The Corrosion Resistance of Aluminum–Bronze Coatings as a Function of Gas Pressure Used in the Thermal Spraying Process
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
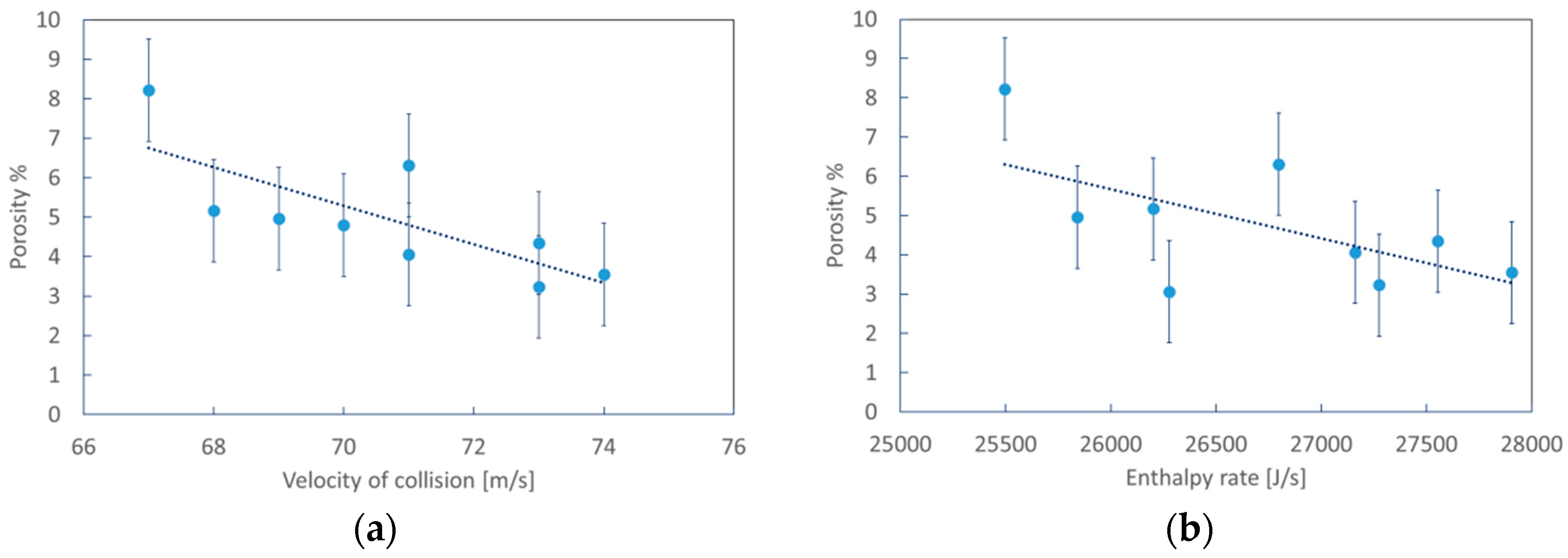
3.1. Numerical Simulation
