Abstract
Gas-liquid membrane contactors that were based on hollow fiber membranes are the example of highly effective hybrid separation processes in the field of membrane technology. Membranes provide a fixed and well-determined interface for gas/liquid mass transfer without dispensing one phase into another while their structure (hollow fiber) offers very large surface area per apparatus volume resulted in the compactness and modularity of separation equipment. In many cases, stated benefits are complemented with high separation selectivity typical for absorption technology. Since hollow fiber membrane contactors are agreed to be one of the most perspective methods for CO2 capture technologies, the major reviews are devoted to research activities within this field. This review is focused on the research works carried out so far on the applications of membrane contactors for other gas-liquid separation tasks, such as water deoxygenation/ozonation, air humidity control, ethylene/ethane separation, etc. A wide range of materials, membranes, and liquid solvents for membrane contactor processes are considered. Special attention is given to current studies on the capture of acid gases (H2S, SO2) from different mixtures. The examples of pilot-scale and semi-industrial implementation of membrane contactors are given.
1. Introduction
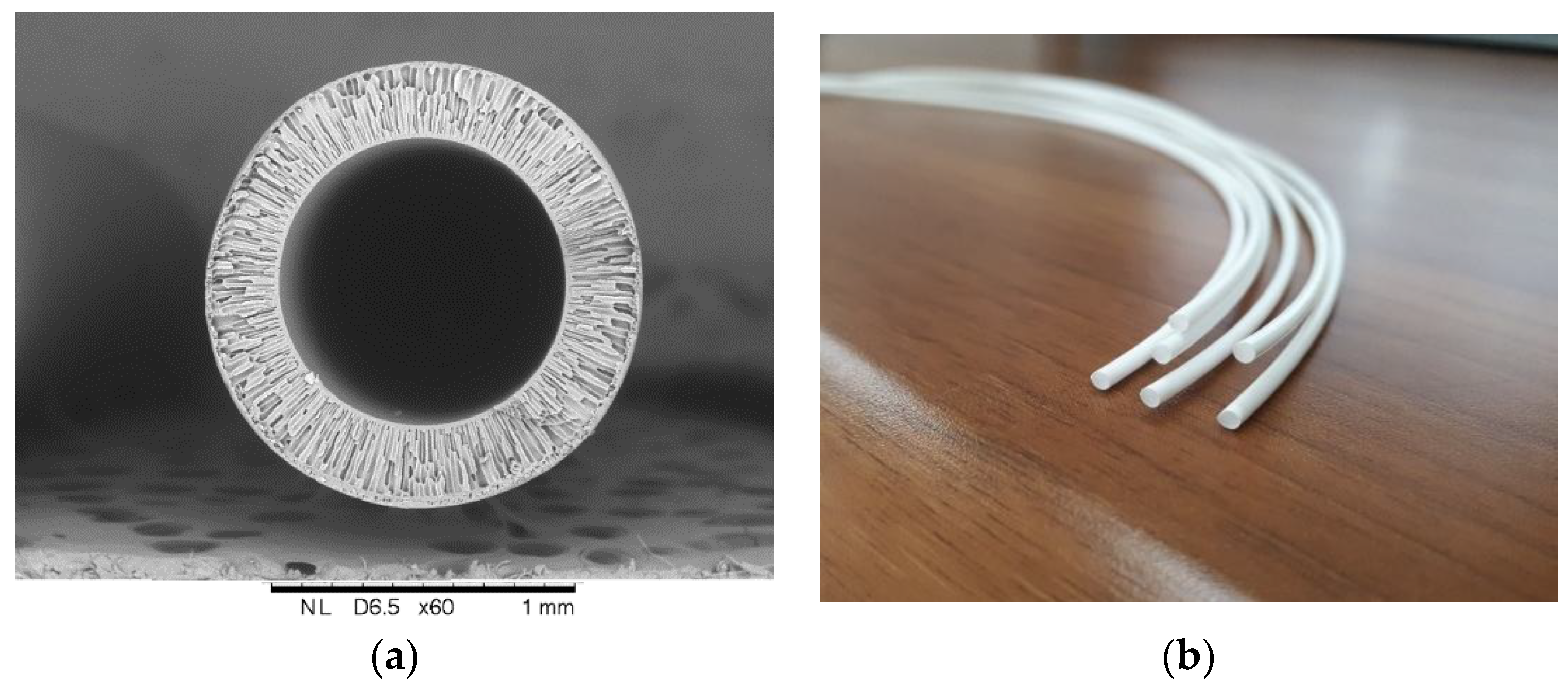
The proposal of Mahon and the group at Dow Chemical [1] to use hollow-fiber membranes as a separation device and their development represents one of the major events in membrane technology. Since then synthetic polymeric hollow-fiber membranes have advanced to play a key role in separation technologies. When compared to the flat-sheet membrane, hollow-fiber configuration has the following advantages: (1) a much larger membrane area per unit volume of membrane module resulting in a higher overall productivity; (2) high self-mechanical support, and (3) good flexibility and easy handling during module fabrication, membrane reparation, and system operation [2,3]. The typical example of hollow fiber membrane made from polysulfone is given in Figure 1.
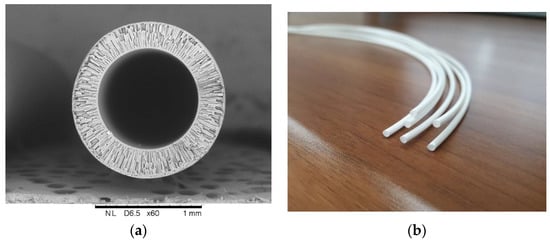
Figure 1.
Asymmetric mesoporous hollow fiber membrane from polysulfone for gas-liquid membrane contactor (developed in A.V. Topchiev institute of petrochemical synthesis): (a) Cross-sectional image; and, (b) General view.
The benefits listed above are efficiently implemented in hollow fiber gas-liquid membrane contactors. Basically, contactors are membrane modules for transport of component between gas and liquid phases. In this case, membranes play the role of interface preventing direct mixing and the dispersion of phases.
In the following review, we tried to summarize extensively studied key separation processes, which can benefit the most from the employment of hollow fiber membrane contactors. The review data are presented in a tabular form providing comprehensive information on the types of hollow fibers used, the principal parameters of gas-liquid membrane contactors, and the experimental conditions in membrane contactor processes. The authors hope that the detailed information on the properties of hollow fiber membranes (geometrical and pore sizes, porosity) and the membrane manufacturers will be of advantage for researchers dealing with general issues of fabrication and the employment of synthetic fibers.
2. Basic Principles, Advantages, and Disadvantages of Hollow Fiber Membrane Contactors
A membrane contactor is a device that is designed to implement a separation or chemical transformation process, employing a membrane as an interface between two phases. While the key function of a membrane is separation by means of selective mass transfer, the membrane contactors do not require membrane selectivity. Generally, a membrane in a gas-liquid contactor is used exclusively as an interface between the gas and liquid phases, providing their efficient contact without direct mixing due to, among other things, high surface area [4,5,6]. The selective properties of membrane contactor are provided by a difference between solubility of the components in the liquid phase used. Therefore, most gas-liquid membrane contactors employ porous membranes that provide high mass transfer properties. However, some tasks necessitate the employment of composite membranes or asymmetric membranes with a thin nonporous polymer layer, e.g., in the case of high-pressure processes [7].
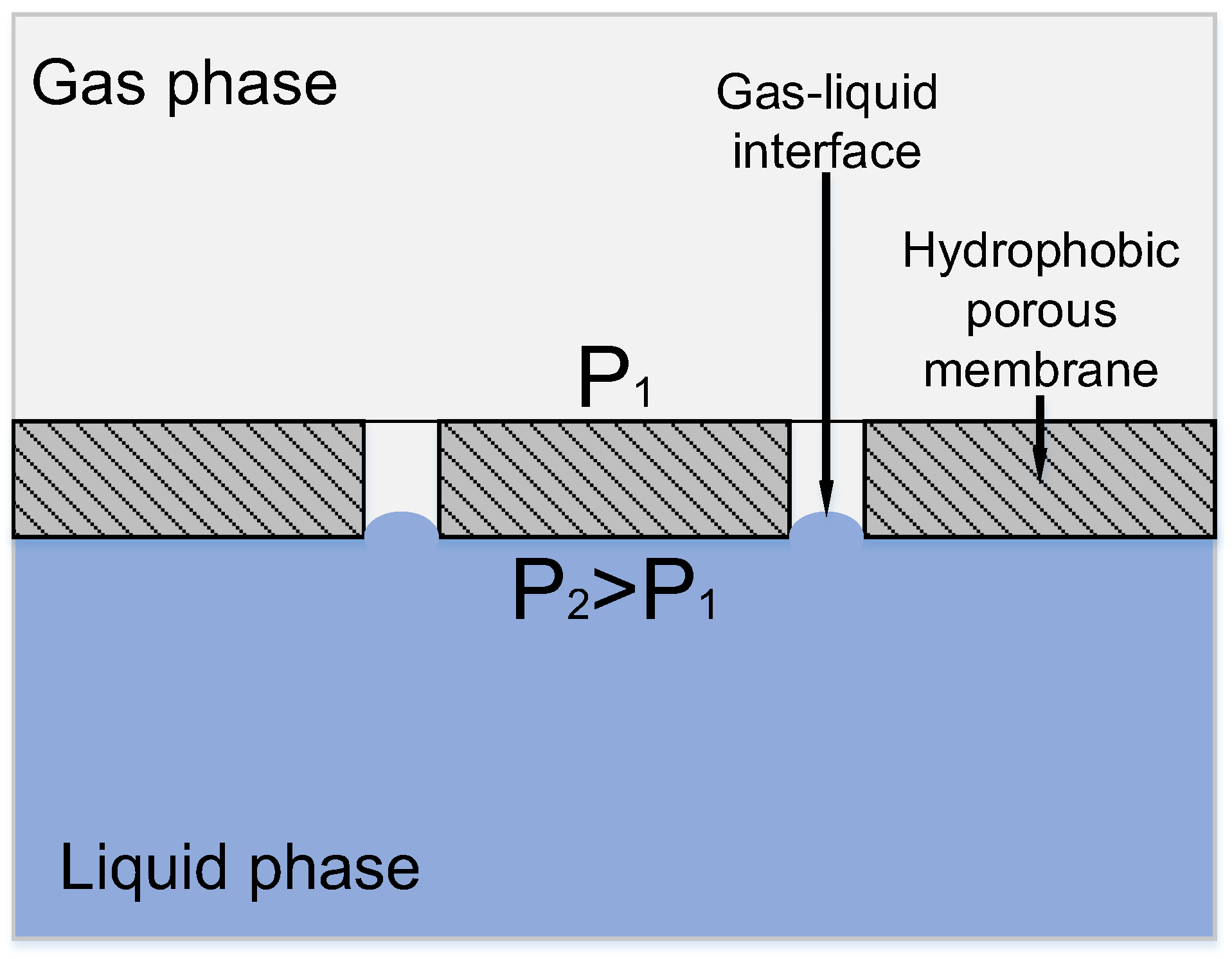
Ideally, a porous hydrophobic membrane excludes the penetration of liquid (particularly, aqueous) phase into the pores; the whole pore volume is filled with gas, and the mass transfer resistance from the membrane side is minimum. Karoor & Sirkar [8] were the first to present the benefits of non-wetted mode (when the membrane pores are filled with gas). Figure 2 shows the interface between the gas and liquid phases in a hydrophobic symmetrical porous membrane.
