Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
2.1.2. Chemical Agents
2.1.3. Finishing Agents
2.1.4. Bacterial Strains
2.2. Methods
2.2.1. Nonwoven Fabrics
2.2.2. Dip-Coating of Nonwoven Fabric and Modification of Phosphonodipeptide
2.2.3. Scanning Electron Microscopy
2.2.4. ATR-FTIR
2.2.5. UV–Vis Analysis
2.2.6. Filtration Parameters
2.2.7. Tensile Testing
2.2.8. Antimicrobial Activity
3. Results and Discussion
3.1. Scanning Electron Microscopy
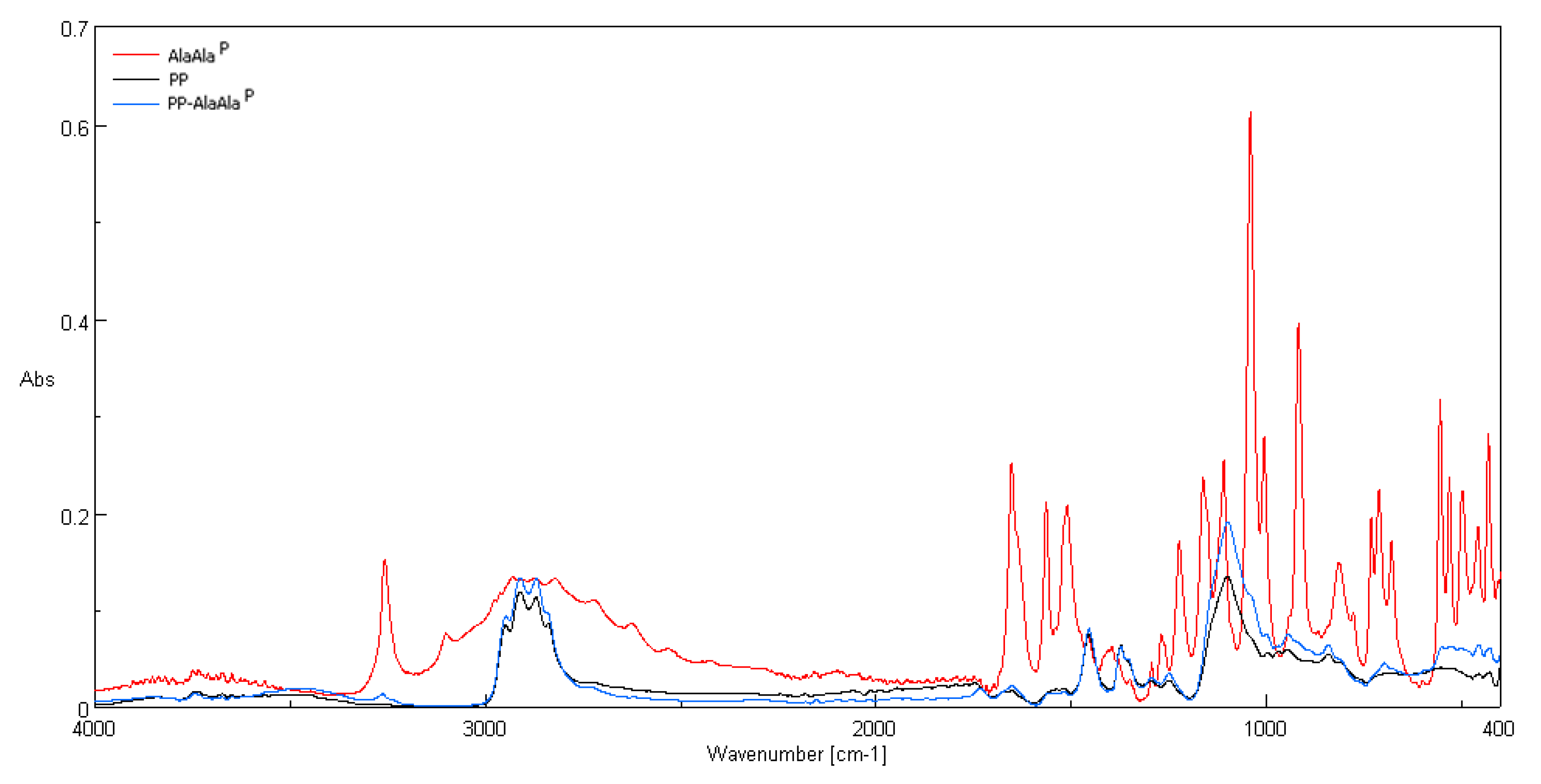
3.2. ATR-FTIR Spectra
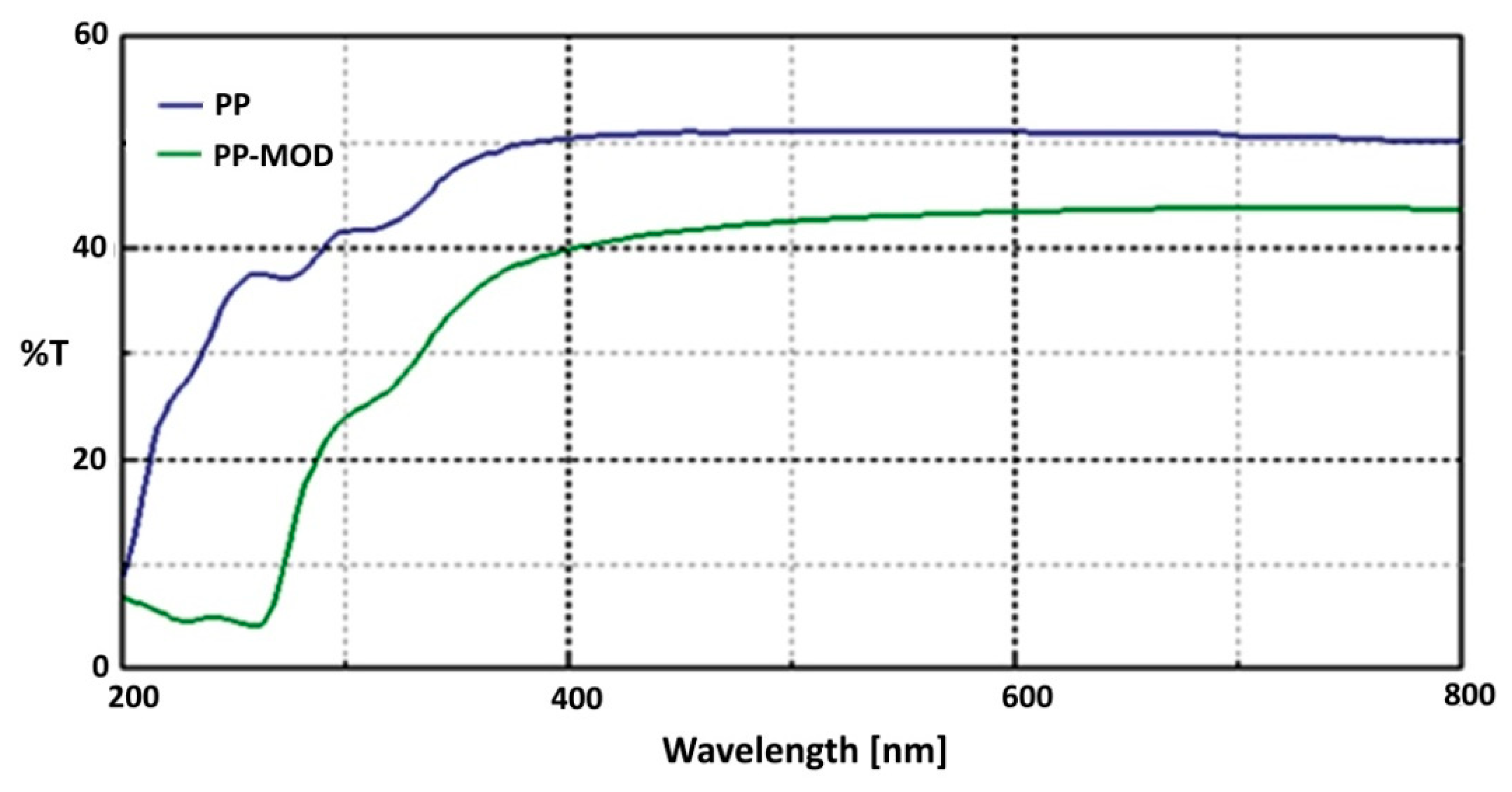
3.3. UV/Vis Transmittance Spectra
3.4. Technical Parameters
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, K.S.; Lian, H.S.; Chen, J.B. Study on the modification of PP nonwoven fabric. Fibres Text. East. Eur. 2011, 3, 82–87. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Simoes, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Wang, C.C.; Su, C.H.; Chen, C.C. Water absorbing and antibacterial properties of N-isopropyl acrylamide grafted and collagen/chitosan immobilized polypropylene nonwoven fabric and its application on wound healing enhancement. J. Biomed. Mater. Res. A 2008, 84, 1006–1017. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, C.C.; Chen, F.L.; Lin, N.-S. An improvement on water absorbing and permeating properties: Heparin immobilizing on acrylic acid-grafted and collagen/chitosan-immobilized wound dressing. J. Appl. Polym. Sci. 2008, 109, 1431–1438. [Google Scholar] [CrossRef]
