Synthesis of Silphenylene-Containing Siloxane Resins Exhibiting Strong Hydrophobicity and High Water Vapor Barriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
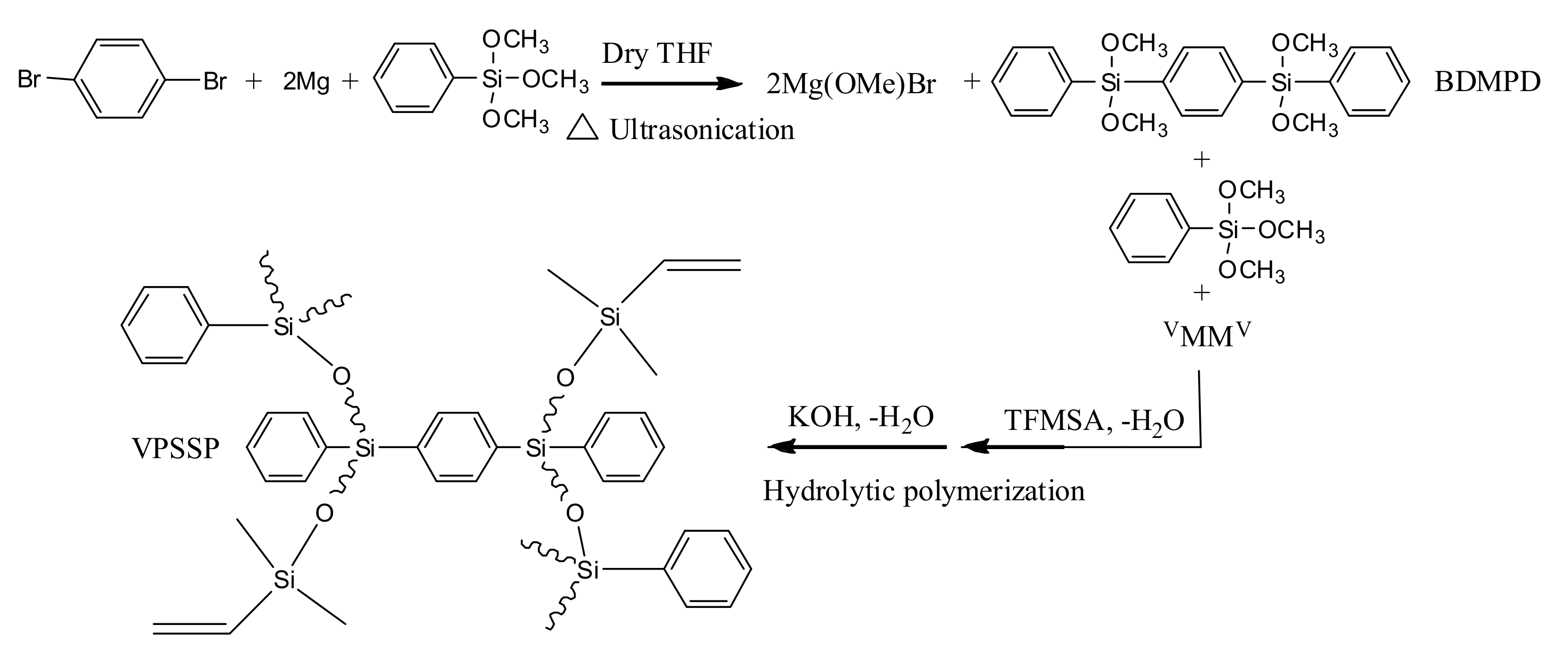
2.2. Synthesis of the 1,4-Bis(Dimethoxyphenylsilyl)Benzene (BDMPD)
2.3. Synthesis of the Vinyl-Terminated Polysiloxanes Containing Silphenylene Units (VPSSP) via the Hydrolytic Polymerization of BDMPD, TMPS, and VMMV
2.4. Preparation of the Fully Cured Siloxane Resins Containing Silphenylene Units (SRS)
2.5. Measurements
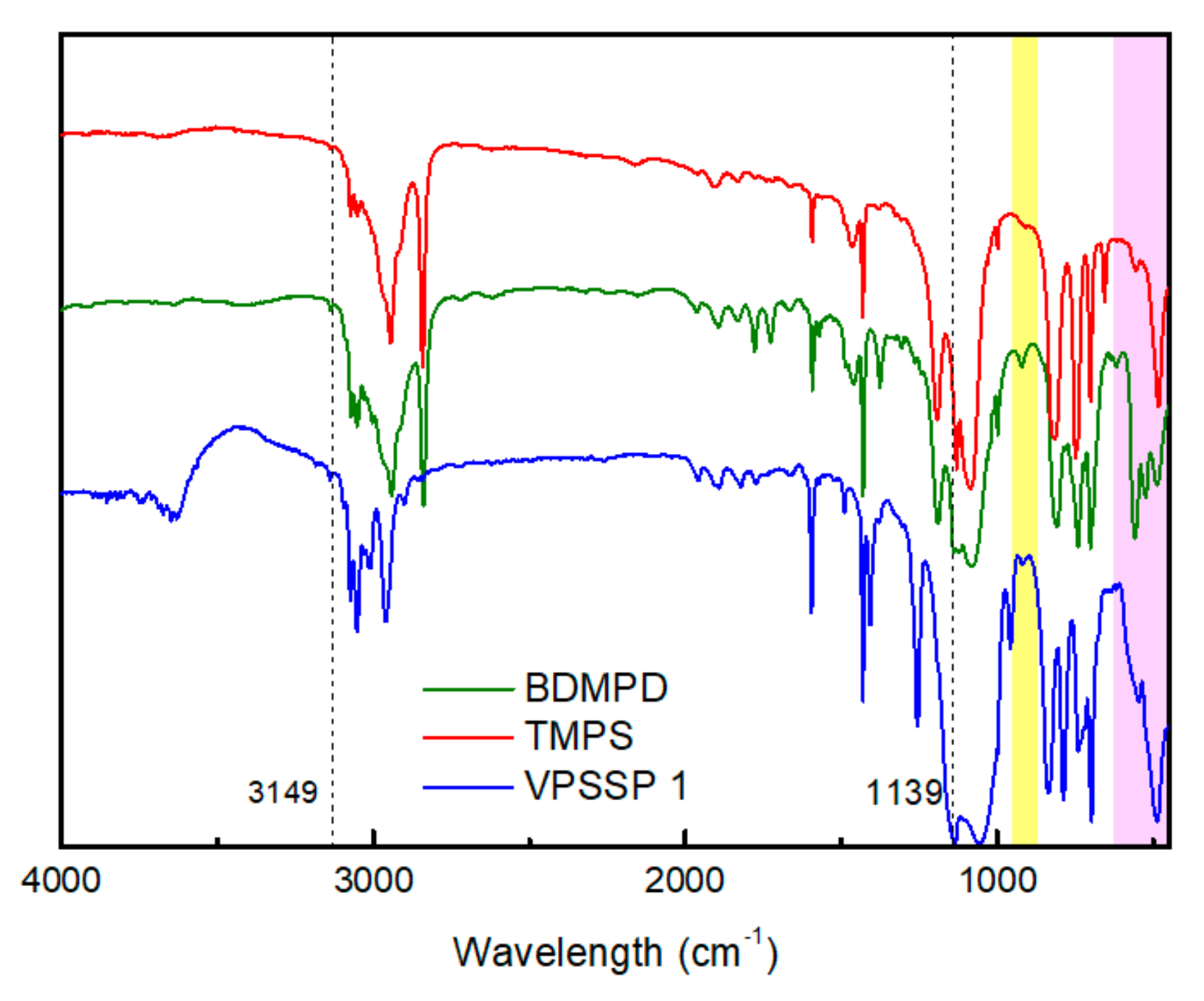
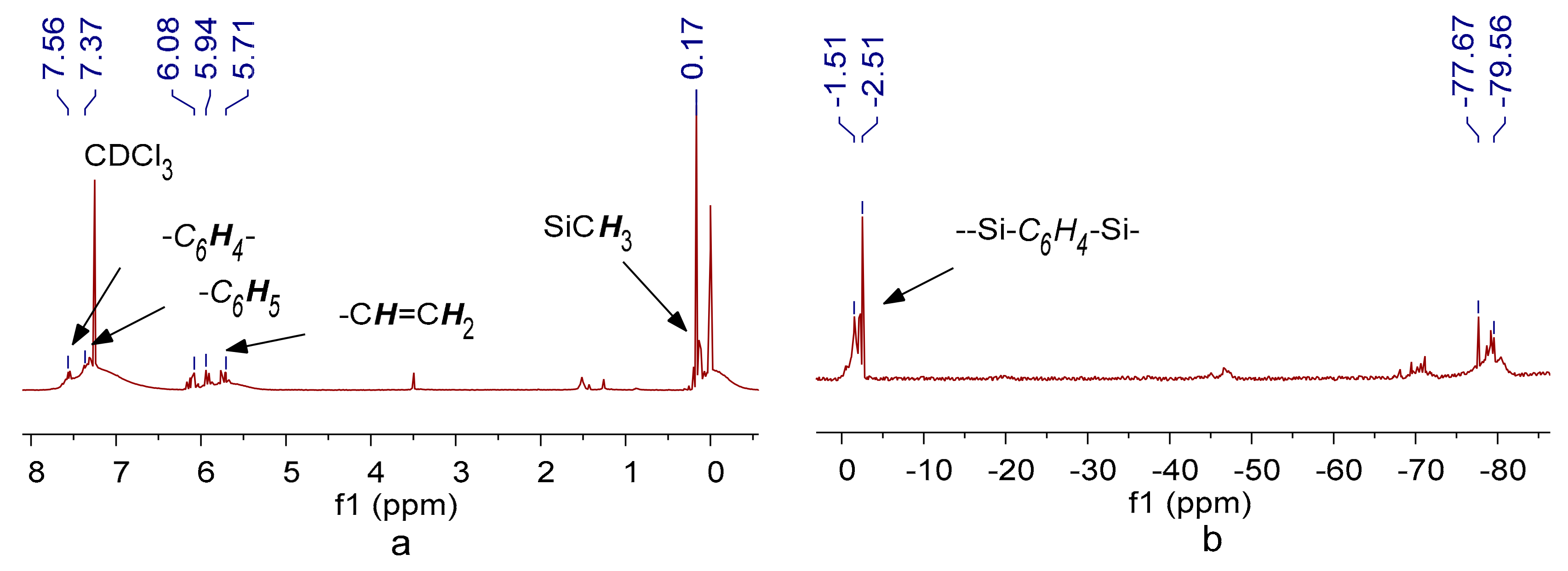
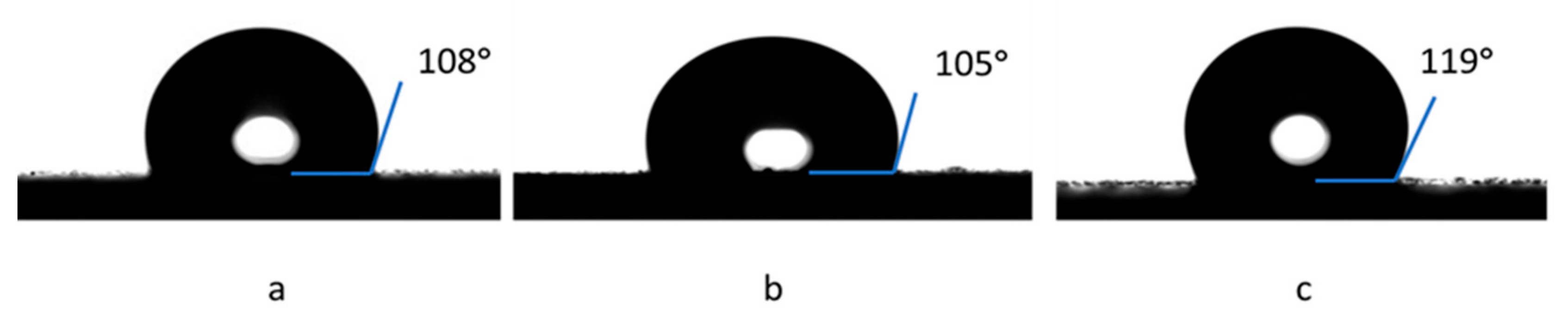
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charifou, R.; Espuche, E.; Gouanvé, F.; Dubost, L.; Monaco, B. SiOx and SiOxCzHw mono-and multi-layer deposits for improved polymer oxygen and water vapor barrier properties. J. Membr. Sci. 2016, 500, 245–254. [Google Scholar] [CrossRef]
- Karasu, F.; Müller, L.; Ridaoui, H.; Ibn Elhaj, M.; Flodberg, G.; Aulin, C.; Axrup, L.; Leterrier, Y. Organic-inorganic hybrid planarization and water vapor barrier coatings on cellulose nanofibrils substrates. Front. Chem. 2018, 6, 571. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yang, D.; Xu, Y.; Wu, D.; Sun, Y. A novel moisture-resistant film for NiSO4 6H2O filter based on isophorone diisocyanate-bridged polysilsesquioxane. Mater. Chem. Phys. 2009, 114, 868–873. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, H.; Xiao, B.; Yan, L.; Lv, H.; Jiang, B. Sol−gel preparation of PDMS/silica hybrid antireflective coatings with controlled thickness and durable antireflective performance. J. Phys. Chem. C 2010, 114, 19979–19983. [Google Scholar] [CrossRef]
