Conversion of Animal-Derived Protein By-Products into a New Dual-Layer Nanofiber Biomaterial by Electrospinning Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Property | Mean Value ± Standard Deviation | |
|---|---|---|
| Collagen Hydrolysate (CH) | Keratin Hydrolysate (KH) | |
| Dry matter (%) | 60.40 ± 0.42 | 9.00 ± 0.05 |
| Ash (%) | 6.24 ± 0.27 | 13.68 ± 0.25 |
| Total nitrogen (%) | 14.67 ± 0.66 | 14.20 ± 0.57 |
| Protein (%) | 82.43 ± 2.66 | 80.65 ± 1.40 |
| pH (pH units) | 8.54 ± 0.10 | 11.85 ± 0.09 |
| Aminic nitrogen (%) | 1.43 ± 0.06 | 1.34 ± 0.06 |
| Electrical conductivity (μs/cm) | 870 ± 0.1 | 13,700 ± 20 |
2.2. Extraction of KH and CH Derived from Animal By-Products
2.3. Preparation of Electrospinning Solutions
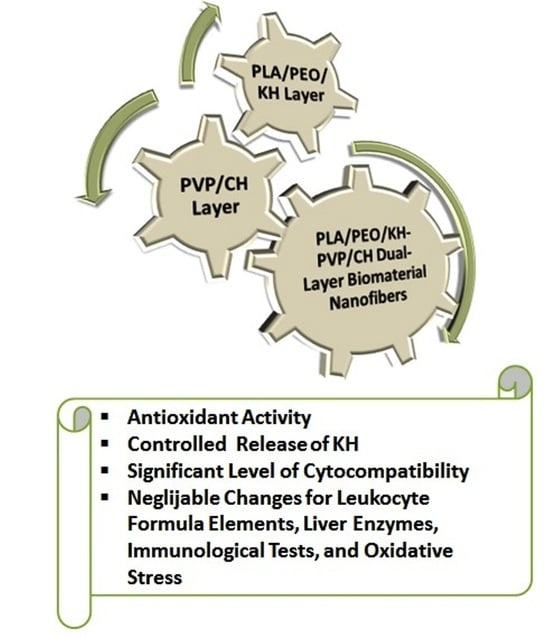
2.4. Fabrication of the Dual-Layer Nanofiber Biomaterial
2.5. Investigation Methods
2.5.1. Morphology Examination
2.5.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR FT-IR)
2.5.3. ABTS Free Radical Scavenging Assay
2.5.4. Controlled Release of KH
2.5.5. In Vitro Cytocompatibility Testing
2.5.6. In Vivo Biocompatibility Testing
2.5.7. Statistical Analysis
3. Results