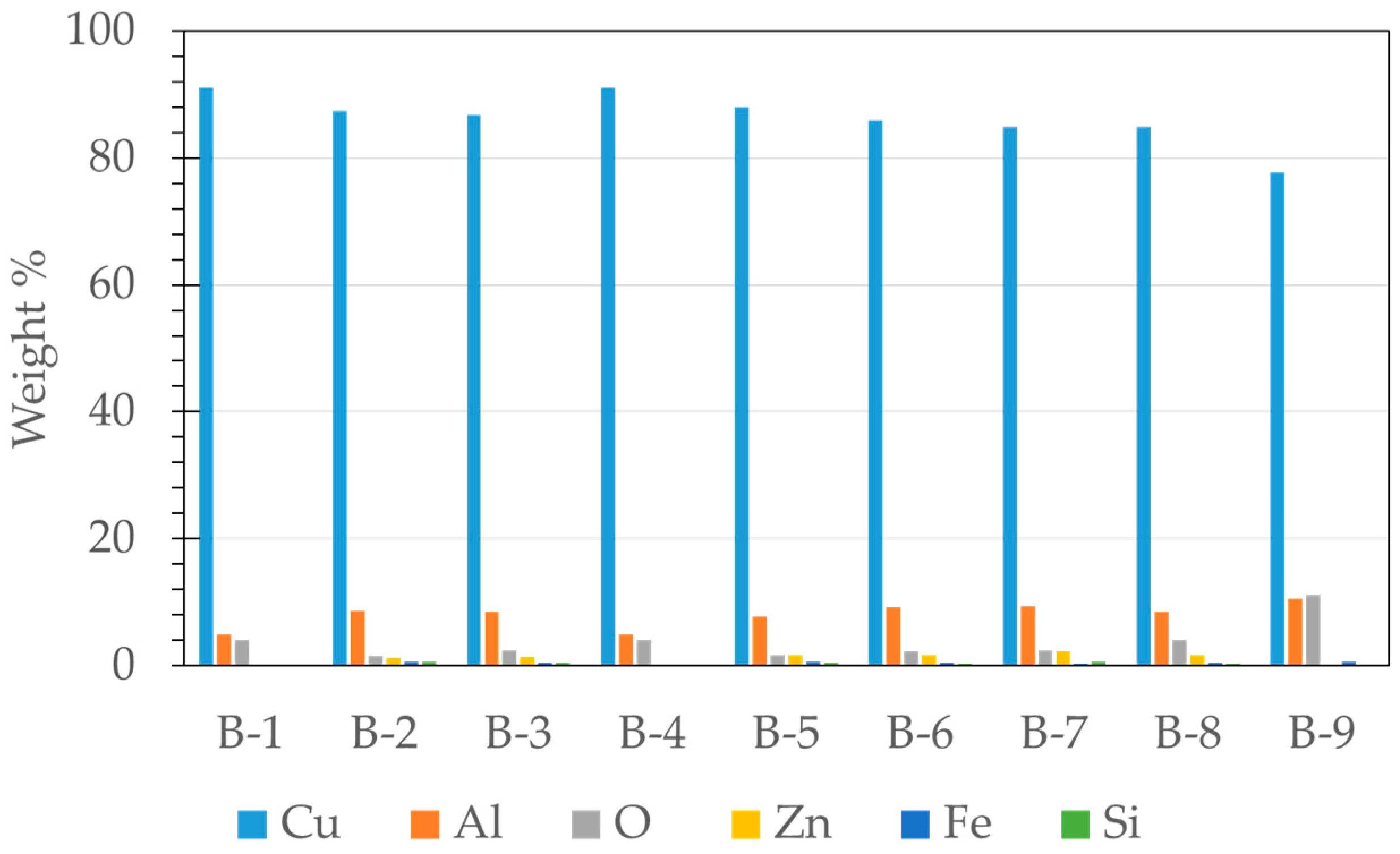
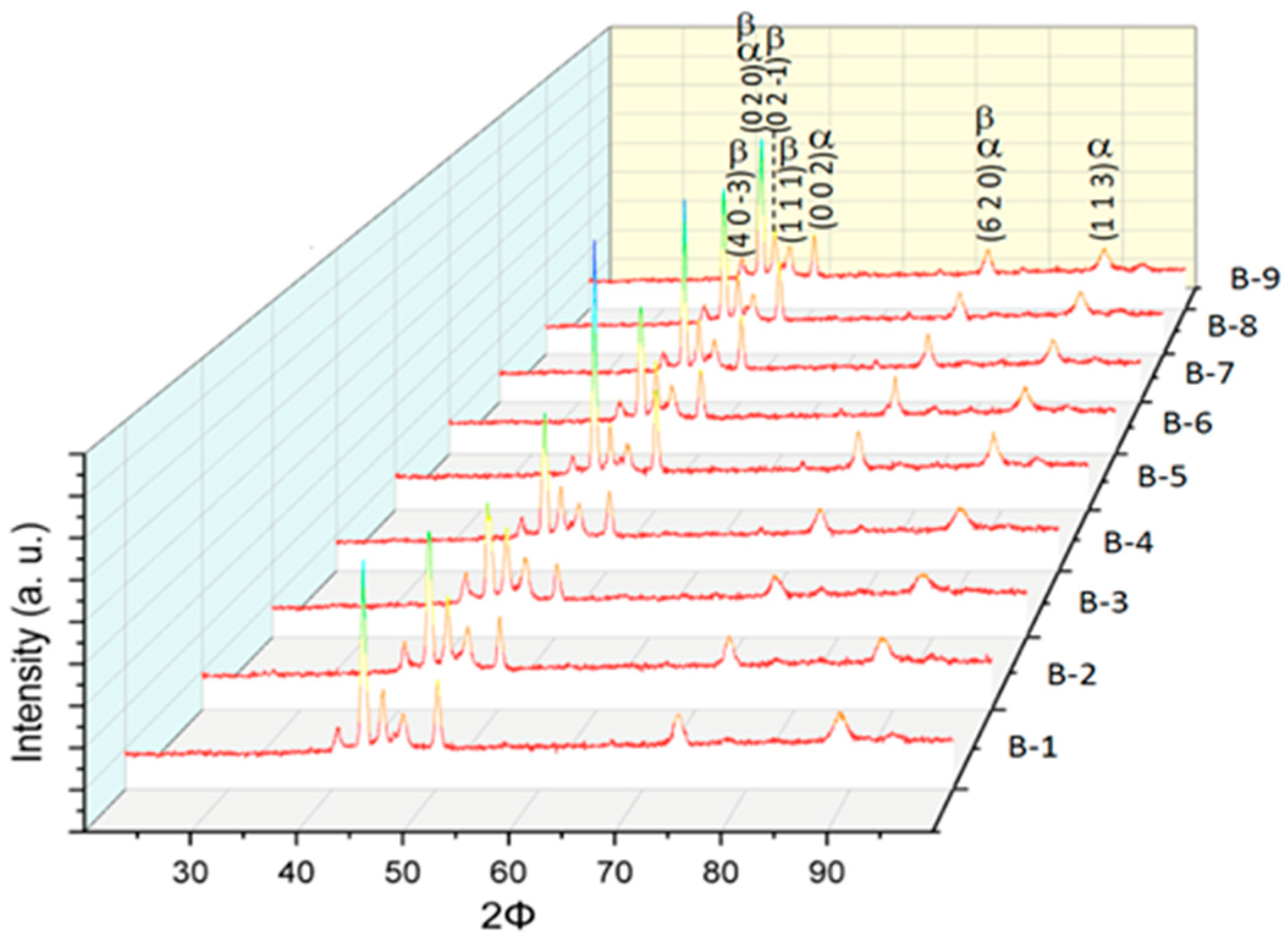
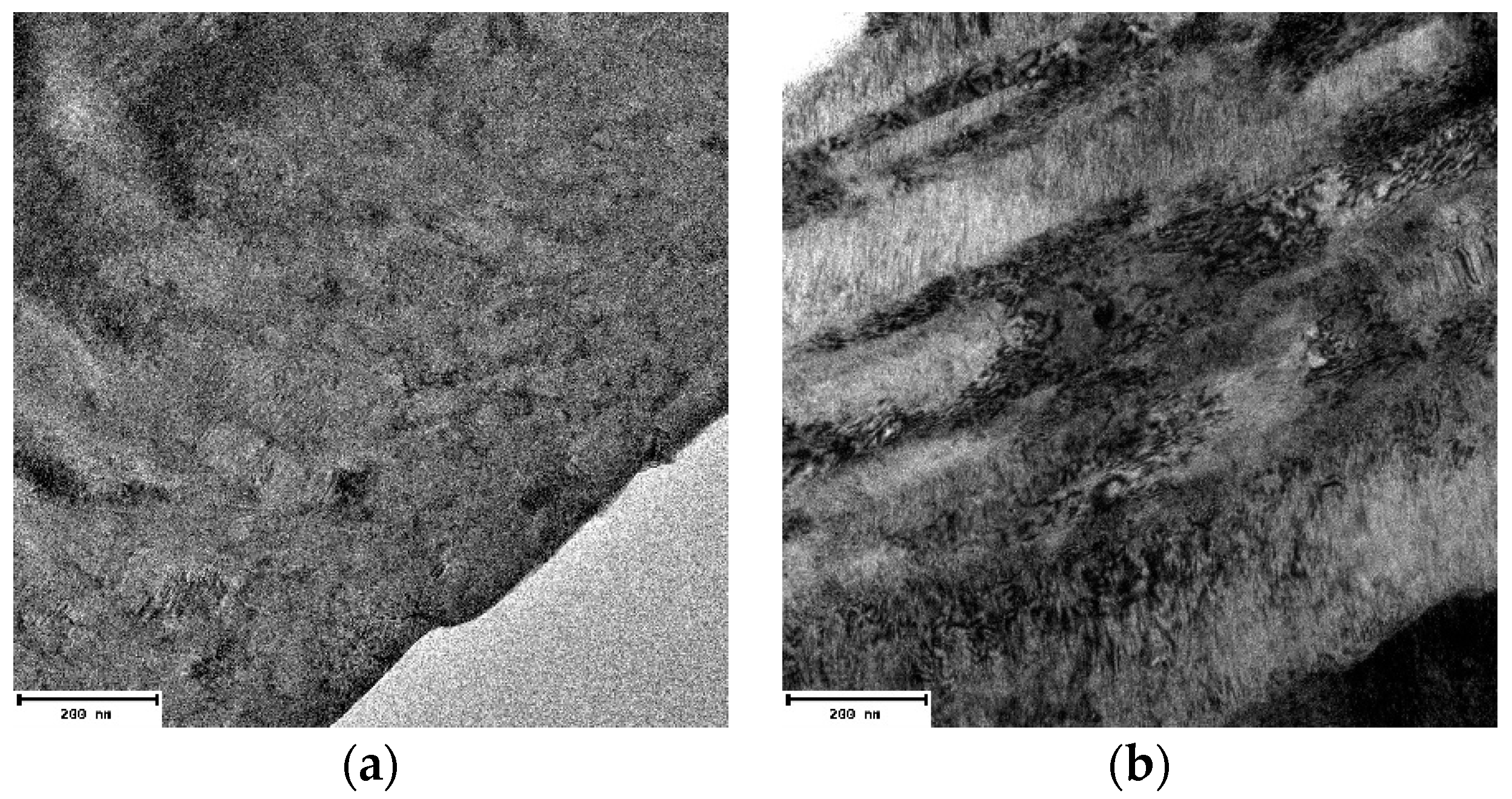
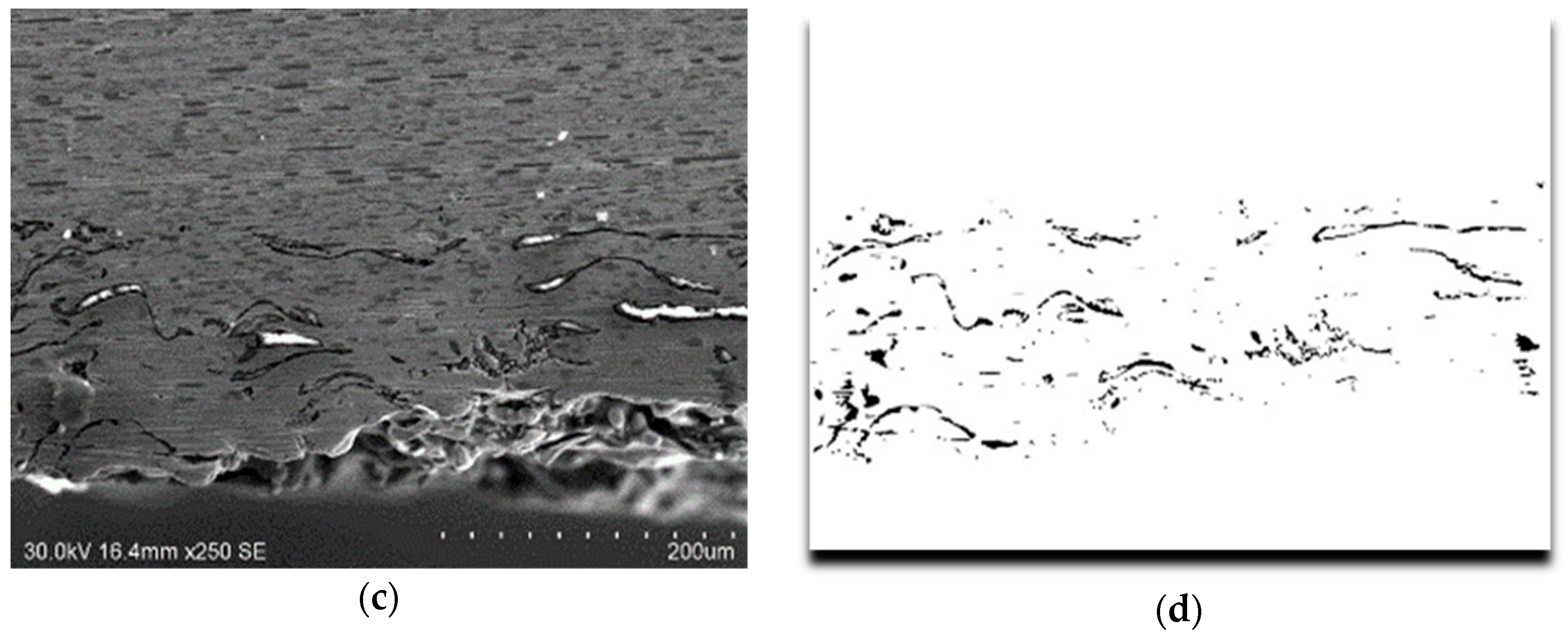
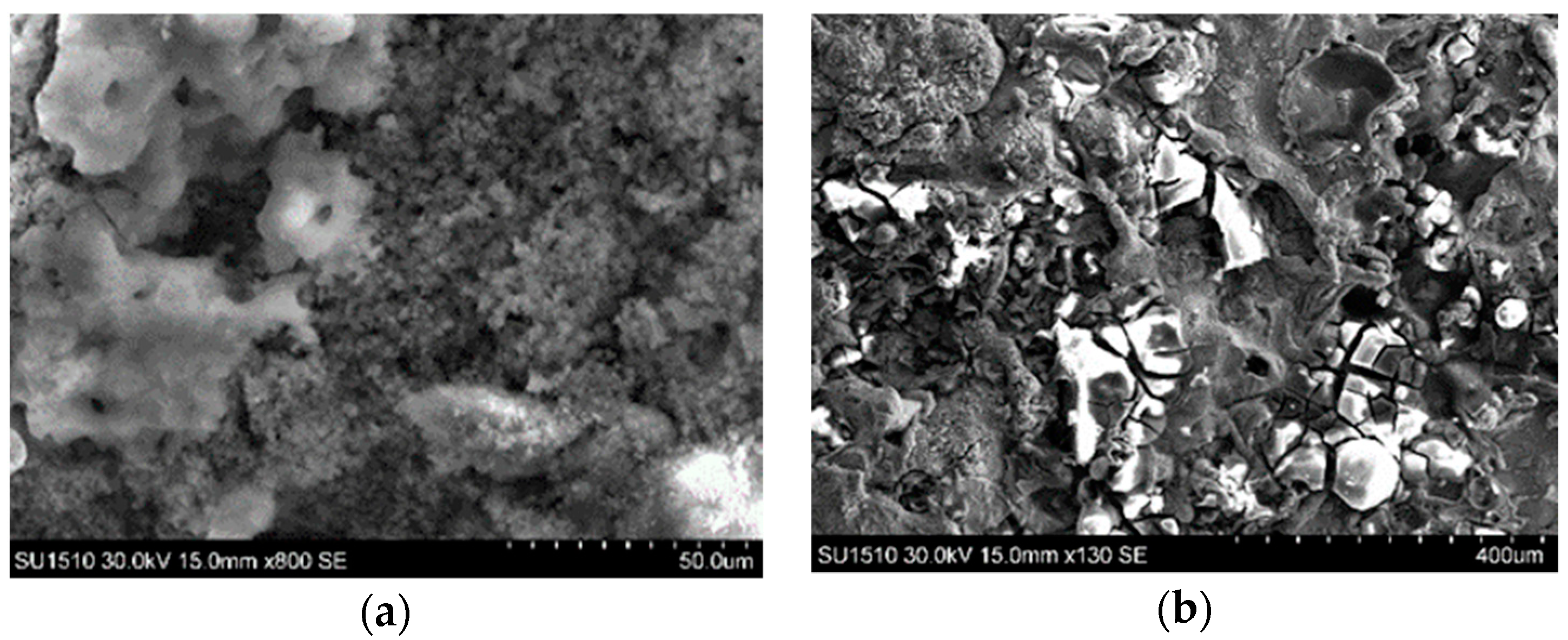
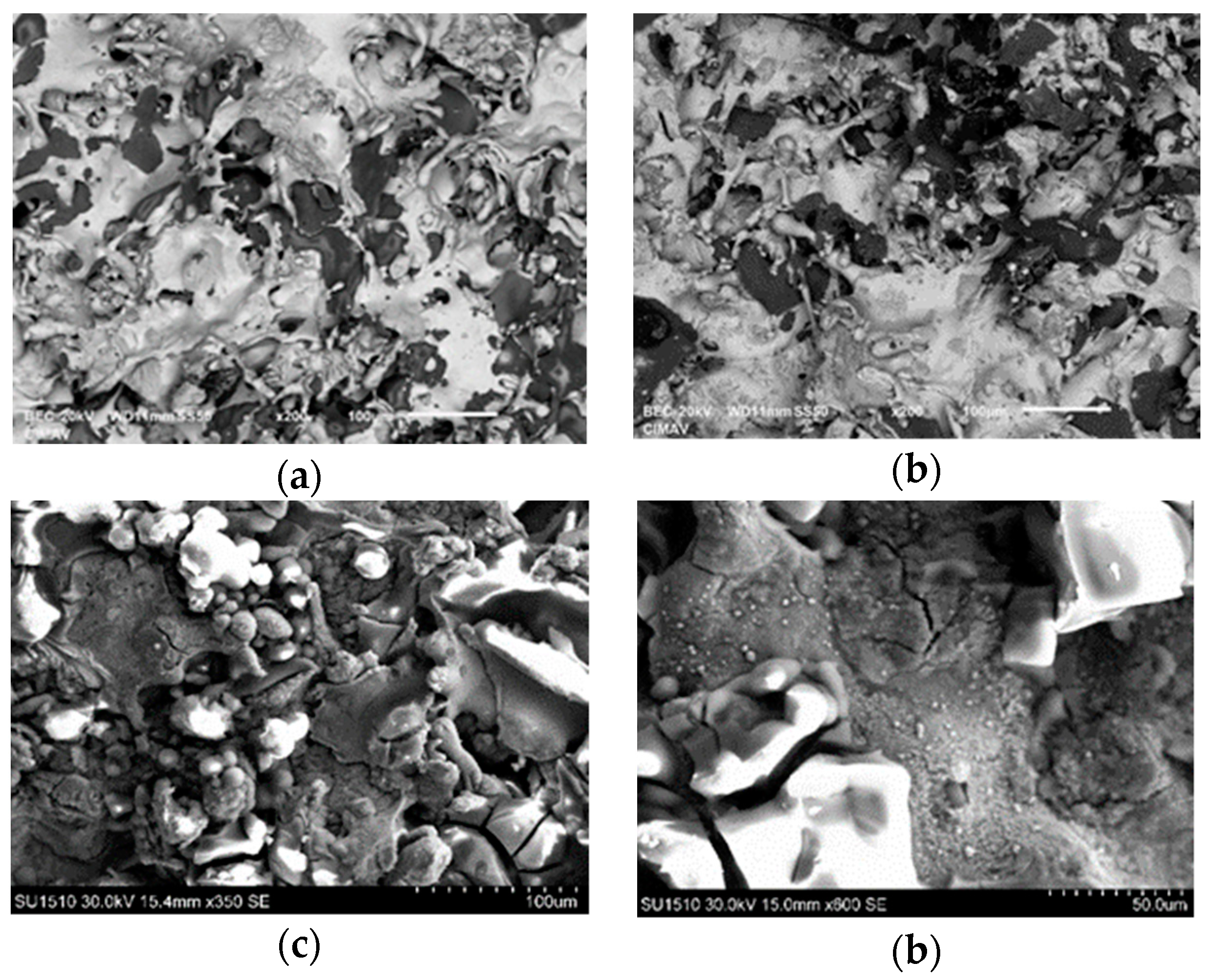