- Hara, T.; Nishimura, S.; Ozawa, S.; Abe, R.; Tokudome, Y.; Takahashi, M. Graphene oxide incorporation in lamellar organosiloxane film for improved water vapor barrier property. J. Sol. Gel Sci. Technol. 2016, 79, 405–409. [Google Scholar] [CrossRef]
- Hao, L.; Gao, T.; Xu, W.; Wang, X.; Yang, S.; Liu, X. Preparation of crosslinked polysiloxane/SiO2 nanocomposite via in-situ condensation and its surface modification on cotton fabrics. Appl. Surf. Sci. 2016, 371, 281–288. [Google Scholar] [CrossRef]
- Tokudome, Y.; Hara, T.; Abe, R.; Takahashi, M. Transparent and robust siloxane-based hybrid lamella film as a water vapor barrier coating. ACS Appl. Mater. Interfaces 2014, 6, 19355–19359. [Google Scholar] [CrossRef]
- Carosio, F.; Colonna, S.; Fina, A.; Rydzek, G.; Hemmerlé, J.; Jierry, L.; Schaaf, P.; Boulmedais, F. Efficient gas and water vapor barrier properties of thin poly(lactic acid) packaging films: Functionalization with moisture resistant nafion and clay multilayers. Chem. Mater. 2014, 26, 5459–5466. [Google Scholar] [CrossRef]
- Gupta, S.; Seethamraju, S.; Ramamurthy, P.C.; Madras, G. Polyvinylbutyral based hybrid organic/inorganic films as a moisture barrier material. Ind. Eng. Chem. Res. 2013, 52, 4383–4394. [Google Scholar] [CrossRef]
- Reddy, J.P.; Varada Rajulu, A.; Rhim, J.W.; Seo, J. Mechanical, thermal, and water vapor barrier properties of regenerated cellulose/nano-SiO2 composite films. Cellulose 2018, 25, 7153–7165. [Google Scholar] [CrossRef]
- Peng, P.; Ke, Q.; Zhou, G.; Tang, T. Fabrication of microcavity-array superhydrophobic surfaces using an improved template method. J. Colloid Interface Sci. 2013, 395, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Rezayi, T.; Entezari, M.H. Achieving to a superhydrophobic glass with high transparency by a simple sol–gel-dip-coating method. Surf. Coat. Technol. 2015, 276, 557–564. [Google Scholar] [CrossRef]
