Protective Mechanism of Silane on Concrete upon Marine Exposure
Abstract
:1. Introduction
2. Experimental
2.1. Concrete Preparation
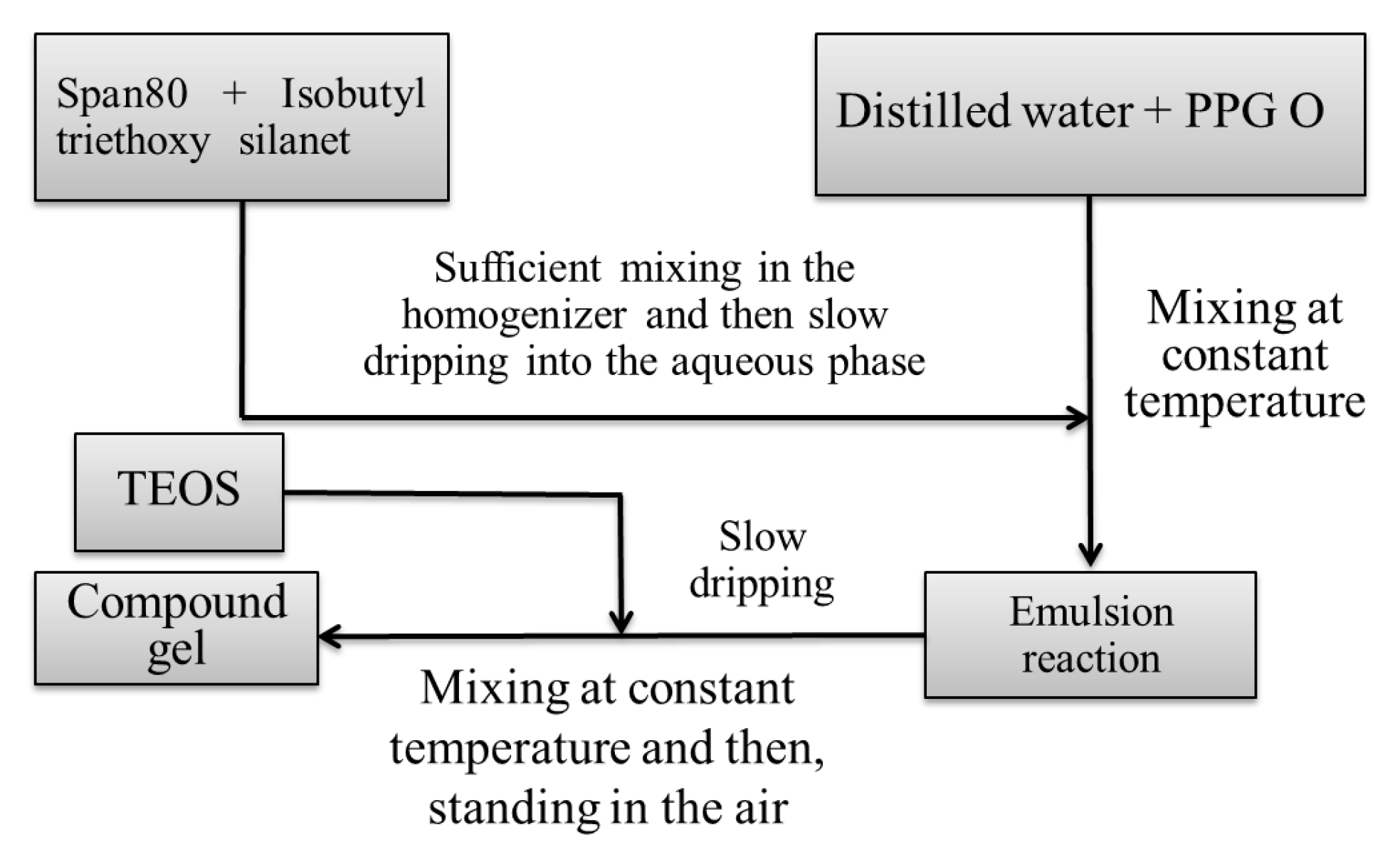
2.2. Raw Materials and Preparation Technology of the Compound Gel
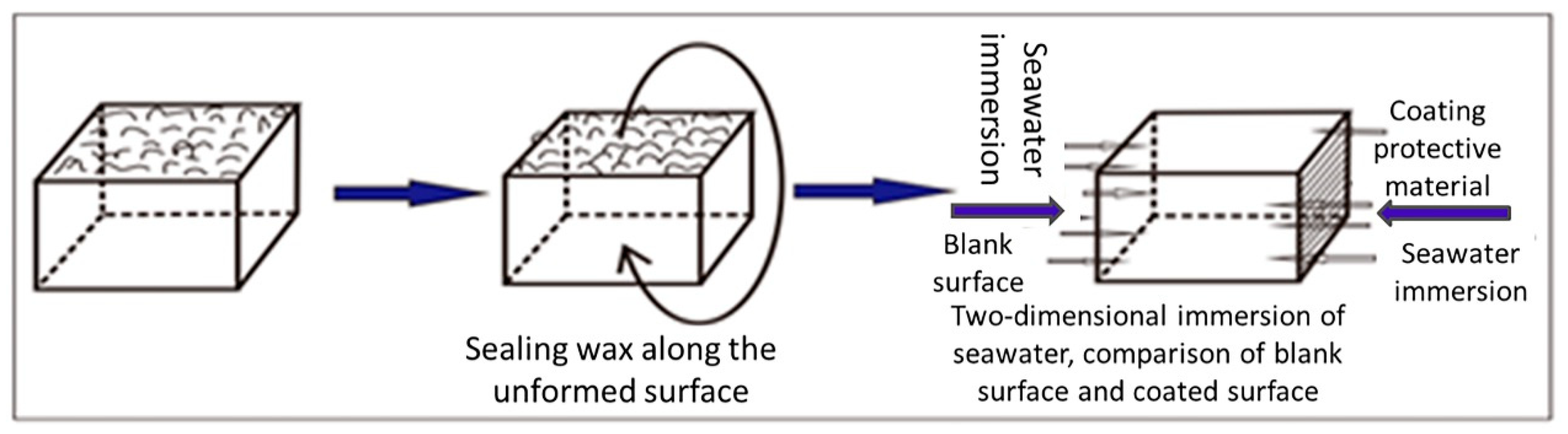
2.3. Marine Exposure Test
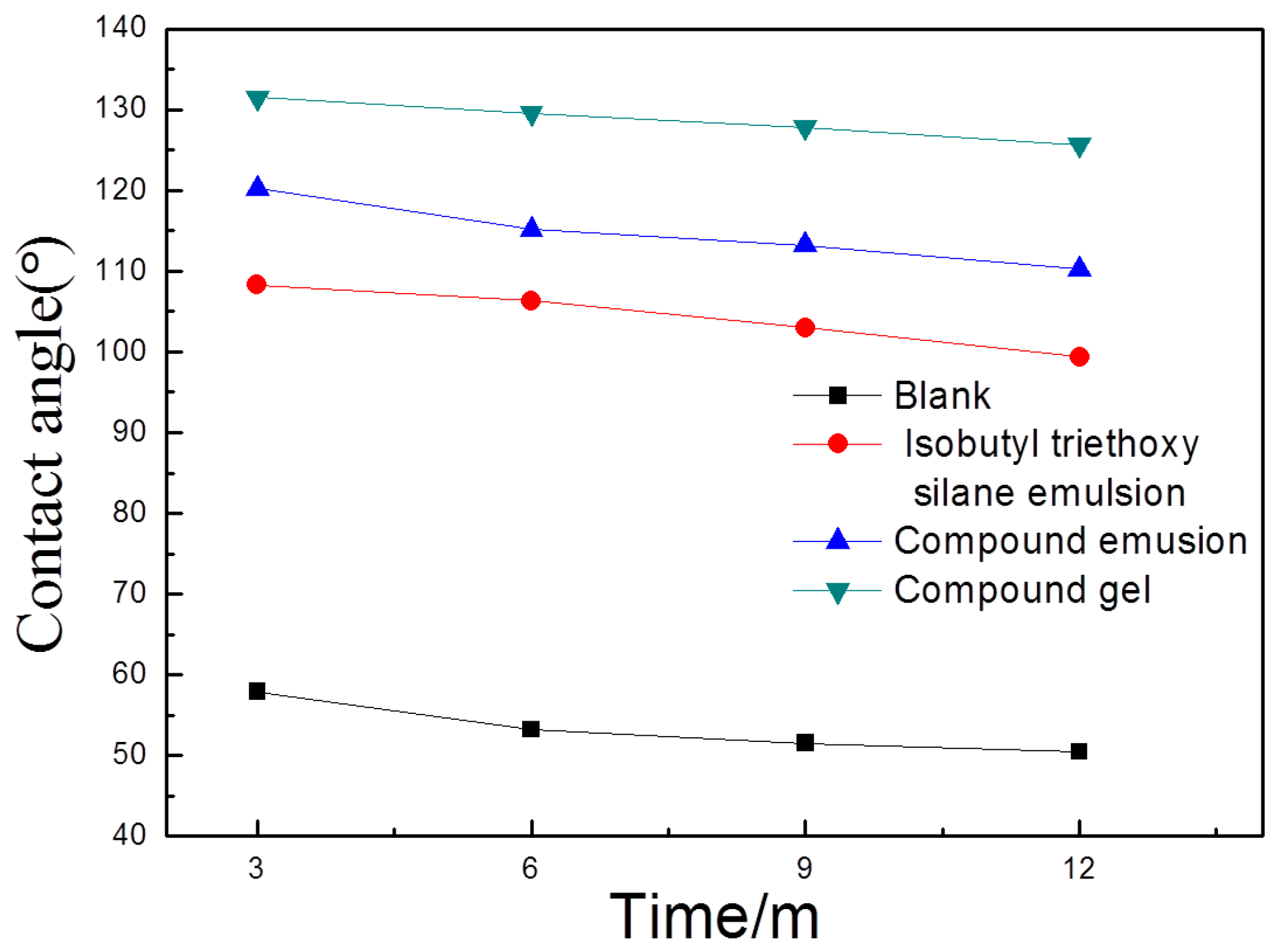
2.4. Contact Angle Determination Test
2.5. Surface Morphology Observation (SEM)
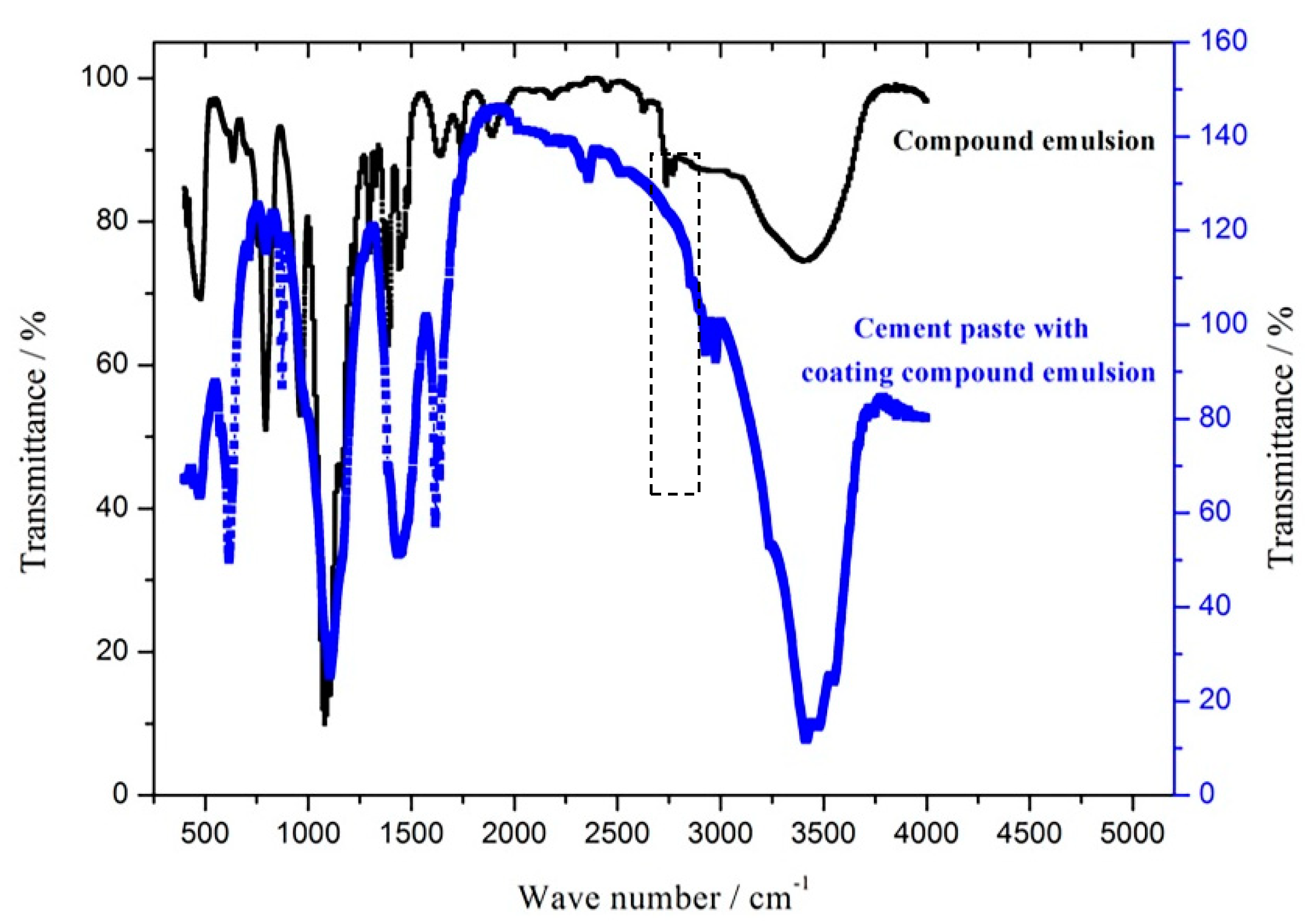
2.6. Infrared Spectrum Analysis
3. Marine Exposure Experiment Results
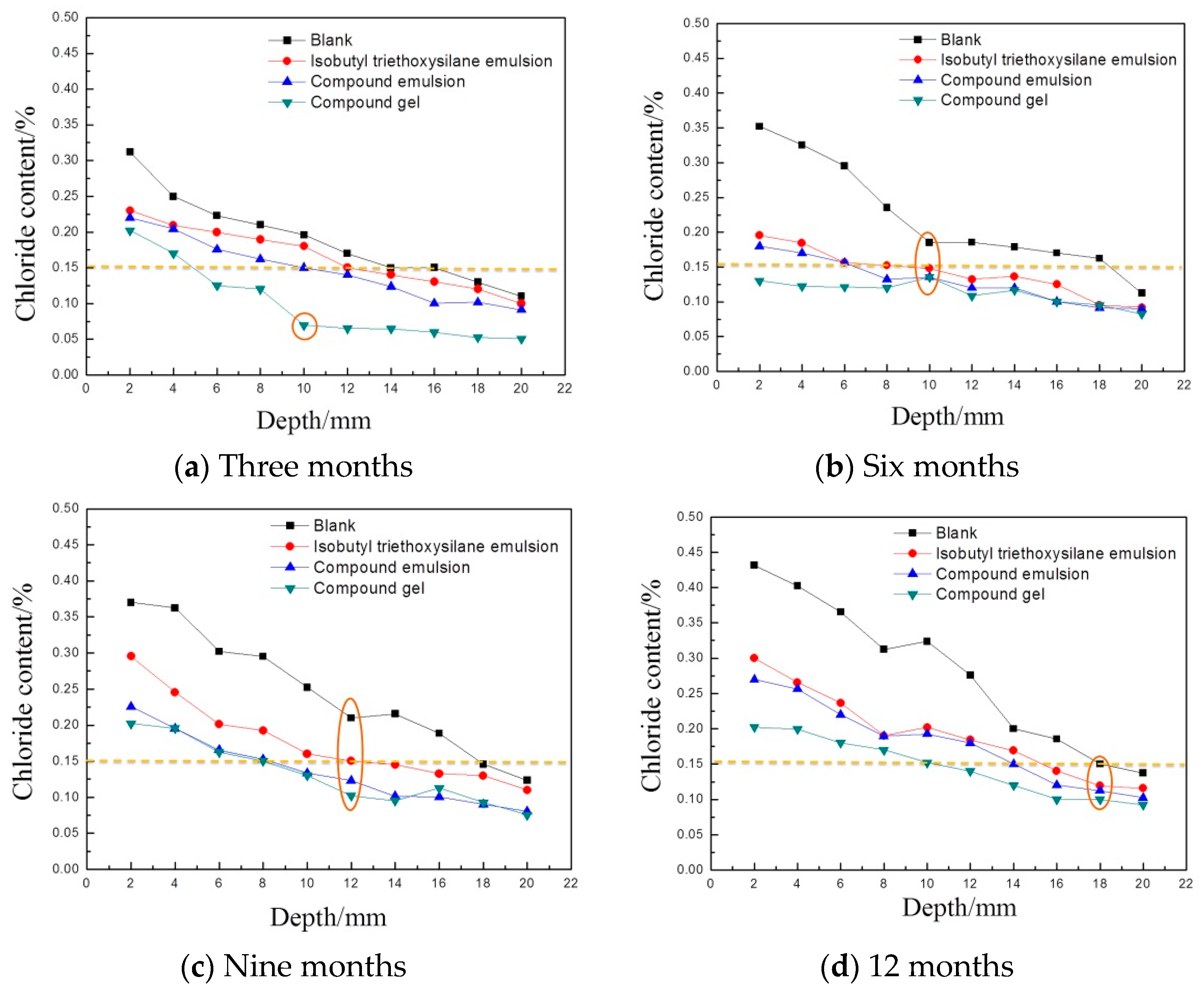
3.1. Results and Analysis in the Full Immersion Zone
3.1.1. Variation of the Chloride Ion Content in the Concrete
3.1.2. Fitting Results of the Chloride Diffusion Coefficients in the Concrete
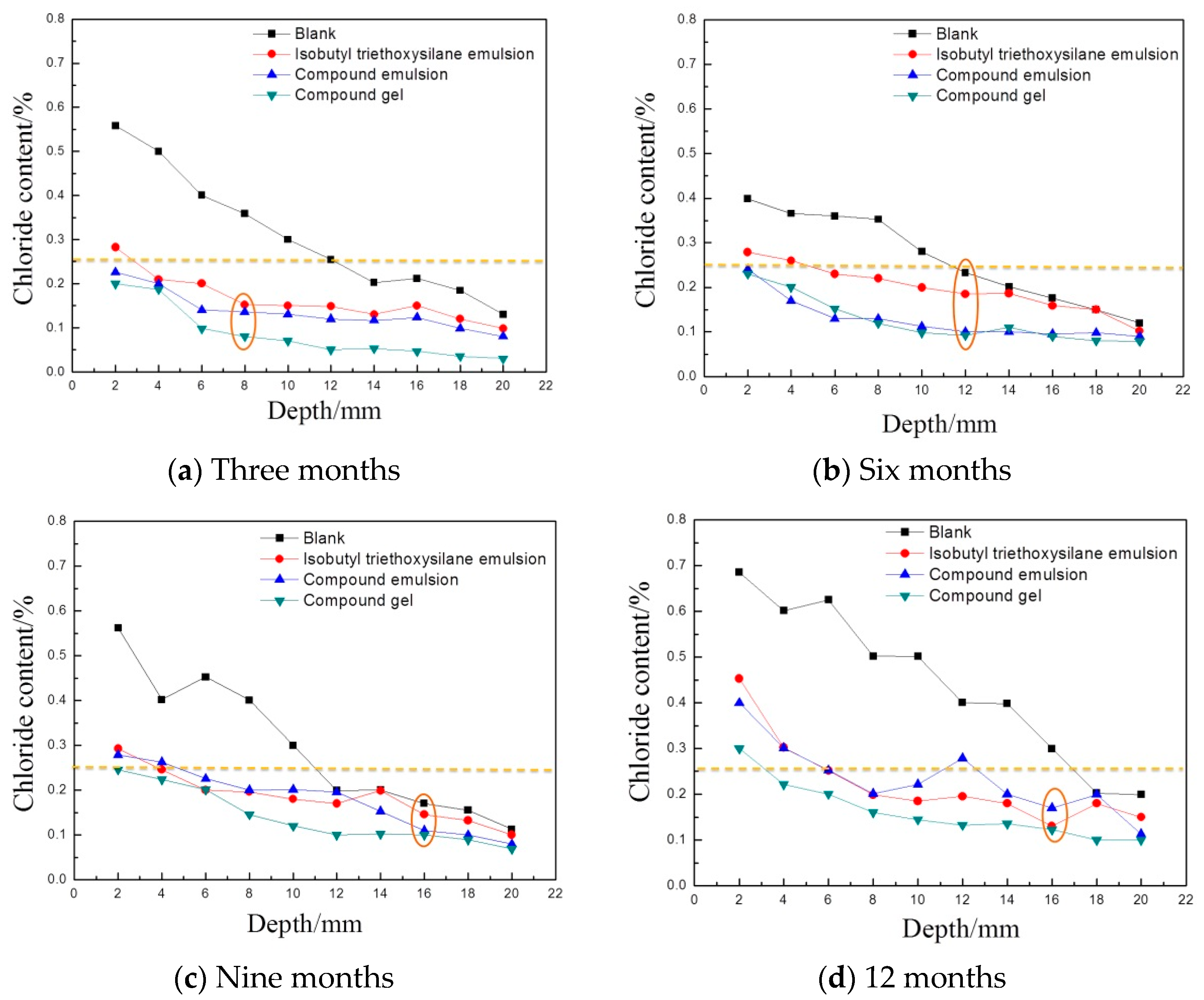
3.2. Results and Analysis in the Tidal Zone
3.2.1. Variation in the Chloride Ion Content in the Concrete
3.2.2. Fitting Results of the Natural Permeability Coefficient in the Concrete
3.3. Results and Analysis in the Splash Zone
3.3.1. Variation of Chloride Ion Content in the Concrete
3.3.2. Fit Results of the Chloride Diffusion Coefficients in the Concrete
3.4. Contact Angle Determination Test
3.5. SEM and Infrared Spectrum Analysis
3.6. Infrared Spectrum Analysis
4. Conclusions
- In the early stage of marine exposure, the three kinds of protective materials had a good ability to resist the chloride ion erosion. With an extension in exposure time, the protective effect of the three protective materials showed a downward trend, to some extent. The compound gel displayed the best stability. Under marine exposure durations, the chloride diffusion coefficients of the concrete test blocks coated with the compound gel were reduced to about 50%. This confirms its excellent ability to resist chloride ion erosion;
