Deposition of TiO2 Thin Films on Wood Substrate by an Air Atmospheric Pressure Plasma Jet
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
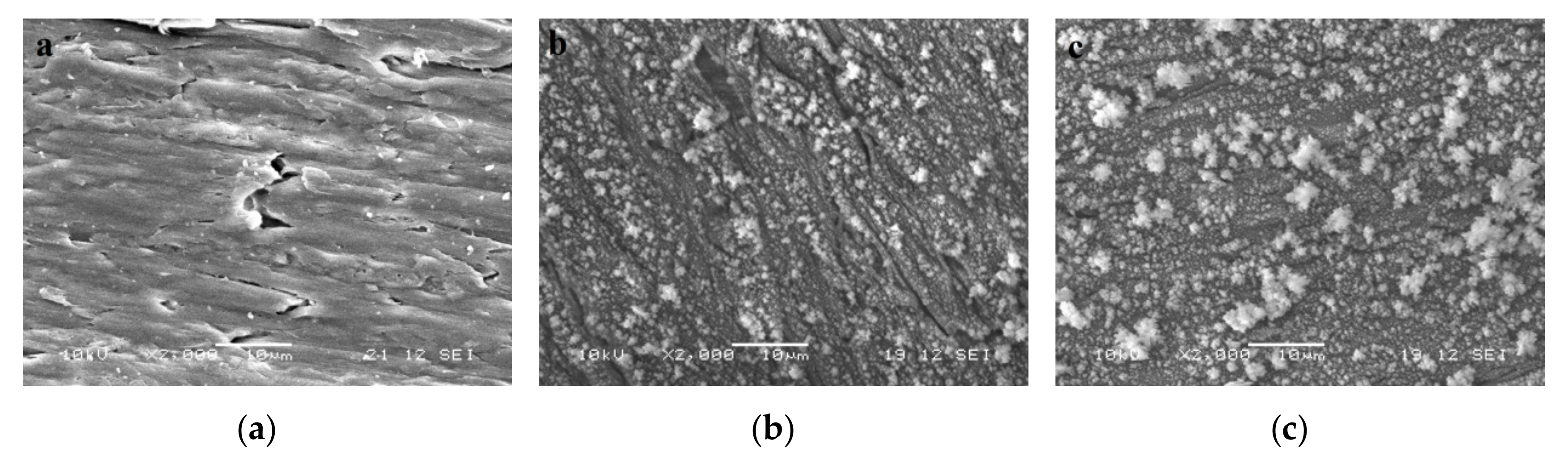
3.1. Characterization of TiO2 Coatings on Wood by Scanning Electron Microscopy (SEM)
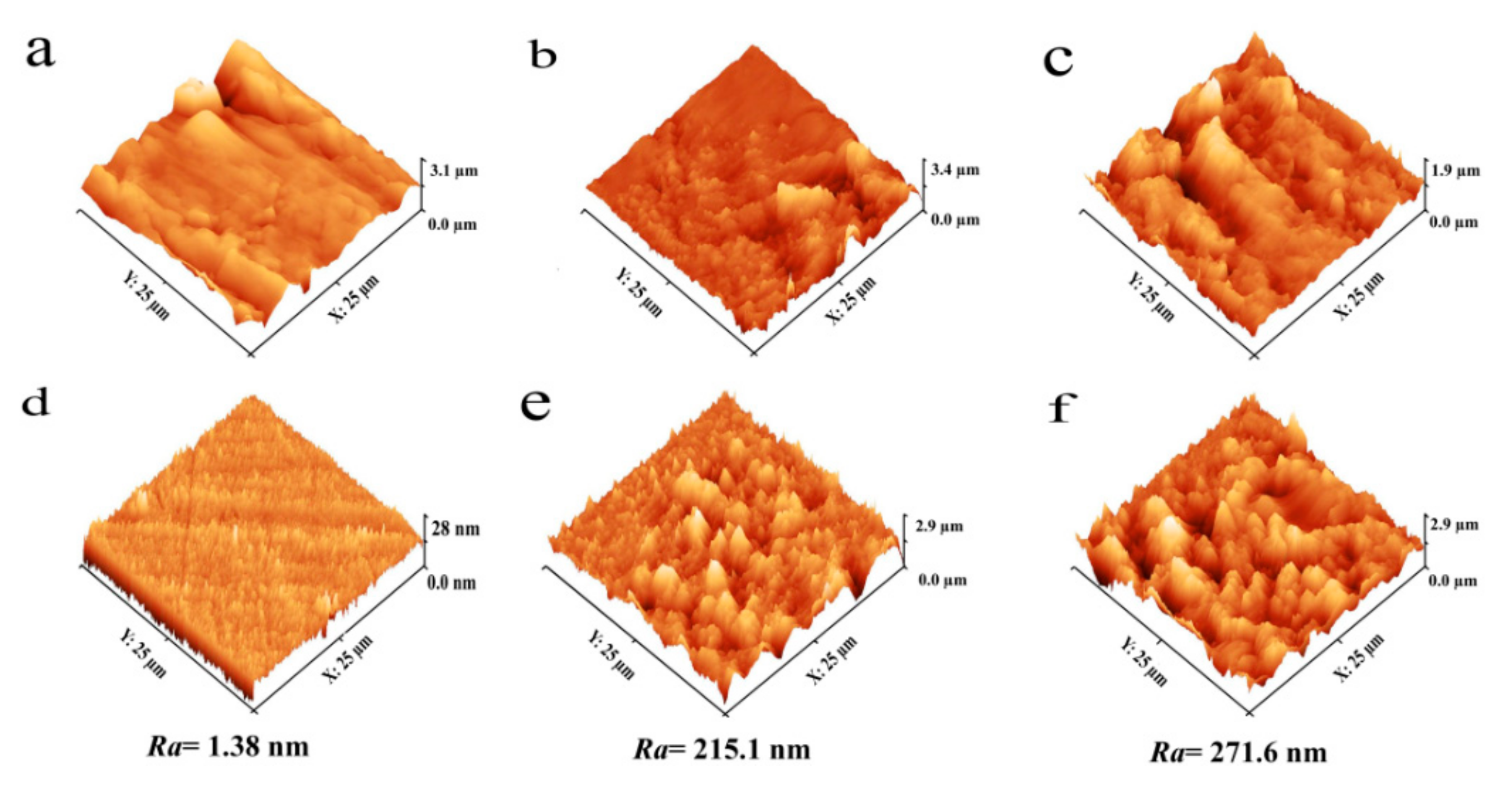
3.2. Analysis of Surface Topography by Atomic Force Microscopy (AFM)
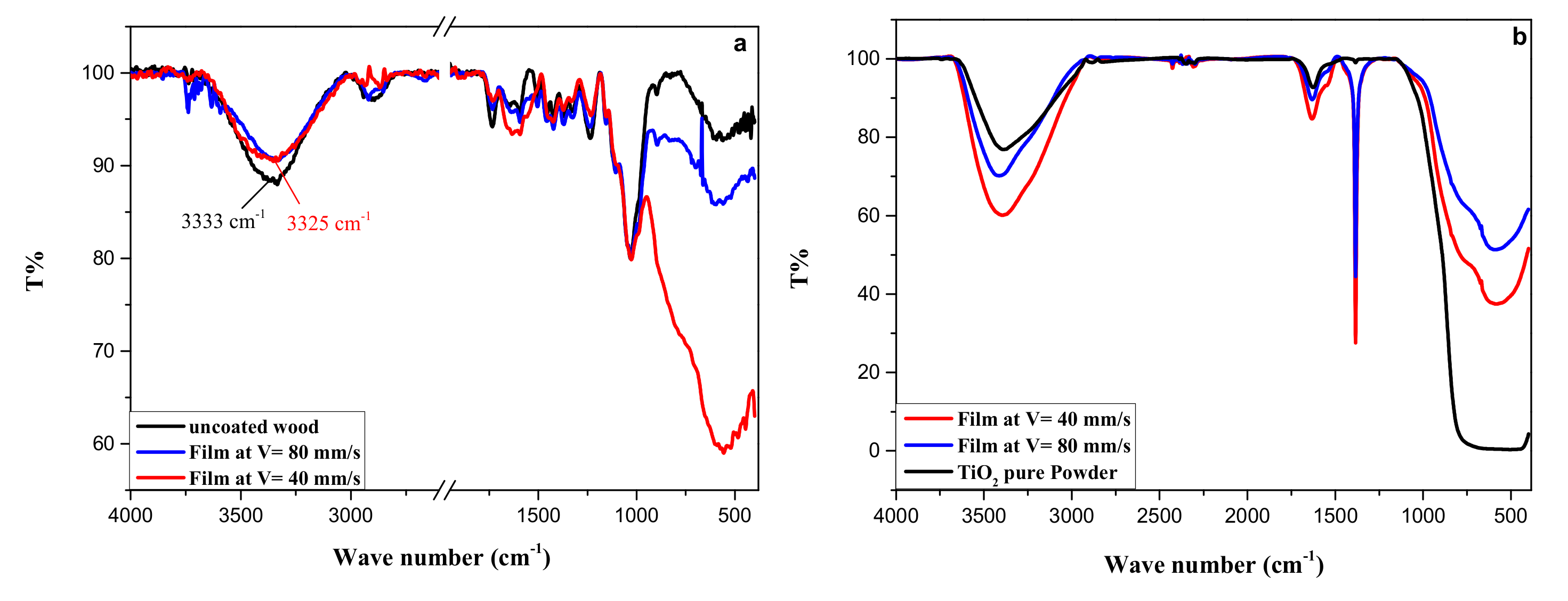
3.3. Characterization of TiO2 Coatings by Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Characterization of TiO2 Coatings by X-ray Photoelectron Spectroscopy (XPS)
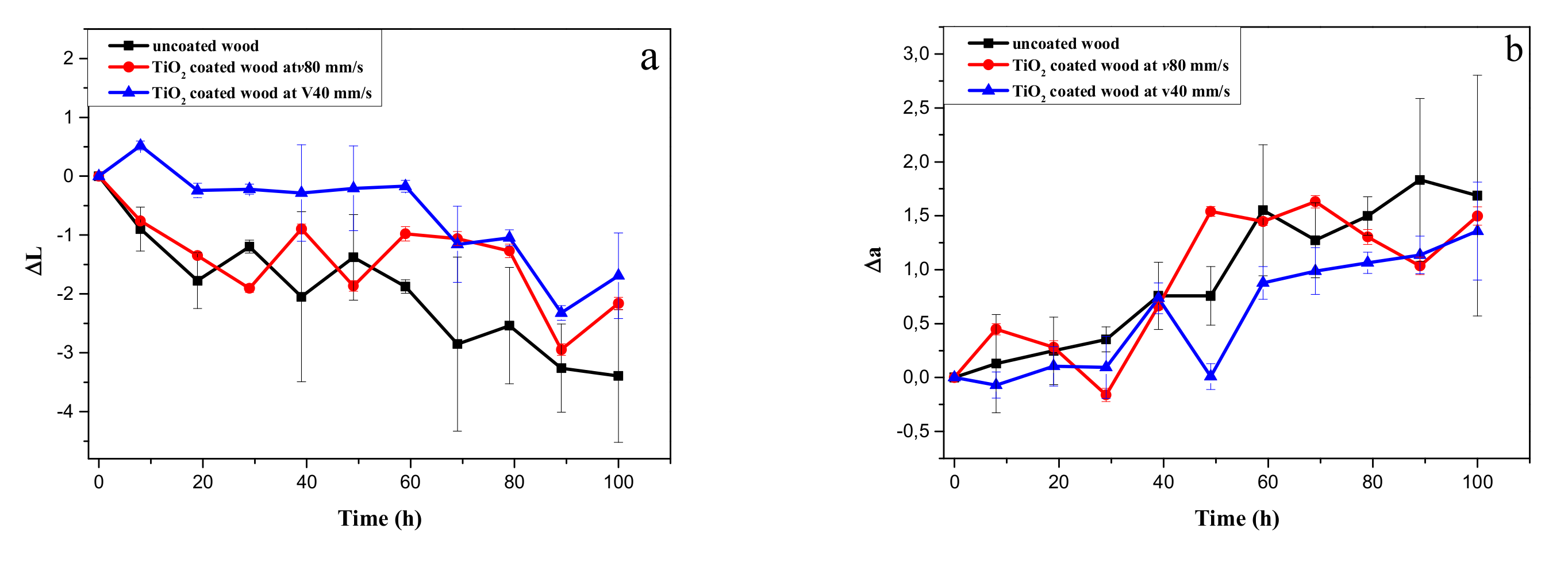
3.5. Ultraviolet (UV) Protection
3.6. Contact Angle Analysis (Water)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Feist, W.C.; Hon, D.S. Chemistry of weathering and protection. In The Chemistry of Solid Wood; Rowell, R.M., Ed.; American Chemical Society: Washington, DC, USA, 1984; Volume 207, pp. 401–451. [Google Scholar]
- Garcia, R.A.; Lopes, J.O.; Nascimento, A.M.; Latorraca, J.V. Color stability of weathered heat-treated teak wood. Maderas Cienc. Tecnol. 2014, 16, 453–462. [Google Scholar] [CrossRef]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhu, S.; Pan, N. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Wallenhorst, L.; Gurău, L.; Gellerich, A.; Militz, H.; Ohms, G.; Viöl, W. UV-blocking properties of Zn/ZnO coatings on wood deposited by cold plasma spraying at atmospheric pressure. Appl. Surf. Sci. 2018, 434, 1183–1192. [Google Scholar] [CrossRef]
- Liu, X.H.; Fu, Y.B. Studies on the structures and optical properties of TiO2 doped with transition metals. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing, Waikoloa, HI, USA, 4–9 August 2013; pp. 295–305. [Google Scholar]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Rassam, G.; Abdi, Y.; Abdi, A. Deposition of TiO2 nano-particles on wood surfaces for UV and moisture protection. J. Exp. Nanosci. 2011, 7, 468–476. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Sun, Q.; Liu, Y.; Cui, Y.; Lu, Y. Growth of TiO2 coating on wood surface using controlled hydrothermal method at low temperatures. Appl. Surf. Sci. 2010, 256, 5046–5050. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Y.; Zhang, H.; Zhao, H.; Yu, H.; Xu, J.; Fu, Y.; Yang, D.; Liu, Y. Hydrothermal fabrication of rutile TiO2 submicrospheres on wood surface: An efficient method to prepare UV-protective wood. Mater. Chem. Phys. 2012, 133, 253–258. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, H.; Liu, Y.; Li, J.; Cui, Y.; Lu, Y. Prolonging the combustion duration of wood by TiO2 coating synthesized using cosolvent-controlled hydrothermal method. J. Mater. Sci. 2010, 45, 6661–6667. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, H.; Liu, Y.; Li, J.; Lu, Y. Improvement of water resistance and dimensional stability of wood through titanium dioxide coating. Holzforschung 2010, 64, 757–761. [Google Scholar] [CrossRef]
- Zheng, R.; Tshabalala, A.M.; Li, Q.; Wang, H. Construction of hydrophobic wood surfaces by room temperature deposition of rutile (TiO2) nanostructures. Appl. Surf. Sci. 2015, 328, 453–458. [Google Scholar] [CrossRef]
- Zanatta, P.; Lazarotto, M.; Cademartori, P.H.; Cava, S.; Moreira, M.; Gatto, D. The effect of titanium dioxide nanoparticles obtained by microwave-assisted hydrothermal method on the color and decay resistance of pinewood. Maderas Cienc. Tecnol. 2017, 19, 495–506. [Google Scholar] [CrossRef]
- Miyafuji, H.; Saka, S. Fire-resisting properties in several TiO2 wood-inorganic composites and their topochemistry. Wood Sci. Technol. 1997, 31, 449–455. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, S.C.; Chang, H.J.; Liu, J.L. Sol-gel deposition of TiO2 nanocoatings on wood surfaces with enhanced hydrophobicity and photostability. Wood Fiber Sci. 2014, 46, 109–117. [Google Scholar]
