Overcoming Challenges and Limitations Regarding the Atomic Force Microscopy Imaging and Mechanical Characterization of Nanofibers
Abstract
1. Introduction
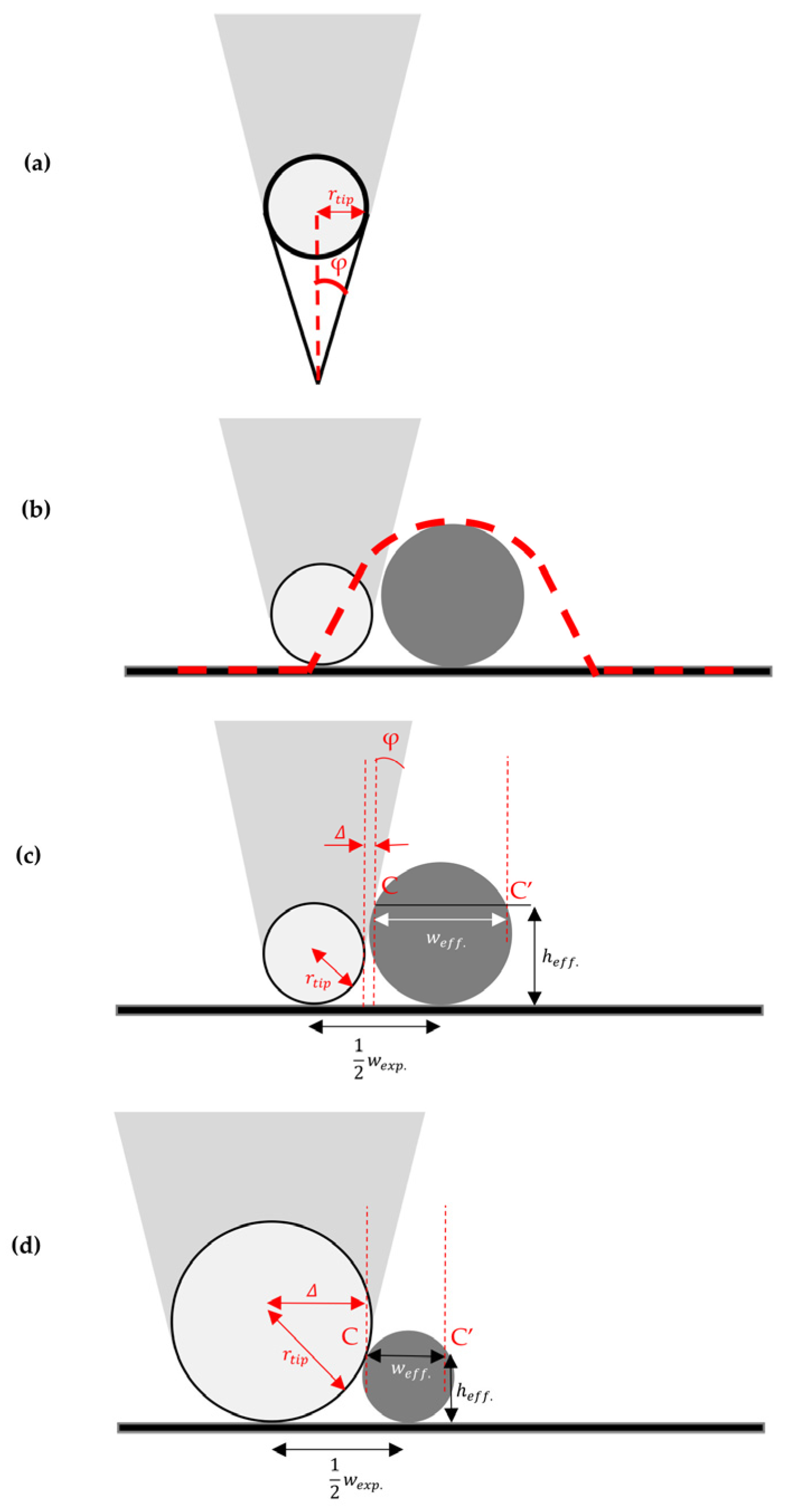
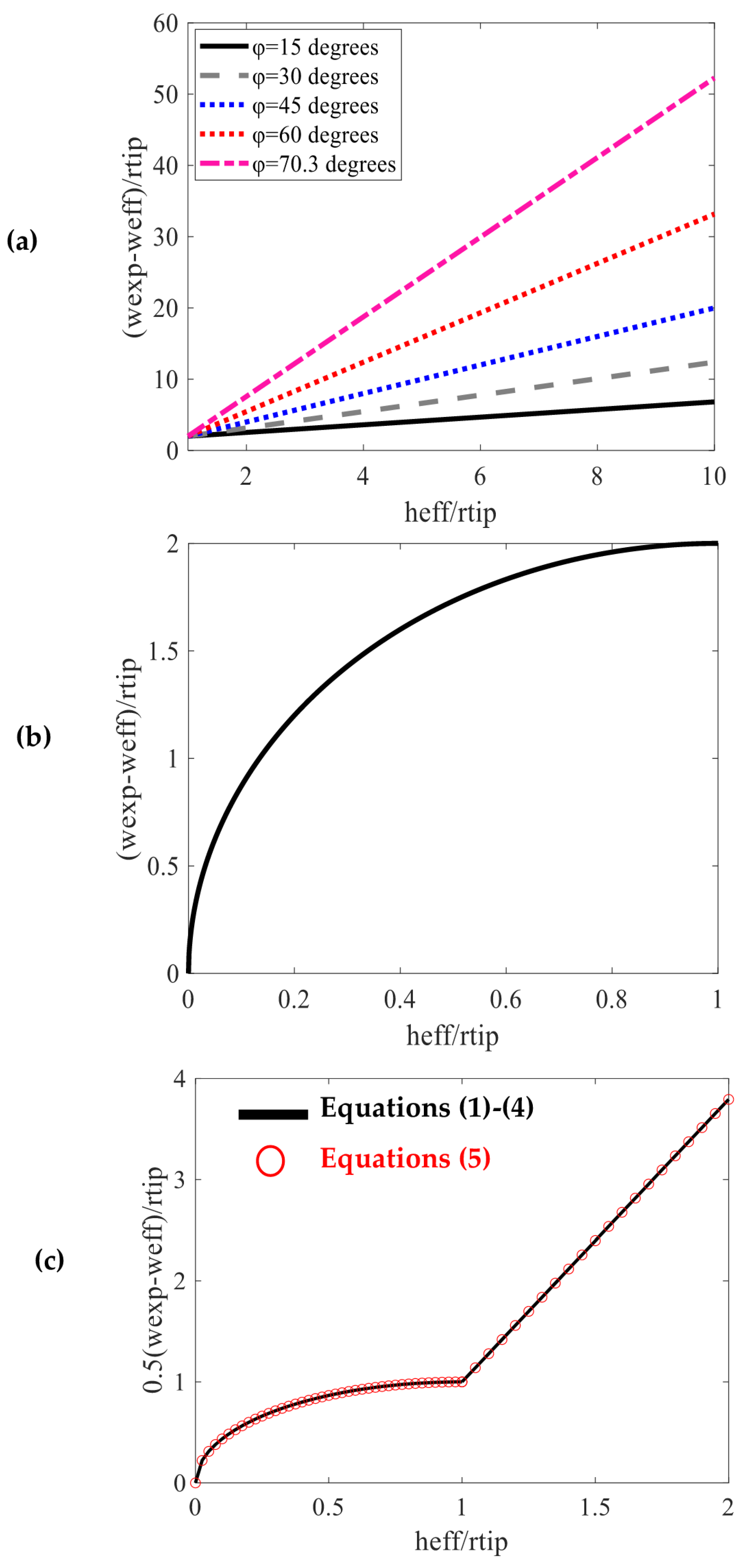
2. AFM Imaging Artifacts
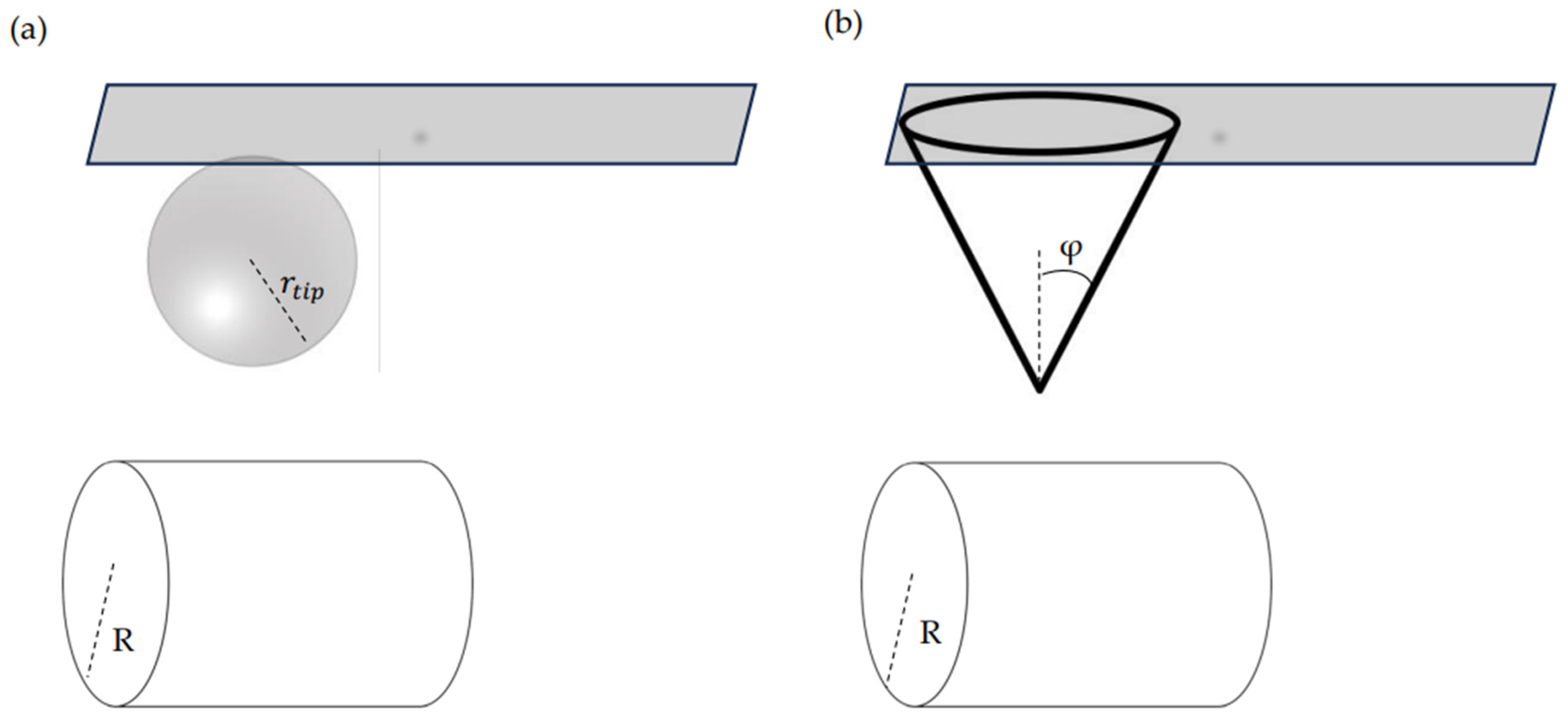
3. Determining the Mechanical Properties of Nanofibers Using AFM
3.1. A Brief Overview of AFM Nanoindentation Method
3.2. Calibration of Probe Parameters
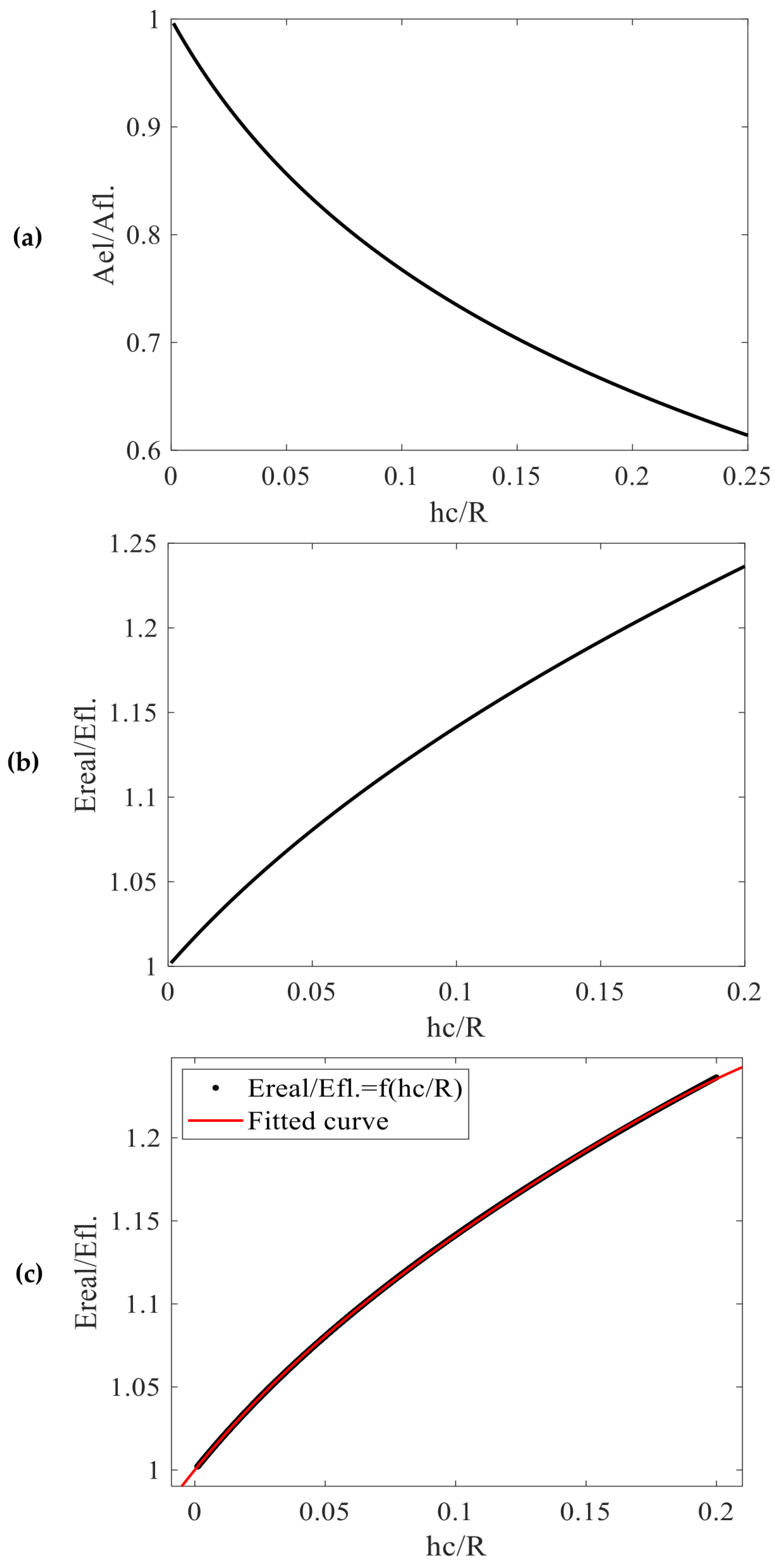
3.3. Elastic Contact
3.4. Elastic–Plastic Contact
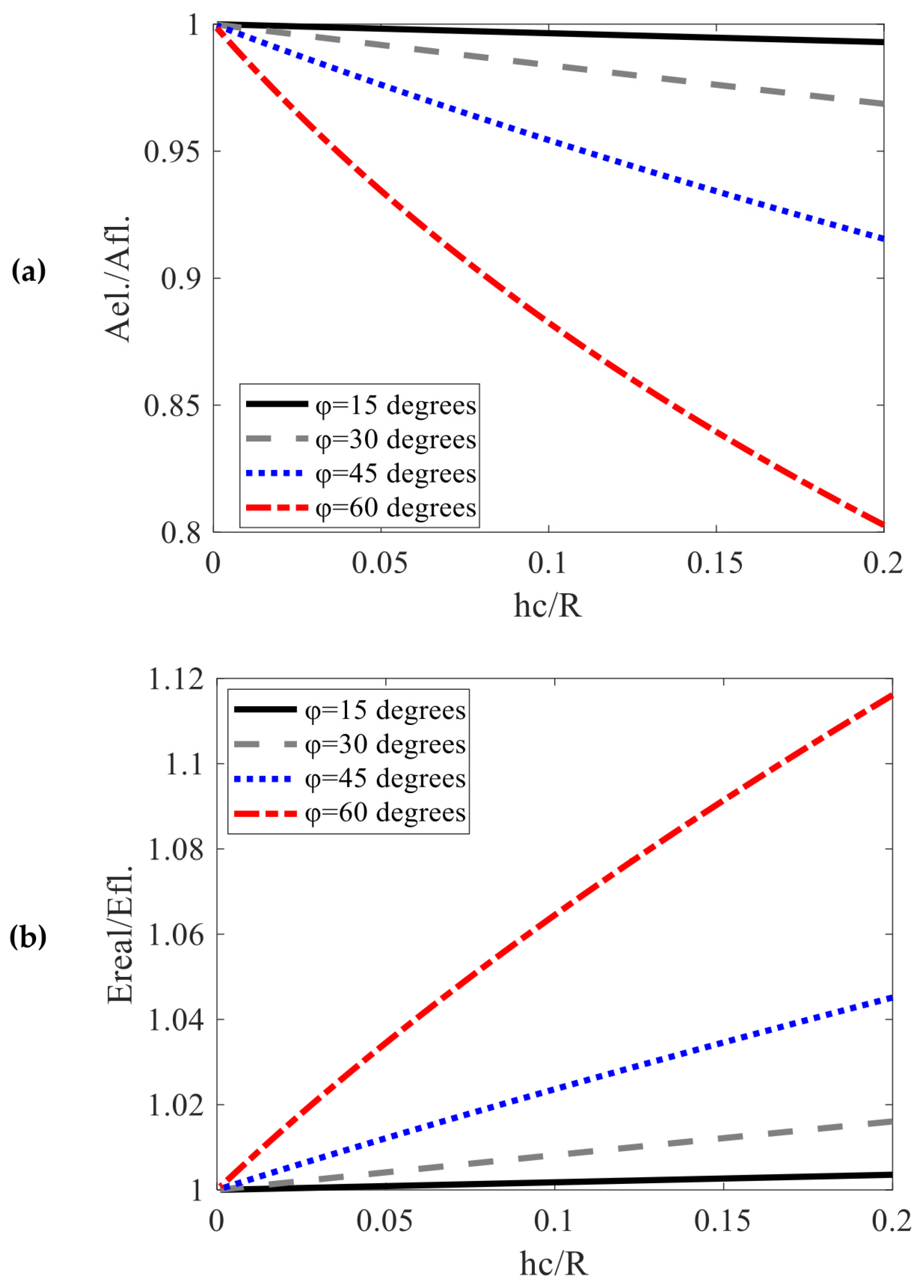
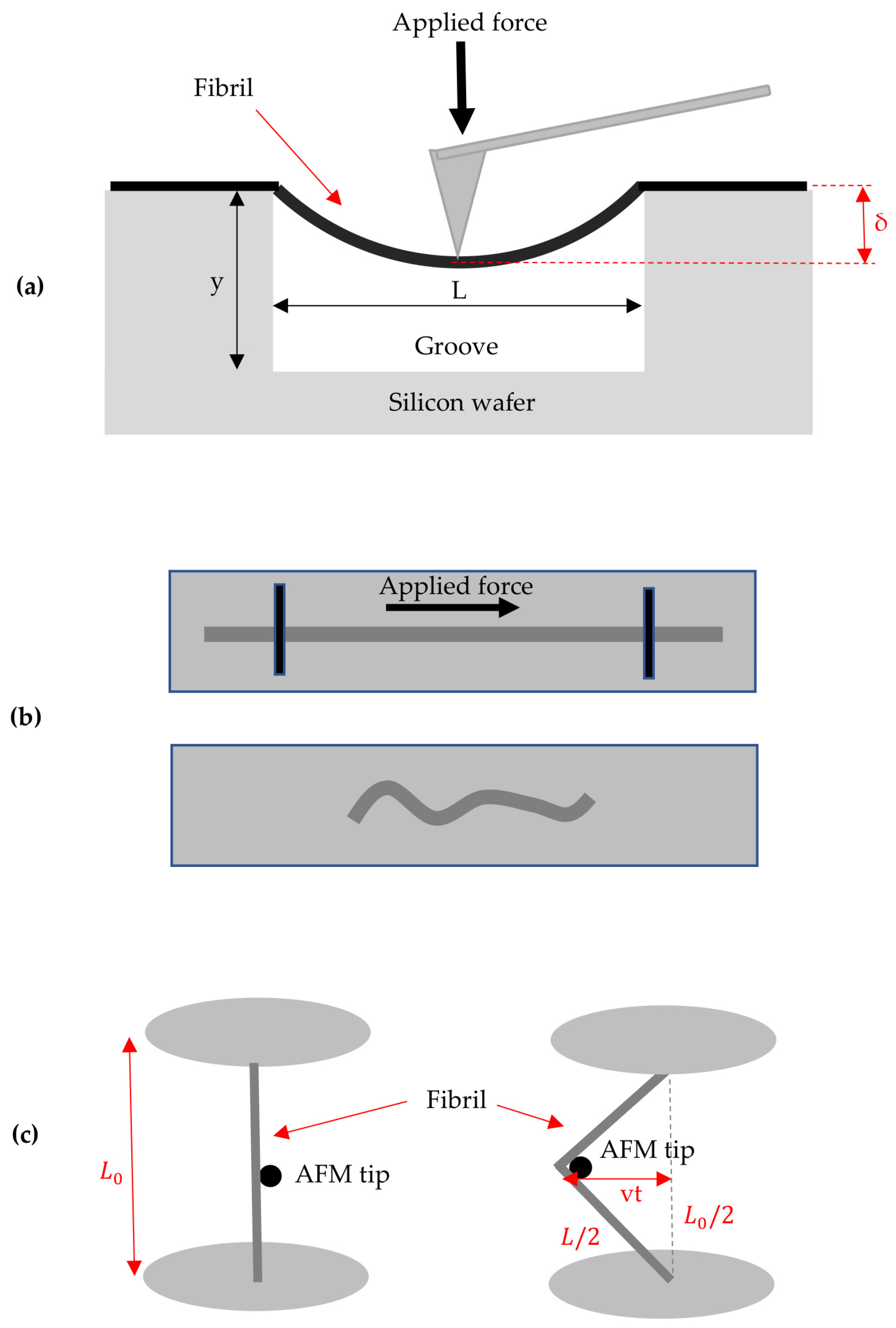
3.5. Other Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Amabili, M.; Asgari, M.; Breslavsky, I.D.; Franchini, G.; Giovanniello, F.; Holzapfel, G.A. Microstructural and mechanical characterization of the layers of human descending thoracic aortas. Acta Biomater. 2021, 134, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Kandapal, S.; Xu, B. Atomic Force Microscopy as a Tool to Study Transport Phenomena in Biological Systems. Processes 2023, 11, 2430. [Google Scholar] [CrossRef]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.L.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Bîrleanu, C.; Pustan, M.; Șerdean, F.; Merie, V. AFM Nanotribomechanical Characterization of Thin Films for MEMS Applications. Micromachines 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications-Possibilities and Limits. Polymers 2022, 21, 1267. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M. Discrimination between Normal and Cancerous Cells Using AFM. BioNanoScience 2016, 6, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, J.; Xu, G.K.; Wang, G.F. Are elastic moduli of biological cells depth dependent or not? Another explanation using a contact mechanics model with surface tension. Soft Matter 2018, 14, 7534–7541. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A. Atomic force microscopy for university students: Applications in biomaterials. Eur. J. Phys. 2017, 38, 033003. [Google Scholar] [CrossRef]
- Stylianou, A.; Kontomaris, S.V.; Grant, C.; Alexandratou, E. Atomic Force Microscopy on Biological Materials Related to Pathological Conditions. Scanning 2019, 2019, 8452851. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Bunch, J.S.; Rhodin, T.N.; McEuen, P.L. Noncontact-AFM imaging of molecular surfaces using single-wall carbon nanotube technology. Nanotechnology 2004, 15, S762004. [Google Scholar] [CrossRef][Green Version]
- de Pablo, P.J. Atomic force microscopy of virus shells. Semin. Cell Dev. Biol. 2018, 73, 199–208. [Google Scholar] [CrossRef]
- Heath, G.R.; Kots, E.; Robertson, J.L.; Lansky, S.; Khelashvili, G.; Weinstein, H.; Scheuring, S. Localization atomic force microscopy. Nature 2021, 594, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mateu, M.G. Mechanical properties of viruses analyzed by atomic force microscopy: A virological perspective. Virus Res. 2012, 168, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Brockwell, D.J.; Radford, S.E.; Thomson, N.H. Effects of hydration on the mechanical response of individual collagen fibrils. Appl. Phys. Lett. 2008, 92, 233902. [Google Scholar] [CrossRef]
- Heim, A.J.; Matthews, W.G.; Koob, T.J. Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl. Phys. Lett. 2006, 89, 181902. [Google Scholar] [CrossRef]