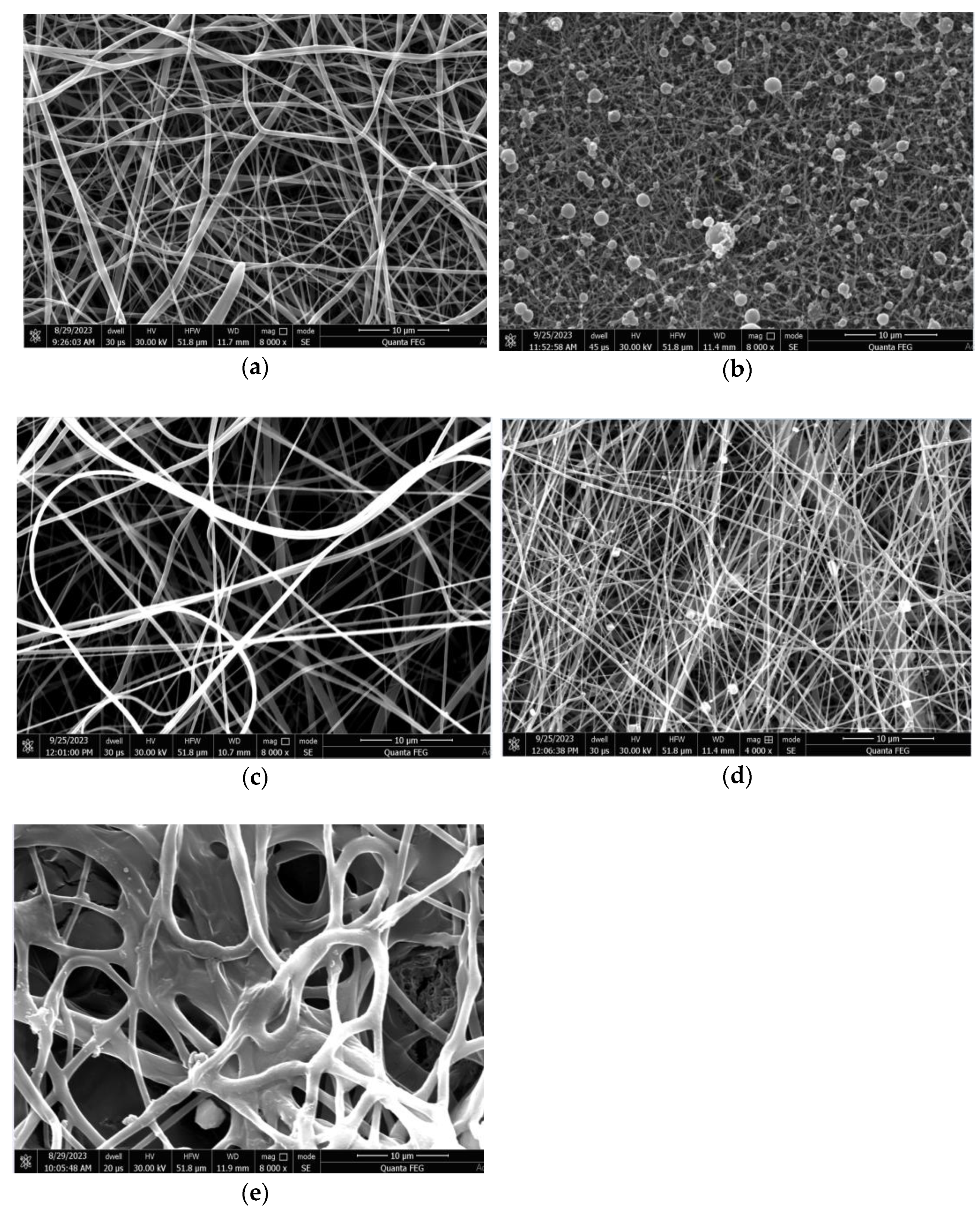
3.1. SEM Morphology
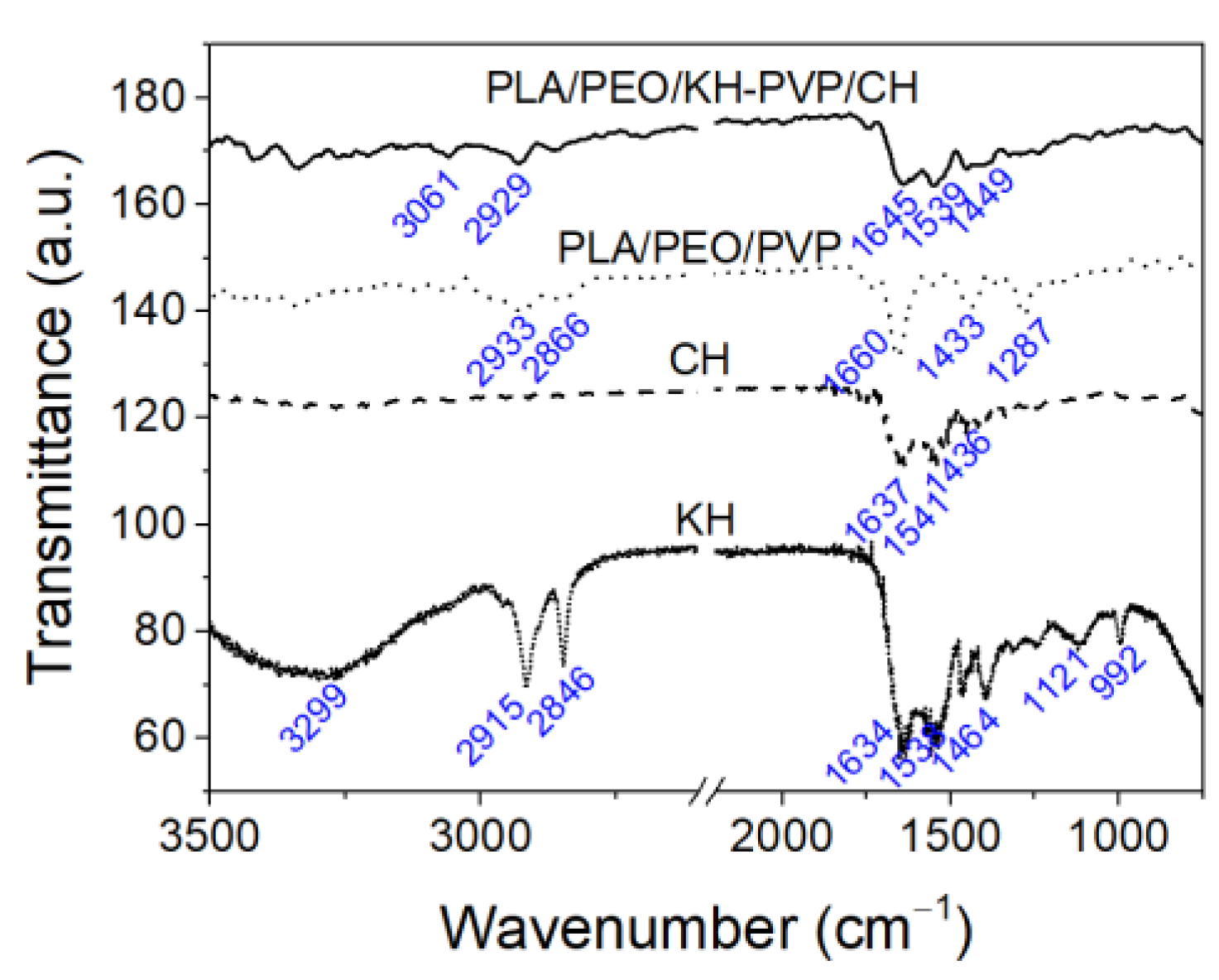
3.2. ATR-FT-IR Measurements
3.3. Antioxidant Activity
3.4. Controlled Release of Proteins
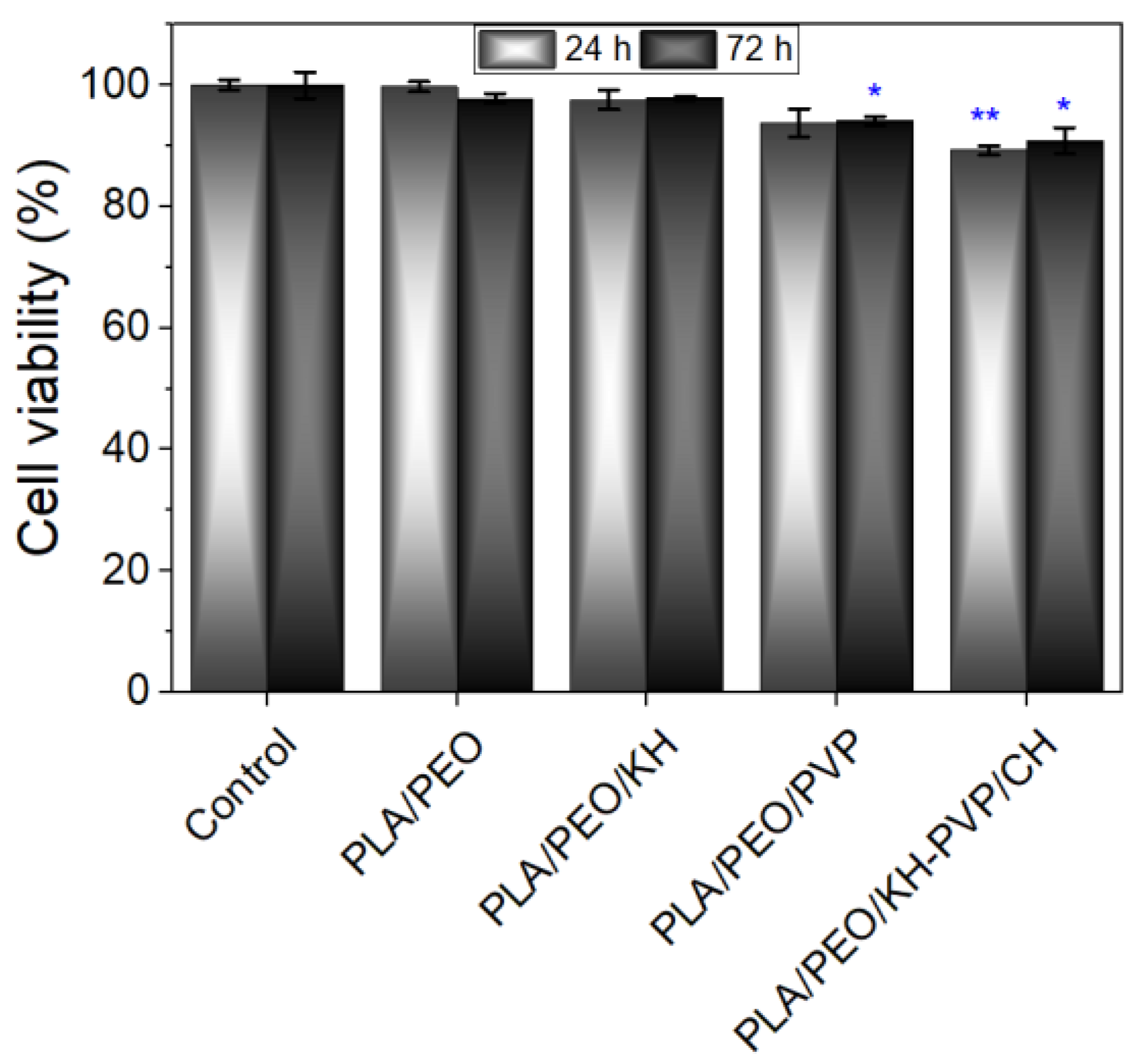
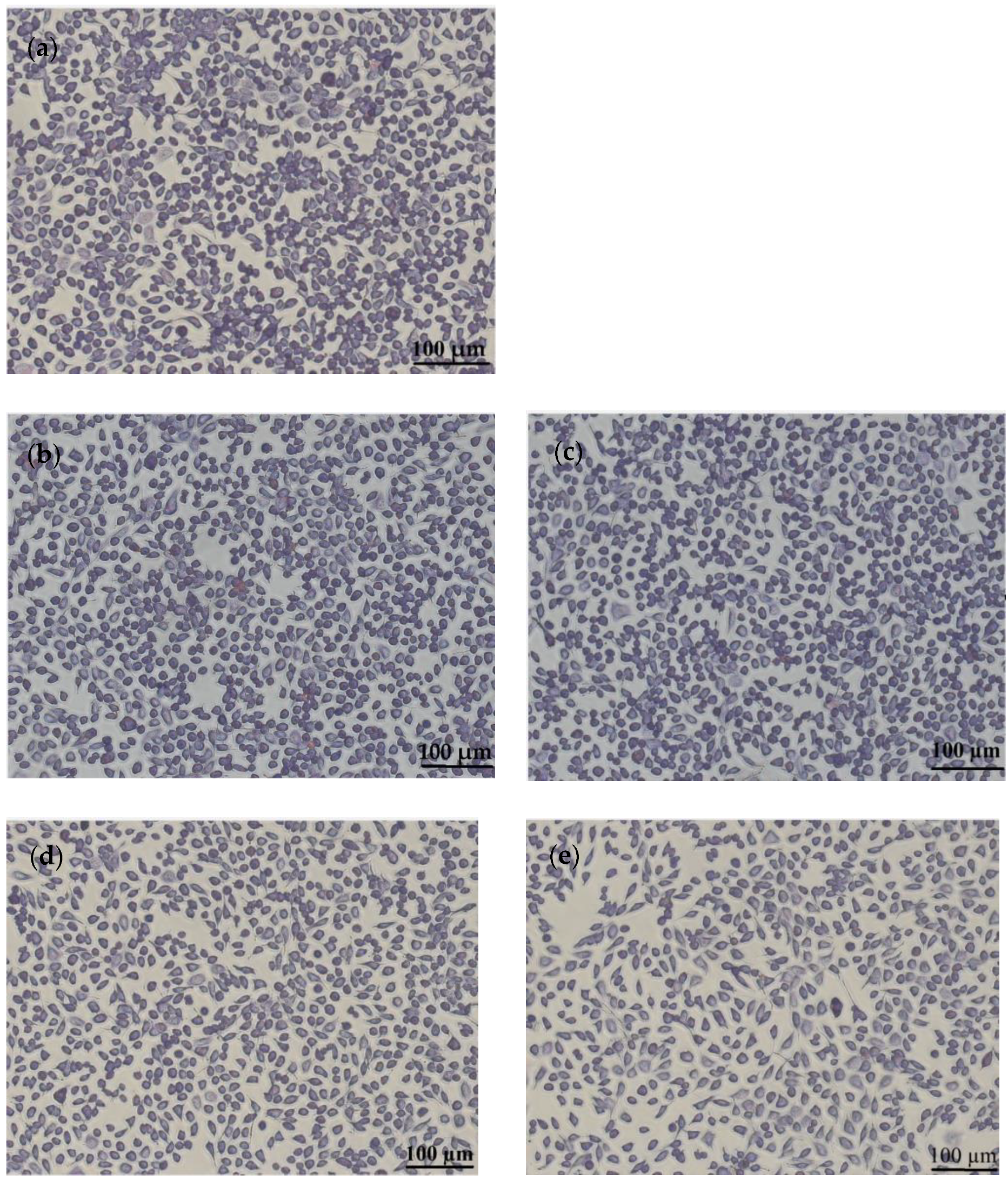
3.5. In Vitro Cytocompatibility
3.6. In Vivo Biocompatibility Tests
3.6.1. Leukocyte Formula Elements
3.6.2. Liver Enzymes
3.6.3. Immunological Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crops and Livestock Products. Available online: http://www.fao.org/faostat/en/#data/QA (accessed on 13 August 2023).