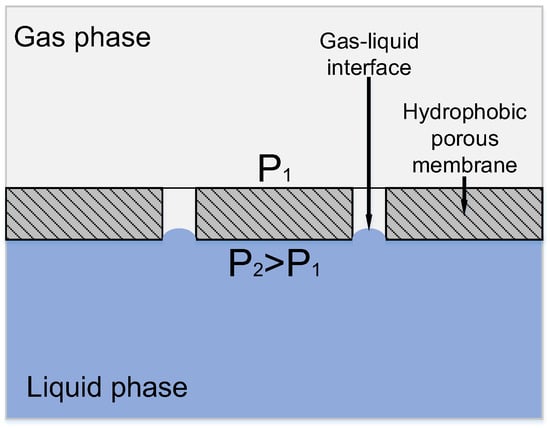
Figure 2.
Principle of mass transfer in a membrane contactor based on a symmetric hydrophobic porous membrane.
In this case, the contact area can be defined as the area of membrane pore mouths, and the operating pressure in the system has to be thoroughly controlled in order to avoid the intermixing of phases. Particularly, the aqueous phase pressure should be equal to or exceed that of the gas phase to completely exclude the possibility of gas bubbles dispersion in the liquid and, therefore, to avoid the undesirable phase mixing mode [9]. On the other hand, the interface area can be defined precisely only if the penetration of aqueous phase into membrane pores is completely excluded. The phenomenon of pore filling with liquid—‘pore wetting’—can derive from exceedingly high pressure from the liquid side, which, at worst, results in transmembrane flux appearance, and, consequently, a drastic decrease in overall gas transport properties. In the case when the liquid layer is immobilized in the pore space, its mass transfer resistance is too high, and the mass-transfer through the membrane becomes a limiting step of the process. As shown in [9,10,11,12], even partial pore filling with liquid leads to a sharp increase in the diffusional resistance in membrane pores, resulting in a drastic decline of membrane gas transport properties. According to Wang et al. [11], 5% wetting of membrane pores leads to a 20% decrease in the overall mass transfer coefficient. On the other hand, as shown in [13], even 2% wetting of membrane pores increases membrane resistance by up to 6%. In some cases, the membrane resistance contribution can reach 90% value [14].
In fact, the hydrophobicity of membrane material does not provide the non-wetted mode if the liquid phase pressure exceeds a critical level (also referred to as breakthrough pressure) [12,15,16]. A breakthrough pressure for each membrane depends on the surface tension of the liquid used as well as on its contact angle value at given operating conditions. The pressure can be quantitively estimated according to Laplace’s equation:
where ΔP—critical breakthrough transmembrane pressure, rp,max—maximum pore radius in the cylindrical approximation, σ—liquid surface tension, and θ—contact angle value.
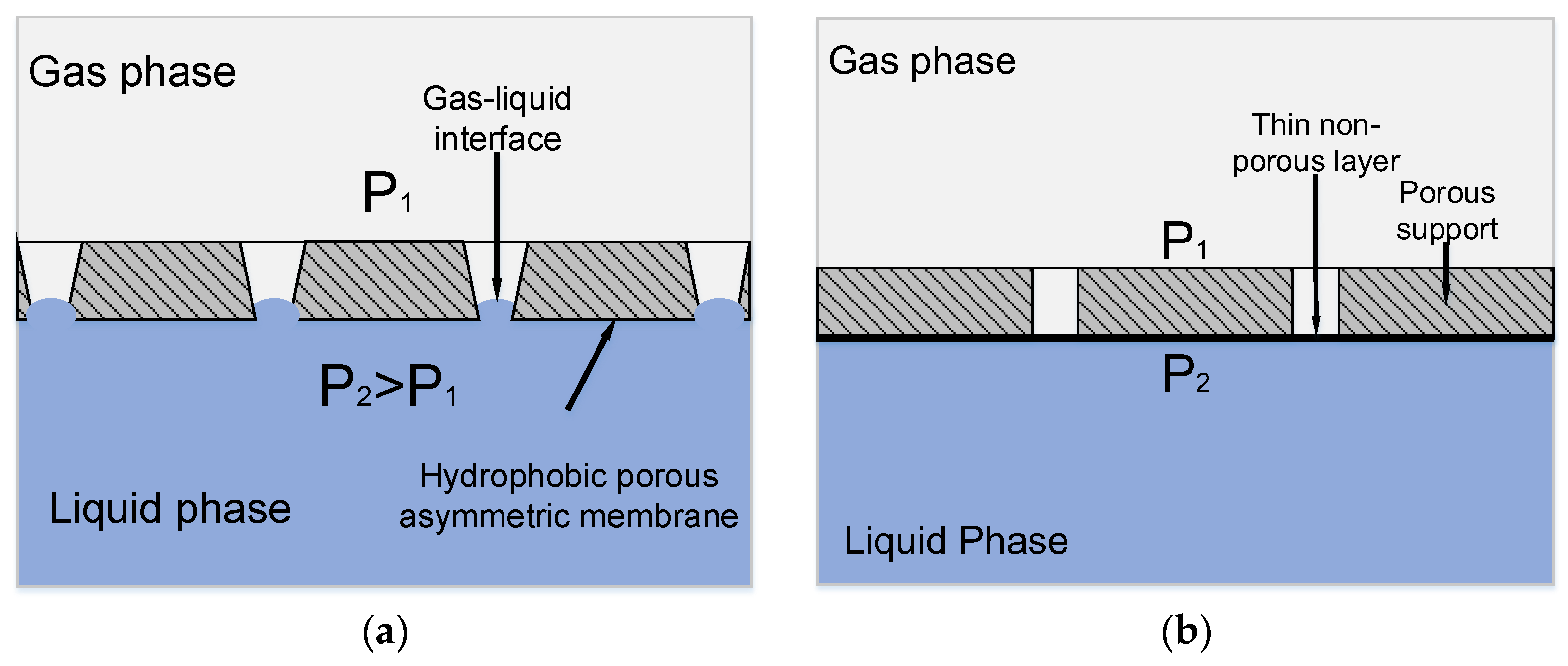
In the case of asymmetrical membranes, the pore size in which decreases gradually when passing from one membrane surface to another, two phases can be brought in contact without dispersion even when operating pressure exceeds the breakthrough pressure on the membrane side with larger pores. Since breakthrough pressure is inversely proportional to membrane pore size, partial wetting can take place only from the side of larger pores, whereas the smaller pores remain resistant to liquid penetration. In this case, the gas-liquid interface appears inside the pore space (see Figure 3a).
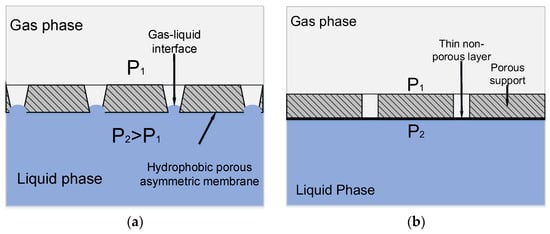
Figure 3.
Principle of mass transfer in a membrane contactor based on: (a) Asymmetric hydrophobic porous membrane; and, (b) Asymmetric composite membrane with a thin nonporous layer.
Another approach aimed to increase the operating pressure of the liquid phase in a membrane contactor is to employ composite membranes with a thin non-porous layer on the porous support. This layer prevents the penetration of the liquid phase into membrane pore space (see Figure 3b) [17,18,19]. A non-porous layer on the porous membrane surface provides a way to extend the range of operating pressures. However, such a layer should be highly permeable; otherwise, membrane resistance to the mass transfer process will increase.