- Via the contact angle determination tests, it can be found that the use of coating protective materials on the coupon surface can effectively reduce the coupon surface tension and increase the contact angle. This makes the coupon surface transform from a hydrophilic surface to anhydrophobic surface. Therefore, coating the coupon surface with protective materials can effectively inhibit the transportation of water molecules into the concrete and reduce the content of chloride ions that are carried by the water molecules into the concrete;
- According to the microscopic test results of the SEM and infrared spectrum analysis, the protective coatings can react with the cement-based materials when they are coated on the surface of cement-based material, and drive the hydrophobic alkyl to form a lamellar protective layer on the coupon surface. This fills the capillary pores and effectively inhibits the entrance of water molecules and ions into the capillary channels of the concrete.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, B.; Qiu, Q.; Gu, Z.; Xiang, J.; Huang, C.; Fang, Y.; Xing, F.; Liu, W. Characterization of carbonation behavior of fly ash blended cement materials by the electrochemical impedance spectroscopy method. Cem. Concr. Compos. 2016, 65, 118–127. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, T.; Ma, H.; Li, Z. Reactive molecular simulation on water confined in the nanopores of the calcium silicate hydrate gel: Structure, reactivity, and mechanical properties. J. Phys. Chem. C 2015, 119, 1346–1358. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, C.; Sun, M.; Li, Z. An innovative corrosion evaluation technique for reinforced concrete structures using magnetic sensors. Constr. Build. Mater. 2017, 135, 68–75. [Google Scholar] [CrossRef]
