
| HVOF | WC83–Co17, WC88–Co12 WC86–Co10Cr4 Cr3C2–40NiCr Cr3C2–25NiCr | C45 steel substrates | Potentiodynamic polarization test Tribocorrosion test | Cr3C2–NiCr coatings showed good barrier protection compared to other coatings. Chemical composition of the Cr3C2–NiCr coating and nickel–chromium matrix allows easier and faster re passivation even though coating is subject to wear. | [89] |
| HVOF | Cr3C2–NiCr WC–Ni | UNS-G41350 steel | Erosion–corrosion test Electrochemical analysis Electrochemical Impedance Spectroscopy (EIS) and Potentiodynamic polarization test | At high erosive conditions, WC–Ni coatings exhibited the lowest material loss and high erosion–corrosion resistance compared to chromium carbide and hard chromium coatings due to its as high hardness. Cr3C2–NiCr coatings showed the best corrosion performance under lower erosive-corrosive conditions compared to hard chromium coatings. Electrochemical measurements showed that Cr3C2–NiCr coatings showed the superior corrosion resistance compared to WC–Ni under both erosive conditions by electrochemical measurements. | [87] |
| HVOF | WC–10Co–4Cr | Stainless steel 1Cr18Ni9Ti | Cavitation-erosion (CE) test | The coating exhibited higher cavitation–corrosion resistance than that of the stainless-steel 1Cr18Ni9Ti in 3.5 wt. % NaCl solution The removal mechanism for the coating was erosion of the binder phase first, followed by brittle detachment of hard phases as a result of the action of corrosion and mechanical effect. | [109] |
| HVOF | Nanostructured Ni–20Cr | SAE213-T22 (T22) and SA 516-Grade 70 steels | Erosion corrosion test (boiler environment) | The investigated nanostructured coating was found to perform better than its conventional (micron-sized Ni–20Cr powder coating) micron-sized counterpart in boiler environment. Nanostructured coating offered better erosion–corrosion resistance under actual boiler conditions, which might be attributed to the presence of protective NiO and Cr2O3 phases in their oxide scales and its superior as-sprayed microhardness. Cyclic formation and erosion of the oxide scale occurred during erosion–corrosion phenomenon for coated samples, whereas SA 516 steel was most likely eroded. | [20] |
| HVOF | Cr3C2–25NiCr | 310S boiler steel | Cyclic oxidation test | The parabolic rate constant value was one-third for the coated specimen during oxidation as compared to uncoated specimen, which indicates that coating shows better oxidation resistance. The splat and globular morphology of coatings responsible for high degradation resistance of the coatings. | [73] |