3.2. Microstructural Characterization of Al–Cu–Fe Coatings
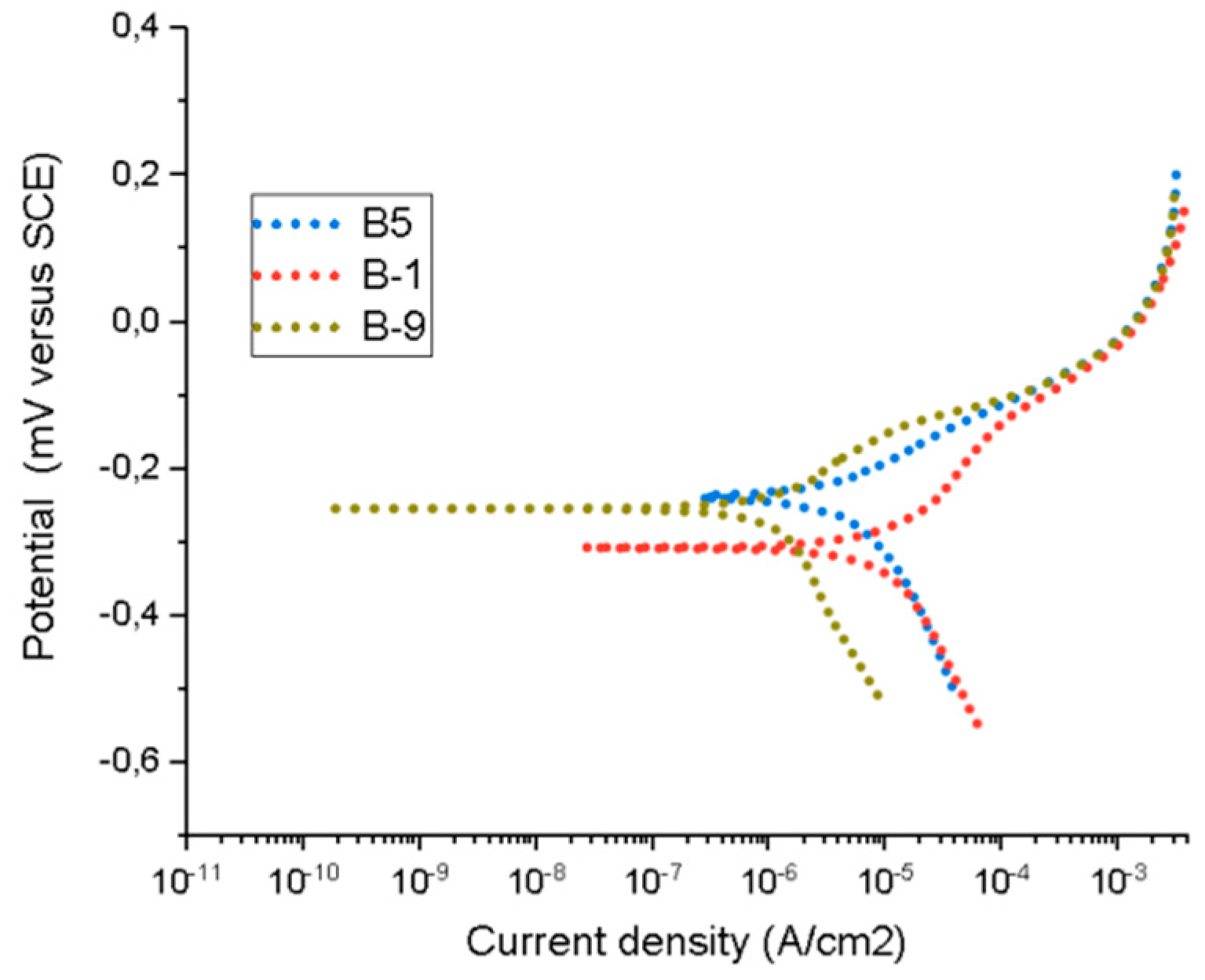
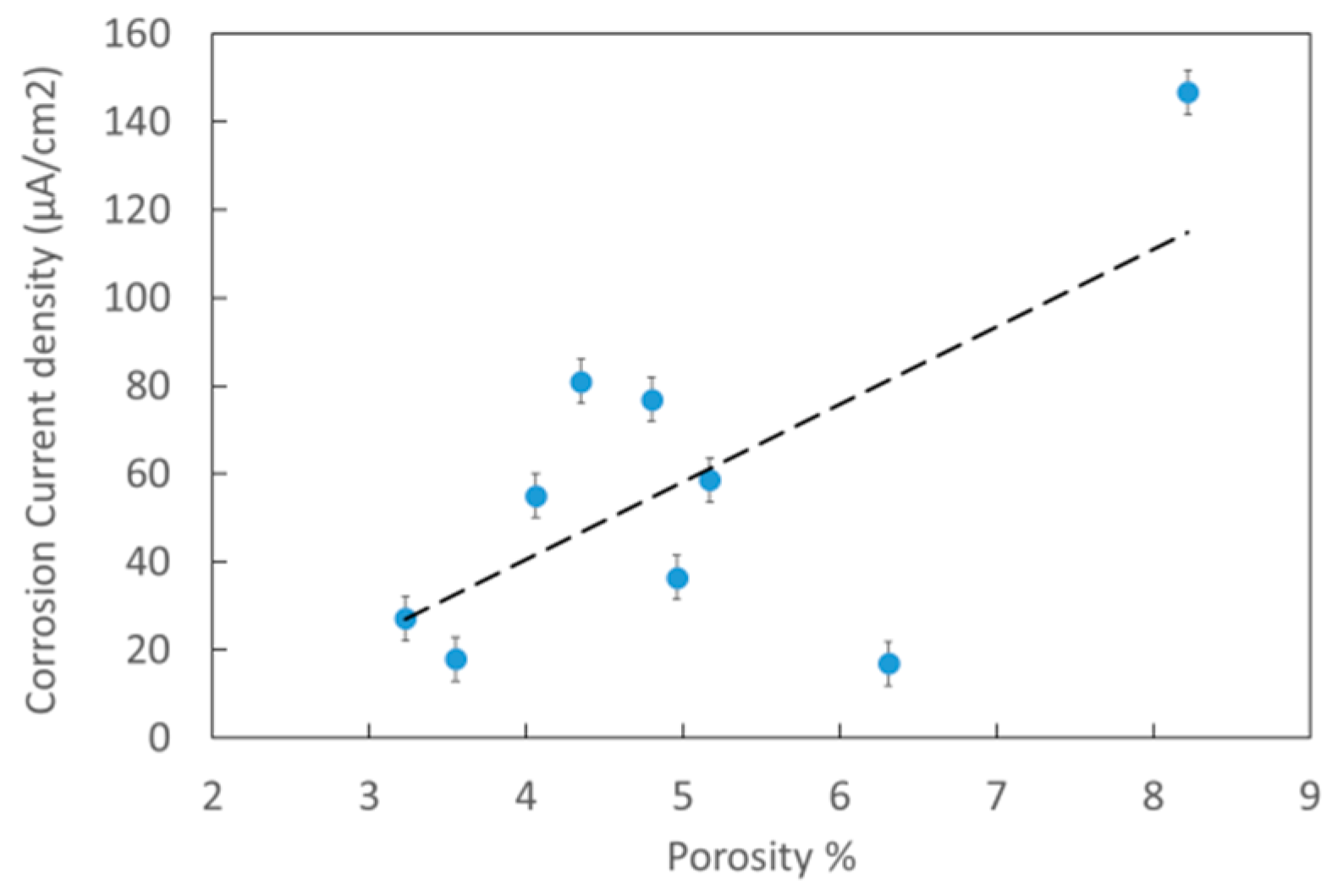
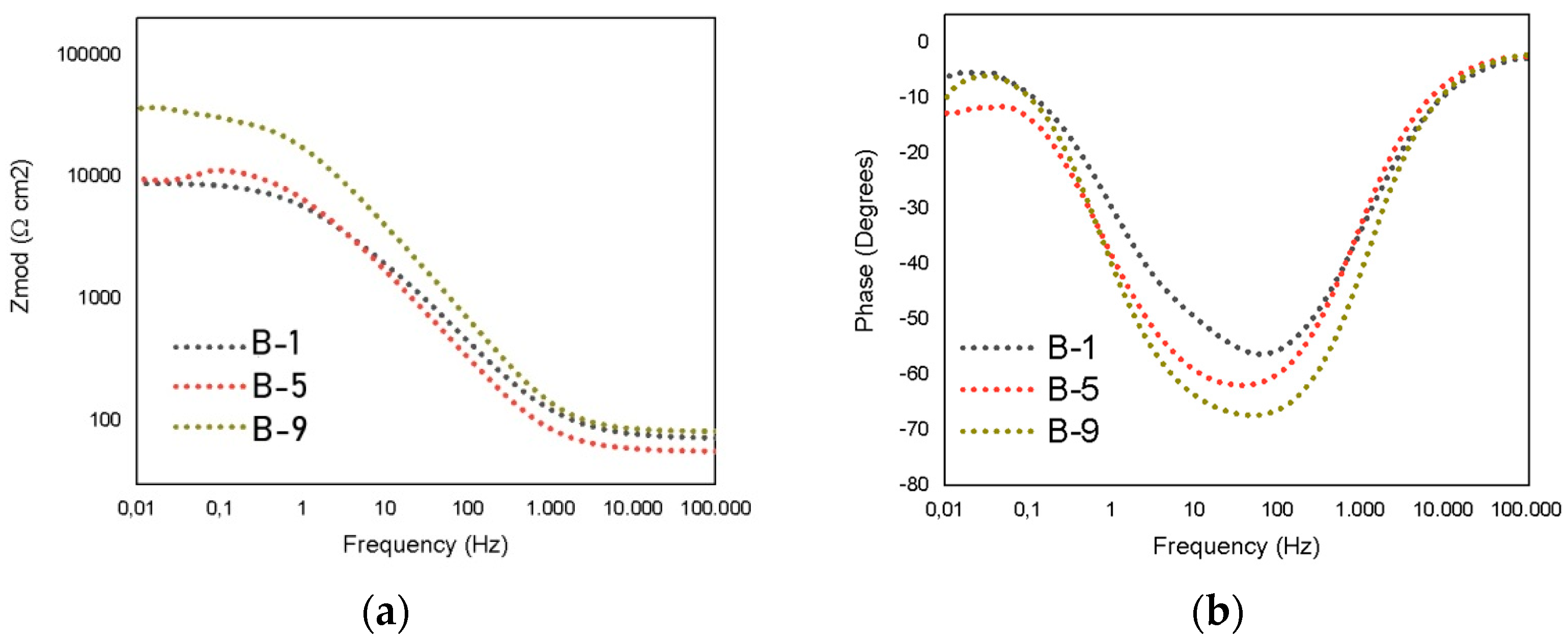
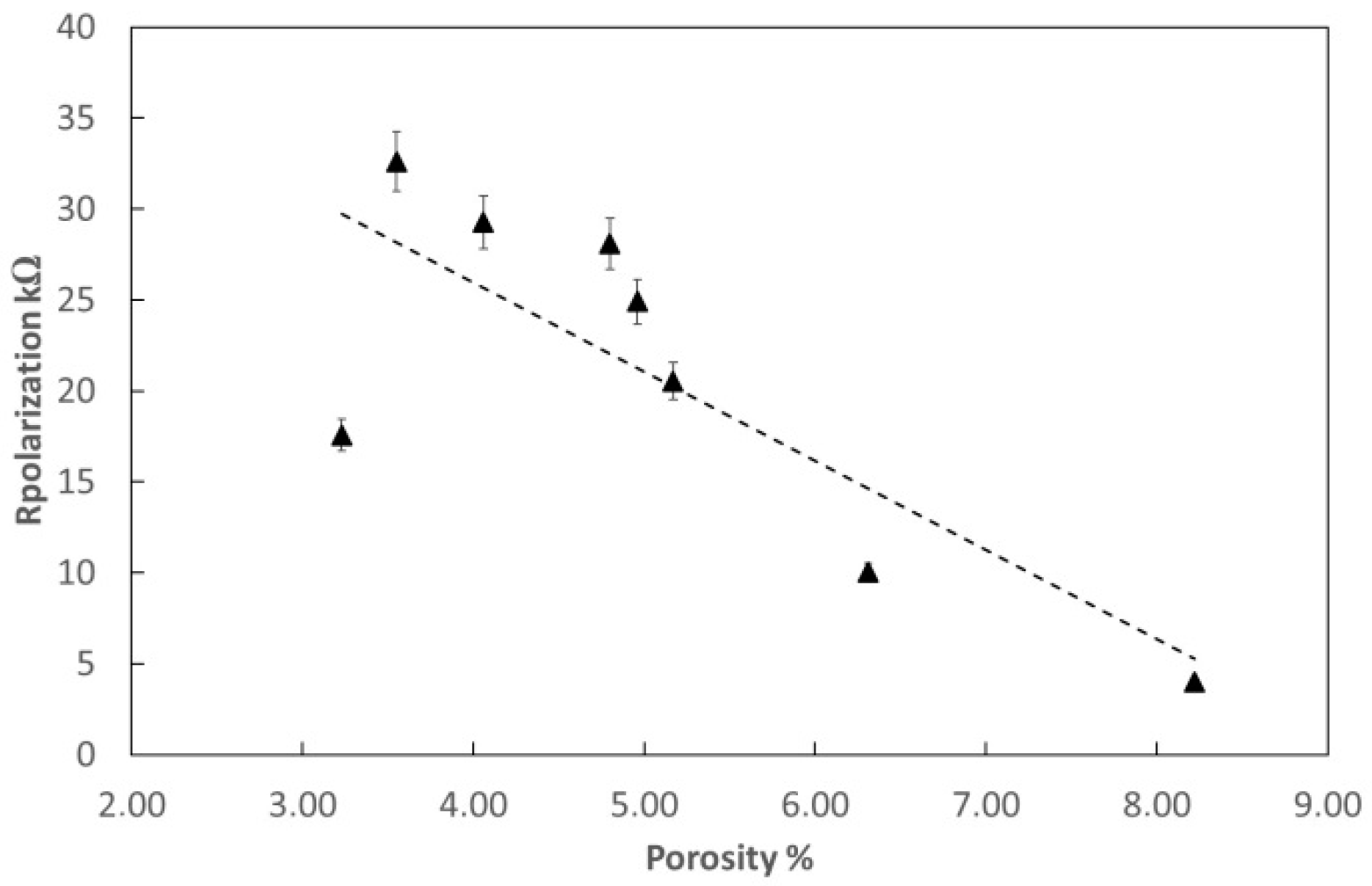
