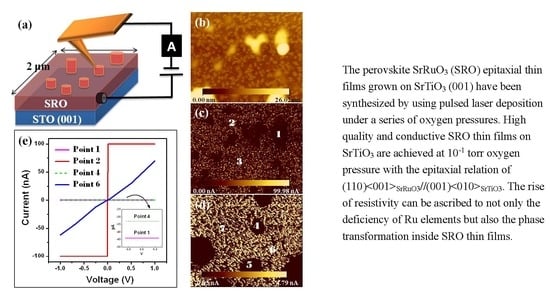
Influence of the Growth Ambience on the Localized Phase Separation and Electrical Conductivity in SrRuO3 Oxide Films
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
3.1. SEM
3.2. TEM
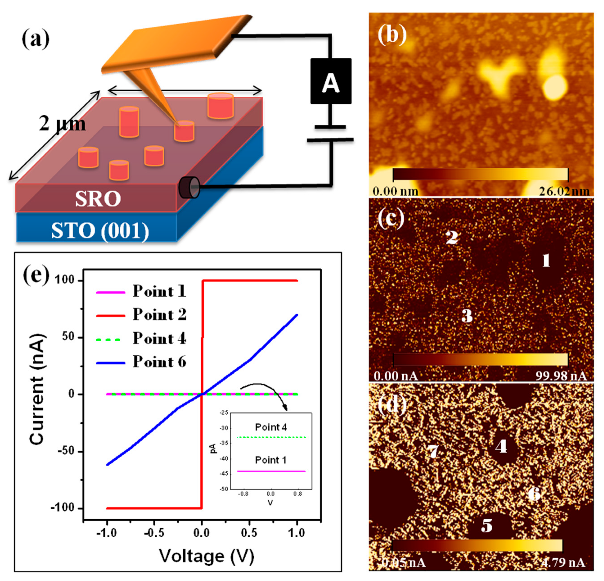
3.3. AFM and C-AFM
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Lichtenberg, F. The story of Sr2RuO4. Prog. Solid State Chem. 2002, 30, 103. [Google Scholar] [CrossRef]
- Tian, W.; Haeni, J.H.; Schlom, D.G.; Hutchinson, E.; Sheu, B.L.; Rosario, M.M.; Schiffer, P.; Liu, Y.; Zurbuchen, M.A.; Pan, X.Q. Epitaxial growth and magnetic properties of the first five members of the layered Srn+1RunO3n+1 oxide series. Appl. Phys. Lett. 2007, 90, 022507. [Google Scholar] [CrossRef]
- Cao, G.; McCall, S.; Crow, J.E. Observation of itinerant ferromagnetism in layered Sr3Ru2O7 single crystals. Phys. Rev. B 1997, 55, R672–R675. [Google Scholar] [CrossRef]
- Klein, L.; Kats, Y.; Marshall, A.F.; Reiner, J.W.; Geballe, T.H.; Beasley, M.R.; Kapitulnik, A. Domain wall resistivity in SrRuO3. Phys. Rev. Lett. 2000, 84, 6090. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.U.; Yamada, H.; Kawasaki, M.; Tokura, Y. Magnetic anisotropy control of SrRuO3 films by tunable epitaxial strain. Appl. Phys. Lett. 2004, 84, 2590–2592. [Google Scholar] [CrossRef]
- Dabrowski, B.; Chmaissem, O.; Klamut, P.W.; Kolesnik, S.; Maxwell, M.; Mais, J.; Ito, Y.; Armstrong, B.D.; Jorgensen, J.D.; Short, S. Reduced ferromagnetic transition temperatures in SrRu1−vO3 perovskites from Ru-site vacancies. Phys. Rev. B 2004, 70, 14423. [Google Scholar] [CrossRef]