| HVOF AC HVAF | FeCrMoMnWBCSi amorphous | 316L SS substrate. | Erosion corrosion test Static electrochemical measurements | AC-HVAF Fe-based amorphous metallic coating exhibits a relatively denser microstructure with high hard phase particles, low porosity, much lower oxide content, and high microhardness compared to HVOF AMC. The AC-HVAF amorphous metallic coating exhibits a higher ability to withstand uniform corrosion and enhanced pitting resistance. The enhanced erosion–corrosion resistance of the AC-HVAF AMC relative to the HVOFAMC could mainly be related to the high hardness and compact structure. | [112] |
| HVOF | Cr3C2–20NiCr | Carbon steel | Wear test Electrochemical corrosion test | The dense surface layer of Cr3C2–20NiCr coating have higher corrosion resistance than carbon steel during testing for 27 days. The carbon steel corroded, and iron oxides were present on the surface. Both polarization and EIS results showed the better corrosion protection properties of the coating. Hardness and tribological properties of the coated sample are more durable and the rate of weight loss is very limited compared to substrate material | [103] |
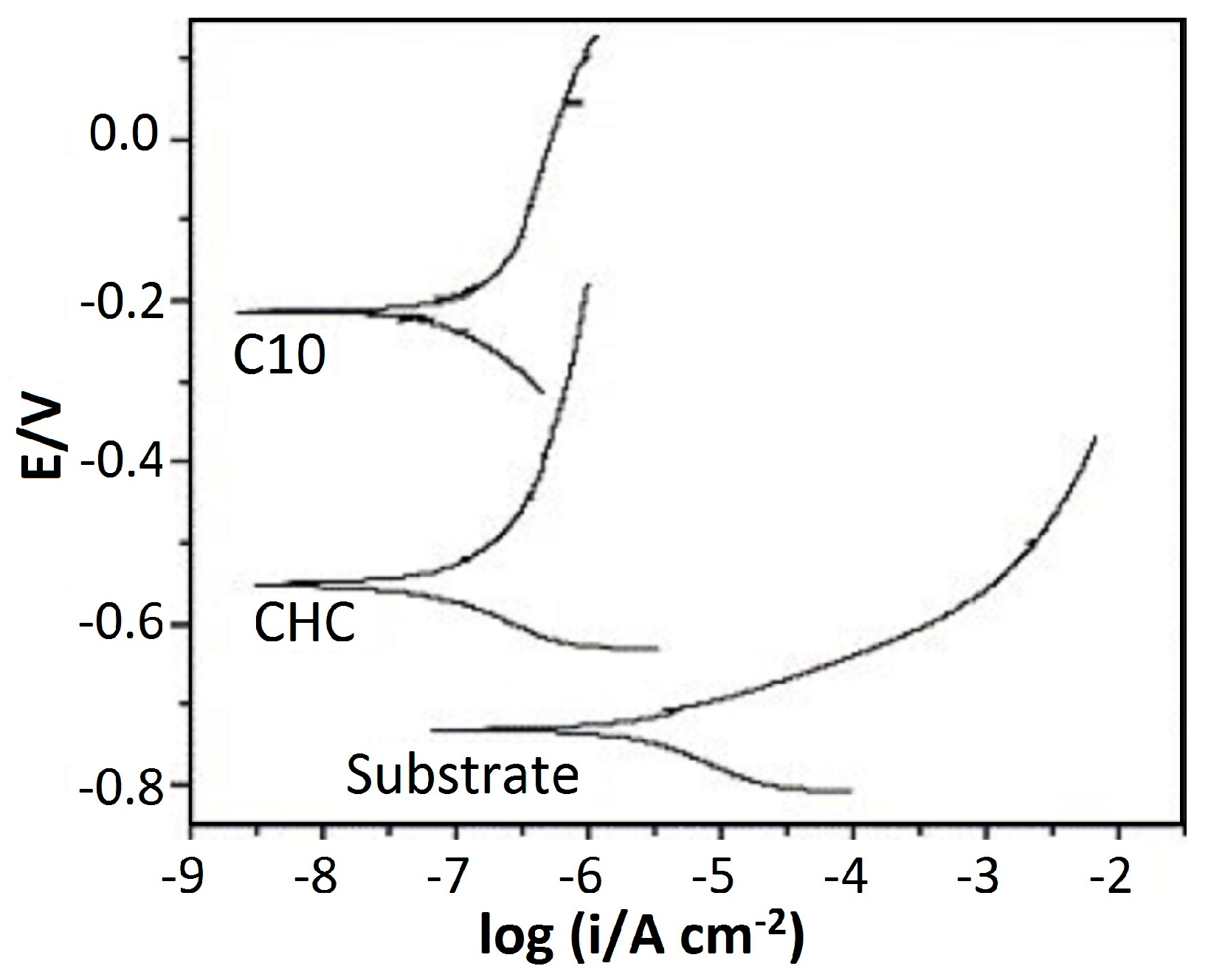
| HVOF | Cr3C2–25NiCr | AISI 304 stainless steel | Tribocorrosion test Electrochemical analysis | No crack or open pores in the HVOF Cr3C2–25NiCr coating results in better corrosion resistance against AISI 304 even in the presence of wear. Coefficient of friction (COF) value changes from 0.28 in cathodic conditions to 0.11 in anodic conditions during the test which can be related to the different composition of the sample surface and also passivation film might be present in anodic conditions. | [104] |
| HVOF Plasma spray | Cr3C2–25NiCr | S45C carbon steel | Electrochemical corrosion test | The electrochemical polarization and EIS results indicated that the HVOF coating has superior corrosion resistance to the plasma coating as well as substrate. Less hole formation due to pitting and crevice on the top of HVOF coated samples were responsible for the superior property against plasma spray. | [77,85] |
| HVOF | Conventional WC–12Co Nanostructured WC–12Co CeO2-modified WC–12Co | AISI 304 stainless steel | Erosion corrosion test | CeO2-modified WC–12Co coating possessed the best erosion–corrosion resistance among the other coatings. Conventional WC–12Co coatings have lowest erosion–corrosion resistance. Formation of microcracks in the Co binder phase and generation of corrosion pits, with propagation of the microcracks over the WC particles to expand forward until arriving at the coating surface or a deeper position. This phenomenon causes WC particle falling off from the coating and revealed the failure mechanism of the CeO2 modified WC–12Co coating. | [21] |