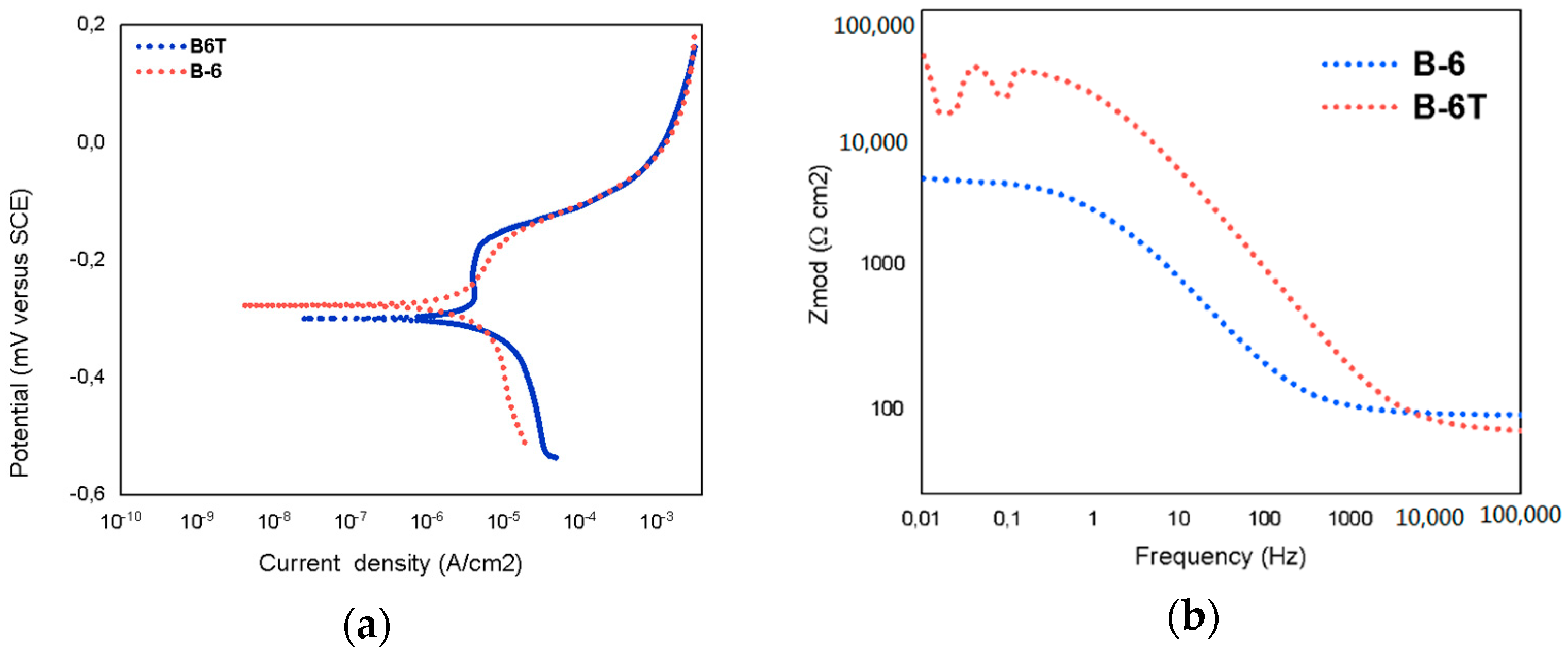
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castolin Eutectic. PROXON® 21071: One Step Alloy Powder for the Cold Spray Process Rebuilding Worn Parts; Castolin Eutectic: Lausanne, Switzerland, 2011. [Google Scholar]
- Morales, J.A.; Olaya, J.J. An Approach to Thermal Spray Technology; UNAL–COTECMAR: Cartagena, Colombia, 2009. [Google Scholar]
- Dimaté, L.M. Resistencia a la Corrosión en Recubrimientos Comerciales METACERAM 25050 y Proxon 21071 Producidos con el Sistema de Proyección Térmica por Llama. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2011. Available online: http://www.bdigital.unal.edu.co/5222/ (accessed on 1 June 2019).
