Optimization of Polyvinyl Alcohol-Based Electrospun Fibers with Bioactive or Electroconductive Phases for Tissue-Engineered Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation
2.3. Electrospinning Process and Post Processing
2.4. Morphology
2.5. Physical and Chemical Characterization
2.6. In Vitro Studies
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2019, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef] [PubMed]
- Dalyan, O.; Öztürk, Ö.F.; Pişkin, M. Toxicity of Polyvinyl Alcohols in Medicinal Chemistry. MANAS J. Eng. 2021, 9, 129–135. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for Application of Electrospinning and Electrospun Nanofibers Technology in Textile Industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Perveen, A.; Matin, M.A.; Arafat, M.T. Effects of Binary Solvent Mixtures on the Electrospinning Behavior of Poly (Vinyl Alcohol). Mater. Res. Express 2018, 5, 115407. [Google Scholar] [CrossRef]
- Parhi, R. Fabrication and Characterization of PVA-Based Green Materials. In Advanced Green Materials; Ahmed, S.B.T.-A.G.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 133–177. ISBN 978-0-12-819988-6. [Google Scholar]
- Waheed, S.; Butcher, A.L.; Oyen, M.L. The Viscoelastic Response of Electrospun Poly(Vinyl Alcohol) Mats. J. Mech. Behav. Biomed. Mater. 2018, 77, 383–388. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber Composites of Polyvinyl Alcohol and Cellulose Nanocrystals: Manufacture and Characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Moradipour, P.; Limoee, M.; Janfaza, S.; Behbood, L. Core-Shell Nanofibers Based on Polycaprolactone/Polyvinyl Alcohol and Polycaprolactone/Collagen for Biomedical Applications. J. Pharm. Innov. 2022, 17, 911–920. [Google Scholar] [CrossRef]
- Renkler, N.Z.; Cruz-Maya, I.; Bonadies, I.; Guarino, V. Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications. Polymers 2022, 14, 4249. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Chen, S.; Wu, S. Designing an Innovative Electrospinning Strategy to Generate PHBV Nanofiber Scaffolds with a Radially Oriented Fibrous Pattern. Nanomaterials 2023, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Mao, Y.; Shen, W.; Wu, S.; Ge, X.; Ao, F.; Ning, Y.; Luo, Y.; Liu, Z. Electrospun Polymers: Using Devices to Enhance Their Potential for Biomedical Applications. React. Funct. Polym. 2023, 186, 105568. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Maleki, H.; Mathur, S.; Klein, A. Antibacterial Ag Containing Core-shell Polyvinyl Alcohol-poly (Lactic Acid) Nanofibers for Biomedical Applications. Polym. Eng. Sci. 2020, 60, 1221–1230. [Google Scholar] [CrossRef]