| HVOF | WC–CoCr Cr3C2–NiCr Al2O3 | S355 steel | Dry erosion test Slurry erosion test | WC–CoCr coating experienced 5 times higher volume loss under dry erosion compared to slurry erosion due to squeeze film effects. Due to high volume loss in all conditions, Cr3C2–NiCr is not a suitable choice to offer increased erosion resistance in dry and slurry environments. WC–CoCr showed a significant reduction in volume loss and wear scar depth over Cr3C2–NiCr and Al2O3 under both dry jet and slurry erosion results in high hardness. | [81] |
| HVOF | 70Ni30Cr | Martensitic stainless-steel SS 410 | Slurry erosion test | The HVOF 70Ni30Cr-coated specimens showed the better combined slurry and cavitation resistance as compared to un coated specimens with 200 µm and 300 µm size sand particle. However, it observed an increase in weight loss with increase in sand particle size and duration of the test. | [113] |
| HVOF | Cr3C2–25NiCr | GH738 Ni-based super alloy | Erosion test | The erosion test results indicate that the volume erosion rate of the coating in addition to, number and depth of the pits on the coating increases with the increase in the air pressure. Above 120 µm coating thickness, the wear rate has negligible variation in the erosion rate. Coating shows better erosion resistance at 90° impact angle as compared to 30° impact angle. Combined behavior of ductile and brittle is the responsible for erosion mechanism of Cr3C2–NiCr samples in which ductile mode of erosion characteristic dominates. | [106] |
| Plasma spray | Ni3Al + Ni–22Cr–10Al–1Y | Superni 75, Superni 600, Superni 718 Superfer 800 H | Erosion corrosion test (boiler environment) | Erosion–corrosion test results in the boiler environment showed that Ni3Al coating layer gets partially oxidized and acts as a perfect barrier against erosion–corrosion of super alloys. Further the partially oxidized coatings remain intact even after 1000 h cycle exposure; thus, it can be presumed that it will enhance the life of boiler tube in the evaluated environment. | [52] |
| HVAF HVOF | 86WC–10Cr–4Co | SS410 | Silt erosion test | HVAF WC–CoCr coating at high velocity revealed superior erosion resistance compared to HVAF coating. It could be attributed to the better adhesion, higher density, hardness, and low oxidation, which enhanced erosion resistance of these samples. The erosion wear mechanism observed to be micro-cutting and plowing at lower impact angle. A low cycle fatigue linked to repeated impact leading to rounding of the edges of the WC grains and promote brittle cracking at higher impact angle. Ductile mode of material removal during erosion was indicated by the Erosion Classification Value Ecv of the coatings. | [84] |