- Papi, M.; Paoletti, P.; Geraghty, B.; Akhtar, R. Nanoscale characterization of the biomechanical properties of collagen fibrils in the sclera. Appl. Phys. Lett. 2014, 104, 103703. [Google Scholar] [CrossRef]
- Yadavalli, V.K.; Svintradze, D.V.; Pidaparti, R.M. Nanoscale measurements of the assembly of collagen to fibrils. Int. J. Biol. Macromol. 2010, 46, 458–464. [Google Scholar] [CrossRef]
- Parvej, M.S.; Wang, X.; Jiang, L. AFM based nanomechanical characterization of cellulose nanofibril. J. Compos. Mater. 2020, 54, 4487–4493. [Google Scholar] [CrossRef]
- Andriotis, O.G.; Manuyakorn, W.; Zekonyte, J.; Katsamenis, O.L.; Fabri, S.; Howarth, P.H.; Davies, D.E.; Thurner, P.J. Nanomechanical assessment of human and murine collagen fibrils via atomic force microscopy cantilever-based nanoindentation. J. Mech. Behav. Biomed. Mater. 2014, 39, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Ottani, V.; Martini, D.; Franchi, M.; Ruggeri, A.; Raspanti, M. Hierarchical structures in fibrillar collagens. Micron 2002, 33, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Vashishth, D. Hierarchy of bone microdamage at multiple length scales. Int. J. Fatigue 2007, 29, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Bechtle, S.; Ang, S.F.; Schneider, G.A. On the mechanical properties of hierarchically structured biological materials. Biomaterials 2010, 31, 6378–6385. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Hierarchical Structure and Nanomechanics of Collagen Microfibrils from the Atomistic Scale Up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J.S. Collagen Diversity, Synthesis and Assembly Collagen; Springer: Boston, MA, USA, 2008; pp. 15–47. [Google Scholar]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P. Collagen Structure and Mechanics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Hasirci, V.A.S.I.F.; Vrana, E.; Zorlutuna, P.; Ndreu, A.; Yilgor, P.I.N.A.R.; Basmanav, F.B.; Aydin, E.R.K.İ.N. Nanobiomaterials: A review of the existing science and technology, and new approaches. J. Biomater. Sci. Polym. Ed. 2006, 17, 1241–1268. [Google Scholar] [CrossRef]
- Zeng, J.; Zeng, Z.; Cheng, Z.; Wang, Y.; Wang, X.; Wang, B.; Gao, W. Cellulose nanofibrils manufactured by various methods with application as paper strength additives. Sci. Rep. 2021, 11, 11918. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Shimizu, M.; Saito, T.; Fukuzumi, H.; Isogai, A. Hydrophobic, ductile, and transparent nanocellulose films with quaternary alkylammonium carboxylates on nanofibril surfaces. Biomacromolecules 2014, 15, 4320–4325. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mascheroni, E.; Piergiovanni, L. The potential of nanocellulose in the packaging field: A review. Packag. Technol. Sci. 2015, 28, 475–508. [Google Scholar] [CrossRef]
- Jin, H.; Kettunen, M.; Laiho, A.; Pynnönen, H.; Paltakari, J.; Marmur, A.; Ikkala, O.; Ras, R.H.A. Superhydrophobic and superoleophobic nanocellulose aerogel membranes as bioinspired cargo carriers on water and oil. Langmuir 2011, 27, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Adamcik, J.; Mezzenga, R. Study of amyloid fibrils via atomic force microscopy. Curr. Opin. Colloid. Interface 2012, 17, 369–376. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, X.; Hu, J.; Zhang, Y. Self-assembly of amyloid-like peptides at interfaces investigated by atomic force microscopy. Sci. Adv. Mater. 2017, 9, 65–76. [Google Scholar] [CrossRef]