- Lencova, S.; Svarcova, V.; Stiborova, H.; Demnerova, K.; Jencova, V.; Hozdova, K.; Zdenkova, K. Bacterial Biofilms on Polyamide Nanofibers: Factors Influencing Biofilm Formation and Evaluation. ACS Appl. Mater. Interfaces 2021, 13, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Jhala, D.; Rather, H.; Vasita, R. Polycaprolactone–Chitosan Nanofibers Influence Cell Morphology to Induce Early Osteogenic Differentiation. Biomater. Sci. 2016, 4, 1584–1595. [Google Scholar] [CrossRef]
- Erickson, A.; Chiarelli, P.A.; Huang, J.; Levengood, S.L.; Zhang, M. Electrospun Nanofibers for 3-D Cancer Models, Diagnostics, and Therapy. Nanoscale Horiz. 2022, 7, 1279–1298. [Google Scholar] [CrossRef]
- Guaccio, A.; Guarino, V.; Perez, M.A.A.; Cirillo, V.; Netti, P.A.; Ambrosio, L. Influence of Electrospun Fiber Mesh Size on HMSC Oxygen Metabolism in 3D Collagen Matrices: Experimental and Theoretical Evidences. Biotechnol. Bioeng. 2011, 108, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-C.; Ito, T.; Kim, K.-O.; Kim, K.-W.; Kim, B.-S.; Khil, M.-S.; Kim, H.-Y.; Kim, I.-S. Electrospun Poly(Vinyl Alcohol) Nanofibers: Effects of Degree of Hydrolysis and Enhanced Water Stability. Polym. J. 2010, 42, 273–276. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Baykara, T.; Taylan, G. Coaxial Electrospinning of PVA/Nigella Seed Oil Nanofibers: Processing and Morphological Characterization. Mater. Sci. Eng. B 2021, 265, 115012. [Google Scholar] [CrossRef]
- Alazab, M.; Mitchell, G.R.; Davis, F.J.; Mohan, S.D. Sustainable Electrospinning of Nanoscale Fibres. Procedia Manuf. 2017, 12, 66–78. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Imre, B.; Pukánszky, B. Compatibilization in Bio-Based and Biodegradable Polymer Blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Mares-Bou, S.; Serrano, M.-A.; Gómez-Tejedor, J.A. Core–Shell Polyvinyl Alcohol (PVA) Base Electrospinning Microfibers for Drug Delivery. Polymers 2023, 15, 1554. [Google Scholar] [CrossRef] [PubMed]