- Wang, C.C.; Su, C.H.; Chen, J.P.; Chen, C.C. An enhancement on healing effect of wound dressing: Acrylic acid grafted and gamma-polyglutamic acid/chitosan immobilized polypropylene non-woven. Mater. Sci. Eng. C 2009, 29, 1715–1724. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, J.P.; Chen, C.C. An enhancement on water absorbing and permeating abilities of acrylic acid grafted and chitosan/collagen immobilized polypropylene non-woven fabric: Chitosan obtained from Mucor. Mater. Sci. Eng. C 2009, 29, 1133–1139. [Google Scholar] [CrossRef]
- Xin, Z.; Du, S.; Zhao, C.; Chen, H.; Sun, M.; Yan, S.; Luan, S.; Yin, J. Antibacterial performance of polypropylene nonwoven fabric wounddressing surfaces containing passive and active components. Appl. Surf. Sci. 2016, 365, 99–107. [Google Scholar] [CrossRef]
- Chen, K.S.; Ku, T.Y.A.; Lee, C.H.; Lin, H.R.; Lin, F.H.; Chen, T.M. Immobilization of chitosan gel with cross-linking reagent on PNIPAAm gel/PP nonwoven composites surface. Mater. Sci. Eng. C 2005, 25, 472–478. [Google Scholar] [CrossRef]
- Simovic, L.M.; Skundric, P.D.; Kostic, M.M.; Tasic, G.M.; Kojic, Z.Z.; Milakovic, B.D.; Medovic, A.H. Efficiency and biocompatibility of antimicrobial textile material of broad spectrum activity. J. Appl. Polym. Sci. 2011, 120, 1459–1467. [Google Scholar] [CrossRef]
- Chen, J.P.; Lee, W.L. Collagen-grafted temperature-responsive nonwoven fabric for wound dressing. Appl. Surf. Sci. 2008, 255, 412–415. [Google Scholar] [CrossRef]
- Lin, F.H.; Tsai, J.C.; Chen, T.M.; Chen, K.S.; Yang, J.M.; Kang, P.L.; Wu, T.H. Fabrication and evaluation of auto-stripped tri-layer wound dressing for extensive burn injury. Mater. Chem. Phys. 2007, 102, 152–158. [Google Scholar] [CrossRef]
- Yang, J.M.; Lin, H.T. Properties of chitosan containing PP-g-AA-g-NIPAAmbigraft nonwoven fabric for wound dressing. J. Membr. Sci. 2004, 243, 1–7. [Google Scholar] [CrossRef]