- Hou, D.; Yan, M.; Zhu, Y.; Jin, L. calcium silicate hydrate from dry to saturated state: Structure, dynamics and mechanical properties. Acta Mater. 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Maes, M.; Snoeck, D.; De Belie, N. Chloride penetration in cracked mortar and the influence of autogenous crack healing. Constr. Build. Mater. 2016, 115, 114–124. [Google Scholar] [CrossRef]
- Yu, D.; Guan, B.; He, R.; Xiong, R. Sulfate attack of Portland cement concrete under dynamic flexural loading: A coupling function. Constr. Build. Mater. 2016, 115, 478–485. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, X.; Zhao, T.; Yang, L. Interaction between compressive load and corrosive-ion attack on reinforced concrete with accelerated potentiostatic corrosion. Constr. Build. Mater. 2016, 113, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sun, M.; Hou, D.; Li, Z. External sulfate attack to reinforced concrete under drying-wetting cycles and loading condition: Numerical simulation and experimental validation by ultrasonic array method. Constr. Build. Mater. 2017, 139, 365–373. [Google Scholar] [CrossRef]
- Liu, T.; Weyers, R. Modeling the dynamic corrosion process in chloride contaminated concrete structures. Cem. Concr. Res. 1998, 28, 365–379. [Google Scholar] [CrossRef]
- Bertolini, L. Corrosion of Steel in Concrete; Wiley Online Library: Hoboken, NJ, USA, 2013; Volume 392. [Google Scholar]
- Li, D.; Wei, R.; Li, L.; Guan, X.; Mi, X. Pitting corrosion of reinforcing steel bars in chloride contaminated concrete. Constr. Build. Mater. 2019, 199, 359–368. [Google Scholar] [CrossRef]
- Lunk, P. Kapillares Eindringen Von Wasser Und Salzlösungen in Beton; ETH Zurich: Zürich, Switzerland, 1997. [Google Scholar]