- Mokrejs, P.; Langmaier, F.; Mladek, M.; Janacova, D.; Kolomaznik, K.; Vasek, V. Extraction of collagen and gelatine from meat industry by-products for food and non food uses. Waste Manag. Res. 2009, 27, 31–37. [Google Scholar] [CrossRef]
- Singaravelu, S.; Ramanathan, G.; Sivagnanam, U.T. Dual-layered 3D nanofibrous matrix incorporated with dual drugs and their synergetic effect on accelerating wound healing through growth factor regulation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 76, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Corchero, J.L.; Favaro, M.T.P.; Marquez-Martinez, M.; Lascorz, J.; Martinez-Torro, C.; Sanchez, J.M.; Lopez-Laguna, H.; de Souza Ferreira, L.C.; Vazquez, E.; Ferrer-Miralles, N.; et al. Recombinant Proteins for Assembling as Nano- and Micro-Scale Materials for Drug Delivery: A Host Comparative Overview. Pharmaceutics 2023, 15, 1197. [Google Scholar] [CrossRef]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 1369. [Google Scholar] [CrossRef]
- Willoughby, C.E.; Batterbury, M.; Kaye, S.B. Collagen corneal shields. Surv. Ophthalmol. 2002, 47, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Shokrani, H.; Shokrani, A.; Sajadi, S.M.; Seidi, F.; Mashhadzadeh, A.H.; Rabiee, N.; Saeb, M.R.; Aminabhavi, T.; Webster, T.J. Cell-Seeded Biomaterial Scaffolds: The Urgent Need for Unanswered Accelerated Angiogenesis. Int. J. Nanomed. 2022, 17, 1035–1068. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Perez-Aguilar, H.; Lacruz-Asaro, M.; Aran-Ais, F. Towards a circular bioeconomy: High added value protein recovery and recycling from animal processing by-products. Sustain. Chem. Pharm. 2022, 28, 100667. [Google Scholar] [CrossRef]
- Broda, J.; Gawlowski, A.; Rom, M.; Kobiela-Mendrek, K. Utilisation of waste wool from mountain sheep as fertiliser in winter wheat cultivation. J. Nat. Fibers 2023, 20, 1–14. [Google Scholar] [CrossRef]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Mahoonak, A.R.S.; Rafipour, F. Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Shahzad, K.; Kettl, K.-H.; Titz, M.; Koller, M.; Schnitzer, H.; Narodoslawsky, M. Comparison of ecological footprint for biobased PHA production from animal residues utilizing different energy resources. Clean Technol. Environ. Policy 2013, 15, 525–536. [Google Scholar] [CrossRef]
- Khodaei, D.; Alvarez, C.; Mullen, A.M. Biodegradable Packaging Materials from Animal Processing Co-Products and Wastes: An Overview. Polymers 2021, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Saad, V.; Gutschmann, B.; Grimm, T.; Widmer, T.; Neubauer, P.; Riedel, S.L. Low-quality animal by-product streams for the production of PHA-biopolymers: Fats, fat/protein-emulsions and materials with high ash content as low-cost feedstocks. Biotechnol. Lett. 2021, 43, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, D.; Madhan, B.; Shanmugam, G.; Palanivel, S.; Narayan, B. Extraction of collagen from raw trimming wastes of tannery: A waste to wealth approach. J. Clean. Prod. 2016, 113, 338–344. [Google Scholar] [CrossRef]
- Tarafdar, A.; Gaur, V.K.; Rawat, N.; Wankhade, P.R.; Gaur, G.K.; Awasthi, M.K.; Sagar, N.A.; Sirohi, R. Advances in biomaterial production from animal derived waste. Bioengineered 2021, 12, 8247–8258. [Google Scholar] [CrossRef]
- Budiatin, A.S.; Gani, M.A.; Nilamsari, W.P.; Ardianto, C.; Khotib, J. The Characterization of Bovine Bone-Derived Hydroxyapatite Isolated Using Novel Non-Hazardous Method. J. Biomim. Biomater. Biomed. Eng. 2020, 45, 49–56. [Google Scholar] [CrossRef]
- Parajuli, K.; Malla, K.P.; Panchen, N.; Ganga, G.C.; Adhikari, R. Isolation of antibacterial nano-hydroxyapatite biomaterial from waste buffalo bone and its characterization. Chem. Chem. Technol. 2022, 16, 133–141. [Google Scholar] [CrossRef]
- Ramesh, S.; Loo, Z.Z.; Tan, C.Y.; Chew, W.J.K.; Ching, Y.C.; Tarlochan, F.; Chandran, H.; Krishnasamy, S.; Bang, L.T.; Sarhan, A.A.D. Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications. Ceram. Int. 2018, 44, 10525–10530. [Google Scholar] [CrossRef]
- Dragusin, D.M.; Curti, F.; Cecoltan, S.; Sarghiuta, D.; Butac, L.M.; Vasile, E.; Marinescu, R.; Stancu, I.C. Biocomposites Based on Biogenous Mineral for Inducing Biomimetic Mineralization. Mater. Plast. 2017, 54, 207–213. [Google Scholar] [CrossRef]
- Horbert, V.; Xin, L.; Foehr, P.; Brinkmann, O.; Bungartz, M.; Burgkart, R.H.; Graeve, T.; Kinne, R.W. In Vitro Analysis of Cartilage Regeneration Using a Collagen Type I Hydrogel (CaReS) in the Bovine Cartilage Punch Model. Cartilage 2019, 10, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Râpă, M.; Gaidău, C.; Stefan, L.M.; Matei, E.; Niculescu, M.; Berechet, M.D.; Stanca, M.; Tablet, C.; Tudorache, M.; Gavrilă, R.; et al. New nanofibers based on protein by-products with bioactive potential for tissue engineering. Materials 2020, 13, 3149. [Google Scholar] [CrossRef]
- Matei, E.; Gaidau, C.; Rapa, M.; Constantinescu, R.; Savin, S.; Berechet, M.D.; Predescu, A.M.; Berbecaru, A.C.; Coman, G.; Predescu, C. Sustainable Rabbit Skin Glue to Produce Bioactive Nanofibers for Nonactive Wound Dressings. Materials 2020, 13, 5388. [Google Scholar] [CrossRef]