- Han, S.W.; Lee, T.H.; Kang, M.S.; Kim, H.J.; Shin, H.K. Probing amyloid β and the antibody interaction using atomic force microscopy. J. Nanosci. Nanotechnol. 2018, 18, 1410–1413. [Google Scholar] [CrossRef]
- Drolle, E.; Hane, F.; Lee, B.; Leonenko, Z. Atomic force microscopy to study molecular mechanisms of amyloid fibril formation and toxicity in Alzheimer’s disease. Drug Metab. Rev. 2014, 46, 207–223. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Aragón, J.; Feoli, S.; Irusta, S.; Mendoza, G. Composite scaffold obtained by electro-hydrodynamic technique for infection prevention and treatment in bone repair. Int. J. Pharm. 2019, 557, 162–169. [Google Scholar] [CrossRef]
- Chou, S.; Wang, J.; Shang, L.; Akhtar, M.U.; Wang, Z.; Shi, B.; Feng, X.; Shan, A. Short, symmetric-helical peptides have narrow-spectrum activity with low resistance potential and high selectivity. Biomater. Sci. 2019, 7, 2394–2409. [Google Scholar] [CrossRef]
- Godakand, V.U.; Li, H.Y.; Alquezar, L.; Zhao, L.X.; Zhu, L.M.; de Silva, R.; de Silva, K.M.N.; Williams, G.R. Tunable drug release from blend poly(vinyl pyrrolidone)-ethyl cellulose nanofibers. Int. J. Pharm. 2019, 562, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chinatangkul, N.; Tubtimsri, S.; Panchapornpon, D.; Akkaramongkolporn, P.; Limmatvapirat, C.; Limmatvapirat, S. Design and characterisation of electrospun shellac-polyvinylpyrrolidone blended micro/nanofibres loaded with monolaurin for application in wound healing. Int. J. Pharm. 2019, 562, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Bukhary, H.; Williams, G.R.; Orlu, M. Electrospun fixed dose formulations of amlodipine besylate and valsartan. Int. J. Pharm. 2018, 549, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Windbergs, M. Controlled dual drug release by coaxial electrospun fibers—Impact of the core fluid on drug encapsulation and release. Int. J. Pharm. 2019, 556, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Neugirg, B.R.; Koebley, S.R.; Schniepp, H.C.; Fery, A. AFM-based mechanical characterization of single nanofibres. Nanoscale 2016, 8, 8414–8426. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V. The Hertz Model in AFM Nanoindentation Experiments: Applications in Biological Samples and Biomaterials. Micro Nanosyst. 2018, 10, 11–22. [Google Scholar] [CrossRef]
- Gołek, F.; Mazur, P.; Ryszka, Z.; Zuber, S. AFM image artifacts. Appl. Surf. Sci. 2014, 304, 11–19. [Google Scholar] [CrossRef]
- Marques-Moros, F.; Forment-Aliaga, A.; Pinilla-Cienfuegos, E.; Canet-Ferrer, J. Mirror effect in atomic force microscopy profiles enables tip reconstruction. Sci. Rep. 2020, 10, 18911. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Doudevski, I.; Erten, S.; Kim, S. Atomic force microscopy (AFM) analysis of an object larger and sharper than the AFM tip. Microsc. Microanal. 2019, 25, 1106–1111. [Google Scholar] [CrossRef]
- Fleischmann, C.; Paredis, K.; Melkonyan, D.; Vandervorst, W. Revealing the 3-dimensional shape of atom probe tips by atomic force microscopy. Ultramicroscopy 2018, 194, 221–226. [Google Scholar] [CrossRef]
- Kondratov, A.V.; Rogov, O.Y.; Gainutdinov, R.V. AFM reconstruction of complex-shaped chiral plasmonic nanostructures. Ultramicroscopy 2017, 181, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fang, Y. Detection of tip convolution effects based on lateral force analysis. In Proceedings of the 2017 IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Shanghai, China, 7–11 August 2017; pp. 13–18. [Google Scholar]