- Tyan, Y.C.; Liao, J.D.; Lin, S.P. Surface properties and in vitro analyses of immobilized chitozan onto polepropylene non-wofen fabric surface using antenna-coupling microwave plasma. J. Mater. Sci. Mater. Med. 2003, 14, 775–781. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Baier, G.; Landfester, K.; Musin, E.; Al-bataineh, S.; Cameron, D.C.; Whittle, J.D.; Sillanpää, M. Attachment of poly(l-lactide) nanoparticles to plasma-treated non-woven polymer fabrics using inkjet printing. Macromol. Biosci. 2015, 15, 1274–1282. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Krumpolec, R.; Homola, T.; Musin, E.; Baier, G.; Landfester, K.; Cameron, D.C.; Černák, M. Ambient air plasma pre-treatment of non-woven fabrics for deposition of antibacterial poly(l-lactide) nanoparticles. Plasma Process. Polym. 2017, 14, 1600231. [Google Scholar] [CrossRef]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Maier, L. Phosphoroorganic detergents. Chimia 1969, 23, 323–330. [Google Scholar]
- Petrov, K.A.; Chauzov, V.A.; Erokhina, T.S. Aminoalky lorganophosphorus compounds. Russ. Chem. Rev. 1974, 43, 984. [Google Scholar] [CrossRef]
- Rizkalla, E.N. Metal chelates of phosphonate-containing ligands. Rev. Inorg. Chem. 1983, 5, 223–304. [Google Scholar]
- Sikorski, J.A.; Logush, E.W. Aliphatic carbon-phosphorus compound as herbicides. In Handbook of Organophosphorus Chemistry, 1st ed.; Engel, R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1992; pp. 751–800. [Google Scholar]
- Neuzil, E.; Cassaigne, A. Natural compounds with biologic value containing a P-C bond and phosphonates. Expos. Annu. Biochim. Med. 1980, 34, 165–210. [Google Scholar]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-aminophosphonate/ phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids-phosphorus analogues of natural amino acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Kudzin, Z.H.; Drabowicz, , J. Thioureidoalkylphosphonates in the synthesis of 1-aminoalkylphosphonic acids. The Ptc-aminophosphonate method. Arkivoc 2011, VI, 227–269. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jakubowski, H.; Kudzin, M.H.; Kudzin, Z.H. Nomenclature of aminoalkylphosphonic acids and derivatives. Evolution of the code system. Acta Biochim. Polon. 2015, 62, 139–150. [Google Scholar] [CrossRef]
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Water Res. 2017, 117, 187–197. [Google Scholar] [CrossRef]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid and methoxyacetic acid induce apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2015, 16, 11750–11765. [Google Scholar] [CrossRef]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid inhibits growth and metastasis of human prostate cancer in an orthotopicxenograft mouse model. Oncotarget 2016, 7, 10616–10626. [Google Scholar] [CrossRef]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature 1959, 184 (Suppl. 12), 901–902. [Google Scholar] [CrossRef]
- Alhadeff, J.A.; Van Bruggen, J.T.; Daves, D., Jr. Biosynthetic studies on 2-aminoethylphosphonic acid in a mammalian (rat) system. Biochim. Biophys. Acta 1972, 286, 103–106. [Google Scholar] [CrossRef]
- Tan, S.A.; Tan, L.G. Distribution of ciliatine (2-aminoethylphosphonic acid) and phosphonoalanine (2-amino-3-phosphonopropionic acid) in human tissues. Clin. Physiol. Biochem. 1989, 7, 303–309. [Google Scholar]
- Watts, M. Glufosinate-Ammonium Monograph. 2008. Available online: http://www.pananz.net/wp-content/uploads/2013/04/Glufosinate-monograph-12-Dec-2008.pdf (accessed on 18 June 2019).