- Zhan, H.; Wittmann, F.; Zhao, T. Chloride barrier for concrete in saline environment established by water repellent treatment. Int. J. Restor. Build. Monum. 2003, 9, 535–550. [Google Scholar]
- Duprat, F. Reliability of RC beams under chloride-ingress. Constr. Build. Mater. 2007, 21, 1605–1616. [Google Scholar] [CrossRef]
- Lambert, P.; Page, C.; Vassie, P. Investigations of reinforcement corrosion. 2. Electrochemical monitoring of steel in chloride-contaminated concrete. Mater. Struct. 1991, 24, 351–358. [Google Scholar] [CrossRef]
- Yin, B.; Liu, T.; Yin, Y. Prolonging the duration of preventing bacterial adhesion of nanosilver-containing polymer films through hydrophobicity. Langmuir 2012, 28, 17019–17025. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Ohama, Y. Handbook of Polymer-Modified Concrete and Mortars: Properties and Process Technology; William Andrew: New York, NY, USA, 1995. [Google Scholar]
- Safiuddin, M. Concrete damage in field conditions and protective sealer and coating systems. Coatings 2017, 7, 90. [Google Scholar] [CrossRef]
- Basheer, L.; Cleland, D.; Long, A. Protection provided by surface treatments against chloride induced corrosion. Mater. Struct. 1998, 31, 459–464. [Google Scholar] [CrossRef]
- De Vries, J.; Polder, R.B. Hydrophobic treatment of concrete. Constr. Build. Mater. 1997, 11, 259–265. [Google Scholar] [CrossRef]
- McGettigan, E. Silicon-based weatherproofing materials. Concr. Int. 1992, 14, 52–56. [Google Scholar]
- Zhang, X.Y.; Li, S.C.; Zhao, T.J.; Jin, Z. Effect of octyl-triethoxysilane emulsion on protection of concrete. Key Eng. Mater. 2015, 629, 504–509. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Bao, Y.; Yu, M. Ultrathin, molecular-sieving graphene oxide membranes for selective hydrogen separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.W.; Sun, Z.P.; Wang, P.M. Effects of silane on anti-corrosion properties of high-performance marine concrete. China Harb. Eng. 2005, 1, 26–27. [Google Scholar]