- Rezaei, S.; Manoucheri, I.; Moradian, R.; Pourabbas, B. One-step chemical vapor deposition and modification of silica nanoparticles at the lowest possible temperature and superhydrophobic surface fabrication. Chem. Eng. J. 2014, 252, 11–16. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.; Chen, X.G. Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties. Appl. Surf. Sci. 2015, 356, 1012–1024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, C.; Cui, X.; Sun, J.; Ding, R.; Zhang, Q.; Xu, Y. Transparent and dense ladder-like alkylene-bridged polymethylsiloxane coating with enhanced water vapor barrier property. ACS Appl. Mater. Interfaces 2015, 7, 22157–22165. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Hancer, M.; Arkaz, H. A facile fabrication of superhydrophobic nanocomposite coating with contact angles approaching the theoretical limit. Appl. Surf. Sci. 2015, 354, 342–346. [Google Scholar] [CrossRef]
- Tian, S.; Li, J.; Cai, Z.; Shi, B.; Chen, W.; Cheng, Y. Piers-Rubinsztajn reaction for BCB functionalized silphenylene/silbiphenylene siloxane oligomers to highly crosslinked low-k thermosets. Eur. Polym. J. 2018, 108, 373–379. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yi, M.; Wu, S.; Tan, L.; Ge, X.; He, M.; Yin, G. Synthesis of structurally precise polysiloxanes via the Piers–Rubinsztajn reaction. Materials 2019, 12, 304. [Google Scholar] [CrossRef]
- Yang, Z.; Han, S.; Zhang, R.; Feng, S.; Zhang, C.; Zhang, S. Effects of silphenylene units on the thermal stability of silicone resins. Polym. Degrad. Stab. 2011, 96, 2145–2151. [Google Scholar] [CrossRef]
- Cao, X.; Wang, L.; Li, B.; Zhang, R. Synthesis and characterization of ladder-like phenylene-bridged polysiloxanes. Polym. Adv. Technol. 1997, 8, 657–661. [Google Scholar] [CrossRef]