- Vacheethasanee, K.; Wang, S.; Qiu, Y.; Marchant, R.E. Poly(Ethylene Oxide) Surfactant Polymers. J. Biomater. Sci. Polym. Ed. 2004, 15, 95–110. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Zuo, B. Structure and Sound Absorption Properties of Spiral Vane Electrospun PVA/PEO Nanofiber Membranes. Appl. Sci. 2018, 8, 296. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural Composite Dressings Based on Collagen, Gelatin and Plant Bioactive Compounds for Wound Healing: A Review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Guarino, V.; Alvarez-Perez, M.A.; Marrese, M.; Ambrosio, L. Optimization of Fully Aligned Bioactive Electrospun Fibers for “In Vitro” Nerve Guidance. J. Mater. Sci. Mater. Med. 2014, 25, 2323–2332. [Google Scholar] [CrossRef]
- Abdul Khodir, W.K.W.; Abdul Razak, A.H.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Vineis, C.; Cruz Maya, I.; Mowafi, S.; Varesano, A.; Sánchez Ramírez, D.O.; Abou Taleb, M.; Tonetti, C.; Guarino, V.; El-Sayed, H. Synergistic Effect of Sericin and Keratin in Gelatin Based Nanofibers for In Vitro Applications. Int. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly Polydisperse Keratin Rich Nanofibers: Scaffold Design and In Vitro Characterization. J. Biomed. Mater. Res. Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive Polymers for Tissue Regeneration: Developments and Perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, P.X.; Guo, B. Conductive Biomaterials for Muscle Tissue Engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Heng, B.C.; Bai, Y.; Li, X.; Lim, L.W.; Li, W.; Ge, Z.; Zhang, X.; Deng, X. Electroactive Biomaterials for Facilitating Bone Defect Repair under Pathological Conditions. Adv. Sci. 2023, 10, 2204502. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, G.; Qin, F.; Hu, L.; Luo, B.; Liu, T.; Yin, X.; Su, Z.; Zeng, Z.; Jiang, Y.; et al. High-Conductivity, Flexible and Transparent PEDOT:PSS Electrodes for High Performance Semi-Transparent Supercapacitors. Polymers 2020, 12, 450. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and Electrically Conducting Polymers for Biomedical Applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Zubair, N.A.; Rahman, N.A.; Lim, H.N.; Zawawi, R.M.; Sulaiman, Y. Electrochemical Properties of PVA–GO/PEDOT Nanofibers Prepared Using Electrospinning and Electropolymerization Techniques. RSC Adv. 2016, 6, 17720–17727. [Google Scholar] [CrossRef]