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: discovery, development, applications, and properties. In Glyphosate Resistance in Crops and Weeds. History, Development and Management; Nandula, V.K., Ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassal, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature 1978, 272, 56–58. [Google Scholar] [CrossRef]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Alaphosphin and related phosphonopeptides. Antimicrob. Agents Chemother. 1979, 15, 684–695. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Rationale, chemistry and structure-activity relationships. Antimicrob. Agents Chemother. 1979, 15, 677–683. [Google Scholar] [CrossRef]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Lloyd, W.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Mechanism of action of alaphosphin. Antimicrob. Agents Chemother. 1979, 15, 696–705. [Google Scholar] [CrossRef]
- Allen, J.G.; Lees, L.J. Pharmacokinetics of alafosfalin, alone and in combination with cephalexin, in humans. Antimicrob. Agents Chemother. 1980, 17, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Atherton, F.R.; Hassall, C.H.; Lambert, R.W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)-phosphonic acid. J. Med. Chem. 1986, 29, 29–40. [Google Scholar] [CrossRef]
- Neuman, H.J. Recent developments in the field of phosphonic acid antibiotics. J. Antimicrob. Chemother. 1984, 14, 309–311. [Google Scholar] [CrossRef]
- Lejczak, B.; Kafarski, P.; Sztajer, H.; Mastalerz, P. Antibacterial activity of phosphono dipeptides related to alafosfalin. J. Med. Chem. 1986, 29, 2212–2217. [Google Scholar] [CrossRef]
- Traub, W.H. In vitro evaluation of alaphosphin (Ro 03-7008) against Serratiamarcescens. Chemotherapy 1980, 26, 103–110. [Google Scholar] [CrossRef]
- Arisawa, M.; Ohshima, J.; Ohsawa, E.; Maruyama, H.B. In vitro potentiation of cephalosporins by alafosfalin against urinary tract bacteria. Antimicrob. Agents. Chemother. 1982, 21, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, E.J.; Graves, E.E. Phosphorylation of cellulose with some phosphonic acid derivatives. Text. Res. J. 2003, 73, 22–26. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Depczyński, R.; Andrijewski, G.; Drabowicz, J.; Łuczak, J. 1-(N-Acylamino)alkanephosphonates. Part IV. N-Acylation of 1-Aminoalkanephosphonic Acids . Pol. J. Chem. 2005, 79, 499–513. [Google Scholar]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J. 1-(N-chloroacetylamino)alkylphosphonic acids-synthetic precursors of phosphonopeptides. Amino Acids 2008, 34, 163–168. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Depczynski, R.; Kudzin, M.H.; Luczak, J.; Drabowicz, J. 1-(N-Trifluoroacetylamino)alkylphosphonic acids: Synthesis and properties. Amino Acids 2007, 33, 663–667. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Urbaniak, P.; Drabowicz, J. Phosphorylation of cellulose. In Proceedings of the 20th International Symposium Advances in the Chemistry of Heteroorganic Compounds, Łódź, Poland, 23–24 November 2017; p. 130. [Google Scholar]
- EN ISO 9237:1998–Textiles–Determination of the Permeability of Fabrics to Air; International Organization for Standardization: Geneva, Switzerland, 1998.
- EN ISO 10319:2015-08–Geosynthetics-Wide-Width Tensile Test; International Organization for Standardization: Geneva, Switzerland, 2015.
- EN ISO 20645:2006–Textile Fabrics–Determination of Antibacterial Activity—Agar Diffusion Plate Test; International Organization for Standardization: Geneva, Switzerland, 2006.