- Hübert, T.; Unger, B.; Bücker, M. Sol-gel derived TiO2 wood composites. J. Sol-Gel Sci. Technol. 2010, 53, 384–389. [Google Scholar] [CrossRef]
- Pavel, P.; Aljaž, V.; Marko, P.; Andrijana, S.; Mohor, M.; Angela, S.; Urban, N.; Boris, O. Structural studies of TiO2/wood coatings prepared by hydrothermal deposition of rutile particles from TiCl4 aqueous solutions on spruce (Picea Abies) wood. Appl. Surf. Sci. 2016, 372, 125–138. [Google Scholar]
- Levasseur, O.; Stafford, L.; Gherardi, N.; Naudé, N.; Blanchard, V.; Blanchet, P.; Riedl, B.; Sarkissian, A. Deposition of hydrophobic functional groups on wood surfaces using atmospheric-pressure dielectric barrier discharge in helium hexamethyldisiloxane gas mixtures. Plasma Process. Polym. 2012, 9, 1168–1175. [Google Scholar] [CrossRef]
- Gerullis, S.; Pfuch, A.; Spange, S.; Kettner, F.; Plaschkies, K.; Kosmachev, P.V.; Volokitin, G.; Grünler, B. Antimicrobial coatings on wood by use of atmospheric pressure chemical vapour deposition (APCVD). Holztechnologie 2016, 57, 16–25. [Google Scholar]
- Köhler, R.; Sauerbier, P.; Militz, H.; Viöl, W. Atmospheric pressure plasma coating of wood and MDF with polyester powder. Coatings 2017, 7, 171. [Google Scholar] [CrossRef]