- Ibrahim, M. Effectiveness of concrete surface treatmentmaterials in reducing chloride-induced reinforcement corrosion. Construct. Build. Mater. 1997, 11, 443–451. [Google Scholar] [CrossRef]
- Christodoulou, C.; Goodier, C.I.; Austin, S.A.; Webb, J.; Glass, G.K. Long-term performance of surface impregnation of reinforced concrete structures with silane. Constr. Build. Mater. 2013, 48, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.L.; Silsbee, M.R.; Gill, P.M.; Scheetz, B.E. Characterization of silicate sealers on concrete. Cem. Concr. Res. 1997, 27, 1561–1567. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhang, W.; Chen, X.; Hou, D.; Zhao, T.; Li, X. Preparation and mechanism of graphene oxide/isobutyltriethoxysilane composite emulsion and its effects on waterproof performance of concrete. Constr. Build. Mater. 2019, 208, 343–349. [Google Scholar] [CrossRef]
- Mózes, G. Paraffin Products; Elsevier: Amsterdam, The Netherlands, 1983; Volume 14. [Google Scholar]
- Chatterji, S. On the applicability of Fick’s second law to chloride ion migration through Portland cement concrete. Cem. Concr. Res. 1995, 25, 299–303. [Google Scholar] [CrossRef]
- Andrade, C. Calculation of chloride diffusion coefficients in concrete from ionic migration measurements. Cem. Concr. Res. 1993, 23, 724–742. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Shekarchi, M.; Hoseini, M. Time-dependent performance of concrete surface coatings in tidal zone of marine environment. Constr. Build. Mater. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Wen, X.; Yu, L.; Tu, J. Analysis of protective performance of gradient structure concrete in marine splash zone. J. Cent. South Univ. (Sci. Technol.) 2013, 44, 792–798. [Google Scholar]