- Urbaniak-Domagala, W. The use of the spectrometric technique FTIR-ATR to examine the polymers surface. In Advanced Aspects of Spectroscopy; Farrukh, M.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 85–112. [Google Scholar]
- Podstawka, E.; Andrzejak, M.; Kafarski, P.; Proniewicz, L.M. Comparison of adsorption mechanism on colloidal silver surface of alafosfalin and its analogs. J. Raman Spectrosc. 2008, 39, 1238–1249. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2012, 114, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]


| Abbrev. | Action/Application | Tested Bacteria | Ref. |
|---|---|---|---|
| PP-NIPAAm-Cg-Cs | Membrane-wound healing enhancement | S. aureus | [5] |
| PP-g-AA-Cg-Cs | |||
| PP-g-AA-Cs-Cg | Various concentrations of heparin | S. aureus | [6] |
| PP-g-AA-Cs-Cg-Hi | |||
| PP-AAg-Cg-Cs | Anti-bacterial property and the effect on accelerating wound healing strong | S. aureus | [7,8] |
| PP-g-AA-PG-Cs | |||
| PP-g-PGMA | Antimicrobial activity; platelet and red blood cell adhesion | E. coli; S. aureus | [9] |
| PP-g-PHMG | |||
| PP-g-PNVP-PGMA | |||
| PP-g-PNVP-PHMG | |||
| PP-g-NIPAAm | Wound dressing applications; good biocompatibility to fibroblast cells | E. coli; S. aureus | [10] |
| PP-g-NIPAAm-Cs | |||
| (PP)-viscose-Cs-Gc(2%–2.5%) | Good biocompatibility; the correlation between efficiency in vitro and in vivo results, qualifies these antimicrobial biomaterials for clinical use | E. coli; S. aureus; Klebsiella aeruginosa; Candida albicans | [11] |
| PP-g-AA-Cg | Wound healing tests | Tests on rats | [12] |
| PP-g-AA-Cg-PNIPAAm | |||
| PP/PNIPAAm/Gelatin-hyaluronan-chodroitin-6-sulfate | Tri-layer membrane as the artificial skin for extensive burn injury | The wound dressing did not induce the tissue inflammatory or immune response. | [13] |
| PP-g-AA-NIPAAm | Potential wound dressing | Pseudomonas aeruginosa; S. aureus | [14] |
| PP-g-AA-NIPAAm-Cs | |||
| PP-g-AA-Cs | Potential wound dressing substitute for second-degree burns | Bioactivity assessments by aPTT; TT and fibrinogen concentration | [15] |
| PP-g-PLLA-Oct-HSA | Active dressings for the treatment of wounds | E. coli; S. aureus | [16] |
| PP-g-PLLA-Oct | Medical dressings specific to infected wounds | E. coli; S. aureus | [17] |
| Abbrev. (Name) | Structure | Action/Application | Ref. |
|---|---|---|---|
| GlyP (phosphoglycine) | 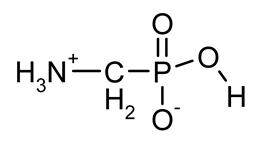 | Primary degradation product of glyphosate, inhibitor of prostate cancer cell growth in vitro, phytotoxin | [18,29,30,31,32,33] |
| β-AlaP (β-phosphono-alanine, 2-AEP, ciliatine) | 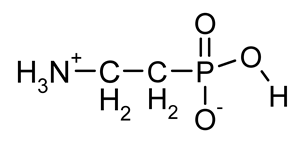 | The most widespread, first AAP isolated in natural sources | [18,34,35,36] |
| GluγP(Me) (phosphinothricin, PPT) | 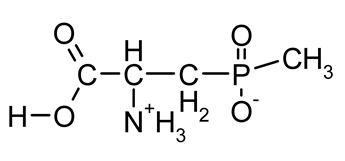 | A naturally occurring broad-spectrum systemic herbicide produced by several species of Streptomyces; inhibiting glutamine synthetase | [18,37] |
| PMG (PhosphonoMethyl-Glycine; Glyphosate) | 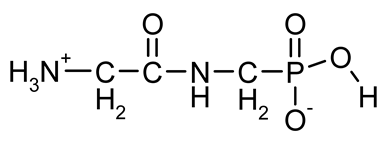 | A broad spectrum, non-selective systemic herbicide, inhibiting the activity of 5-enolpyruvyl-shikimic acid-3-phosphate synthase | [18,38,39] |
| Ala-AlaP (alaphosphin; alafosfalin) | 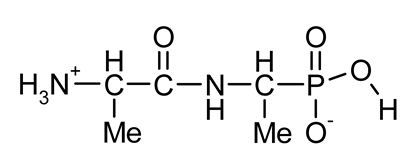 | Ala-AlaP selectively inhibited peptidoglycan biosynthesis in both Gram-negative and Gram-positive bacteria. The mechanism of action of Ala-AlaP involve three stages: (i) active transport by peptide permeases; (ii) intracellular peptidase cleavage; and (iii) action of Ala-AlaP on alanine racemase (EC 5.1.1.1) | [18,40,41,42,43,44,45,46,47,48,49] |