- Lin, Y.C.; Komatsu, H.; Ma, J.; Axelsenb, P.H.; Fakhraai, Z. Quantitative analysis of amyloid polymorphism using height histograms to correct for tip convolution effects in atomic force microscopy imaging. RSC Adv. 2016, 6, 114286–114295. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Han, Z.; Xu, X.; Li, S.; Wu, A. A reconstruction method of AFM tip by using 2 µm lattice sample. Optoelectron. Lett. 2022, 18, 440–443. [Google Scholar] [CrossRef]
- Kim, S.; Moon, D.; Jeon, B.R.; Yeon, J.; Li, X.; Kim, S. Accurate Atomic-Scale Imaging of Two-Dimensional Lattices Using Atomic Force Microscopy in Ambient Conditions. Nanomaterials 2022, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; Batziou, K. Artifacts and Practical Issues in Atomic Force Microscopy. Methods Mol. Biol. 2019, 1886, 3–28. [Google Scholar] [PubMed]
- Shen, J.; Zhang, D.; Zhang, F.H.; Gan, Y. AFM tip-sample convolution effects for cylinder protrusions. Appl. Surf. Sci. 2017, 422, 482–491. [Google Scholar] [CrossRef]
- Díaz, S.R. On the propagation of methodological uncertainties in Depth Sensing Indentation data analysis: A brief and critical review. Mech. Res. Commun. 2020, 105, 103516. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Chung, K.H. Effect of tip shape on nanomechanical properties measurements using AFM. Ultramicroscopy 2019, 202, 1–9. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. The truncated cone effect in AFM nanoindentation on soft samples. Micro Nanosyst. 2023, 15, 153–158. [Google Scholar] [CrossRef]
- Guillonneau, G.; Wheeler, J.M.; Wehrs, J.; Philippe, L.; Baral, P.; Höppel, H.W.; Göken, M. Determination of the true projected contact area by in situ indentation testing. J. Mater. Res. 2019, 34, 2859–2868. [Google Scholar] [CrossRef]
- Jakes, J.E.; Stauffer, D. Contact area correction for surface tilt in pyramidal nanoindentation. J. Mater. Res. 2021, 36, 2189–2197. [Google Scholar] [CrossRef]
- Andriotis, O.G.; Elsayad, K.; Smart, D.E.; Nalbach, M.; Davies, D.E.; Thurner, P.J. Hydration and nanomechanical changes in collagen fibrils bearing advanced glycation end-products. Biomed. Opt. Express 2019, 10, 1841–1855. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Stylianou, A.; Nikita, K.S.; Malamou, A. A discussion regarding the application of the Hertz contact theory on biological samples in AFM nanoindentation experiments. Micro Nanosys. 2021, 13, 42–48. [Google Scholar] [CrossRef]
- Minary-Jolandan, M.; Yu, M.F. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 2009, 10, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Kontomaris, S.V.; Yova, D. Assessing Collagen Nanoscale Thin Films Heterogeneity by AFM Multimode Imaging and Nanoindetation for NanoBioMedical Applications. Micro Nanosyst. 2014, 6, 95–102. [Google Scholar] [CrossRef]
- Allison, D.P.; Mortensen, N.P.; Sullivan, C.J.; Doktycz, M.J. Atomic force microscopy of biological samples. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Rabe, U.; Kopycinska, M.; Hirsekorn, S.; Arnold, W. Evaluation of the contact resonance frequencies in atomic force microscopy as a method for surface characterization. Ultrasonics 2002, 40, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Chena, Z.; Chenb, F.; Wang, D.; Zhou, L. Tapping modes in the Atomic Force Microscope model with Lennard-Jones force and slow-fast base motion. Chaos Solit. Fractals 2021, 144, 110696. [Google Scholar] [CrossRef]