- Yoo, Y.Z.; Chmaissem, O.; Kolesnik, S.; Dabrowski, B.; Maxwell, M.; Kimball, C.W.; McAnelly, L.; Haji-Sheikh, M.; Genis, A.P.J. Contribution of oxygen partial pressures investigated over a wide range to SrRuO3 thin-film properties in laser deposition processing. Appl. Phys. 2005, 97, 103525. [Google Scholar] [CrossRef]
- Chang, Y.J.; Kim, C.H.; Phark, S.-H.; Kim, Y.S.; Yu, J.; Noh, T.W. Fundamental thickness limit of itinerant ferromagnetic SrRuO3 thin films. Phys. Rev. Lett. 2009, 103, 057201. [Google Scholar] [CrossRef]
- Xia, J.; Siemons, W.; Koster, G.; Beasley, M.R.; Kapitulni, A. Critical thickness for itinerant ferromagnetism in ultrathin films of SrRuO3. Phys. Rev. B 2009, 79, 140407. [Google Scholar] [CrossRef]
- Grutter, A.; Wong, F.; Arenholz, E.; Liberati, M.; Vailionis, A.; Suzuki, Y. Enhanced magnetism in epitaxial SrRuO3 thin films. Appl. Phys. Lett. 2010, 96, 082509. [Google Scholar] [CrossRef]
- Panchal, G.; Rawat, R.; Bagri, A.; Mandal, A.K.; Choudharya, R.J.; Phase, D.M. Effect of oxygen partial pressure on the electronic and magnetic properties of epitaxial SrRuO3 thin films. Phys. B Phys. Condens. Matter 2019, 572, 190–194. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, N.; Zhang, Y.-Y.; Qi, R.-J.; Huang, R.; Tang, X.-D.; Yang, P.-X.; Xiang, P.-H.; Duan, C.-G. Structure and electrical properties of epitaxial SrRuO3 thin films controlled by oxygen partial pressure. J. Appl. Phys. 2016, 120, 235108. [Google Scholar] [CrossRef]
- Hiratani, M.; Okazaki, C.; Imagawa, K.; Takagi, K. SrRuO3 thin films grown under reduced oxygen pressure. Jpn. J. Appl. Phys. 1996, 35, 6212. [Google Scholar] [CrossRef]