- Giteru, S.G.G.; Ramsey, D.H.H.; Hou, Y.; Cong, L.; Mohan, A.; El-Din Ahmed Bekhit, A. Wool keratin as a novel alternative protein: A comprehensive review of extraction, purification, nutrition, safety, and food applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 643–687. [Google Scholar] [CrossRef]
- Athwal, S.; Sharma, S.; Gupta, S.; Nadda, A.K.; Gupta, A.; Husain, M.S.B. Sustainable Biodegradation and Extraction of Keratin with Its Applications. In Handbook of Biopolymers; Thomas, S., AR, A., Jose Chirayil, C., Thomas, B., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2022; p. 25302740. [Google Scholar]
- Perta-Crisan, S.; Ursachi, C.S.; Gavrilas, S.; Oancea, F.; Munteanu, F.-D. Closing the Loop with Keratin-Rich Fibrous Materials. Polymers 2021, 13, 1896. [Google Scholar] [CrossRef]
- Majeed, Z.; Farhat, H.; Ahmad, B.; Iqbal, A.; Faiz, A.U.H.; Mahnashi, M.H.; Alqarni, A.O.; Alqahtani, O.; Ali, A.A.; Momenah, A.M. Process optimization, antioxidant, antibacterial, and drug adjuvant properties of bioactive keratin microparticles derived from porcupine (Hystrix indica) quills. PeerJ 2023, 11, e15653. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R.; Rameli, M.A.P.; Al Jeffri, A.; Ismail, W.I.W. Bovine Based Collagen Dressings in Wound Care Management. J. Pharm. Res. Int. 2020, 32, 48–63. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Chen, S.; Wu, S. Designing an Innovative Electrospinning Strategy to Generate PHBV Nanofiber Scaffolds with a Radially Oriented Fibrous Pattern. Nanomaterials 2023, 13, 1150. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Gomaa, S.F.; Madkour, T.M.; Moghannem, S.; El-Sherbiny, I.M. New polylactic acid/ cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int. J. Biol. Macromol. 2017, 105, 1148–1160. [Google Scholar] [CrossRef]
- Yang, Q.B.; Li, Z.Y.; Hong, Y.L.; Zhao, Y.Y.; Qiu, S.L.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J. Polym. Sci. Part B-Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef]
- Gaudreault, R.; Whitehead, M.A.; van de Ven, T.G.M. Molecular orbital studies of gas-phase interactions between complex molecules. J. Phys. Chem. A 2006, 110, 3692–3702. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, K.; Siddaiah, T.; Gopal, N.O.; Kumar, Y.M.; Ramu, C. Optical and electrical conductivity studies of VO2+ doped polyvinyl pyrrolidone (PVP) polymer electrolytes. J. Sci.-Adv. Mater. Devices 2019, 4, 230–236. [Google Scholar] [CrossRef]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef]
- Goh, P.S.; Othman, M.H.D.; Matsuura, T. Waste Reutilization in Polymeric Membrane Fabrication: A New Direction in Membranes for Separation. Membranes 2021, 11, 782. [Google Scholar] [CrossRef]
- Gaidau, C.; Niculescu, M.; Stepan, E.; Taloi, D.; Filipescu, L. Additives and Advanced Biomaterials Obtained from Leather Industry by-products. Rev. Chim. 2009, 60, 501–507. [Google Scholar]
- Gaidau, C.; Stanca, M.; Niculescu, M.D.; Alexe, C.A.; Becheritu, M.; Horoias, R.; Cioineag, C.; Rapa, M.; Stanculescu, I.R. Wool Keratin Hydrolysates for Bioactive Additives Preparation. Materials 2021, 14, 4696. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Darie-Nita, R.N.; Rapa, M.; Frackowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Karacelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydogan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Rashedi, S.M.; Khajavi, R.; Rashidi, A.; Rahimi, M.K.; Bahador, A. Nanocomposite-Coated Sterile Cotton Gas Based on Polylactic Acid and Nanoparticles (Zinc Oxide and Copper Oxide) and Tranexamic Acid Drug with the Aim of Wound Dressing. Regen. Eng. Transl. Med. 2021, 7, 200–217. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Barbosa, W.S.; Rangel, W.S.P.; Moura, I.; Matos, A.P.D.; Melgaco, F.G.; Dias, M.L.; Ricci, E.; da Silva, L.C.P.; de Abreu, L.C.L.; et al. Polymeric membrane based on polyactic acid and babassu oil for wound healing. Mater. Today Commun. 2021, 26, 102173. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Cheng, Z.; Yang, G.; Zhao, C.; Gao, F.; Zhang, Z.; Qian, Y. Sandwich structure Aloin-PVP/Aloin-PVP-PLA/PLA as a wound dressing to accelerate wound healing. Rsc Adv. 2022, 12, 27300–27308. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, N.M.; Moore, D.M.; Zimmerman, K.; Smith, S.A. Hematologic Assessment in Pet Rats, Mice, Hamsters, and Gerbils Blood Sample Collection and Blood Cell Identification. Clin. Lab. Med. 2015, 35, 629–640. [Google Scholar] [CrossRef]
- Wolf, M.F.; Anderson, J.M. Practical approach to blood compatibility assessments: General considerations and standards. Biocompat. Perform. Med. Devices 2012, 50, 159–200. [Google Scholar]
- Zou, W.S.; Yang, Y.Q.; Gu, Y.; Zhu, P.F.; Zhang, M.J.; Cheng, Z.; Liu, X.Y.; Yu, Y.J.; Peng, X.H. Repeated Blood Collection from Tail Vein of Non-Anesthetized Rats with a Vacuum Blood Collection System. Jove-J. Vis. Exp. 2017, 10, 5852. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood Sample Collection in Small Laboratory Animals (vol 1, pg 87, 2010). J. Pharmacol. Pharmacother. 2017, 8, 153. [Google Scholar] [CrossRef]
- Tranquilli, W.J.; Thurmon, J.C.; Grimm, K.A.; Lumb, W.V. Lumb & Jones’ Veterinary Anesthesia and Analgesia; Ames, T.E., Ed.; Blackwell Pub.: Ames, IA, USA, 2007; Available online: https://books-library.net/files/books-library.online_noob5781bfd0cd351b04a09bb-58889 (accessed on 26 August 2023).