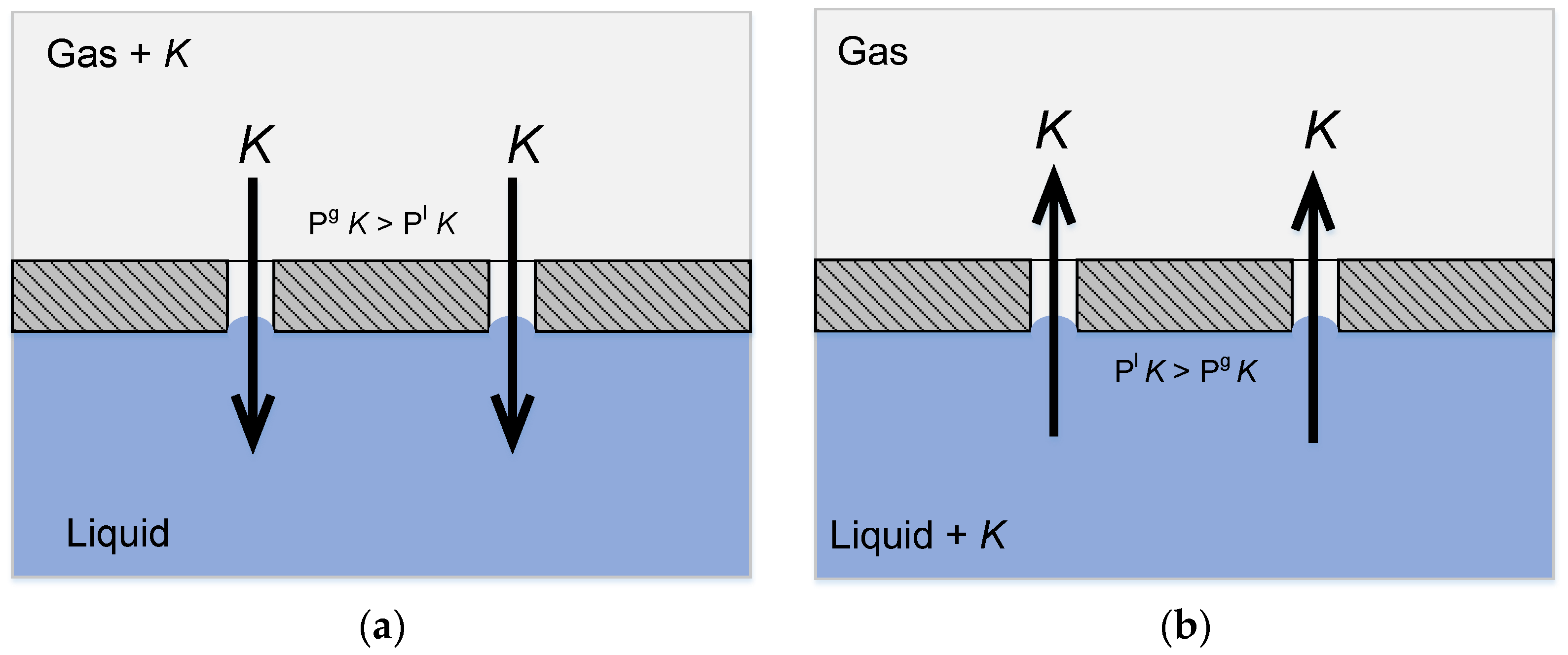
Gas-liquid membrane contactors may be employed for absorption of the desired component in a liquid absorbent (see Figure 4a), as well as for reverse process—desorption from the liquid phase (see Figure 4b). Furthermore, membrane contactors possess a number of essential properties due to the hollow fiber membrane configuration, thus being promising contact devices.
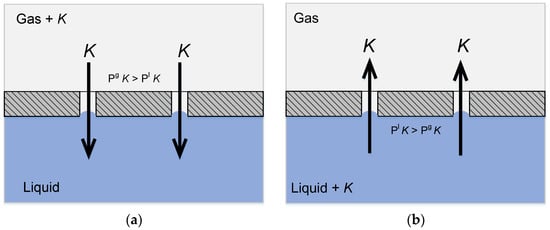
Figure 4.
Principle of mass transfer in a membrane contactor: (a) Absorption of a component from the gaseous phase; and, (b) Desorption of a component from the liquid phase.
As can be seen from Figure 4a,b, the ideal case of membrane contactor operation provides a way to implement both absorption and desorption process at the clearly defined gas-liquid membrane interface. This means that the area of contact and mass transfer remains constant, and all of the equipment operates with equal productivity, even when the process conditions or the liquid properties are changed.
As noted above, hollow fiber membranes provide large surface area per apparatus volume (up to 10,000 m2/m3), thus making the equipment compact (with small dimensions and weight) [4,5]. As shown in [20], the dimensions and weight of the membrane contactor can be decreased by 72% and 66%, respectively, as compared to conventional absorption columns. Furthermore, theoretical estimations indicate [21] that the dimensions of an absorber can be reduced to 10 times when replaced with a membrane contactor.
It is important to emphasize that a large phase contact area is the key factor providing high efficiency of hollow fiber membrane contactors compared to conventional techniques and equipment (e.g., packed columns). Furthermore, the mass transfer coefficient values for membrane contactors and packed columns are usually comparable. It was proved experimentally that the overall mass transfer coefficient of a membrane contactor could be increased by 40–50% [22], which corresponds to a decrease in absorber dimensions by the same 40–50%.
An important advantage of membrane contactors is the absence of dispersion between two phases. Therefore, there is no need to separate the phases in output and also no droplet carry-out or foaming in liquid phase; gas and liquid flows can be controlled independently within the wide range of process rates [6].
Like all membrane processes, membrane contactors provide operating flexibility and scale-up simplicity due to the modular nature. They do not have any moving parts or elements [23] and, in general, operate with low pressure drops (not more than 1.2 bar [24]).
However, membrane contactors have a number of disadvantages. First of all, the membrane itself contributes to the overall mass transfer resistance. Other disadvantages are the follows: decreasing with time mass transport properties as a result of wetting in porous hollow fiber membrane [11,25] or physical aging of top layer material in composite membrane [18,26]; process sensitivity to impurities in gas mixture [27,28] or liquid phase [29], which affect chemical resistance of a membrane material; and, limited temperature and pressure range for polymeric hollow fiber membranes [25].
3. Design of Membrane Contactors
3.1. Lab-Scale and Pilot Membrane Contactors
The development of hollow fiber membrane contactors began with conventional ‘shell-and-tube’ modules, with hollow fiber membranes playing the role of tubes. In this case, the contacting phases are brought into fiber lumen and shell side in a co-current or counter-current flow regime [30,31,32]. In the configuration considered initially, the liquid phase was brought into fiber lumen to provide equal distribution of liquid phase and to achieve operation of all fibers in the contactor. However, it caused large pressure drop due to high hydraulic resistance with a decreased diameter of hollow fibers. Consequently, it significantly restrained the possibility of increasing liquid phase flow in these devices and the development of compact industrial contactors [30].
In the case when water is brought into the shell side, the hydraulic resistance of the module decreases, thus decreasing the liquid pressure drop. As shown by Wang and Cussler [31], if the liquid in the shell side flows normally to the axis of the fibers (and gas is brought inside the fibers), then the mass transfer coefficient increases significantly. From this perspective, transverse-flow modules were designed and constructed [6], and the most of modern gas-liquid contactors originate from these modules.
The transverse-flow was investigated by Bhaumik et al. [33] for the development of a device based on a mat of hollow fibers wound around the central porous tube (liquid dispenser). The authors proved the efficiency of these systems for CO2 absorption in water.
TNO Company (Den Haag, The Netherlands) was the first who patented a cubical module containing fibers aligned in a certain order providing good flow distribution [34]. These systems are notable for high mass transfer coefficients, low-pressure drop, and scale-up simplicity. Furthermore, successful pilot-scale experiments were performed for various fields of application [35]. TNO also patented a novel type of membrane module providing gas flows operation at high pressures [36]. The module that was based on hollow fibers in a high-pressure shell is designed for absorption of different gases, such as CO2 and H2S for natural gas stripping or the purification of petrochemical flows.