- Dankowicz, H. Nonlinear dynamics as an essential tool for non-destructive characterization of soft nanostructures using tapping-mode atomic force microscopy. Phil. Trans. R. Soc. A 2006, 364, 3505–3520. [Google Scholar] [CrossRef]
- Bellotti, R.; Picotto, G.B.; Ribotta, L. AFM Measurements and Tip Characterization of Nanoparticles with Different Shapes. Nanomanuf. Metrol. 2022, 5, 127–138. [Google Scholar] [CrossRef]
- Canet-Ferrer, J.; Coronado, E.; Forment-Aliaga, A.; Pinilla-Cienfuegos, E. Correction of the tip convolution effects in the imaging of nanostructures studied through scanning force microscopy. Nanotechnology 2014, 25, 395703. [Google Scholar] [CrossRef] [PubMed]
- Reiss, G.; Schneider, F.; Vancea, J.; Hoffmann, H. Scanning tunneling microscopy on rough surfaces: Deconvolution of constant current images. Appl. Phys. Lett. 1990, 57, 867. [Google Scholar] [CrossRef][Green Version]
- Keller, D. Reconstruction of STM and AFM images distorted by finite-size tips. Surf. Sci. 1991, 253, 353. [Google Scholar] [CrossRef]
- Villarubia, J.S. Morphological estimation of tip geometry for scanned probe microscopy. Surf. Sci. 1994, 321, 287. [Google Scholar] [CrossRef]
- Villarubia, J.S. Algorithms for scanned probe microscopy: Image simulation, surface reconstruction and tip estimation. J. Res. Natl. Inst. Stand. Technol. 1997, 102, 425. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Niu, D.X.; Jiang, C.R.; Yang, X.J. The conductive properties of single DNA molecules studied by torsion tunneling atomic force microscopy. Nanotechnology 2013, 25, 025707. [Google Scholar] [CrossRef] [PubMed]
- Winzer, A.T.; Kraft, C.; Bhushan, S.; Stepanenko, V.; Tessmer, I. Correcting for AFM tip induced topography convolutions in protein–DNAsamples. Ultramicroscopy 2012, 121, 8. [Google Scholar] [CrossRef]
- Wang, C.; Itoh, H. Evaluation of errors in the measurement of surface roughness at high spatial frequency by atomic force microscopy on a thin film. J. Appl. Phys. 2012, 51, 08KB11. [Google Scholar] [CrossRef]
- Severin, N.; Dorn, M.; Kalachev, A.; Rabe, J.P. Replication of Single macromolecules with graphene. Nano Lett. 2011, 11, 2436. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Lacaze, E.; Jupille, J. Probing the probe: AFM tip-profiling via nanotemplates to determine Hamaker constants from phase–distance curves. Ultramicroscopy 2012, 121, 25. [Google Scholar] [CrossRef]
- Alle, M.J.; Hud, N.V.; Balooch, M.; Tench, R.J.; Siekhaus, W.J.; Balhorn, R. Tip-radius-induced artifacts in AFM images of protamine-complexed DNA fibers. Ultramicroscopy 1992, 42, 1095. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Malamou, A.; Stylianou, A. The Hertzian theory in AFM nanoindentation experiments regarding biological samples: Overcoming limitations in data processing. Micron 2022, 155, 103228. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Tsuboi, K.; Yokota, S.; Tagawa, S.; Kondo, T. Characterization of an Amphiphilic Janus-Type Surface in the Cellulose Nanofibril Prepared by Aqueous Counter Collision. Biomacromolecules 2021, 22, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Sambani, K.; Kontomaris, S.V.; Yova, D. Atomic Force Microscopy Imaging of Elastin Nanofibers Self-Assembly. Materials 2023, 16, 4313. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.D.; Tardy, B.L.; Rojas, O.J. Accounting for Substrate Interactions in the Measurement of the Dimensions of Cellulose Nanofibrils. Biomacromolecules 2019, 20, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.J.; Shiflett, O.; Shah, S.; Renner, T.; Soulas, N.; Scharre, D.; McTigue, D.; Agarwal, G. Artifacts in magnetic force microscopy of histological sections. J. Magn. Magn. Mater. 2022, 564, 170116. [Google Scholar] [CrossRef]