- Siemons, W.; Koster, G.; Vailionis, A.; Yamamoto, H.; Blank, D.H.A.; Beasley, M.R. Dependence of the electronic structure of SrRuO3 and its degree of correlation on cation off-stoichiometry. Appl. Phys. Lett. 2007, 76, 075126. [Google Scholar]
- Vailionis, A.; Siemons, W.; Koster, G. Room temperature epitaxial stabilization of a tetragonal phase in ARuO3 (A=Ca and Sr) thin films. Appl. Phys. Lett. 2008, 93, 051909. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Äkäslompolo, L.; Qin, Q.H.; Dijken, S.V. Toward all-oxide magnetic tunnel junctions: Epitaxial growth of SrRuO3/CoFe2O4/La2/3Sr1/3MnO3 trilayers. Cryst. Growth Des. 2012, 12, 954–959. [Google Scholar] [CrossRef]
- Velev, J.P.; Duan, C.G.; Burton, D.J.; Smogunov, A.; Niranjan, M.K.; Tosatti, E.; Jaswal, S.S.; Tsymbal, E.Y. Magnetic tunnel junctions with ferroelectric barriers: Prediction of four resistance states from first principles. Nano Lett. 2009, 9, 427–432. [Google Scholar] [CrossRef]
- Herranz, G.; Sánchez, F.; Fontcuberta, J. Domain structure of epitaxial SrRuO3 thin films. Phys. Rev. B 2005, 71, 174411. [Google Scholar] [CrossRef]
- Choi, K.J.; Baek, S.H.; Jang, H.W.; Belenky, J.L.; Lyubchenko, M.; Eom, C.B. Phase-transition temperatures of strained single-crystal SrRuO3 thin films. Adv. Mater. 2010, 22, 759–762. [Google Scholar] [CrossRef]
- Kan, D.; Shimakawa, Y. Strain effect on structural transition in SrRuO3 epitaxial thin films. Cryst. Growth Des. 2011, 11, 5483–5487. [Google Scholar] [CrossRef]
- Singh, D.J.J. Electronic and magnetic properties of the 4d itinerant ferromagnet SrRuO3. Appl. Phys. 1996, 79, 4818. [Google Scholar] [CrossRef]
- Zayak, A.T.; Huang, X.J.; Neaton, B.; Rabe, K.M. Structural, electronic, and magnetic properties of SrRuO3 under epitaxial strain. Phys. Rev. B 2006, 74, 094104. [Google Scholar] [CrossRef]