| Laser cladding Detonation spraying Plasma spraying | Chromium carbide-NiCrMoNb powder | Steel with 0.27% carbon | Erosion test Nano impact test | Detonation sprayed coating had the lowest erosion rate followed by laser clad coating. Due to brittle nature and poor splat bonding of plasma sprayed coating showed the highest erosion rate. Both dispersion strengthening and solid solution strengthening are found to be effective in enhancing the hardness of Chromium carbide-Ni-rich alloy system. However, excessive dissolution of carbide leads to embrittlement of the Ni rich matrix. | [53] |
| HVOF | WC−12Co WC−10Co−4Cr Cr3C2–25NiCr | 316 stainless steel | Wear test Solid particle erosion test Abrasive wear test | Change in erosion mechanism from plastic deformation and micro fracture of carbides to carbide pull out, splat exfoliation, subsurface splat removal, and subsurface cracking occurs due to the transition in erosion rate. The abrasive wear rates of the coatings changes from a mild oxidational wear regime at a load of 20 N to severe wear regime at a load of 40 N. Formation of tribo films and its composition influenced the frictional behavior of the coatings. | [41] |
| HVOF | WC–10Co–4Cr | 1Cr18Ni9Ti stainless steel | Slurry erosion test Microbial corrosion electrochemical measurement Electrochemical Impedance Spectroscopy (EIS) and Potentiodynamic polarization test | WC–10Co–4Cr coating exhibited a superior slurry erosion–corrosion resistance compared to the substrate material in both distilled water and 3.5 wt. % NaCl slurries. Slurry erosion mechanism in distilled water through formation of cracks and microchipping, whereas in 3.5 wt. % NaCl slurry detachment of binder phase of the coating, microchipping and fragments present in the slurry. Potentiodynamic polarization and EIS results revealed that WC–10Co–4Cr coating had a comparable microbial influenced corrosion resistance in seawater with SRB compared to substrate. | [15] |
| Thermo-reactive deposition | Cr7C3 | Medium carbon steel | Erosion corrosion test Potentiodynamic polarization test | No significant dependency to impact angle variation in polarization curves and due to high chemical stability of the Cr7C3, there is a slight difference between coated and uncoated samples. However, uncoated sample corrosion potential decreases with increasing impact angle. Cracking and chipping off is the dominant failure mechanism at low impact angle, flake fragmentation, and platelet formation are the main one at 60° for the coated samples. | [54] |