- Fauchais, M.I.; Heberlein, P.L.; Boulos, J.V.R. Thermal Spray Fundamentals, from Powder to Part; Springer: New York, NY, USA, 2014; pp. 228–238. [Google Scholar]
- Djebali, M.; Pateyron, R.; ElGanaoui, B. Scrutiny of plasma spraying complexities with case study on the optimized conditions toward coating process control. Case Stud. Therm. Eng. 2015, 6, 171–181. [Google Scholar] [CrossRef]
- JETS and POUDRES. Available online: http://jets.poudres.free.fr/en/home.html (accessed on 15 July 2019).
- Delluc, G.; Mariaux, G.; Vardelle, A.; Fauchais, P.; Pateyron, B. A Numerical Tool for Plasma Spraying Part I: Modelling of Plasma Jet and Particle Behaviour. In Proceedings of the 16th International Symposium on Plasma Chemistry, Taormina, Italy, 22–27 June 2003. [Google Scholar]
- Ortiz, J.L.; Manzano, A.; Olvera, O.; Perez, M.A. Principios Básicos de Corrosión y Sus Prácticas de Laboratorio, 1st ed.; Limusa: Ciudad de Mexico, Mexico, 2009; pp. 21–40. [Google Scholar]
- Cadavid, E.; Parra, C.; Vargas, F. Estudio de llamas oxiacetilénica usadas en la proyección térmica. Rev. Colomb. Mater. 2016, 9, 15–16. Available online: http://aprendeenlinea.udea.edu.co/revistas/index.php/materiales/article/view/326490/20783786 (accessed on 1 July 2019).
- LI, W.S.; Yi, L.I.U.; Wang, Z.P.; Chao, M.; Wang, S.C. Effects of Ce in novel bronze and its plasma sprayed coating. Trans. Nonferrous Met. Soc. China 2012, 22, 2139–2145. [Google Scholar] [CrossRef]
- Gonzales, A.G. Estudio de la Influencia de Las Propiedades Físicas y Mecánicas en el Comportamiento Tribológico de Recubrimientos Duros Para Herramientas de Corte y Procesamiento de Polietileno. Master’s Thesis, Universidad de Antioquia, Medellin, Columbia, 2008. Available online: http://tesis.udea.edu.co/handle/10495/48 (accessed on 1 June 2019).
- Koivuluoto, H.; Honkanen, M.; Vuoristo, P. Cold-sprayed copper and tantalum coatings—Detailed FESEM and TEM analysis. Surf. Coat. Technol. 2010, 204, 2353–2361. [Google Scholar] [CrossRef]
- Hincapie-campos, W.S.; Olaya, J.J.; Alfonso-Orjuela, J.E. Microstructural, mechanical and wear characterization of CuxAly coatings deposited via thermal projection. DYNA 2017, 84, 155–163. [Google Scholar] [CrossRef]
- Richer, P.; Zúñiga, A.; Yandouzi, M.; Jodoin, B. CoNiCrAl Y microstructural changes induced during Cold Gas Dynamic Spraying. Surf. Coat. Technol. 2008, 203, 364–371. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Miyazaki, F.; Yamasaki, M.; Yamagata, Y.; Kobayashi, N.; Muraoka, K. Coating Qualities Deposited Using Three Different Thermal Spray Technologies in Relation with Temperatures and Velocities of Spray Droplets. Coatings 2017, 7, 27. [Google Scholar] [CrossRef]
- Richardson, I. Guide to Nickel Aluminium Bronze for Engineers. Copp. Dev. Assoc. 2016, 222, 8–9. Available online: https://www.copper.org/applications/marine/nickel_al_bronze/pub-222-nickel-al-bronze-guide-engineers.pdf (accessed on 1 June 2019).