- Bailey, S.I.; Li, X. Corrosion of stainless steels in the marine splash zone. Adv. Mater. Res. 2013, 610–613, 272–275. [Google Scholar] [CrossRef]
- Wen, X.D.; Tu, J.L.; Gan, W.Z. Durability protection of the functionally graded structure concrete in the splash zone. Constr. Build. Mater. 2013, 41, 246–251. [Google Scholar] [CrossRef]
- Kunpu, Z.; Zongmin, L.; Jize, M. Modeling and simulation of chloride transport in concrete in intertidal and splash zone conditions. J. Harbin Eng. Univ. 2016, 7, 950–954. [Google Scholar] [CrossRef]
- Wang, B.Z. Capillary Absorption of Chloride Ions in Unsaturated Concrete; Shanghai Institute of Corrosion Science and Technology: Shanghai, China, 2012. [Google Scholar]
- Hou, D. Insights on capillary adsorption of aqueous sodium chloride solution in the nanometer calcium silicate channel: A molecular dynamics study. J. Phys. Chem. C 2017, 121, 13786–13797. [Google Scholar] [CrossRef]
- Medeiros, M.H.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Zhang, P. Steel reinforcement corrosion in concrete under combined actions: The role of freeze-thaw cycles, chloride ingress, and surface impregnation. Constr. Build. Mater. 2017, 148, 113–121. [Google Scholar] [CrossRef]
- Slowik, V.; Schmidt, M.; Fritzsch, R. Capillary pressure in fresh cement-based materials and identification of the air entry value. Cem. Concr. Compos. 2008, 30, 557–565. [Google Scholar] [CrossRef]
- Hanžič, L.; Kosec, L.; Anžel, I. Capillary absorption in concrete and the Lucas–Washburn equation. Cem. Concr. Compos. 2010, 32, 84–91. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Kou, S.C.; Poon, C.S.; Dai, J.G.; Li, Q.Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Zhao, T.; Jin, Z. Effect of silane polymers and silane emulsions on carbonization and freezing-thawing cycle concrete resistance. Concrete 2015, 3, 69–73. [Google Scholar]