- Devi, C.; Gellanki, J.; Pettersson, H.; Kumar, S. High Sodium Ionic Conductivity in PEO/PVP Solid Polymer Electrolytes with InAs Nanowire Fillers. Sci. Rep. 2021, 11, 20180. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Garcia, W.; Cruz-Maya, I.; Melendez-Zambrana, A.; Ramos-Colon, I.; Pinto, N.J.; Thomas, S.W.; Guarino, V. Optimization of PVDF-TrFE Based Electro-Conductive Nanofibers: Morphology and In Vitro Response. Materials 2023, 16, 3106. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Zuppolini, S.; Zarrelli, M.; Mazzotta, E.; Borriello, A.; Malitesta, C.; Guarino, V. Polydopamine-Coated Alginate Microgels: Process Optimization and In Vitro Validation. J. Funct. Biomater. 2022, 14, 2. [Google Scholar] [CrossRef]
- Saracino, E.; Zuppolini, S.; Guarino, V.; Benfenati, V.; Borriello, A.; Zamboni, R.; Ambrosio, L. Polyaniline Nano-Needles into Electrospun Bio Active Fibres Support In Vitro Astrocyte Response. RSC Adv. 2021, 11, 11347–11355. [Google Scholar] [CrossRef]
- Sarip, M.N.; Noor, M.F.H.M.; Ahmad, Z.; Shuhaime, N.; Dahan, R.M.; Arshad, A.N.; Ismail, W.I.N.W. Conductivity Study of Polyvinyl Alcohol/Polyvinyl Pyrrolidone (PVA/PVP)-KOH Coatings System. AIP Conf. Proc. 2018, 2031, 20022. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Lett. 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.Y.; Chang, N.Y.; Li, C.; Chan, V.; Hsieh, J.H.; Tsai, Y.-H.; Lin, T. Fabrication of Gelatin Nanofibers by Electrospinning—Mixture of Gelatin and Polyvinyl Alcohol. Polymers 2022, 14, 2610. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ming, J.; Zhang, H.; Zuo, B. EDC/NHS Crosslinked Electrospun Regenerated Tussah Silk Fibroin Nanofiber Mats. Fibers Polym. 2012, 13, 613–617. [Google Scholar] [CrossRef]
- Yousefzadeh, M. Modeling and Simulation of the Electrospinning Process. In Electrospun Nanofibers; Afshari, M.B.T.-E.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 277–301. [Google Scholar]
- Sharma, S.K.; Prakash, J.; Pujari, P.K. Effects of the Molecular Level Dispersion of Graphene Oxide on the Free Volume Characteristics of Poly(Vinyl Alcohol) and Its Impact on the Thermal and Mechanical Properties of Their Nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 29201–29209. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, J.; Zhang, S.; Guan, F.; Yu, Y.; Feng, S.; Yao, Q.; Bao, D. Improved Biomedical Bioactivity of Polyvinyl Alcohol/Polyethylene Oxide Composite System-Based Nanofiber Membranes via Incorporating Antarctic Krill Protein. Eur. Polym. J. 2023, 187, 111888. [Google Scholar] [CrossRef]
- Jia, L.; Qin, X. The Effect of Different Surfactants on the Electrospinning Poly(Vinyl Alcohol) (PVA) Nanofibers. J. Therm. Anal. Calorim. 2013, 112, 595–605. [Google Scholar] [CrossRef]