- Toft, M.F.; Petersen, M.H.; Dragsted, N.; Hansen, A.K. The impact of different blood sampling methods on laboratory rats under different types of anaesthesia. Lab. Anim. 2006, 40, 261–274. [Google Scholar] [CrossRef]
- Tartau, L.; Cazacu, A.; Melnig, V. Ketoprofen-liposomes formulation for clinical therapy. J. Mater. Sci.-Mater. Med. 2012, 23, 2499–2507. [Google Scholar] [CrossRef]
- Directive 2010/63 / EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Used Animals for Scientific Purposes. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 (accessed on 20 September 2021).
- AVMA Guidelines on Euthanasia (Formerly the Report of the AVMA Panel on Euthanasia). 2007. Available online: https://olaw.nih.gov/sites/default/files/Euthanasia2007.pdf (accessed on 20 September 2023).
- Ranjbar-Mohammadi, M.; Nouri, M. Production and in vitro analysis of catechin incorporated electrospun gelatin/poly (lactic acid) microfibers for wound dressing applications. J. Ind. Text. 2022, 51, 7529S–7544S. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.J.; Wang, Y.X.; Wang, Q. Second-Order Derivation Fourier Transform Infrared Spectral Analysis of Regenerated Wool Keratin Structural Changes. Aatcc J. Res. 2022, 9, 43–48. [Google Scholar] [CrossRef]
- Berechet, M.D.; Niculescu, M.D.; Gaidau, C.; Ignat, M.; Epure, D.G. Alkaline-Enzymatic Hydrolysis of Wool Waste for Different Applications. Rev. Chim. 2018, 69, 1649–1654. [Google Scholar] [CrossRef]
- Leroy, M.; Labbe, J.-F.; Ouellet, M.; Jean, J.; Lefevre, T.; Laroche, G.; Auger, M.; Pouliot, R. A comparative study between human skin substitutes and normal human skin using Raman microspectroscopy. Acta Biomater. 2014, 10, 2703–2711. [Google Scholar] [CrossRef]
- Hajikhani, M.; Emam-Djomeh, Z.; Askari, G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021, 172, 143–153. [Google Scholar] [CrossRef]
- Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Rapa, M.; Stanca, M.; Ditu, L.M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618. [Google Scholar] [CrossRef]
- Roberto Medina-Medrano, J.; Alexandra Quinones-Munoz, T.; Arce-Ortiz, A.; Gabriel Torruco-Uco, J.; Hernandez-Martinez, R.; Alejandro Lizardi-Jimenez, M.; Varela-Santos, E. Antioxidant Activity of Collagen Extracts Obtained from the Skin and Gills of Oreochromis sp. J. Med. Food 2019, 22, 722–728. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepanska, B.; Polkowska, I.; Malek, M.; Kluczynski, J.; Pazdzior-Czapula, K.; Wekwejt, M.; Michno, A.; Ronowska, A.; Palubicka, A.; Nowicka, B.; et al. The characterization of collagen-based scaffolds modified with phenolic acids for tissue engineering application. Sci. Rep. 2023, 13, 9966. [Google Scholar] [CrossRef]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004, 25, 3259–3266. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Rapa, M.; Stefan, L.M.; Preda, P.; Darie-Nita, R.N.; Gaspar-Pintiliescu, A.; Seciu, A.M.; Vasile, C.; Matei, E.; Predescu, A.M. Effect of hydrolyzed collagen on thermal, mechanical and biological properties of poly(lactic acid) bionanocomposites. Iran. Polym. J. 2019, 28, 271–282. [Google Scholar] [CrossRef]