- Rijnders, G.; Blank, D.H.; Choi, J.; Eom, C.-B. Enhanced surface diffusion through termination conversion during epitaxial SrRuO3 growth. Appl. Phys. Lett. 2004, 84, 505–507. [Google Scholar] [CrossRef]
- Hong, W.; Lee, H.N.; Yoon, M.; Christen, H.M.; Lowndes, D.H.; Suo, Z.; Zhang, Z. Persistent step-flow growth of strained films on vicinal substrates. Phys. Rev. Lett. 2005, 95, 095501. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, R.; Sánchez, F.; Santiso, J.; Fontcuberta, J. Reversible growth-mode transition in SrRuO3 epitaxy. Appl. Phys. Lett. 2008, 93, 151916. [Google Scholar] [CrossRef]
- Estève, D.; Maroutian, T.; Pillard, V.; Lecoeur, P. Step velocity tuning of SrRuO3 step flow growth on SrTiO3. Phys. Rev. B 2011, 83, 193401. [Google Scholar] [CrossRef]
- Yuan, G.L.; Uedono, A. Behavior of oxygen vacancies in BiFeO3/SrRuO3/SrTiO3(100) and DyScO3(100) heterostructures. Appl. Phys. Lett. 2009, 94, 132905. [Google Scholar] [CrossRef]
- Shin, J.; Kalinin, S.V.; Lee, H.N.; Christen, H.M.; Moore, R.G.; Plummer, E.W.; Baddorf, A.P. Surface stability of epitaxial SrRuO3 films. Surf. Sci. 2005, 581, 118–132. [Google Scholar] [CrossRef]
- Lee, H.N.; Christen, H.M.; Chisholm, M.F.; Rouleau, C.M.; Lowndes, D.H. Thermal stability of epitaxial SrRuO3 films as a function of oxygen pressure. Appl. Phys. Lett. 2004, 84, 4107. [Google Scholar] [CrossRef]
- Lee, S.A.; Oh, S.; Hwang, J.-Y.; Choi, M.; Youn, C.; Kim, J.W.; Chang, S.H.; Woo, S.; Bae, J.-S.; Park, S.; et al. Enhanced electrocatalytic activity via phase transitions in strongly correlated SrRuO3 thin films. Energy Environ. Sci. 2017, 10, 924–930. [Google Scholar] [CrossRef]