- Vokoun, D.; Samal, S.; Stachiv, I. Magnetic Force Microscopy in Physics and Biomedical Applications. Magnetochemistry 2022, 8, 42. [Google Scholar] [CrossRef]
- Passeri, D.; Dong, C.; Angeloni, L.; Pantanella, F.; Natalizi, T.; Berlutti, F. Thickness Measurement of Soft Thin Films on Periodically Patterned Magnetic Substrates by Phase Difference Magnetic Force Microscopy. Ultramicroscopy 2014, 136, 96–106. [Google Scholar] [CrossRef]
- Newacheck, S.; Huynh, N.U.; Youssef, G. Colossal Crystal in P3HT:PCBM Blends for Enhanced Organic Magnetism. Cryst. Growth Des. 2021, 21, 5300–5305. [Google Scholar] [CrossRef]
- Nocera, T.M.; Zeng, Y.; Agarwal, G. Distinguishing Ferritin from Apoferritin Using Magnetic Force Microscopy. Nanotechnology 2014, 25, 461001. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Cinar, I.; Lacour, D.; Montaigne, F. Artifacts in magnetic force microscopy under in-plane applied magnetic field: Magnetic bubble as a case study. J. Magn. Magn. Mater. 2020, 500, 166296. [Google Scholar] [CrossRef]
- Angeloni, L.; Passeri, D.; Reggente, M.; Mantovani, D.; Rossi, M. Removal of electrostatic artifacts in magnetic force microscopy by controlled magnetization of the tip: Application to superparamagnetic nanoparticles. Sci. Rep. 2016, 6, 26293. [Google Scholar] [CrossRef] [PubMed]
- Ciuta, G.; Dumas-Bouchiat, F.; Dempsey, N.; Fruchart, O. Some aspects of Magnetic Force Microscopy of hard magnetic films. IEEE Trans. Magn. 2016, 52, 6500408. [Google Scholar] [CrossRef]
- Sifford, J.; Walsh, K.J.; Tong, S.; Bao, G.; Agarwal, G. Indirect magnetic force microscopy. Nanoscale Adv. 2019, 1, 2348. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Stylianou, A.; Malamou, A. Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils. Materials 2022, 15, 2477. [Google Scholar] [CrossRef] [PubMed]
- Creasey, R.G.; Gibson, C.T.; Voelcker, N.H. Characterization of fiber-forming peptides and proteins by means of atomic force microscopy. Curr. Protein Pept. Sci. 2012, 13, 232–257. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Patrickios, C.S.; Stylianopoulos, T. Exploring the Nano-Surface of Collagenous and Other Fibrotic Tissues with AFM. In Fibrosis: Methods and Protocols; Rittié, L., Ed.; Springer: New York, NY, USA, 2017; pp. 453–489. [Google Scholar]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.; Bobrowska, J.; et al. Standardized nanomechanical atomic force microscopy procedure (SNAP) for measuring soft and biological samples. Sci. Rep. 2017, 7, 5117. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Malamou, A.; Stylianopoulos, T. A discussion regarding the approximation of cylindrical and spherical shaped samples as half spaces in AFM nanoindentation experiments. Mater. Res. Express 2018, 5, 085402. [Google Scholar] [CrossRef]
- Vojta, J.H.V.; Ilavsky, M. Penetration Behavior of the System Sphere-Cylinder. Polym. Eng. Sci. 1980, 20, 402–405. [Google Scholar]