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical Corrosion of Unalloyed Copper in Chloride media—A Critical Review. Corros. Sci. 2004, 49, 109–135. [Google Scholar] [CrossRef]
- Wharton, J.A.; Stokes, K.R. The Influence of Nickel-aluminium Bronze Microstructure and Crevice Solution on the Nitiation of Crevice Corrosion. Electrochim. Acta 2008, 53, 2463–2473. [Google Scholar] [CrossRef]
- Kabasakaloğlu, A.A.M.; Kiyak, T.; Şendil, O. Electrochemical Behavior of Brass in 0.1 M NaCl. Appl. Surf. Sci. 2002, 193, 167–174. [Google Scholar] [CrossRef]
- Walsh, F.C.; De León, C.P.; Kerr, C.; Court, S.; Barker, B.D. Electrochemical characterisation of the porosity and corrosion resistance of electrochemically deposited metal coatings. Surf. Coat. Technol. 2008, 202, 5092–5102. [Google Scholar] [CrossRef]
- Gavrila, M.; Millet, J.P.; Mazille, H.; Marchandise, D.; Cuntz, J.M. Corrosion behaviour of zinc-nickel coatings, electrodeposited on steel. Surf. Coat. Technol. 2000, 123, 164–172. [Google Scholar] [CrossRef]
- Bendezú, R.P.; Gonçalves, R.P.; Neiva, A.C.; De Melo, H.G. EIS and microstructural characterization of artificial nitrate patina layers produced at room temperature on copper and bronze. J. Braz. Chem. Soc. 2007, 18, 54–64. [Google Scholar] [CrossRef]
- Pombo Rodriguez, R.M.H.; Paredes, R.S.C.; Wido, S.H.; Calixto, A. Comparison of aluminum coatings deposited by flame spray and by electric arc spray. Surf. Coat. Technol. 2007, 202, 172–179. [Google Scholar] [CrossRef]
- Sadawy, M.M.; Ghanem, M.; Zohdy, K.M. Corrosion and Electrochemical Behavior of Leaded-Bronze Alloys in 3.5 wt% NaCl Solution. Am. J. Chem. Appl. 2014, 1, 1–6. [Google Scholar]
- Wharton, J.A.; Barik, R.C.; Kear, G.; Wood, R.J.K.; Stokes, K.R.; Walsh, F.C. The corrosion of nickel-aluminium bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H.R.; Farahany, S.; Abdolahi, A.; Taheri, M.M. Influence of Silver nanoparticles addition on the phase transformation, mechanical properties and corrosion behaviour of Cu–Al–Ni shape memory alloys. J. Alloys Compd. 2014, 612, 471–478. [Google Scholar] [CrossRef]
- Dermaj, A.; Hajjaji, N.; Joiret, S.; Rahmouni, K.; Srhiri, A.; Takenouti, H.; Vivier, V. Electrochemical and spectroscopic evidences of corrosion inhibition of bronze by a triazole derivative. Electrochim. Acta 2007, 52, 4654–4662. [Google Scholar] [CrossRef]
- Rahmouni, K.; Keddam, M.; Srhiri, A.; Takenouti, H. Corrosion of copper in 3% NaCl solution polluted by sulphide ions. Corros. Sci. 2005, 47, 3249–3266. [Google Scholar] [CrossRef]
- Xu, C.; Du, L.; Yang, B.; Zhang, W. Study on salt spray corrosion of Ni-graphite abradable coating with 80Ni20Al and 96NiCr-4Al as bonding layers. Surf. Coat. Technol. 2011, 205, 4154–4161. [Google Scholar] [CrossRef]
- Epelboin, I.; Keddam, M.; Takenouti, H. Use of impedance measurements for the determination of the instant rate of metal corrosion. J. Appl. Electrochem. 1972, 2, 71–79. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, D.M.; Wang, H.P.; Xu, Z.; Yi, C.X.; Zhang, Z. Corrosion behavior of aluminum bronze under thin electrolyte layers containing artificial seawater. Int. J. Electrochem. Sci. 2015, 10, 9056–9072. Available online: http://www.electrochemsci.org/papers/vol10/101109056.pdf (accessed on 1 June 2019).
- Lister, T.E.; Wright, R.N.; Pinhero, P.J.; Swank, W.D. Corrosion of thermal spray hastelloy C-22 coatings in dilute HCl. J. Therm. Spray Technol. 2002, 11, 530–535. [Google Scholar] [CrossRef]





| Testing | Oxygen Pressure [kPa] | Oxygen Volumetric Flow [L/min] | Acetylene Pressure [kPa] | Acetylene Volumetric Flow [L/min] |
|---|---|---|---|---|
| B-1 | 331 | 33 | 69 | 27 |
| B-2 | 345 | 35 | 69 | 27 |
| B-3 | 359 | 37 | 69 | 27 |
| B-4 | 331 | 33 | 83 | 28 |
| B-5 | 345 | 35 | 83 | 28 |
| B-6 | 359 | 37 | 83 | 28 |
| B-7 | 331 | 33 | 97 | 29 |
| B-8 | 345 | 35 | 97 | 29 |
| B-9 | 359 | 37 | 97 | 29 |
| Testing | Rsoln [Ω] | Rct [Ω] | Rpo [Ω] | CPE1 | n | CPE2 | m | Rp [Ω] |
|---|---|---|---|---|---|---|---|---|
| B-1 | 54.06 | 3157 | 819 | 1.39 × 10−4 | 0.672 | 2.93 × 10−4 | 0.556 | 3977 |
| B-2 | 80.84 | 16,650 | 3878 | 2.66 × 10−8 | 0.998 | 1.89 × 10−5 | 0.786 | 20,528 |
| B-3 | 64.16 | 13,280 | 15,990 | 5.58 × 10−6 | 0.954 | 8.32 × 10−6 | 0.836 | 29,270 |
| B-4 | 80.74 | 10,310 | 14,590 | 6.90 × 10−6 | 0.831 | 6.05 × 10−6 | 0.845 | 24,900 |
| B-5 | 56.21 | 4595 | 5413 | 9.73 × 10−6 | 0.99 | 1.80 × 10−5 | 0.804 | 10,008 |
| B-6 | 84.26 | 9236 | 56,620 | 3.10 × 10−6 | 0.99 | 2.87 × 10−6 | 0.869 | 65,856 |
| B-7 | 57.66 | 18,960 | 9118 | 6.22 × 10−6 | 0.740 | 6.60 × 10−6 | 0.849 | 28,078 |
| B-8 | 59.46 | 9676 | 7897 | 5.73 × 10−6 | 0.970 | 6.77 × 10−6 | 0.822 | 17,573 |
| B-9 | 81.51 | 25,050 | 7567 | 5.12 × 10−6 | 0.661 | 6.17 × 10−6 | 0.848 | 32,617 |
| Testing | Acetylene Pressure [kPa] | Oxygen Pressure [kPa] | Porosity [%] | Rp [Ω] | Current Density [μA/cm2] |
|---|---|---|---|---|---|
| B-6 | 69 | 345 | 4.35 | 4847 | 2.93 × 10−4 |
| B-6T | 69 | 345 | 2.65 | 24,850 | 1.89 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, A.; Piamba, O.; Olaya, J. The Corrosion Resistance of Aluminum–Bronze Coatings as a Function of Gas Pressure Used in the Thermal Spraying Process. Coatings 2019, 9, 507. https://doi.org/10.3390/coatings9080507
Morales A, Piamba O, Olaya J. The Corrosion Resistance of Aluminum–Bronze Coatings as a Function of Gas Pressure Used in the Thermal Spraying Process. Coatings. 2019; 9(8):507. https://doi.org/10.3390/coatings9080507
Chicago/Turabian StyleMorales, Alfredo, Oscar Piamba, and Jhon Olaya. 2019. "The Corrosion Resistance of Aluminum–Bronze Coatings as a Function of Gas Pressure Used in the Thermal Spraying Process" Coatings 9, no. 8: 507. https://doi.org/10.3390/coatings9080507
APA StyleMorales, A., Piamba, O., & Olaya, J. (2019). The Corrosion Resistance of Aluminum–Bronze Coatings as a Function of Gas Pressure Used in the Thermal Spraying Process. Coatings, 9(8), 507. https://doi.org/10.3390/coatings9080507