- Zheng, J.-Y.; Zhuang, M.-F.; Yu, Z.-J.; Zheng, G.-F.; Zhao, Y.; Wang, H.; Sun, D.-H. The Effect of Surfactants on the Diameter and Morphology of Electrospun Ultrafine Nanofiber. J. Nanomater. 2014, 2014, 689298. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication Challenges and Trends in Biomedical Applications of Alginate Electrospun Nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Hirsch, E.; Pantea, E.; Vass, P.; Domján, J.; Molnár, M.; Suhajda, Á.; Andersen, S.K.; Vigh, T.; Verreck, G.; Marosi, G.J.; et al. Probiotic Bacteria Stabilized in Orally Dissolving Nanofibers Prepared by High-Speed Electrospinning. Food Bioprod. Process. 2021, 128, 84–94. [Google Scholar] [CrossRef]
- Shen, W.; Hsieh, Y.-L. Biocompatible Sodium Alginate Fibers by Aqueous Processing and Physical Crosslinking. Carbohydr. Polym. 2014, 102, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Salihu, R.; Abd Razak, S.I.; Zawawi, N.A.; Kadir, M.R.; Ismail, N.I.; Jusoh, N.; Mohamad, M.R.; Nayan, N.H. Citric Acid: A Green Cross-Linker of Biomaterials for Biomedical Applications. Eur. Polym. J. 2021, 146, 110271. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking Electrospun Poly (Vinyl) Alcohol Fibers with Citric Acid to Impart Aqueous Stability for Medical Applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- Lian, Z.; Ye, L. Structure and Properties of PVA/PEO Hydrogel Prepared by Freezing/Thawing Method. J. Thermoplast. Compos. Mater. 2011, 26, 912–922. [Google Scholar] [CrossRef]
- Liu, H.; Zuo, B. Sound Absorption Property of PVA/PEO/GO Nanofiber Membrane and Non-Woven Composite Material. J. Ind. Text. 2019, 50, 512–525. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wu, H.; Wang, C.; Salleh, K.M.; Li, H.; Zakaria, S. Thermally Treated Berberine-Loaded SA/PVA/PEO Electrospun Microfiber Membranes for Antibacterial Wound Dressings. Polymers 2022, 14, 4473. [Google Scholar] [CrossRef]
- Yu, D.; Feng, Y.-Y.; Xu, J.-X.; Kong, B.-H.; Liu, Q.; Wang, H. Fabrication, Characterization, and Antibacterial Properties of Citric Acid Crosslinked PVA Electrospun Microfibre Mats for Active Food Packaging. Packag. Technol. Sci. 2021, 34, 361–370. [Google Scholar] [CrossRef]
- Pathan, S.G.; Fitzgerald, L.M.; Ali, S.M.; Damrauer, S.M.; Bide, M.J.; Nelson, D.W.; Ferran, C.; Phaneuf, T.M.; Phaneuf, M.D. Cytotoxicity Associated with Electrospun Polyvinyl Alcohol. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1652–1662. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, M.; Li, Y.; Li, K.; Chen, S.; Zhao, W.; Wu, S.; Han, Y. Electrospun Chitosan–Polyvinyl Alcohol Nanofiber Dressings Loaded with Bioactive Ursolic Acid Promoting Diabetic Wound Healing. Nanomaterials 2022, 12, 2933. [Google Scholar] [CrossRef]