| HVOF | Cr3C2–25NiCr | Carbon steel | Impact test | No adhesive failure was observed under the given test conditions excluding under a loading force of F = 1000 N. Generation of small crater volumes due to plastic deformation, tribo-reaction, and micro-abrasion under impact loading. At critical load conditions with higher forces, induced crack initiation and growth addition to increases the generated crater volume rapidly and the applied force has a decisive influence on the possible failure modes and mechanism. The compressive residual stresses in the HVOF-sprayed coating were important to delay the crack initiation and growth because the effective tensile stresses were reduced during the test. | [54] |
| HVOF | WC–12Co WC–12Co–10Al2O3 WC–12Co–15Al2O3 | 304 stainless Steel | Wear test Potentiodynamic polarization and EIS test | The addition of Al2O3 in WC–Co coating enhanced its microhardness and wear properties decrease the wear rate. It is observed that all the coatings showed higher Icorr values than substrate which indicates that HVOF coatings have poor corrosion resistance and this is due to the porous nature of the coatings. Thus, it is inferred that the hardness, wear rate, and COF values of the developed WC–12Co–Al2O3 HVOF coatings are comparable with hard chrome coating. | [85] |
| HVOF | Cr3C2–25NiCr Cr3C2–WC–NiCoCrMo | Plain carbon steel | High temperature wear test | The Cr3C2–WC–NiCoCrMo coating exhibits lower porosity and higher hardness compared to the Cr3C2–NiCr coating. The Cr3C2–WC–NiCoCrMo coating produces relatively higher friction coefficients (COF) compared to the Cr3C2–NiCr coating at high temperatures (450, 550, 650 °C) in this testing conditions. Cr3C2–WC–NiCoCrMo coatings showed lower wear rates and increased by nearly two times when the temperature increased from 450 to 650 °C, but the wear rates of the Cr3C2–NiCr coating are roughly the same, indicating that the microstructure and properties of the Cr3C2–WC–NiCoCrMo coating are prone to affect at higher temperature (550–650 °C) compared to the Cr3C2–NiCr coating. | [83] |
| HVOF | Nanostructured WC–10Co4Cr (200–500 nm) Conventional Cr3C2–25NiCr NiCrWSiFeB | AISI 1020 steel | Slurry erosion test | Nanostructure coating showed low erosion wear compared to conventional coatings due to higher microhardness value, lower porosity, higher fracture toughness, and lower roughness. Improvements in properties are related to size of the powder particles, density of the coating, and more uniform reinforcement carbides (WC) distribution. | [19] |