- Kontomaris, S.V.; Malamou, A. An extension of the general nanoindentation equation regarding cylindrical—Shaped samples and a simplified model for the contact ellipse determination. Mater. Res. Express 2018, 5, 125403. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquidaz, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Bouzakis, K.D.; Pappa, M.; Maliaris, G.; Michailidis, N. Fast determination of parameters describing manufacturing imperfections and operation wear of nanoindenter tips. Surf. Coat. Technol. 2013, 215, 218–223. [Google Scholar] [CrossRef]
- McAllister, Q.P.; Gillespie, J.W.; VanLandingham, M.R. Nonlinear indentation of fibers. J. Mater. Res. 2012, 27, 197–213. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. Hertz model or Oliver & Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Mater. Res. Express 2020, 7, 033001. [Google Scholar]
- Cheng, Q.; Wang, S. A method for testing the elastic modulus of single cellulose fibrils via atomic force microscopy. Compos. Part A Appl. Sci. 2008, 39, 1838–1843. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Stylios, G.K. The tensile properties of electrospun nylon 6 single nanofibers. J. Polym. Sci. B Polym. Phys. 2010, 48, 1719–1731. [Google Scholar] [CrossRef]
- Kluge, D.; Singer, J.C.; Neubauer, J.W.; Abraham, F.; Schmidt, H.-W.; Fery, A. Influence of the Molecular Structure and Morphology of Self-Assembled 1,3,5-Benzenetrisamide Nanofibers on their Mechanical Properties. Small 2012, 8, 2563–2570. [Google Scholar] [CrossRef]
- Tanur, A.E.; Wang, J.; Reddy, A.L.; Lamont, D.N.; Yap, Y.K.; Walker, G.C. Diameter-dependent bending modulus of individual multiwall boron nitride nanotubes. J. Phys. Chem. B 2013, 117, 4618–4625. [Google Scholar] [CrossRef]
- Chen, Y.; Dorgan, B.L., Jr.; McIlroy, D.N.; Aston, D.E. On the importance of boundary conditions on nanomechanical bending behavior and elastic modulus determination of silver nanowires. J. Appl. Phys. 2006, 100, 10430. [Google Scholar] [CrossRef]
- Ling, X.Y.; Phang, I.Y.; Schonherr, H.; Reinhoudt, D.N.; Vancso, G.J.; Huskens, J. Freestanding 3D supramolecularparticle bridges: Fabrication and mechanical behavior. Small 2009, 5, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Stachewicz, U.; Bailey, R.J.; Wang, W.; Barber, A.H. Size dependent mechanical properties of electrospun polymer fibers from a composite structure. Polymer 2012, 53, 5132–5137. [Google Scholar] [CrossRef]
- Wu, B.; Heidelberg, A.; Boland, J.J. Mechanical properties of ultrahigh-strength gold nanowires. Nat. Mater. 2005, 4, 525–529. [Google Scholar] [CrossRef]
- Tao, X.Y.; Dong, L.X.; Wang, X.N.; Zhang, W.K.; Nelson, B.J.; Li, X.D. B4C-nanowires/carbon-microfiber hybrid structures and composites from cotton T-shirts. Adv. Mater. 2010, 22, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Gachon, E.; Mesquida, P. Mechanical properties of collagen fibrils determined by buckling analysis. Acta Biomater. 2022, 149, 60–68. [Google Scholar] [CrossRef]
- Xiao, J.; Ryu, S.Y.; Huang, Y.; Hwang, K.C.; Paik, U.; Rogers, J.A. Mechanics of nanowire/nanotube in-surface buckling on elastomeric substrates. Nanotechnology 2010, 21, 85708. [Google Scholar] [CrossRef]
- Khang, D.Y.; Xiao, J.; Kocabas, C.; MacLaren, S.; Banks, T.; Jiang, H.; Huang, Y.Y.; Rogers, J.A. Molecular scale buckling mechanics in individual aligned single-wall carbon nanotubes on elastomeric substrates. Nano. Lett. 2008, 8, 124–130. [Google Scholar] [CrossRef]