3.2. Commercial Membrane Contactors
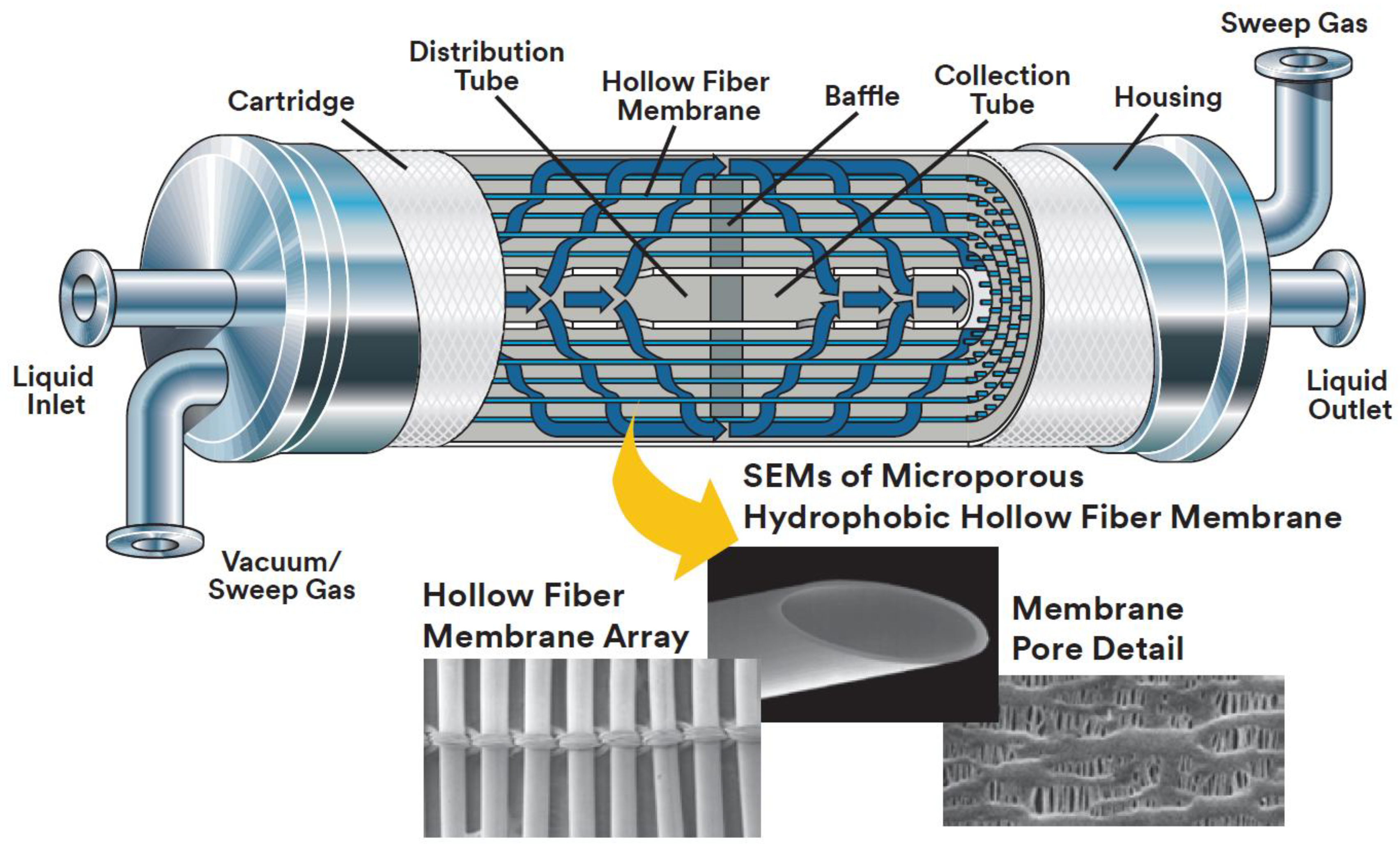
Memrbana-Charlotte Company (Charlotte, NC, USA, formerly a division of Celgard LLC, today a division of 3M) is a leading manufacturer of membrane contactors. It offers a line of membrane modules of various performance, designed for mass transfer tasks in gas-liquid systems. Figure 5 shows commercial membrane contactor Liqui-Сel® (Liqui-Cel™ Extra-Flow membrane contactor, 3M, Maplewood, MN, USA) [37].
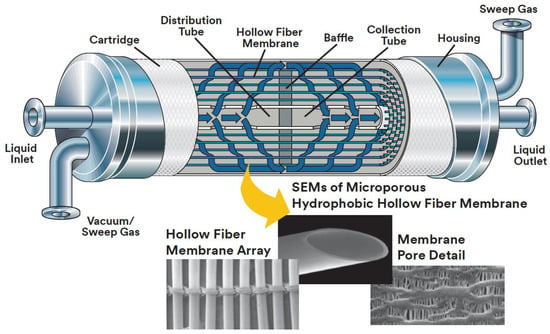
Figure 5.
Module design and polypropylene hollow fibers in typical Liqui-Cel® membrane contactor (adapted with permission from [37], Liqui-Cel™, 2017 3M Company).
Liqui-Сel® contactors are equipped with porous polypropylene membranes Celgard X50, which are supplied in a fiber mat form: parallel hollow fibers are connected to each other by a polymer thread (see down part of Figure 5). Such a configuration is designed to simplify packing of membranes into commercial Liqui-Cel® contactor housings. A membrane sheet is wound around the axial porous tube (liquid dispenser), and the membranes are parallel to the tube axis. A baffle, located in the middle of the axial tube and module, bisects the module and directs the liquid into fiber shell side in the first (left) compartment of the module. Further, the liquid, reaching the outer boundary of the fibers and passing through the gap between the baffle and the wall of the module housing, enters the second (right) compartment of the module and it moves perpendicular to the fibers in the direction of the axial tube through which it exits the apparatus. Consequently, transverse liquid flow (in relation to gas flow in the fiber lumen) is achieved in each compartment of the module [37]. Transverse-flow hollow fiber contactors possess the following advantages:
- liquid flows perpendicularly to the fibers, forming local turbulence and thus increasing the mass transfer coefficient in the liquid phase in fiber shell side; and,
- due to the equal distance between the fibers and to the baffle, a liquid flow channel in the fiber shell side minimizes.
These membrane modules were designed mostly for outgassing of water (to obtain ultra-pure water for electronics and energy industries), carbonation of beverages, etc. More detailed information on case studies can be found on the manufacturer website [38].
4. Membrane Materials for Hollow Fiber Membrane Contactors
Currently, a wide range of hollow fiber membranes is introduced to the market. The membranes differ in their morphology, transport properties, and separation mechanisms. All of the characteristics mentioned are mainly determined by membrane material properties and fabrication techniques. Generally, synthetic membranes comprise organic (polymeric) membranes and inorganic membranes, but the most of membrane contactors employ polymeric membranes. As mentioned above, membranes can be symmetrical (porous or non-porous, or dense) or asymmetrical, the latter possessing pore size gradient across membrane thickness, which can include thin non-porous layer on the membrane surface. Asymmetrical membranes can be fabricated from the same polymeric material or else from different polymeric materials—in the case of composite membranes.
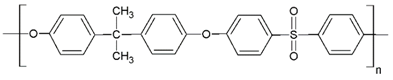
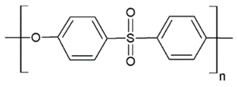
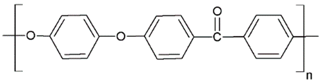
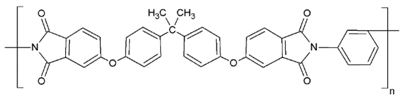
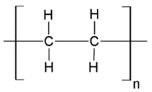
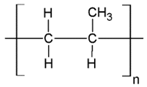
Fabrication of porous membranes assumes that selection of main membrane polymer should be conditioned mainly by high chemical and thermal stability of the membrane. Porous polymeric hollow fiber membranes are fabricated by different techniques. For example, membranes from chemically stable PP or PTFE are prepared by melting extrusion, followed by stretching to form the pores. Fibers from the other polymers (PVDF, PSF, PEI, etc.) can be prepared with different non-solvent induced or temperature-induced phase separation techniques. Selection of efficient polymeric material should also consider its specific operating conditions and operational features of the membrane materials obtained. Table 1 lists main membrane materials used for the fabrication of hollow fiber membranes in membrane contactors.

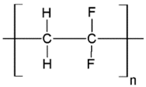
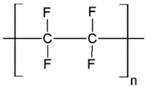
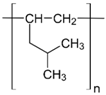
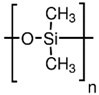
Table 1.
Chemical structures of polymer materials used for hollow fibers in membrane contactors.
5. Membrane Contactor Applications
5.1. Removal of Acid Components from Gas Mixtures
Perhaps the first place in the number of works devoted to hollow fiber membrane contactors is occupied by the acid gas removal: carbon and sulfur dioxides (CO2, SO2) and hydrogen sulfide (H2S) capture processes. These components are formed in a number of technological operations. For instance, CO2 and SO2 are formed in large quantities during the burning of fossil fuels and also during steel and cement production. The removal of these gases is very important from the ecological point of view, since CO2 is one of the key greenhouse gases, and SO2 emissions result in acid rain problems. In natural gas treatment processes, CO2 and H2S removal are crucial both economically and technologically, since H2S is extremely toxic and it also poisons catalysts used in some further natural gas treatment processes, while CO2 decreases its combustion heat. In addition, both CO2 and H2S corrode industrial equipment. The same factors determine the necessity of H2S and CO2 removal from the product of hydrogen or methane biomass fermentation—so-called biogas.