- Lin, O.H.; Akil, H.M.; Ishak, M.Z. Surface-activated nanosilica treated with silane coupling agents/polypropylene composites: Mechanical, morphological, and thermal studies. Polym. Compos. 2011, 32, 1568–1583. [Google Scholar] [CrossRef]
- Wong, K.; Weyers, R.; Cady, P. The retardation of reinforcing steel corrosion by alkyl-alkoxyl silane. Cem. Concr. Res. 1983, 13, 778–788. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The effect of silane-based hydrophobic admixture on corrosion of reinforcing steel in concrete. Cem. Concr. Res. 2008, 38, 1354–1357. [Google Scholar] [CrossRef]





| Material | Dosage/(kg·m−3) | Habitat |
|---|---|---|
| Cement | 380 | ShanLv Co., Ltd., Qingdao, China |
| Sand | 579 | River sand in Qingdao, China |
| Macadam | 1269 | Yuanping Stone Co., Qingdao, China |
| Water | 152 | Qingdao, China |
| Water reducer | 54.9 | Sobute New Materials Co., Ltd., Nanjing, China |
| Raw Materials | Molecular Formulas | Manufacturer |
|---|---|---|
| Isobutyl triethoxysilane (ITES) | (CH3)2CHCH2Si(OC2H5)3 | QuanzhouSicong Chemical Co., Ltd., Quanzhou, China |
| Tetraethoxysilane (TEOS) | Si(OC2H5)4 | Shanghai Aibi Chemistry Preparation Co., Ltd., Shanghai, China |
| Peregal O (PPG O) | RO-(CH2CH2O)n-HR | Shanghai Aibi Chemistry Preparation Co., Ltd., Shanghai, China |
| Polyethylene glycol (PEG 2000) | HO(C2H4O)nH | Tianjin Guangfu Fine Chemical Research Institute, Tianjin, China |
| Span80 | C24H44O6 | Tianjin Ruijinte Chemical Co., Ltd., Tianjin, China |
| Distilled water | H2O | Qingdao University of Technology, Qingdao, China |
| Exposure Time (Month) | Chloride Diffusion Coefficients/(×10−12 m2·s−1) | |||