- Buske, C. Environmentally friendly and cost-saving Atmospheric-pressure plasma technology. Innov. Surf. Technol. 2009, 2, 18–22. [Google Scholar]
- Dong, S.; Zhao, Z.; Dauskardt, R.H. Highly transparent bilayer organosilicate multifunctional coatings on plastics deposited by atmospheric plasma deposition with dual organic and inorganic precursors in air. ACS Appl. Mater. Interf. 2015, 7, 17929–17934. [Google Scholar] [CrossRef] [PubMed]
- Pappas, D. Status and potential of atmospheric plasma processing of materials. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 020801. [Google Scholar] [CrossRef]
- Fakhouri, H.; Ben Salem, D.; Carton, O.; Pulpytel, J.; Arefi-Khonsari, F. Highly effcient photocatalytic TiO2 coatings deposited by open air atmospheric pressure plasma jet with aerosolized TTIP precursor. J. Phys. D Appl. Phys. 2014, 47, 265301. [Google Scholar] [CrossRef]
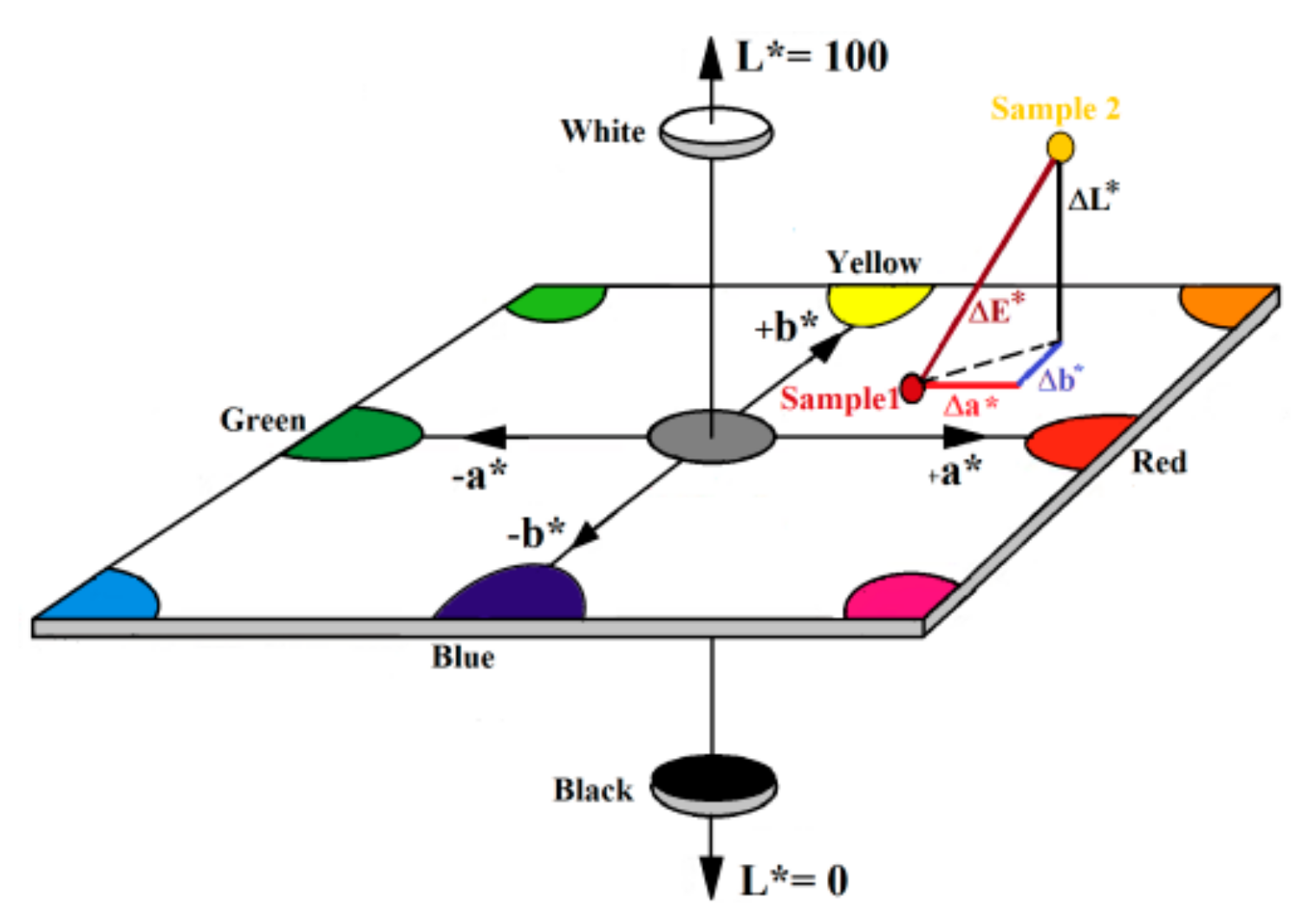
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey. J. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Ozen, E.; Yeniocak, M.; Colak, M.; Koca, I. Colorability of wood material with punica granatum and morus nigra extracts. Bioresources 2014, 9, 2797–2807. [Google Scholar] [CrossRef]