- Quigley, A.S.; Veres, S.P.; Kreplak, L. Bowstring Stretching and Quantitative Imaging of Single Collagen Fibrils via Atomic Force Microscopy. PLoS ONE 2016, 11, e0161951. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Georgakopoulos, A.; Malamou, A. Is it mathematically correct to fit AFM data (obtained on biological materials) to equations arising from Hertzian mechanics? Micron 2023, 164, 103384. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Stylianou, A.; Chliveros, G.; Malamou, A. AFM Indentation on Highly Heterogeneous Materials Using Different Indenter Geometries. Appl. Mech. 2023, 4, 460–475. [Google Scholar] [CrossRef]
- Sweers, K.; Van Der Werf, K.; Bennink, M.; Subramaniam, V. Nanomechanical properties of α-synuclein amyloid fibrils: A comparative study by nanoindentation, harmonic force microscopy, and Peakforce QNM. Nanoscale Res. Lett. 2011, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Adamcik, J.; Berquand, A.; Mezzenga, R. Single-step direct measurement of amyloid fibrils stiffness by peak force quantitative nanomechanical atomic force microscopy. Appl. Phys. Lett. 2011, 98, 193701. [Google Scholar] [CrossRef]
- Adamcik, J.; Lara, C.; Usov, I.; Jeong, J.S.; Ruggeri, F.S.; Dietler, G.; Lashuel, H.A.; Hamley, I.W.; Mezzenga, R. Measurement of intrinsic properties of amyloid fibrils by the peak force QNM method. Nanoscale 2012, 4, 4426–4429. [Google Scholar] [CrossRef] [PubMed]
- Lamour, G.; Yip, C.K.; Li, H.; Gsponer, J. High Intrinsic Mechanical Flexibility of Mouse Prion Nanofibrils Revealed by Measurements of Axial and Radial Young’s Moduli. ACS Nano 2014, 8, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Adamcik, J.; Jeong, J.S.; Lashuel, H.A.; Mezzenga, R.; Dietler, G. Influence of the β-sheet content on the mechanical properties of aggregates during amyloid fibrillization. Angew. Chem. Int. Ed. Engl. 2015, 54, 2462–2466. [Google Scholar] [CrossRef] [PubMed]
- Sweers, K.K.M.; van der Werf, K.O.; Bennink, M.L.; Subramaniam, V. Atomic force microscopy under controlled conditions reveals structure of C-terminal region of α-synuclein in amyloid fibrils. ACS Nano. 2012, 6, 5952–5960. [Google Scholar] [CrossRef]
- Persch, G.; Born, C.; Utesch, B. Nano-hardness investigations of thin films by an atomic force microscope. Microelectron. Eng. 1994, 24, 113–121. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Nikita, K.S.; Malamou, A. Determination of the linear elastic regime in AFM nanoindentation experiments on cells. Mater. Res. Express 2019, 11, 115410. [Google Scholar] [CrossRef]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontomaris, S.V.; Stylianou, A.; Chliveros, G.; Malamou, A. Overcoming Challenges and Limitations Regarding the Atomic Force Microscopy Imaging and Mechanical Characterization of Nanofibers. Fibers 2023, 11, 83. https://doi.org/10.3390/fib11100083
Kontomaris SV, Stylianou A, Chliveros G, Malamou A. Overcoming Challenges and Limitations Regarding the Atomic Force Microscopy Imaging and Mechanical Characterization of Nanofibers. Fibers. 2023; 11(10):83. https://doi.org/10.3390/fib11100083
Chicago/Turabian StyleKontomaris, Stylianos Vasileios, Andreas Stylianou, Georgios Chliveros, and Anna Malamou. 2023. "Overcoming Challenges and Limitations Regarding the Atomic Force Microscopy Imaging and Mechanical Characterization of Nanofibers" Fibers 11, no. 10: 83. https://doi.org/10.3390/fib11100083
APA StyleKontomaris, S. V., Stylianou, A., Chliveros, G., & Malamou, A. (2023). Overcoming Challenges and Limitations Regarding the Atomic Force Microscopy Imaging and Mechanical Characterization of Nanofibers. Fibers, 11(10), 83. https://doi.org/10.3390/fib11100083