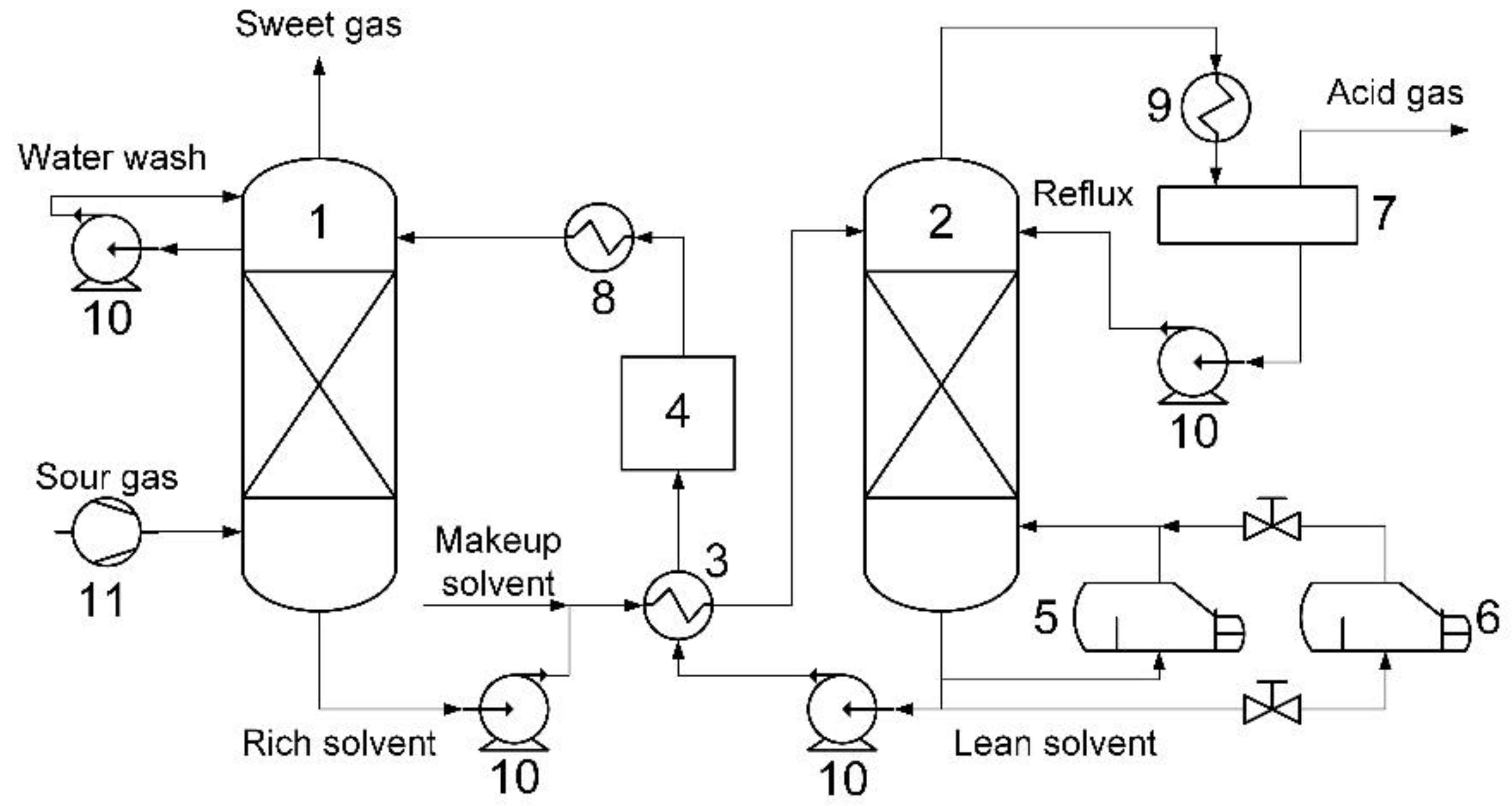
The conventional technique is the absorptive removal of acid components employing various absorption liquids. The principle of the technique is shown in Figure 6.
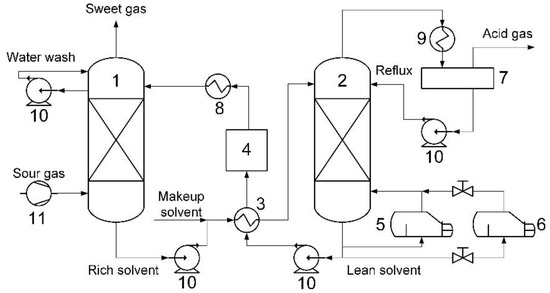
Figure 6.
Principle of absorption-based acid gas capture technology: 1—absorber; 2—stripper; 3—rich/lean heat-exchanger; 4—filter; 5—reboiler; 6—solvent reclaiming unit; 7—reflux drum; 8—cooler; 9—condenser; 10—pump; and, 11—compressor.
The main components of the system are devices for absorption and direct interaction between acid gases and selective absorbent (absorber) as well as the reverse process—desorption of acid gases and regeneration of absorbent (stripper). The devices are large metal-consuming columns with lots of contact elements. These devices are meant to be replaced with hollow fiber membrane contactors—more compact and cheap module systems.
5.1.1. Removal of CO2
Removal of CO2 from gaseous media is the most studied area in the field of hollow fiber contactors. Not only novel membrane materials and membranes are extensively developed, but also CO2-selective absorption liquids that are compatible with the fibers are under investigation. Process design for membrane contactors improves, while carbon dioxide removal is the object of numerous physical, chemical, and technological simulation studies. In terms of the present review, this aspect is not considered in details, since literature provides a number of special overviews [16,23,49]. Two review articles were published recently: one of them is focused on post-combustion carbon capture [50]. In another paper, we analyzed the recent research results considering development and fabrication of porous and composite hollow fiber membranes for contactors. The most popular solvents for carbon dioxide capture were also described, with a particular focus on the pilot tests of membrane contactors [51].
5.1.2. Removal of H2S
Table 2 provides a review of recent works on hydrogen sulfide removal. The key fields of application for membrane contactors are H2S odor control, H2S removal from natural gas, and biogas purification. The latter two research areas are the most extensively studied. Natural gas sweetening employs mostly hollow fiber membranes of various configurations made from perfluorinated polymers—PVDF [52], PTFE [53], poly(tetrafluoroethylene-co-perfluorinated alkyl vinyl ether) (PFA) [54], but there is also an example of employing PSF fibers [52]. This is determined mainly by the combination of two factors: (1) absorbents are alkaline agents in aqueous solution form (solutions of NaOH, K2CO3 [54], monoethanolamine (MEA), diethanolamine (DEA) [55], and methyldiethnolamine (MDEA) [52]) able to chemical bonding with H2S; in relation to the agents, hydrophobic fibers are relatively inert and are poorly wetted by them; (2) natural gas sweetening takes place at high pressures (approx. 50 bar), and thus absorbent breakthrough pressure values that are typical for perfluorinated fibers become important [55]. A feature of this approach is the fact that natural gas often includes CO2 also, and both acidic components have to be removed—the works mentioned above focus on this.

Table 2.
Studies of hydrogen sulfide removal.
Researchers studying H2S removal from biogas employ not only hydrophobic porous hollow fibers (e.g., from PVDF [56,57]), but also non-porous fibers from PDMS. The works [48,58] should be noted where authors used a single PDMS hollow fiber having atypical large sizes (7.0 mm/9.0 mm) in immersion-type membrane contactor and achieved high H2S removal degrees with low methane loss. The result was achieved through a combination of hollow fiber operation and the employment of sulfur-oxidizing microorganisms in the liquid phase.
5.1.3. Removal of SO2
The only field comprising absorptive removal of SO2 in membrane contactors is the Flue-Gas Desulfurization (FGD) process (see Table 3). Researchers employ a number of membrane materials: PSF [62], PP [44,63], PVDF [64], and even ceramic hollow fiber membranes from aluminum oxide [56,57]. The latter were extensively studied by A. Irabien and co-authors: the researchers employed commercial modules containing 280 fibers for SO2 capture using organic solvent N,N-dimethylaniline [65,66]. The alternative absorbent used belongs to a novel generation of solvents—ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate [67,68]. As widely known, ionic liquids (ILs), which are organic salts with melting temperatures less than 100 °C [69,70], might also possess relatively high acid gas solubility and noticeable selectivity over other gases, and were already proposed for CO2 capture/stripping in membrane gas-liquid contactors [71,72] as an alternative to conventional solvents due to extremely low volatility, good thermal stability, lower heat duty at desorption stage as a result of physical bonding of CO2 molecules, low corrosiveness, and even biodegradability [73,74].

Table 3.
Studies on sulfur dioxide removal.
The works of the last five years in the field are mainly devoted to computational fluid dynamics (CFD) simulation of SO2 absorption in hollow fibers [63,75,76,77], while there are virtually no experimental works using the novel hollow fiber membranes.
The only published pilot-scale study of FGD process was performed in the early 00’s by TNO company, as mentioned in [78,79]. The authors declare that they implemented the PP-fibers based system with sodium bisulfite solution inside the fibers that allows for obtaining a SO2 recovery of over 95% at a capacity of 120 m3/h of SO2-containing flue gas from combustion of H2S-biogas of potato starch production plant of AVEBE (Veendam, The Netherlands). Authors stated that during the experiments (two production seasons, each being six months long) no fouling of the membranes or decline in the mass transfer were observed.
5.2. Membrane Oxygenation/Deoxygenation
Membrane degassing of liquids, particularly the removal of dissolved oxygen from water, is the second most important field of membrane contactor studies. Removal of dissolved oxygen (DO) from water is a necessary process in many industries, including pharmaceutical, food, power, and semiconductor. For example, in the power industry removal of DO to levels of around 5 ppm is required to prevent corrosion in boilers and pipes. In comparison, ultra-pure water, as used in the washing of silicon wafers in the semiconductor industry, is perhaps the most demanding in terms of DO level with some applications requiring extremely low DO levels of around 0.1 ppb [81]. This field of application was the first to develop and optimize hollow fiber membrane modules. Successful pilot-scale and industrial tests of commercial membrane contactors were performed.