| Applied names are in accordance with the general rules elaborated by Kudzin et al. [24,25]. | |||
| Processing Parameters | |
|---|---|
| Temperature of the extruder in zone 1 | 240 °C |
| Temperature of the extruder in zone 2 | 285 °C |
| Temperature of the extruder in zone 3 | 290 °C |
| Head temperature | 240 °C |
| Air heater temperature | 270 °C |
| Air flow rate | 7–8 m3/h |
| Polymer yields | 4 g/min |
| Mass per unit area of nonwovens | 80 g/m2 |
| Styrene-Acrylic Resin | Wetting Agent | Thickening Agent | Water |
|---|---|---|---|
| 5 g | 5 g | 1 g | 89 g |
| 5% | 5% | 1% | 89% |
| Polypropylene-IR according to Urbaniak-Domagala, 2012 [58] | |||||||||
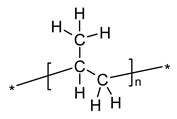 | |||||||||
| Vibrations | |||||||||
| Wave number (cm−1) | 2916 | 2959 | 2881 | 2841 | 1460 | 1376 | 1357 | 1170, 1153 | 975, 899 |
| Vibration type (group) | ν(CH2) | ν(CH3) | ν(CH3) | ν(CH2) | δ(CH3) | δ(CH3) | γ(CH2–CH) | γ(CH3), δ(CH2), δ(CH) | γ(CH3), ν(CH2), ν(CH) |
| Ala-AlaP-IR according toPodstawka et al., 2008 [59] | |||||||||
 | |||||||||
| Vibrations | |||||||||
| Wave number, (cm−1) | 3273 | 3100 | 2982 | 2940, 2885 | 1664 | 1081 | 930 | 700 | 558 |
| Vibration type (group) | ν(N–H) | ν(C–H) | ν(CS–H) | ν(C–H)CH3 | ν(C=O),ν(C–N)A,Δ(CCA(=O)NA) | ν(C–N),ν(C–C)Ala,δ(CCH3)Ala,ρb(C–N+H3) | δ(C–C)Ala,ν(C–N+H3) | ν(CS–P), ν(C–CA) | Δ(NACS(P) C) δ(NACS(P) C) |
| Ala-AlaP/PP | |||||||||
| Wave number, (cm−1) | 3273 | – | – | – | 1664 | 1081 | 930 | 700 | 558 |
| Vibration type (group) | ν(N–H) | – | – | – | ν(C=O), ν(C–N)A,Δ((CCA(=O)NA) | ν(C–N), ν(C–C)Ala,δ(CCH3),ρb(C–N+H3) | δ(C–C)Ala, ν(C–N+H3) | ν(CS–P), ν(C–CA) | Δ(NACS(P)C) δ(NACS(P)C) |
| Parameter | PP | PP-Ala-AlaP (%Ala-AlaP Paste Concentrations) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 10 | 15 | |||
| Average air permeability (mm/s), pressure decrease: | 100 Pa | 250 | 196 | 198 | 185 | 196 | 193 | 191 |
| 200 Pa | 482 | 369 | 355 | 322 | 351 | 360 | 355 | |
| Parameter | PP | PP-Ala-AlaP (%Ala-AlaP Paste Concentrations) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 10 | 15 | ||
| Tensile strength (kN/m) | 0.32 | 0.40 | 0.41 | 0.39 | 0.38 | 0.37 | 0.37 |
| Relative elongation at maximum load (%) | 35.0 | 38.7 | 38.1 | 38.2 | 37.5 | 37.3 | 37.2 |
| Sample No. | Ala-AlaP on Polypropylene Nonwovens [Ala-AlaP/PP] | Average Inhibition Zone (ZoI) of Bacterial Growth for Bacteria (mm) | |
|---|---|---|---|
| Ala-AlaP Coating Pastes Concentrations (%) | E. coli | S. aureus | |
| 1 | 0 | 0 | 0 |
| 2 | 0.005 | 0 | 0 |
| 3 | 0.01 | 12 | 0 |
| 4 | 0.05 | 12 | 12 |
| 5 | 0.1 | 14 | 14 |
| 6 | 0.15 | 14 | 14 |
| Surface of applied disks with 1 cm diameter equals 0.785 cm2 Concentration of inoculum (bacterial suspension) amount of live bacteria CFU/mL = 0.4 × 108 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudzin, M.H.; Mrozińska, Z.; Walawska, A.; Sójka-Ledakowicz, J. Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin. Coatings 2019, 9, 412. https://doi.org/10.3390/coatings9070412
Kudzin MH, Mrozińska Z, Walawska A, Sójka-Ledakowicz J. Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin. Coatings. 2019; 9(7):412. https://doi.org/10.3390/coatings9070412
Chicago/Turabian StyleKudzin, Marcin H., Zdzisława Mrozińska, Anetta Walawska, and Jadwiga Sójka-Ledakowicz. 2019. "Biofunctionalization of Textile Materials.1. Biofunctionalization of Poly(Propylene) (PP) Nonwovens Fabrics by Alafosfalin" Coatings 9, no. 7: 412. https://doi.org/10.3390/coatings9070412