- Asiri, A.; Saidin, S.; Sani, M.H.; Al-Ashwal, R.H. Epidermal and Fibroblast Growth Factors Incorporated Polyvinyl Alcohol Electrospun Nanofibers as Biological Dressing Scaffold. Sci. Rep. 2021, 11, 5634. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Mirzadeh, H. Electrospinning, Mechanical Properties, and Cell Behavior Study of Chitosan/PVA Nanofibers. J. Biomed. Mater. Res. A 2015, 103, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Zhao, Y.; Liang, S.; Xu, A.; Gao, X.; Deng, F.; Fang, J.; Wei, S. Peptide-Decorated Polyvinyl Alcohol/Hyaluronan Nanofibers for Human Induced Pluripotent Stem Cell Culture. Carbohydr. Polym. 2014, 101, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular Interactions in Gelatin/Chitosan Composite Films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of Cell Binding to Collagen and Gelatin: A Study of the Effect of 2D and 3D Architecture and Surface Chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef]
- Banner, J.; Dautzenberg, M.; Feldhans, T.; Hofmann, J.; Plümer, P.; Ehrmann, A. Water Resistance and Morphology of Electrospun Gelatine Blended with Citric Acid and Coconut Oil. Tekstilec 2018, 61, 129–135. [Google Scholar] [CrossRef]
- Mahnama, H.; Dadbin, S.; Frounchi, M.; Rajabi, S. Preparation of Biodegradable Gelatin/PVA Porous Scaffolds for Skin Regeneration. Artif. Cells Nanomed. Biotechnol. 2017, 45, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, Z.; Slaughter, G. Cross-Linked Electrospun Gelatin Nanofibers for Cell-Based Assays. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 6088–6091. [Google Scholar] [CrossRef]
- Damayanti, R.; Alfian, Z.; Zulfajri, M. Biosynthesis of Silver Nanoparticles Loaded PVA/Gelatin Nanocomposite Films and Their Antimicrobial Activities. Inorg. Chem. Commun. 2022, 144, 109948. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of Gelatin Fibers Using Solutions with Low Acetic Acid Concentration: Effect of Solvent Composition on Both Diameter of Electrospun Fibers and Cytotoxicity. J. Appl. Polym. Sci. 2015, 132, 42115. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA Proton Exchange Membranes: Correlation between Physiochemical Properties and Free Volume Determined by Positron Annihilation Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef] [PubMed]