- Kawakita, T.; Oh, H.S.; Moon, J.Y.; Liu, Y.; Imae, I.; Kawakami, Y.; Oh, H.; Moon, J. Synthesis, characterization and thermal properties of phenylene-disiloxane polymers obtained from catalytic cross-dehydrocoupling polymerization of bis(dimethylsilyl)benzene isomers and water. Polym. Int. 2001, 50, 1346–1351. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, L.; Diao, S.; Feng, S.; Zhang, C. Study on the synthesis and thermal degradation of silicone resin containing silphenylene units. Thermochim. Acta 2011, 521, 170–175. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, J.; Zhao, X.; Yang, S.; Fan, L. Synthesis and characterization of siloxane resins derived from silphenylene-siloxane copolymers bearing benzocyclobutene pendant groups. J. Polym. Sci. Part. A Polym. Chem. 2008, 46, 7868–7881. [Google Scholar] [CrossRef]
- So, B.K.; Lee, K.S.; Lee, S.M.; Lee, M.K.; Lim, T.K. Synthesis and linear/nonlinear optical properties of new polyamides with dans chromophore and silphenylene groups. Opt. Mater. 2003, 21, 87–92. [Google Scholar] [CrossRef]
- Jia, M.; Wu, C.; Li, W.; Gao, D. Synthesis and characterization of a silicone resin with silphenylene units in Si-O-Si backbones. J. Appl. Polym. Sci. 2009, 114, 971–977. [Google Scholar] [CrossRef]
- Bahadur, M.; Norris, A.W.; Zarisfi, A.; Alger, J.S.; Windiate, C.C. Silicone materials for LED packaging. In Proceedings of the Sixth International Conference on Solid State Lighting, San Diego, CA, USA, 12 September 2006; Volume 6337, 63370F. [Google Scholar]
- Wang, J.; Zhou, J.; Jin, K.; Wang, L.; Sun, J.; Fang, Q. A new fluorinated polysiloxane with good optical properties and low dielectric constant at high frequency based on easily available tetraethoxysilane (teos). Macromolecules 2017, 50, 9394–9402. [Google Scholar] [CrossRef]