- Keeble, A.J.; Wicklein, S.; Dittmann, R.; Ravelli, L.; Mackie, R.A.; Egger, W. Identification of A- and B-site cation vacancy defects in perovskite oxide thin films. Phys. Rev. Lett. 2010, 105, 226102. [Google Scholar] [CrossRef] [PubMed]
- Chmielowski, R.; Madigou, V.; Ferrandis, P.; Zalecki, R.; Blicharski, M.; Leroux, C. Ferroelectric Bi3.25La0.75Ti3O12 thin films on a conductive Sr4Ru2O9 electrode obtained by pulsed laser deposition. Thin Solid Films 2007, 515, 6314–6318. [Google Scholar] [CrossRef]

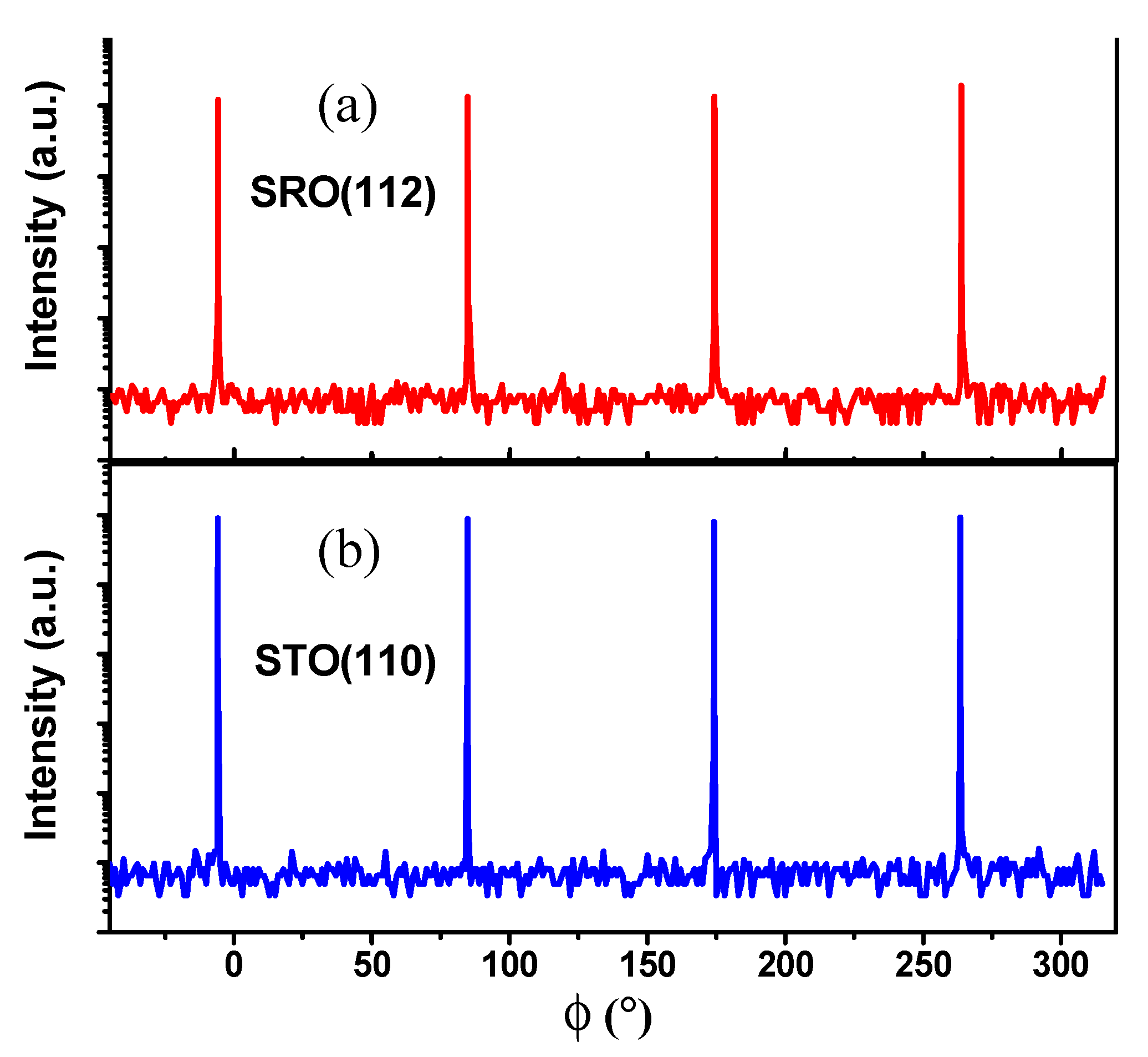
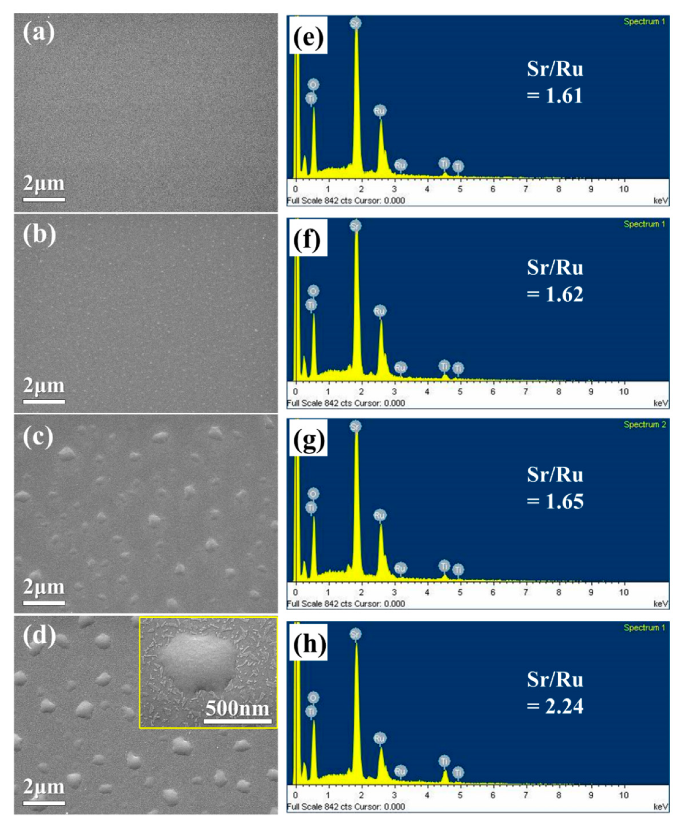
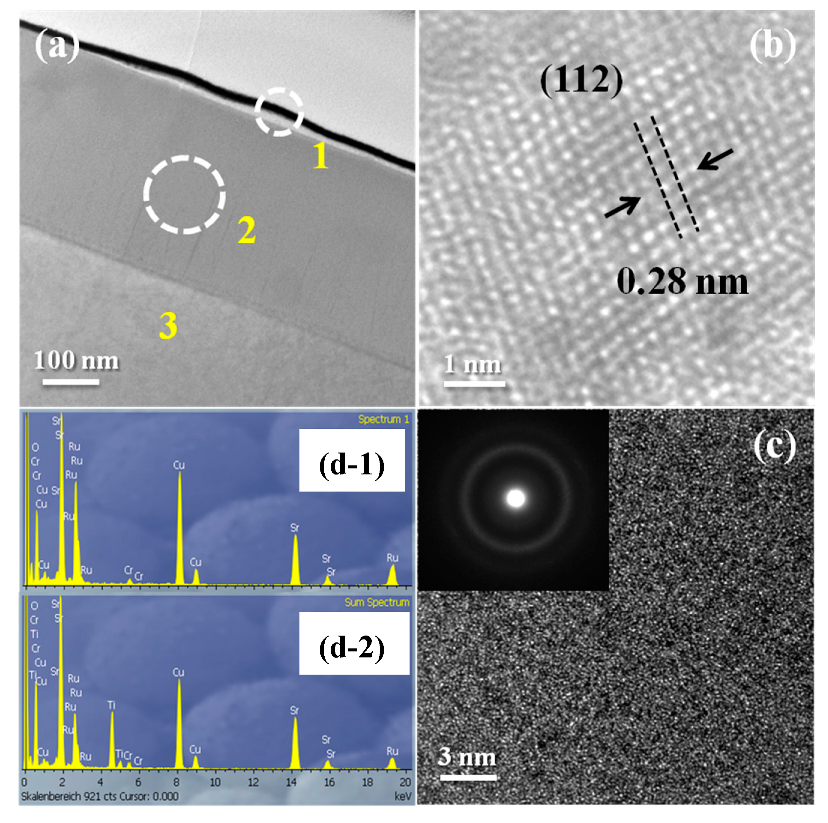
| Sample | A | B | C | D |
|---|---|---|---|---|
| Oxygen pressure (Torr) | 10−1 | 10−4 | 10−6 | 10−7 |
| d-spacing of (110) (Å) | 3.928 | 3.947 | 3.959 | 3.963 |
| Secondary domain d (Å) | – | – | 3.987 | 4.076 |
| FWHM (Degree) | 0.0741 | 0.2725 | 0.3190 | 0.4045 |
| Resistivity (μΩ-cm) | 224 | 600 | 4380 | 61,440 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-M. Influence of the Growth Ambience on the Localized Phase Separation and Electrical Conductivity in SrRuO3 Oxide Films. Coatings 2019, 9, 589. https://doi.org/10.3390/coatings9090589
Cheng H-M. Influence of the Growth Ambience on the Localized Phase Separation and Electrical Conductivity in SrRuO3 Oxide Films. Coatings. 2019; 9(9):589. https://doi.org/10.3390/coatings9090589
Chicago/Turabian StyleCheng, Hsin-Ming. 2019. "Influence of the Growth Ambience on the Localized Phase Separation and Electrical Conductivity in SrRuO3 Oxide Films" Coatings 9, no. 9: 589. https://doi.org/10.3390/coatings9090589