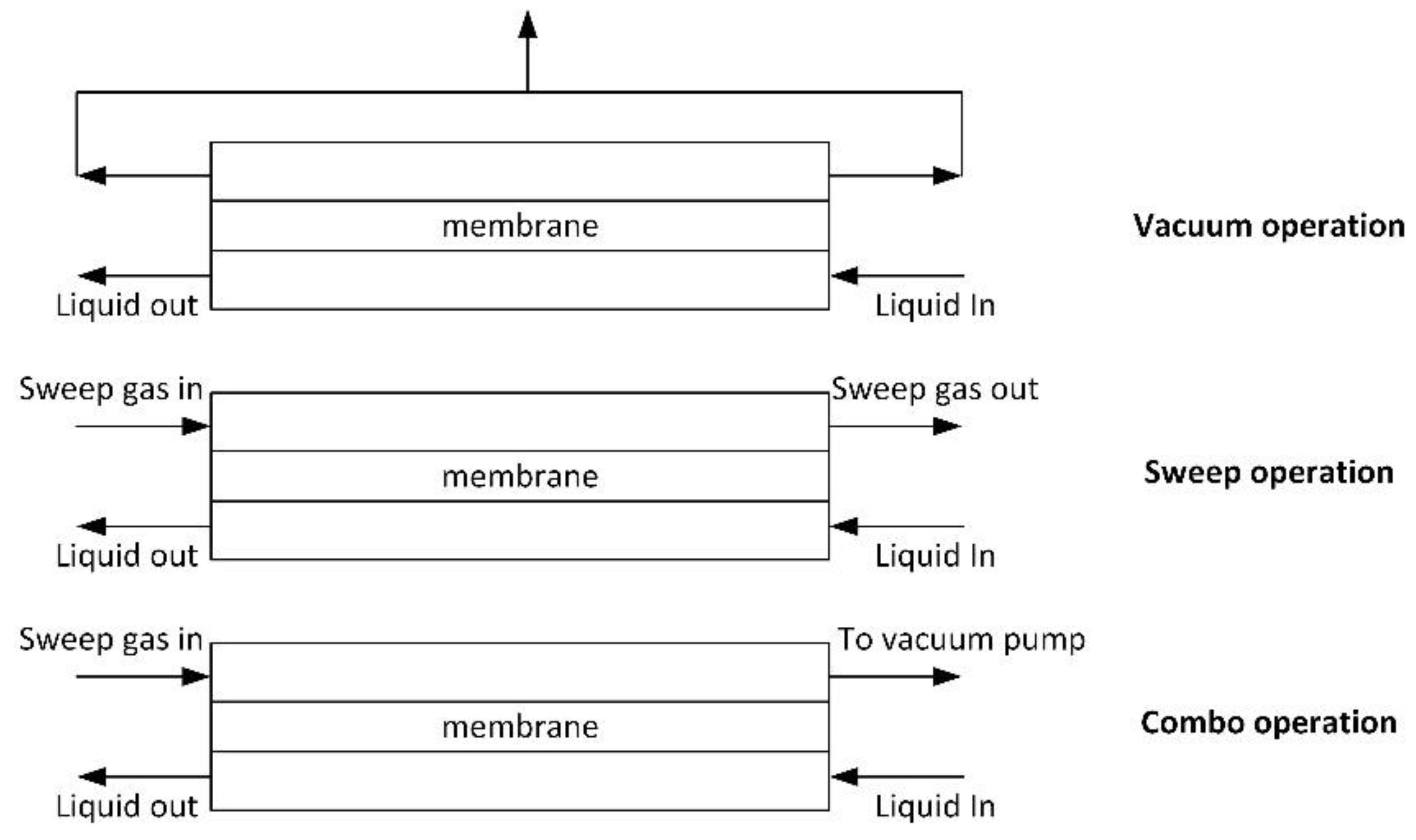
Removal of dissolved oxygen from water requires driving force for transmembrane transport; therefore, the oxygen content in the gas phase has to be reduced. The following approaches are mostly used:
- vacuum operation [43,81,82,83,84,85,86,87];
- sweep operation [32,39,82,88]; and,
- combo operations (combines the first two) [82,89].
Process schemes are given in Figure 7.
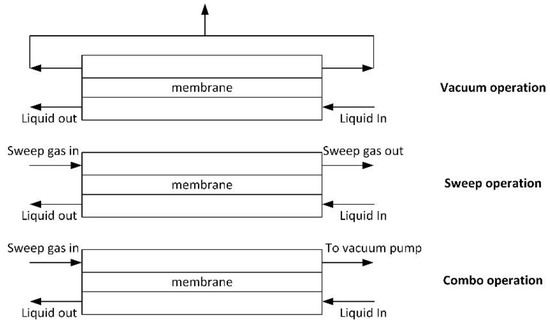
Figure 7.
Different methods for dissolved oxygen removal from water (adapted from [82]).
In the case of the vacuum method, the best separation is achieved when vacuum is applied from the both sides of the membrane module. The advantage of the vacuum method is the simultaneous removal of all gases, but a deep vacuum has to be maintained in order to achieve high water purification degree. In case of sweep or combo operation, gas and liquid should flow in the counter-flow mode to increase separation degree. One of the advantages of the membrane contactor is the ability of the module to operate, regardless of its position (vertical or horizontal). Nitrogen is often used as a sweep gas. The method is quite efficient, but has some disadvantages: (1) purified water saturates with nitrogen; (2) deep purification of water requires nitrogen of high purity; and, (3) a large amount of water evaporates during deep purification, thus affecting the process energetics (energy consumption related to the latent heat of evaporation). The combo method allows for controlling the residual content of sweep gas in water.
As can be seen from Table 4, dissolved oxygen removal from aqueous media employs mostly hydrophobic PP porous hollow fiber membranes of different configuration. Since the membrane is hydrophobic, water does not fill the pores in normal conditions. However, other hollow fiber membranes are also used: hydrophobic porous hollow fibers from PE [43] and PVDF [90], PSF porous hollow fibers [39], hydrophobic dense membranes from silicon rubber [82], and composite membranes with a selective layer from perfluorodimethyldioxole-tetrafluoroethylene (PDD-TFE) on PP support [91].

Table 4.
Studies on membrane oxygenation/deoxygenation.
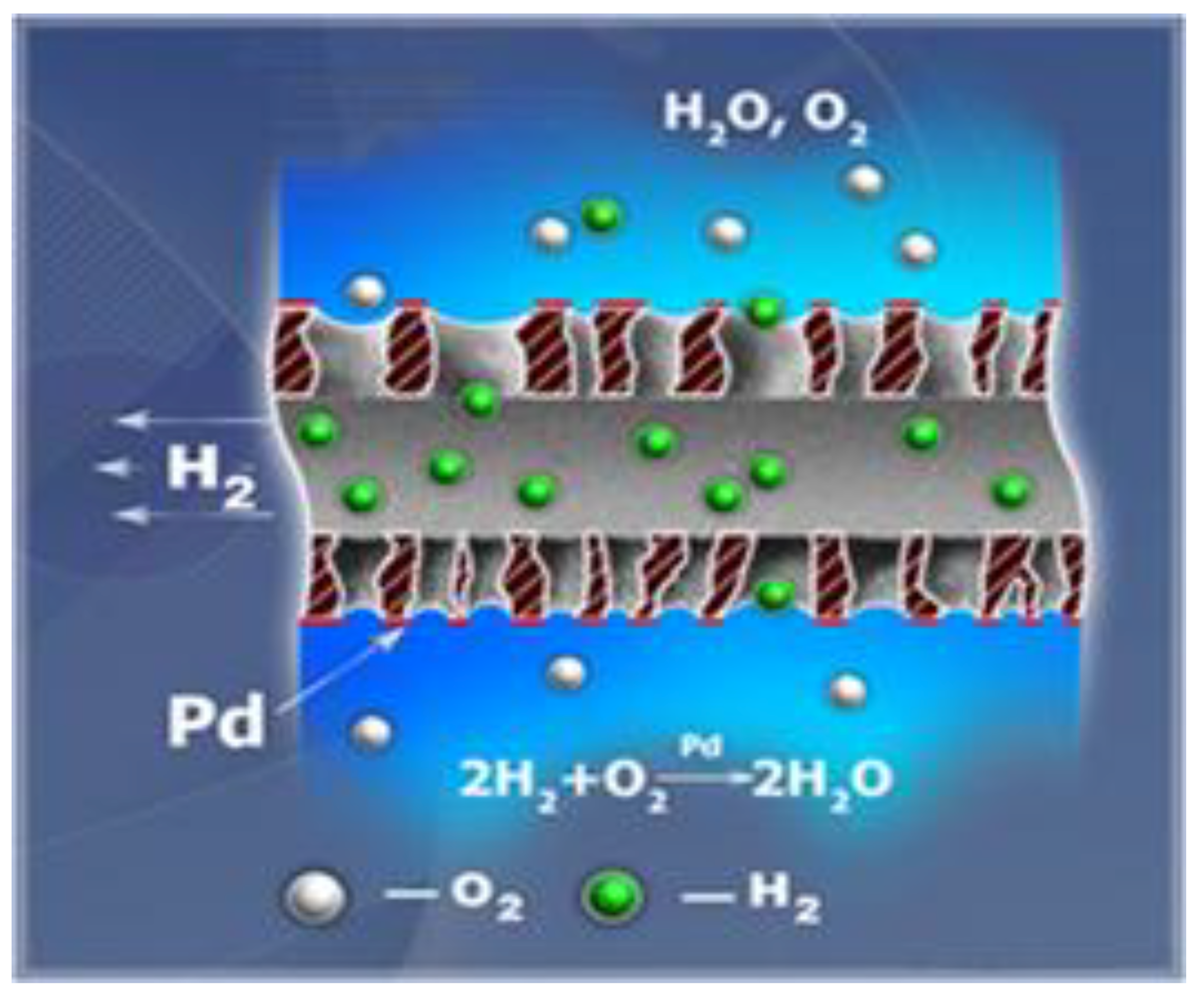
An important step in the evolution of the technology was the development of catalytic hollow fiber membranes by researchers from TIPS RAS and TNO [92,93,94]. The membranes are commercial porous hollow fibers made from PP with diverse configuration, coated by palladium nanoparticles. Water containing DO flows over the outer surface of the Pd-loaded hydrophobic hollow fiber membrane, whereas hydrogen is supplied into the lumen side of hollow fibers and it approaches the working surface of catalyst through membrane pores. Due to the catalytic activation of hydrogen adsorbed on the palladium surface, the heterogeneous reaction of the reduction of DO takes place, and water is formed according to the reaction, as presented in Figure 8.