- Watanabe, H.; Takahashi, M.; Kihara, H.; Yoshida, M. Biobased coatings based on eugenol derivatives. ACS Appl. Bio Mater. 2018, 1, 808–813. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, J.; Fang, L.; Wang, Y.; Chen, X.; Chen, X.; Hou, J.; Sun, J.; Fang, Q. Fluoro-containing polysiloxane thermoset with good thermostability and acid resistance based on the renewable multifunctional vanillin. ACS Sustain. Chem. Eng. 2019, 7, 7304–7311. [Google Scholar] [CrossRef]



| Sample | BDMPD/TMPS (mol %) | Glass Transition Temperature (Tg/°C) | n | Yield (%) | Mw (kDa) | Mz/Mw |
|---|---|---|---|---|---|---|
| VPSSP 1 | 20 | −23.6 | 1.542 | 64.96 | 65.72 | 21.03 |
| VPSSP 2 | 15 | −28.2 | 1.539 | 76.46 | 3.309 | 2.00 |
| VPSSP 3 | 10 | −29.8 | 1.537 | 79.15 | 4.165 | 2.09 |
| VPSSP 4 | 5 | −34.4 | 1.532 | 51.56 | 2.919 | 1.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yi, M.; Wu, S.; Tan, L.; Xu, Y.; Guan, Z.; Ge, J.; Yin, G. Synthesis of Silphenylene-Containing Siloxane Resins Exhibiting Strong Hydrophobicity and High Water Vapor Barriers. Coatings 2019, 9, 481. https://doi.org/10.3390/coatings9080481
Chen X, Yi M, Wu S, Tan L, Xu Y, Guan Z, Ge J, Yin G. Synthesis of Silphenylene-Containing Siloxane Resins Exhibiting Strong Hydrophobicity and High Water Vapor Barriers. Coatings. 2019; 9(8):481. https://doi.org/10.3390/coatings9080481
Chicago/Turabian StyleChen, Xunjun, Minghao Yi, Shufang Wu, Lewen Tan, Yixin Xu, Zhixing Guan, Jianfang Ge, and Guoqiang Yin. 2019. "Synthesis of Silphenylene-Containing Siloxane Resins Exhibiting Strong Hydrophobicity and High Water Vapor Barriers" Coatings 9, no. 8: 481. https://doi.org/10.3390/coatings9080481