- Azeez Betti, N. Thermogravimetric Analysis on PVA / PVP Blend Under Air Atmosphere. Eng. Technol. J. 2016, 34, 2433–2442. [Google Scholar] [CrossRef]
- Bello, J.S.; Cruz-Maya, I.; González-Alva, P.; Alvarez-Perez, M.A.; Guarino, V. Electro- and Nonelectro-Assisted Spinning Technologies for In Vitro and In Vivo Models. In Nanomaterials for Theranostics and Tissue Engineering; Rossi, F., Rainer, A.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–204. ISBN 978-0-12-817838-6. [Google Scholar]
- Cruz-Maya, I.; Varesano, A.; Vineis, C.; Guarino, V. Comparative Study on Protein-Rich Electrospun Fibers for In Vitro Applications. Polymers 2020, 12, 1671. [Google Scholar] [CrossRef]
- García-Hernández, A.B.; Morales-Sánchez, E.; Calderón-Domínguez, G.; de la, P.; Salgado-Cruz, M.; Farrera-Rebollo, R.R.; Vega-Cuellar, M.Á.; García-Bórquez, A. Hydrolyzed Collagen on PVA-Based Electrospun Membranes: Synthesis and Characterization. J. Appl. Polym. Sci. 2021, 138, 51197. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.W.; Guarino, V. New Insights to Design Electrospun Fibers with Tunable Electrical Conductive–Semiconductive Properties. Sensors 2023, 23, 1606. [Google Scholar] [CrossRef] [PubMed]
- Cassu, S.N.; Felisberti, M.I. Poly(Vinyl Alcohol) and Poly(Vinylpyrrolidone) Blends: 2. Study of Relaxations by Dynamic Mechanical Analysis. Polymer 1999, 40, 4845–4851. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y.; Chai, W.; Yang, M. Measurement and Modeling of the Effect of Composition Ratios on the Properties of Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) Membranes. Mater. Des. 2016, 103, 249–258. [Google Scholar] [CrossRef]
- Virginia, C.; Khasanah, A.; Jauhari, J.; Sriyanti, I. Electrospinning and Characterization Nanofibers and Nano Particle of Polyvinylpyrrolidone. IOP Conf. Ser. Mater. Sci. Eng. 2020, 850, 12039. [Google Scholar] [CrossRef]
- Basha, S.K.; Sundari, G.; Vijay Kumar, K. Electrical Conductivity, Transport and Discharge Characteristics of a Sodium Acetate Trihydrate Complexed with Polyvinyl Alcohol for Electrochemical Cell. Int. J. ChemTech Res. 2015, 8, 803–810. [Google Scholar]
- Gökmeşe, F.; Uslu, İ.; Aytimur, A. Preparation and Characterization of PVA/PVP Nanofibers as Promising Materials for Wound Dressing. Polym. Plast. Technol. Eng. 2013, 52, 1259–1265. [Google Scholar] [CrossRef]
- Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.; Ramakrishna, S. Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Appl. Sci. 2021, 11, 2779. [Google Scholar] [CrossRef]
- Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.A.; Hatami-Marbini, H.; Smith, B.J.; Ricci, J.L.; Madihally, S.V.; Vashaee, D.; Tayebi, L. 3D Conductive Nanocomposite Scaffold for Bone Tissue Engineering. Int. J. Nanomed. 2014, 9, 167–181. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive Biomaterials: Vehicles for Controlled Delivery of Therapeutic Agents for Drug Delivery and Tissue Regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, H.; Lee, E.K. New Opportunities for Organic Semiconducting Polymers in Biomedical Applications. Polymers 2022, 14, 2960. [Google Scholar] [CrossRef] [PubMed]
- Latonen, R.-M.; Cabrera, J.A.W.; Lund, S.; Kosourov, S.; Vajravel, S.; Boeva, Z.; Wang, X.; Xu, C.; Allahverdiyeva, Y. Electrospinning of Electroconductive Water-Resistant Nanofibers of PEDOT–PSS, Cellulose Nanofibrils and PEO: Fabrication, Characterization, and Cytocompatibility. ACS Appl. Bio Mater. 2021, 4, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Pisesweerayos, P.; Dangtip, S.; Supaphol, P.; Srikhirin, T. Electrically Conductive Ultrafine Fibers of PVA-PEDOT/PSS and PVA-AgNPs by Means of Electrospinning. Adv. Mater. Res. 2014, 1033–1034, 1024–1035. [Google Scholar] [CrossRef]


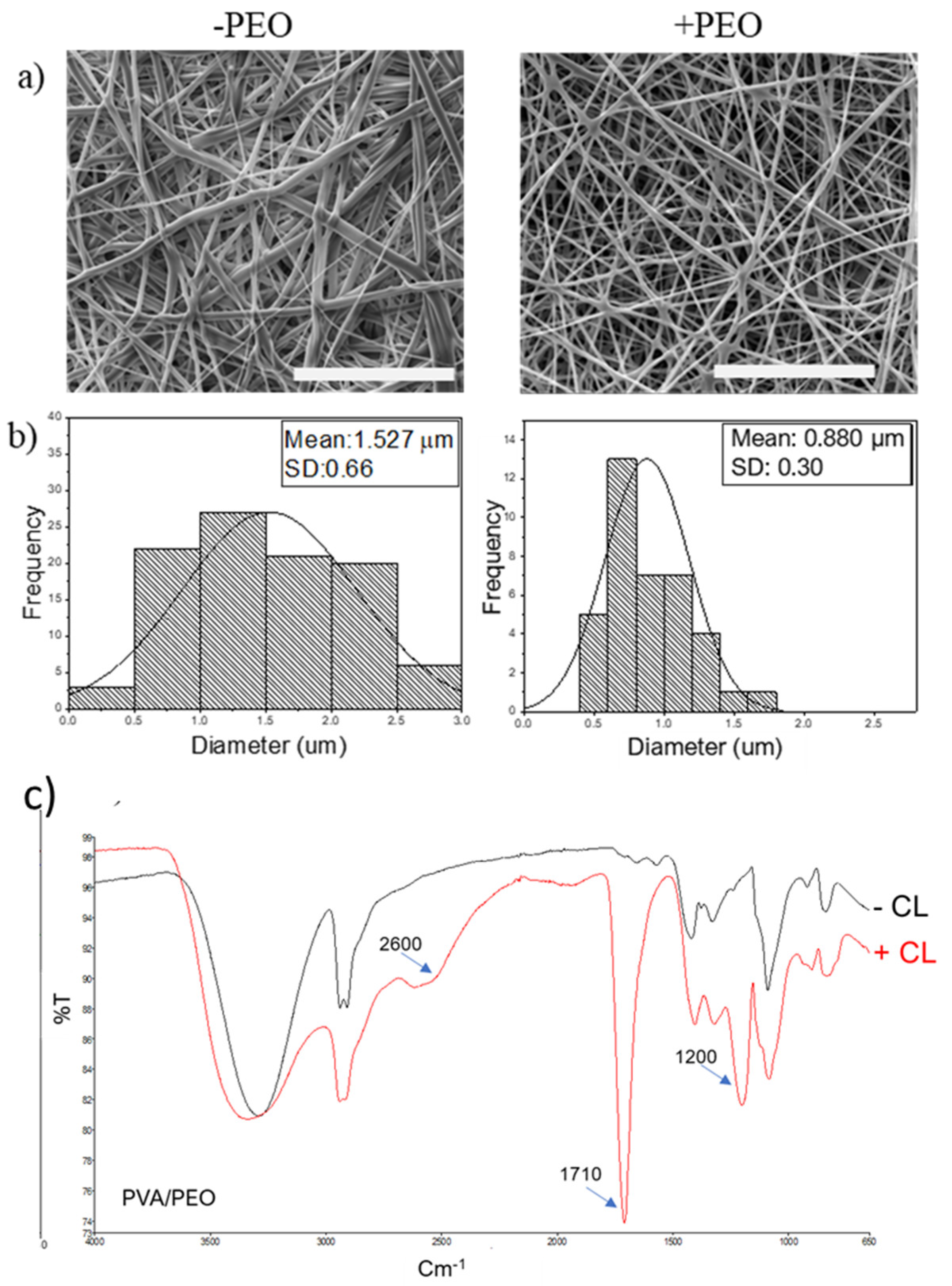
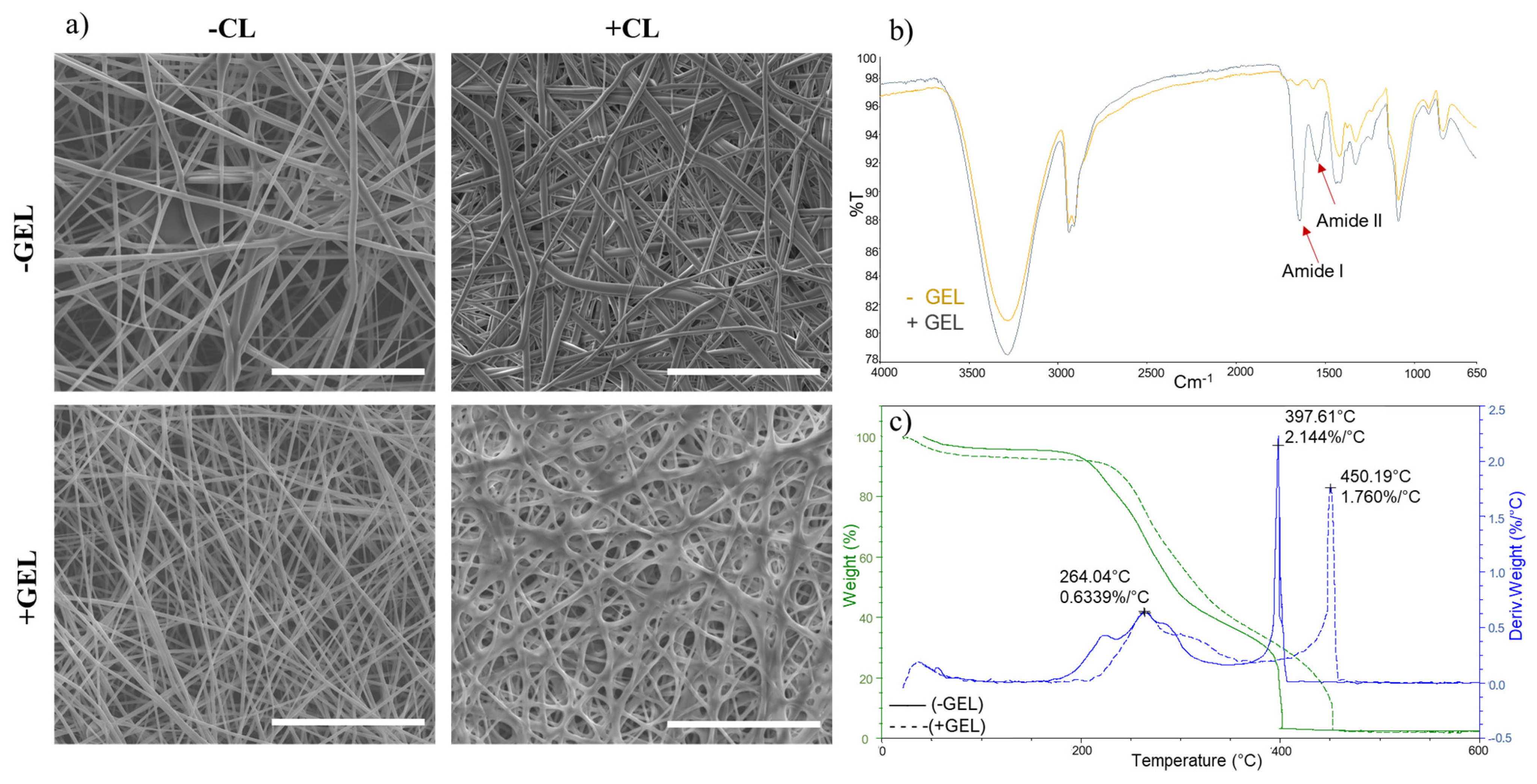

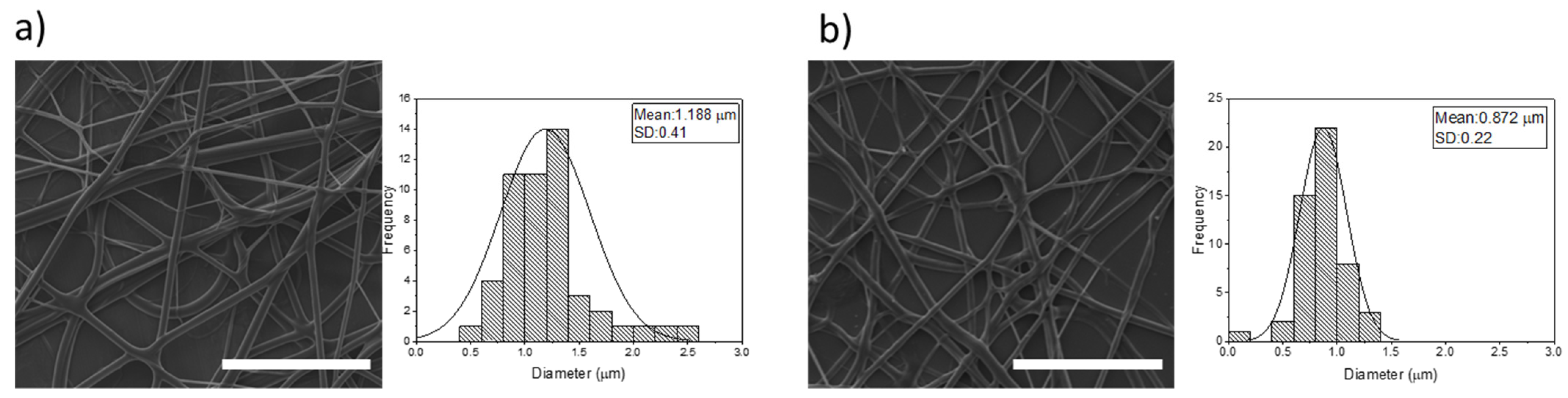
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renkler, Z.; Cruz Maya, I.; Guarino, V. Optimization of Polyvinyl Alcohol-Based Electrospun Fibers with Bioactive or Electroconductive Phases for Tissue-Engineered Scaffolds. Fibers 2023, 11, 85. https://doi.org/10.3390/fib11100085
Renkler Z, Cruz Maya I, Guarino V. Optimization of Polyvinyl Alcohol-Based Electrospun Fibers with Bioactive or Electroconductive Phases for Tissue-Engineered Scaffolds. Fibers. 2023; 11(10):85. https://doi.org/10.3390/fib11100085
Chicago/Turabian StyleRenkler, Zeynep, Iriczalli Cruz Maya, and Vincenzo Guarino. 2023. "Optimization of Polyvinyl Alcohol-Based Electrospun Fibers with Bioactive or Electroconductive Phases for Tissue-Engineered Scaffolds" Fibers 11, no. 10: 85. https://doi.org/10.3390/fib11100085
APA StyleRenkler, Z., Cruz Maya, I., & Guarino, V. (2023). Optimization of Polyvinyl Alcohol-Based Electrospun Fibers with Bioactive or Electroconductive Phases for Tissue-Engineered Scaffolds. Fibers, 11(10), 85. https://doi.org/10.3390/fib11100085