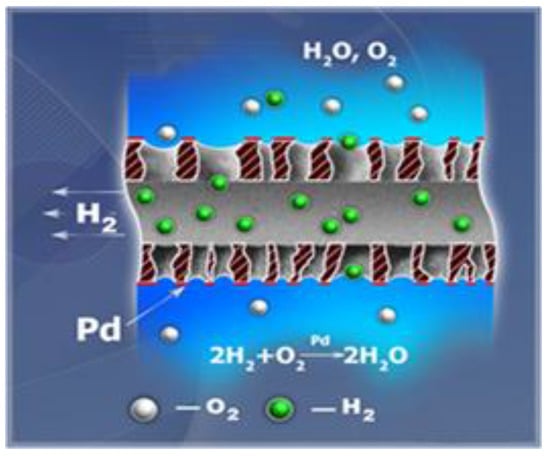
Figure 8.
The principle of dissolved oxygen removal in catalytic gas-liquid hollow fiber membrane contactor (adapted from [92]).
In contrast to conventional membrane modules, these membrane contactors significantly increase the process kinetics, and a fast oxygen removal system is obtained that has the potential of maintaining removal rate, even at very low oxygen concentrations.
Current research studies in the field of water deoxygenation are focused on the employment of novel tri-bore hollow fiber membranes from PVDF [45,95,96]. The novelty of form factor is complemented by the fact that the authors employ a relatively large amount of membrane in the module (200). However, the degree of oxygen removal from water is relatively low and membrane fouling is observed under real aquaculture deoxygenation.
Comprehensive laboratory research and success achieved in the field during the 90’s moved hollow fiber membrane contactors to pilot-scale and semi-industrial level: it was shown that the contactors could be employed to obtain ultrapure water [82,84] and low-oxygen boiler feed water [86,89]. All of the research employed commercial modules that were based on porous PP fibers, particularly Liqui-Cel® contactors by 3M Company (formerly Celgard LLC). Phase contact areas were up to 130 m2 and the modules purified substantial water flows—up to 50 m3/h (see Table 4). The researchers were able to reduce the oxygen content in water by three orders of magnitude in semi-industrial conditions [82], while the price of membrane treated water was twice lower than that for thermal degassed water [89].
Table 4 lists some additional works unlocking the potential of membrane degassing: membrane bubbleless aeration and membrane contactors as “artificial gills”, providing the oxygen from water for human subsea closed life systems. Furthermore, we decided to include another important section: the process that is, to a certain extent, reverse to those mentioned above, namely, absorptive removal of oxygen from gaseous mixtures, particularly, from the air. Implementation of the process in the membrane contactors is considered to replace the existing technology for obtaining pure oxygen via cryogenic separation of air. The main focus in this field is a selection of oxygen-selective absorbents and their employment in commercially available membrane modules. The researchers use pseudo-plastic non-Newtonian sodium carboxymethylcellulose (CMC) solutions [97], polyethyleneimine (PEI)-cobalt complex solution in water [98], as well as surprising absorbent based on slaughterhouse wastewaters that contain 90% of blood [99].
Finally, Table 4 provides some examples of modern research in the field of membrane blood oxygenation. This field was the first to employ the approach based on the selective transport of gases between gas and liquid phases through porous hollow fiber. The membrane oxygenators based on fibers from siloxane-polycarbonate copolymer were proposed in the 1970’s [100]. In this case, membrane contactor-oxygenator is a device used to add oxygen to, and remove carbon dioxide from the blood: O2 diffuses from the gas side through the membrane into the blood, and CO2 diffuses through the membrane from the blood into the gas for disposal. It can be used in two principal modes: to imitate the function of the lungs in cardiopulmonary bypass (CPB), and to oxygenate blood in longer-term life support, termed extracorporeal membrane oxygenation, ECMO.
Interested readers are advised to read reviews in the field, particularly [101]. Currently, novel hollow fiber membranes are being developed, including plasma-activated PMP fibers modified by grafting of 2-methacryloyloxyethyl phosphorylcholine with improved hemocompatibility [47]; the simulation of the process is performed using glycerol [102] or its aqueous solutions [103] as blood simulator.
5.3. Membrane Ozonation
Membrane ozonation is, in some way, the successor of membrane oxygenation, in which oxygen is replaced with more active ozone (O3)—triatomic oxygen molecule with great oxidative power, characteristic odor and colorless, which can easily react with and destroy a great number of organic compounds. Due to its activity ozone has been widely used for contaminants removal and disinfection in the water treatment process.
Because of the ozone reactivity, the applicability of polymeric hollow fiber membranes is limited, since many polymeric materials are susceptible to destruction in the O3 medium. As shown in [110], hollow fibers from PES and PEI degrade under the O3 exposure, PP and PDMS are more resistant, but undergo structural modifications with extended contact time, whereas flats-sheet membranes from PVDF and PTFE showed maximum resistance to ozone. Therefore, researchers employ membranes from ceramic materials [111,112,113] or glasses [114]. These membranes are tubular (with inner diameter >4 mm) and therefore are not discussed in the present review.
However, it is noteworthy that the first membrane contactors employed for ozonation tasks were hollow fiber modules produced by W.L. Gore & Associates (Elkton, MD, USA) under trademark ‘DISSO3LVETM’. These modules were based on hollow fibers from expanded PTFE (i.d. 1.7 mm/o.d. 2.7 mm, pore size 0.003 μm, length 0.8 m). Each module contained 100 membranes, typical gas and flow rates were 3 and 10–20 L/min) [115,116]. As indicated in [6], these modules were successfully implemented in the semiconductor industry and they had become the technology of choice for ozonation in Japan.
Table 5 provides a review of modern research in the field of membrane ozonation employing polymeric hollow fiber membrane contactors. Obviously, as before, the focus of research is on hydrophobic hollow fibers from chemically resistant perfluorinated PVDF or PTFE. They are most widely used for the decolorization of waste streams containing different dyes, particularly streams from textile industry [117,118,119]. Membrane contactors are also used for ozonation of effluents containing organic contaminants and volatile organic compounds [120,121].

Table 5.
Studies on membrane ozonation.
5.4. Gas Humidity Control
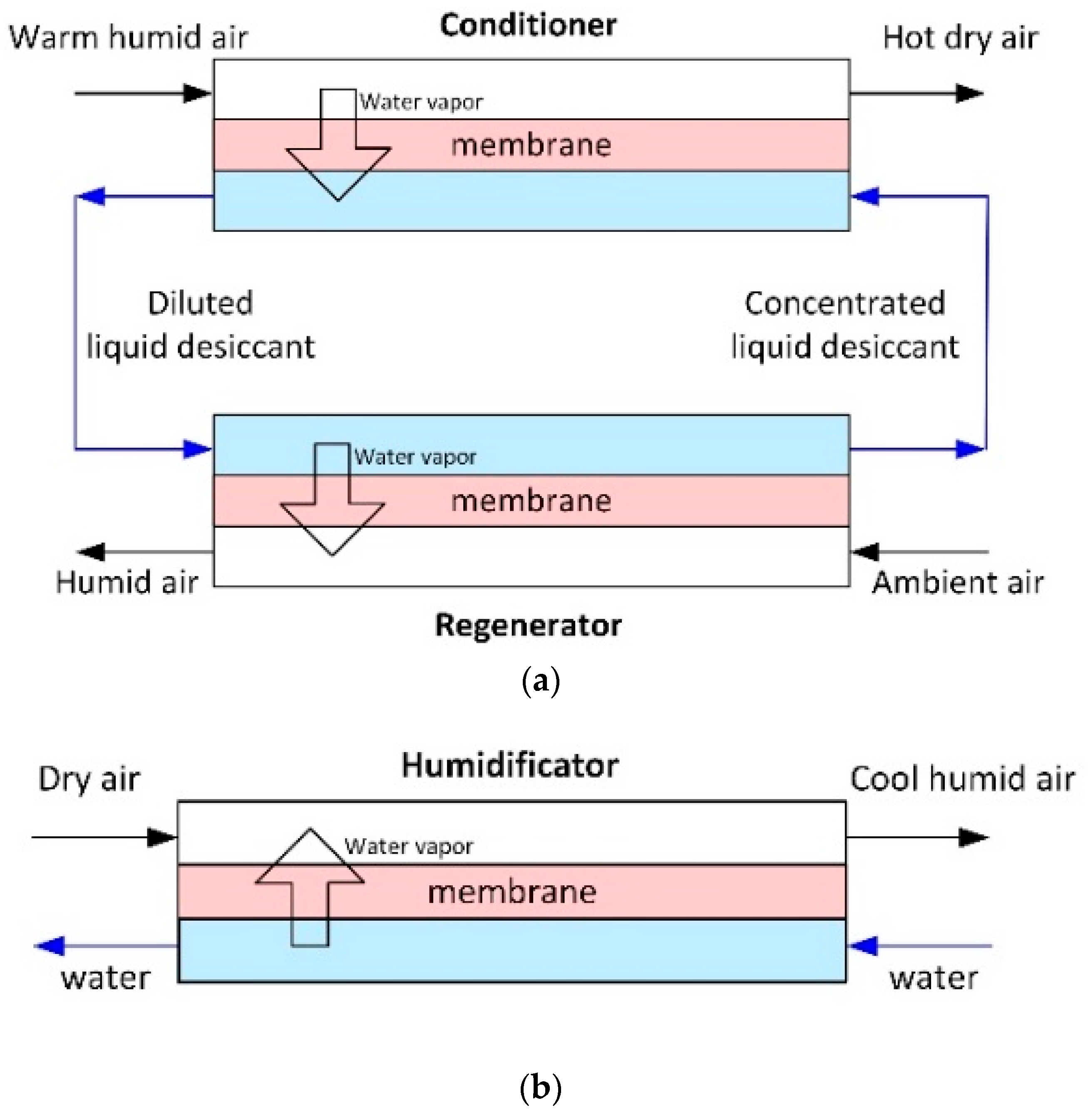
Membrane gas humidity control, particularly from air, is one more field of membrane contactors application. The humidity control of the air is of great importance in air conditioning systems as applied, e.g., in buildings, vehicles, and containers for storage and the transport of perishable products [42]. A direct process—dehumidification (the removal of water vapor from the air)—is more common, since it is responsible for a significant amount of the energy consumption during mechanical cooling of air in humid climates. In fact, energy consumption for air dehumidification accounts for 20–40% of the total energy use for air conditioning, and it can be even higher when 100% fresh air ventilation is required for indoor environmental control [124]. Membrane air dehumidification process is based on absorption of water vapors from the air by liquid desiccant through porous hollow fiber membrane, with following regeneration of desiccant (Figure 9a). A reverse process—evaporative cooling/humidification that differ only in the goal of the process (evaporative cooling is used as an energy efficient means of cooling air to control temperatures and humidification to humidify the dry air, see Figure 9b)—also becomes the subject of membrane contactor research. Table 6 provides a review of the works in the field.