| HVOF | Conventional Cr3C2–25NiCr Nanostructured Cr3C2–25NiCr | AISI 1045 steel | Tribo-corrosion tests | Electrochemical data suggested that the corrosion rate of chromium coatings is increased compared to nanostructured coating by almost one order of magnitude by the mechanical damage. The use of nano-sized powders improves the good behavior of the coating due to decrease of the interconnected porosity, a lower roughness, and a better distribution of the chromium carbides in the metal matrix. | [6] |
| HVOF | Nanostructured WC–Co Conventional WC–Co | UNS G41350 steel | Wear test Potentiodynamic polarization and EIS test | The friction coefficient of the nanostructured WC–Co coating was 30% lower than that of the conventional coating and the wear path was also thinner. The nanostructured coating showed better sliding wear behavior and the higher microhardness seems to be the factor controlling the sliding wear resistance. Nanostructured coating showed a 3.5 times higher corrosion protection due to sealing properties of the coating. | [18] |
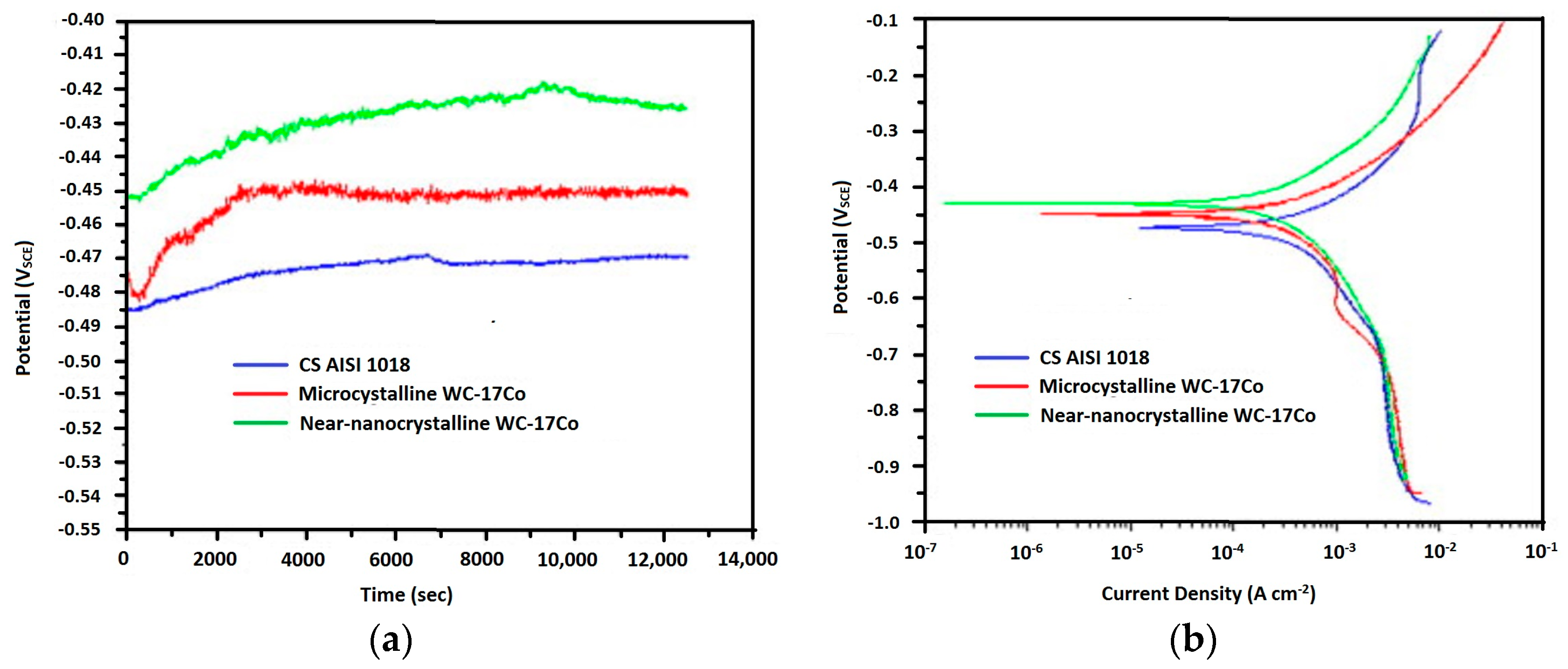
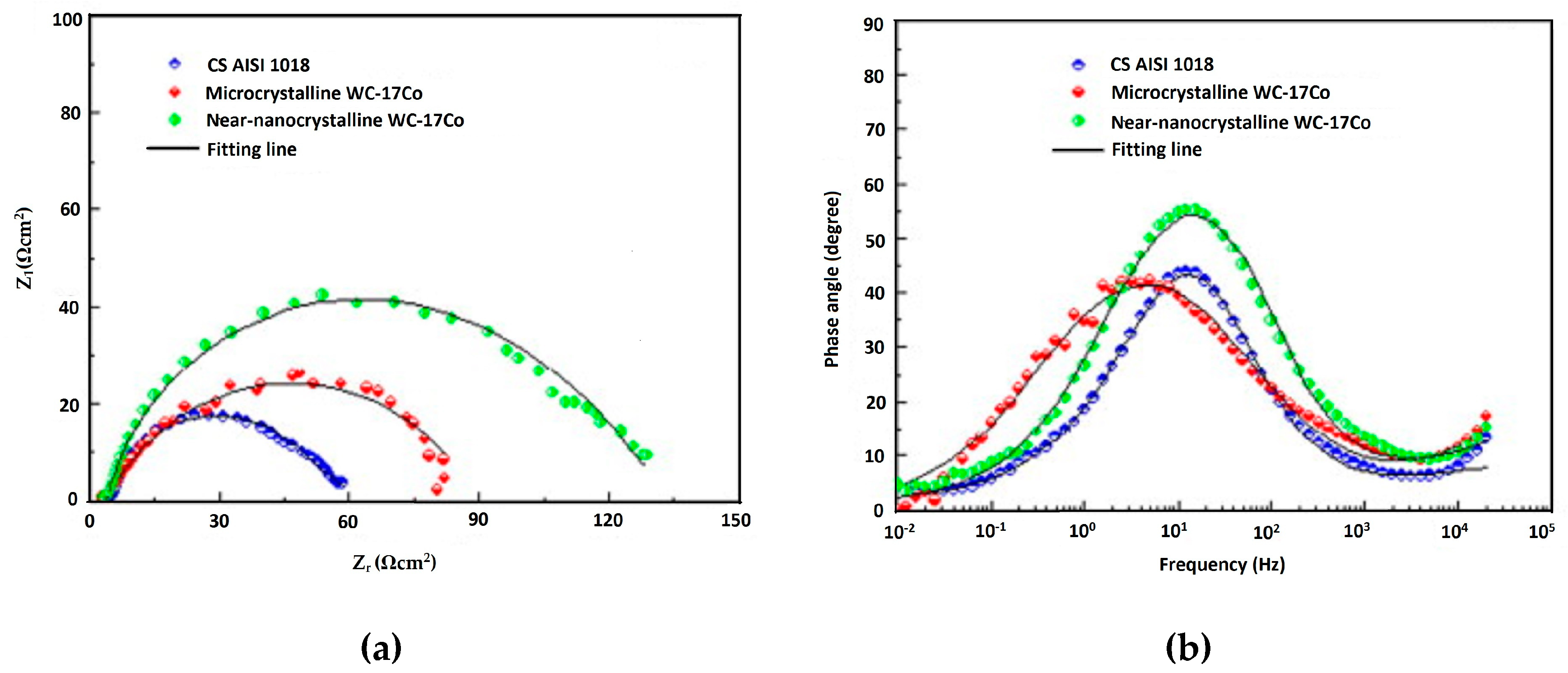
| HVOF | Near-nanocrystalline WC–17Co Microcrystalline WC–Co | AISI 1018 steel | Erosion corrosion test Potentiodynamic polarization test | Near-nanocrystalline coating showed approximately 1/3 lower erosion–corrosion rate than that of the microcrystalline coating and the erosion–corrosion mechanism in the coating was dominated by pure erosion in the microcrystalline coating and the corrosion-enhanced erosion in the near-nanocrystalline coating. | [114] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Seman, S.; Singh, G.; Jayaganthan, R. Nanocrystalline Cermet Coatings for Erosion–Corrosion Protection. Coatings 2019, 9, 400. https://doi.org/10.3390/coatings9060400
Tiwari A, Seman S, Singh G, Jayaganthan R. Nanocrystalline Cermet Coatings for Erosion–Corrosion Protection. Coatings. 2019; 9(6):400. https://doi.org/10.3390/coatings9060400
Chicago/Turabian StyleTiwari, Abhishek, Saravanan Seman, Gaurav Singh, and Rengaswamy Jayaganthan. 2019. "Nanocrystalline Cermet Coatings for Erosion–Corrosion Protection" Coatings 9, no. 6: 400. https://doi.org/10.3390/coatings9060400
APA StyleTiwari, A., Seman, S., Singh, G., & Jayaganthan, R. (2019). Nanocrystalline Cermet Coatings for Erosion–Corrosion Protection. Coatings, 9(6), 400. https://doi.org/10.3390/coatings9060400