- Oberhofnerová, E.; Pánek, M.; García-Cimarras, A. The effect of natural weathering on untreated wood surface. Maderas Cienc. Tecnol. 2017, 19, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, Q.; Hubert, J.; Huang, W.; Baert, K.; Wallaert, G.; Terryn, H.; Delplancke-Ogletree, M.; Reniers, F. Deposition of photocatalytic anatase titanium dioxide films by atmospheric dielectric barrier discharge. Surf. Coat. Technol. 2017, 310, 173–179. [Google Scholar] [CrossRef]
- Olay, M.G.; González-Salgado, F.; Cruz, G.J.; Gómez, L.; García-Rosales, G.; Gonzalez-Torres, M.; Lopez-Gracia, O.G. Chemical structure of TiO organometallic particles obtained by plasma. Adv. Nanopart. 2013, 2, 229–235. [Google Scholar] [CrossRef]
- Nakamura, M.; Korzec, D.; Aoki, T.; Engemann, J.; Hatanaka, Y. Characterization of TiOx film prepared by plasma enhanced chemical vapor deposition using a multi-jet hollow cathode plasma source. Appl. Surf. Sci. 2001, 175, 697–702. [Google Scholar] [CrossRef]
- Borca, E.; Bercu, M.; Georgescu, S.; Hodorogea, S.; Cotoi, E. XRD and FTIR characterization of nanocrystalline YVO4:Eu derived by coprecipitation process. Z. Krist. Suppl. 2008, 27, 457–462. [Google Scholar]