- Râpă, M.; Zaharescu, T.; Stefan, L.M.; Gaidău, C.; Stănculescu, I.; Constantinescu, R.R.; Stanca, M. Bioactivity and Thermal Stability of Collagen–Chitosan Containing Lemongrass Essential Oil for Potential Medical Applications. Polymers 2022, 14, 3884. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.S.O.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef]


| Composition | Electrospinning | Flow Rate (mL/h) | Voltage (kV) | Distance between Needle and Collector (cm) | Collection Time (min) |
|---|---|---|---|---|---|
| PLA/PEO | Monoaxial | 2.8 | 22.79 | 13 | 180 |
| PLA/PEO/KH | 1st layer, monoaxial | 5.7 | 22.96 | 13 | 180 |
| PLA/PEO/PVP | Monoaxial | 4.0 | 19.29 | 13 | 180 |
| PVP/CH | 2nd layer, coaxial | 3.5; 2.5 | 21.10 | 13 | 180 |
| PLA/PEO/KH-PVP-CH | Assembled structure | 90-90 |
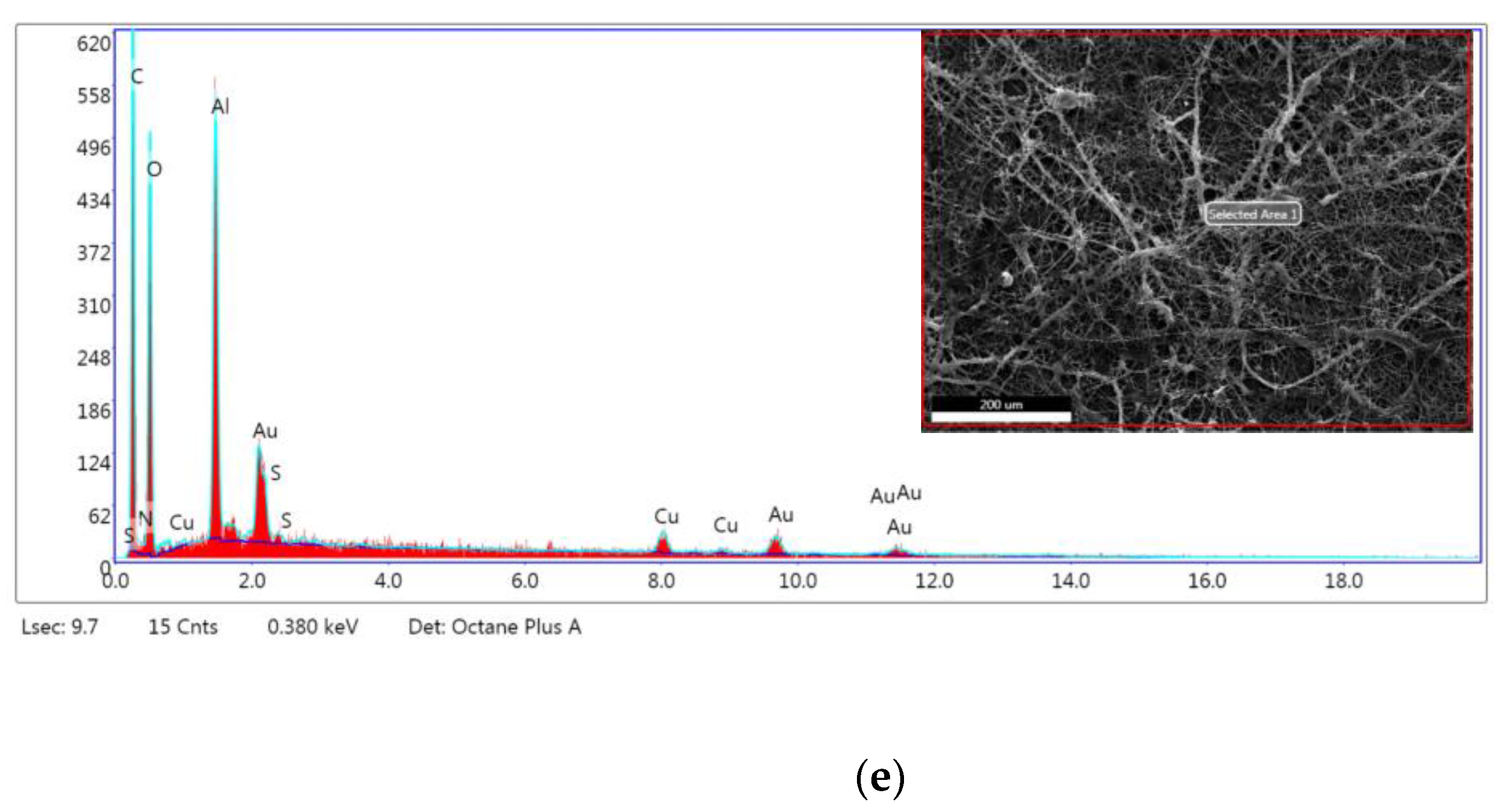
| Element | PLA/PEO | PLA/PEO/KH | PLA/PEO/PVP | PVP/CH | PLA/PEO/KH-PVP/CH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | |
| Carbon (C) | 65.81 | 76.55 | 49.54 | 67.18 | 58.51 | 70.83 | 37.77 | 55.81 | 49.46 | 60.41 |
| Nitrogen (N) | 9.63 | 9.60 | 0.38 | 0.44 | 11.19 | 11.61 | 5.94 | 7.53 | 0.71 | 0.74 |
| Oxygen (O) | 10.37 | 9.05 | 10.33 | 10.52 | 11.21 | 10.19 | 4.20 | 4.66 | 37.17 | 34.08 |
| Natrium (Na) | 1.69 | 1.31 | ||||||||
| Aluminum (Al) | 8.17 | 4.23 | 35.28 | 21.30 | 12.33 | 6.64 | 44.34 | 29.17 | 7.72 | 4.20 |
| Chloride (Cl) | 1.61 | 0.81 | 3.76 | 0.28 | ||||||
| Sulfur (S) | 0.04 | 0.02 | 0.04 | 0.02 | ||||||
| Iron (Fe) | 0.30 | 0.08 | 0.50 | 0.16 | ||||||
| Copper (Cu) | 0.92 | 0.20 | 1.01 | 0.26 | 1.11 | 0.25 | 1.03 | 0.29 | 1.14 | 0.26 |
| Gold (Au) | 5.10 | 0.36 | 3.42 | 0.28 | 5.36 | 0.40 | 2.92 | 0.26 | 3.76 | 0.28 |
| Duration | Leukocyte Differential Count (%) | |||||
|---|---|---|---|---|---|---|
| PMN | Ly | E | M | B | ||
| Witness | 24 h | 29.4 ± 7.7 | 63.9 ± 18.3 | 0.1 ± 0.01 | 6.1 ± 1.1 | 0.2 ± 0.05 |
| 7 d | 28.6 ± 8.9 | 64.8 ± 19.5 | 0.2 ± 0.05 | 6.2 ± 1.1 | 0.2 ± 0.05 | |
| PLA/PEO | 24 h | 28.5 ± 8.5 | 65.0 ± 17.5 | 0.2 ± 0.05 | 6.1 ± 1.3 | 0.2 ± 0.05 |
| 7 d | 28.7 ± 9.1 | 64.6 ± 18.7 | 0.2 ± 0.01 | 6.3 ± 1.3 | 0.2 ± 0.1 | |
| PLA/PEO/KH | 24 h | 29.2 ± 8.3 | 64.1 ± 18.9 | 0.1 ± 0.01 | 6.4 ± 0.5 | 0.2 ± 0.1 |
| 7 d | 28.9 ± 9.3 | 64.5 ± 18.3 | 0.1 ± 0.01 | 6.3 ± 1.1 | 0.2 ± 0.05 | |
| PLA/PEO/PVP | 24 h | 29.3 ± 7.9 | 64.3 ± 17.9 | 0.1 ± 0.05 | 6.1 ± 1.1 | 0.2 ± 0.1 |
| 7 d | 29.1 ± 8.7 | 64.4 ± 19.1 | 0.1 ± 0.01 | 6.2 ± 1.3 | 0.2 ± 0.1 | |
| PVP/CH | 24 h | 28.6 ± 8.5 | 64.8 ± 18.5 | 0.2 ± 0.01 | 6.2 ± 1.1 | 0.2 ± 0.1 |
| 7 d | 27.8 ± 8.9 | 64.5 ± 18.7 | 0.1 ± 0.05 | 6.3 ± 1.3 | 0.2 ± 0.05 | |
| PLA/PEO/KH-PVP/CH | 24 h | 28.6 ± 8.7 | 65.6 ± 18.9 | 0.1 ± 0.01 | 6.3 ± 1.1 | 0.2 ± 0.05 |
| 7 d | 28.8 ± 9.1 | 64.4 ± 17.7 | 0.2 ± 0.05 | 6.4 ± 0.5 | 0.2 ± 0.1 | |
| Duration | TGP (U/mL) | TGO (U/mL) | LDH (U/L) | |
|---|---|---|---|---|
| Witness | 24 h | 39.6 ± 10.3 | 158.6 ± 31.5 | 328.28 ± 64.33 |
| 7 d | 40.2 ± 10.5 | 160.4 ± 30.7 | 333.56 ± 70.67 | |
| PLA/PEO | 24 h | 40.5 ± 10.9 | 161.7 ± 32.9 | 332.34 ± 71.13 |
| 7 d | 40.7 ± 10.7 | 163.2 ± 33.7 | 335.83 ± 66.83 | |
| PLA/PEO/KH | 24 h | 39.4 ± 9.7 | 159.8 ± 35.3 | 330.45 ± 69.45 |
| 7 d | 40.3 ± 10.5 | 161.3 ± 32.7 | 334.32 ± 58.83 | |