|---|---|---|---|---|
| Blank | Silane Emulsion | ST Compound Emulsion * | ST Compound Gel | |
| 3 | 7.85 | 3.35 | 3.15 | 1.62 |
| 6 | 3.56 | 2.01 | 1.85 | 1.32 |
| 9 | 2.75 | 2.12 | 1.69 | 1.42 |
| 12 | 2.50 | 2.06 | 1.62 | 1.21 |
| Exposure Time (Month) | Chloride Diffusion Coefficients/(×10−12 m2·s−1) | |||
|---|---|---|---|---|
| Blank | Silane Emulsion | ST Compound Emulsion * | ST Compound Gel | |
| 3 | 8.52 | 3.92 | 2.72 | 1.89 |
| 6 | 5.82 | 3.12 | 3.01 | 1.51 |
| 9 | 3.72 | 3.31 | 1.82 | 1.41 |
| 12 | 2.72 | 1.98 | 1.92 | 1.35 |
| Exposure Time (Month) | Chloride Diffusion Coefficients/(×10−12 m2·s−1) | |||
|---|---|---|---|---|
| Blank | Silane Emulsion | ST Compound Emulsion * | ST Compound Gel | |
| 3 | 13.99 | 5.82 | 5.62 | 3.01 |
| 6 | 6.45 | 4.36 | 3.03 | 2.32 |
| 9 | 4.95 | 3.35 | 2.96 | 2.45 |
| 12 | 4.56 | 3.06 | 2.82 | 1.99 |
| Coating Method | Silicon Content (at.%) | |
|---|---|---|
| Spot Scanning | Map Scanning | |
| Blank | 3.98 | 4.75 |
| Silane emulsion | 9.56 | 9.23 |
| Compound emulsion | 12.65 | 11.78 |
| Compound gel | 14.26 | 13.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, W.; Liu, J.; Hou, D.; Geng, Y.; Chen, X.; Gao, Y.; Jin, Z.; Yin, B. Protective Mechanism of Silane on Concrete upon Marine Exposure. Coatings 2019, 9, 558. https://doi.org/10.3390/coatings9090558
Li S, Zhang W, Liu J, Hou D, Geng Y, Chen X, Gao Y, Jin Z, Yin B. Protective Mechanism of Silane on Concrete upon Marine Exposure. Coatings. 2019; 9(9):558. https://doi.org/10.3390/coatings9090558
Chicago/Turabian StyleLi, Shaochun, Wenjuan Zhang, Jun Liu, Dongshuai Hou, Yongjuan Geng, Xu Chen, Yan Gao, Zuquan Jin, and Bing Yin. 2019. "Protective Mechanism of Silane on Concrete upon Marine Exposure" Coatings 9, no. 9: 558. https://doi.org/10.3390/coatings9090558