- Dou, B.; Wang, Y.; Zhang, T.; Liu, B.; Shao, Y.; Meng, G.; Wang, F. Growth behaviors of layered double hydroxide on microarc oxidation film and anti-corrosion performances of the composite film. J. Electrochem. Soc. 2016, 163, 917–927. [Google Scholar] [CrossRef]
- Kang, S.; Mauchauffé, R.; You, Y.S.; Moon, S.Y. Insights into the role of plasma in atmospheric pressure chemical vapor deposition of titanium dioxide thin films. Sci. Rep. 2018, 8, 16684. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood—The state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Schulte, K. Application of micronized titanium dioxide as inorganic UV absorber. In Proceedings of the 11th Asia Pacific Coating Conference, Bangkok, Thailand, 26–27 June 2001. [Google Scholar]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M.; Nekahi, A. Properties and applications of superhydrophobic coatings in high voltage outdoor insulation: A review. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3630–3646. [Google Scholar]
- Gao, L.; Lu, Y.; Cao, J.; Li, J.; Sun, Q. Reversible photocontrol of wood-surface wettability between superhydrophilicity and superhydrophobicity based on a TiO2 film. J. Wood Chem. Technol. 2015, 35, 365–373. [Google Scholar] [CrossRef]
- Chen, F.; Yang, X.; Wu, Q. Antifungal capability of TiO2 coated film on moist wood. Build. Environ. 2009, 44, 1088–1093. [Google Scholar] [CrossRef]

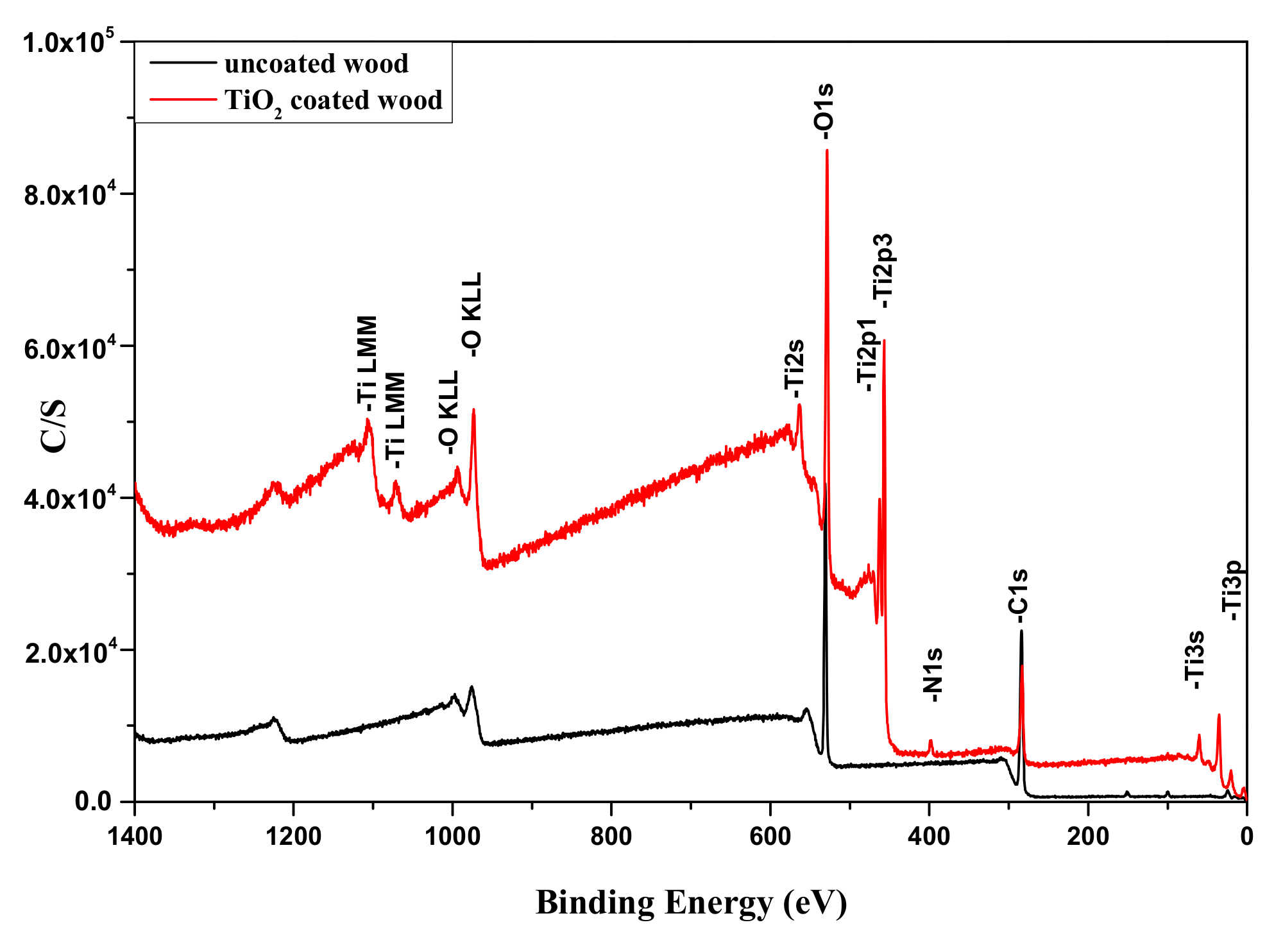
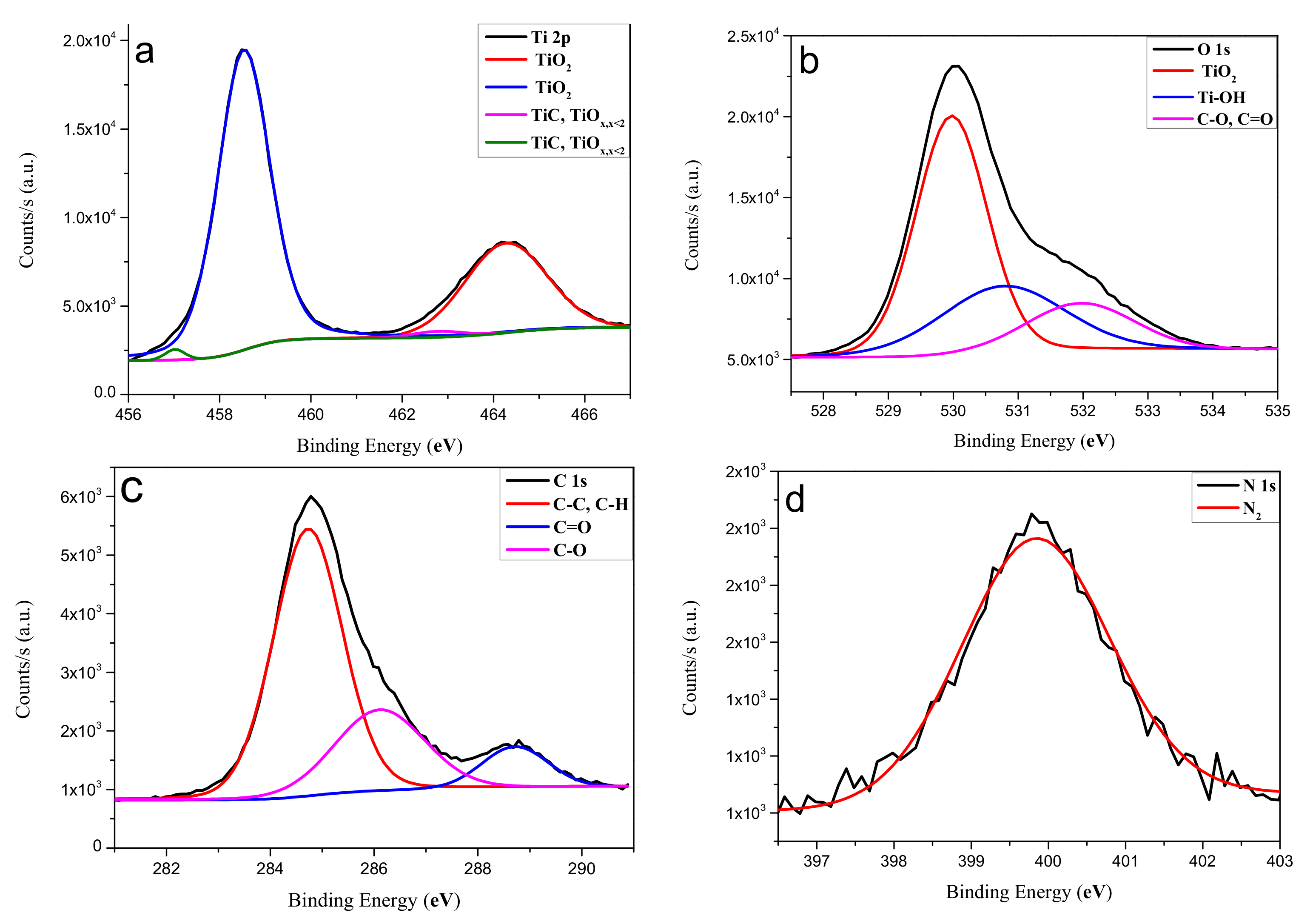


| Element | Binding Energy (eV) | Assignment | Atomic Percentage (at.%) |
|---|---|---|---|
| Ti | 458.53 and 464.31 | TiO2 | 15.7 |
| 457.01 and 462.44 | Ti–C or TiOx < 2 | 0.8 | |
| O | 530.01 | TiO2 | 31.3 |
| 531.33 | Ti–OH | 10.8 | |
| 532.35 | C–O or C=O | 3.5 | |
| C | 284.75 | C–C or C-H | 24.1 |
| 286.18 | C–O | 8.3 | |
| 288.76 | C=O | 3.5 | |
| N | 399.82 | N2 | 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jnido, G.; Ohms, G.; Viöl, W. Deposition of TiO2 Thin Films on Wood Substrate by an Air Atmospheric Pressure Plasma Jet. Coatings 2019, 9, 441. https://doi.org/10.3390/coatings9070441
Jnido G, Ohms G, Viöl W. Deposition of TiO2 Thin Films on Wood Substrate by an Air Atmospheric Pressure Plasma Jet. Coatings. 2019; 9(7):441. https://doi.org/10.3390/coatings9070441
Chicago/Turabian StyleJnido, Ghiath, Gisela Ohms, and Wolfgang Viöl. 2019. "Deposition of TiO2 Thin Films on Wood Substrate by an Air Atmospheric Pressure Plasma Jet" Coatings 9, no. 7: 441. https://doi.org/10.3390/coatings9070441