| PLA/PEO/PVP | 24 h | 40.6 ± 11.3 | 158.5 ± 33.5 | 331.27 ± 72.13 |
| 7 d | 39.8 ± 9.9 | 162.7 ± 31.7 | 335.19 ± 71.33 | |
| PVP/CH | 24 h | 39.5 ± 10.7 | 160.6 ± 34.1 | 332.53 ± 67.67 |
| 7 d | 39.8 ± 10.3 | 163.8 ± 33.5 | 336.13 ± 69.27 | |
| PLA/PEO/KH-PVP/CH | 24 h | 39.7 ± 10.5 | 160.4 ± 35.3 | 330.67 ± 71.33 |
| 7 d | 40.5 ± 11.1 | 161.3 ± 30.9 | 333.21 ± 70.67 |
| Duration | Urea (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|
| Witness | 24 h | 27.7 ± 3.7 | 0.7 ± 0.01 |
| 7 d | 28.3 ± 4.3 | 0.7 ± 0.03 | |
| PLA/PEO | 24 h | 26.9 ± 4.7 | 0.8 ± 0.03 |
| 7 d | 28.5 ± 4.5 | 0.9 ± 0.05 | |
| PLA/PEO/KH | 24 h | 29.1 ± 5.3 | 0.8 ± 0.01 |
| 7 d | 29.5 ± 4.9 | 0.8 ± 0.01 | |
| PLA/PEO/PVP | 24 h | 29.1 ± 5.1 | 0.7 ± 0.01 |
| 7 d | 28.9 ± 3.7 | 0.9 ± 0.03 | |
| PVP/CH | 24 h | 29.3 ± 4.5 | 0.8 ± 0.03 |
| 7 d | 29.7 ± 4.9 | 0.8 ± 0.05 | |
| PLA/PEO/KH-PVP/CH | 24 h | 29.5 ± 4.7 | 0.9 ± 0.01 |
| 7 d | 28.7 ± 5.3 | 0.8 ± 0.03 |
| Duration | SOD (U/mg Protein) | GPX (µm/mg Protein) | |
|---|---|---|---|
| Witness | 24 h | 103.6 ± 16.5 | 13.1 ± 2.3 |
| 7 d | 105.2 ± 19.7 | 12.3 ± 1.7 | |
| PLA/PEO | 24 h | 103.5 ± 18.3 | 12.7 ± 1.3 |
| 7 d | 104.6 ± 18.5 | 13.3 ± 1.9 | |
| PLA/PEO/KH | 24 h | 104.7 ± 17.7 | 13.5 ± 2.1 |
| 7 d | 104.4 ± 19.3 | 12.7 ± 1.5 | |
| PLA/PEO/PVP | 24 h | 104.2 ± 19.1 | 12.9 ± 1.7 |
| 7 d | 105.8 ± 20.3 | 12.3 ± 1.1 | |
| PVP/CH | 24 h | 103.1 ± 18.7 | 13.1 ± 2.5 |
| 7 d | 105.5 ± 17.9 | 13.3 ± 1.7 | |
| PLA/PEO/KH-PVP/CH | 24 h | 104.8 ± 19.1 | 12.7 ± 1.5 |
| 7 d | 105.4 ± 19.5 | 13.5 ± 1.3 |
| Duration | OC (Colonies/mL) | PC (Colonies/mL) | BC (Colonies/mL) | |
|---|---|---|---|---|
| Witness | 7 d | 769.83 ± 65.27 | 529.37 ± 41.17 | 713.56 ± 62.21 |
| PLA/PEO | 7 d | 773.13 ± 61.43 | 531.41 ± 39.33 | 718.34 ± 65.37 |
| PLA/PEO/KH | 7 d | 785.45 ± 67.45 | 525.29 ± 38.25 | 717.55 ± 60.33 |
| PLA/PEO/PVP | 7 d | 794.33 ± 70.21 | 522.13 ± 40.21 | 714.21 ± 53.67 |
| PLA/PEO/KH-PVP/CH | 7 d | 788.29 ± 62.83 | 530.67 ± 33.83 | 721.82 ± 57.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaidău, C.; Râpă, M.; Stefan, L.M.; Matei, E.; Berbecaru, A.C.; Predescu, C.; Mititelu-Tartau, L. Conversion of Animal-Derived Protein By-Products into a New Dual-Layer Nanofiber Biomaterial by Electrospinning Process. Fibers 2023, 11, 87. https://doi.org/10.3390/fib11100087
Gaidău C, Râpă M, Stefan LM, Matei E, Berbecaru AC, Predescu C, Mititelu-Tartau L. Conversion of Animal-Derived Protein By-Products into a New Dual-Layer Nanofiber Biomaterial by Electrospinning Process. Fibers. 2023; 11(10):87. https://doi.org/10.3390/fib11100087
Chicago/Turabian StyleGaidău, Carmen, Maria Râpă, Laura Mihaela Stefan, Ecaterina Matei, Andrei Constantin Berbecaru, Cristian Predescu, and Liliana Mititelu-Tartau. 2023. "Conversion of Animal-Derived Protein By-Products into a New Dual-Layer Nanofiber Biomaterial by Electrospinning Process" Fibers 11, no. 10: 87. https://doi.org/10.3390/fib11100087
APA StyleGaidău, C., Râpă, M., Stefan, L. M., Matei, E., Berbecaru, A. C., Predescu, C., & Mititelu-Tartau, L. (2023). Conversion of Animal-Derived Protein By-Products into a New Dual-Layer Nanofiber Biomaterial by Electrospinning Process. Fibers, 11(10), 87. https://doi.org/10.3390/fib11100087