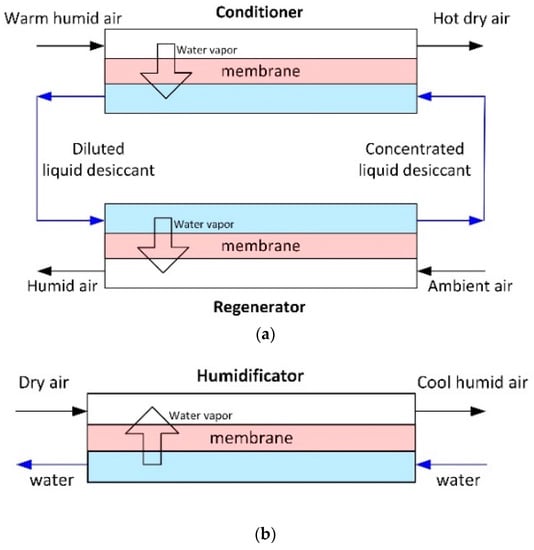
Figure 9.
Membrane contactors for air humidity control: (a) Membrane air dehumidification; and, (b) Evaporative cooling/membrane humidification (adapted from [125]).

Table 6.
Studies on gas humidity control.
As can be seen from Table 6, air dehumidification employs mostly hollow fibers from hydrophobic PVDF, since the desiccants employed are based on aqueous media. However, there are works on dense hydrophilic hollow fibers [126] or composite membranes with a thin non-porous layer from PDMS [42] or polyvinyl alcohol [127]. It is notable that the reverse process employs a wider range of membrane materials—in addition to PVDF, membrane humidificators operate also with hollow fibers from PP [128,129] and PES [40].
Liquid desiccants for air dehumidification can be divided into two broad ranges: (1) aqueous solution of salt desiccants—for example, lithium chloride (LiCl) [42,130,131] or calcium or magnesium chloride (CaCl2 or MgCl2) [132,133] that usually used for residential and commercial building applications, (2) glycols, like tri-ethylene glycol (TEG), which are frequently used for industrial applications [134]. The work [135] is worth of being noted individually; it employs chilled water as a desiccant for air control in spacecraft applications.
As in the fields discussed above, humidity control employing hollow fiber membrane contactors comes to a pilot-scale level. For instance, a pilot rectangular transverse-flow contactor is developed. It contains 2900 composite PVDF fibers with a thin dense outer layer from polyvinyl alcohol and shows the productivity of 100 m3/h by humidified air and 50 kg/h by water wed into fiber lumen [127]. The same group of authors developed an alternative design of stacked industrial membrane contactor, consisting of 25 smaller elements, each containing 100–150 PVDF membranes. Such a design provides a way to simplify scale-up of the contactor for industrial applications [136].
Modern research in the field is determined by a search of an optimal combination of hollow fiber membranes and liquid desiccant. Some researchers propose to employ novel hollow fibers combined with conventional desiccants. Authors of the work [132] propose employing tri-bore hollow fibers from PVDF with CaCl2 solution. In contrast, it is proposed to employ membrane contactors that are based on commercial fibers from Pebax®1074 and novel alkoxylated siloxane liquid desiccant (Dow CorningXX-8810) with a significantly reduced vapor pressure dependence on temperature [126].
Unfortunately, it is not possible to describe all the data on gas-liquid membrane contactors for gas humidity control in the present review. Interested readers can familiarize with the theme in detail by referring to modern overviews [125,137,138,139,140].
Almost 90% of the works in the field of gas humidity control are related to dehumidification/humidification of air. However, works considering dehumidification of other gas mixtures, e.g., natural gas, appeared recently [141]. In this case, the authors propose employing membrane contactors for subsea natural gas dehumidification using triethylene glycol as a liquid desiccant. The membranes proposed within this concept are hollow fibers from PTFE.
Within the framework of this section, we would also like to mention membrane condensation as a new field of membrane contactors application. In this process, contactors are employed as condensers to recover process water from waste gases, and hollow fibers play the role of condensation surface. The first works in the field were published in 2012–2014 [142,143] by E. Drioli group. The applicability of this concept for the recovery of water from flue gases of post-combustion CO2 capture processes is currently being estimated [144,145].
5.5. Olefin/Paraffin Separation
Another important field of application for membrane contactors is a separation of unsaturated hydrocarbons from their mixtures with saturated hydrocarbons. Olefin/paraffin separation is one of the most important processes in the petrochemical industry, because ethylene and propylene are the most popular chemical precursors for chemical syntheses and industrial processes. The main difficulty in the separation of olefin from paraffin having same carbon number is the low difference in components boiling temperatures, e.g., for ethylene and ethane mixture, this value is 14.7 °C. Traditional olefin/paraffin separation technology is low-temperature distillation [148], where distillation columns having trays number 100 and higher are used. The technology disadvantages are high capital costs and large metal consumption due to the application of low temperatures and high pressures. Chemical absorption with membrane contactors is a perspective alternative. In this case, transition metals and salts solutions are used as chemical absorbents [149]. Bonding force in the transition metal ion and olefin π-complex is mainly defined by the metal electronegativity value and the lattice energy of its salt [150]. The most studied liquid absorbent is an aqueous silver nitrate solution while using of Cu+ salts is less versatile because, in the presence of water vapors or oxidants, copper salts are susceptible to oxidizing or disproportionating [151].
Generally, researchers in the field of olefin/paraffin separation focus on removal of ethylene from ethane mixtures or propylene from propane mixtures (see Table 7). In the case of ethylene mixtures, Tsou et al. [152] were the first to investigate the effect of some parameters (liquid flow rate, absorbent composition), including the morphology of PSF hollow fibers, on the results of ethylene/ethane separation. The results proved the exploitability of hollow fiber membrane contactors for this task. Important research studies were carried out by K. Nymejier et al. This scientific group focused on fabrication of composite hollow fiber membranes based on porous polypropylene fibers resistant to wetting by absorption liquid. The authors not only fabricated such membranes [153,154], but also developed the strategy for the selection of non-porous layer material and thus increased ethylene removal selectivity by several orders of magnitude [41]. An alternative approach employed mesoporous hollow fibers from PSF with the hydrophobized surface, which possessed high gas transport properties in contrast to composite membranes [155]. The authors showed that the combination of ethylene permeance and removal degree was the maximum amongst the published works.

Table 7.
Studies on olefin/paraffin separation.
The field of propane/propylene separation offers a wider range of research studies: for instance, the employed absorption liquids are not only aqueous silver nitrate solutions (as in case of ethane/ethylene mixtures), but also ionic liquids [156] and their mixtures with silver salts [157]. According to Table 7, the most widely used hollow fibers are made from PP, PVDF, PTFE, with diverse configuration; they may be both commercially available and home-made. Especially worth of note is the work [158], employing a membrane contactor based on asymmetric ceramic Al2O3 fibers modified with 1H,1H,2H,2H-perfluorooctylethoxysilane to increase hydrophobicity. The authors describe the technique of membrane fabrication and they also specify stability of the membranes under conditions of long-term contact with silver salts.
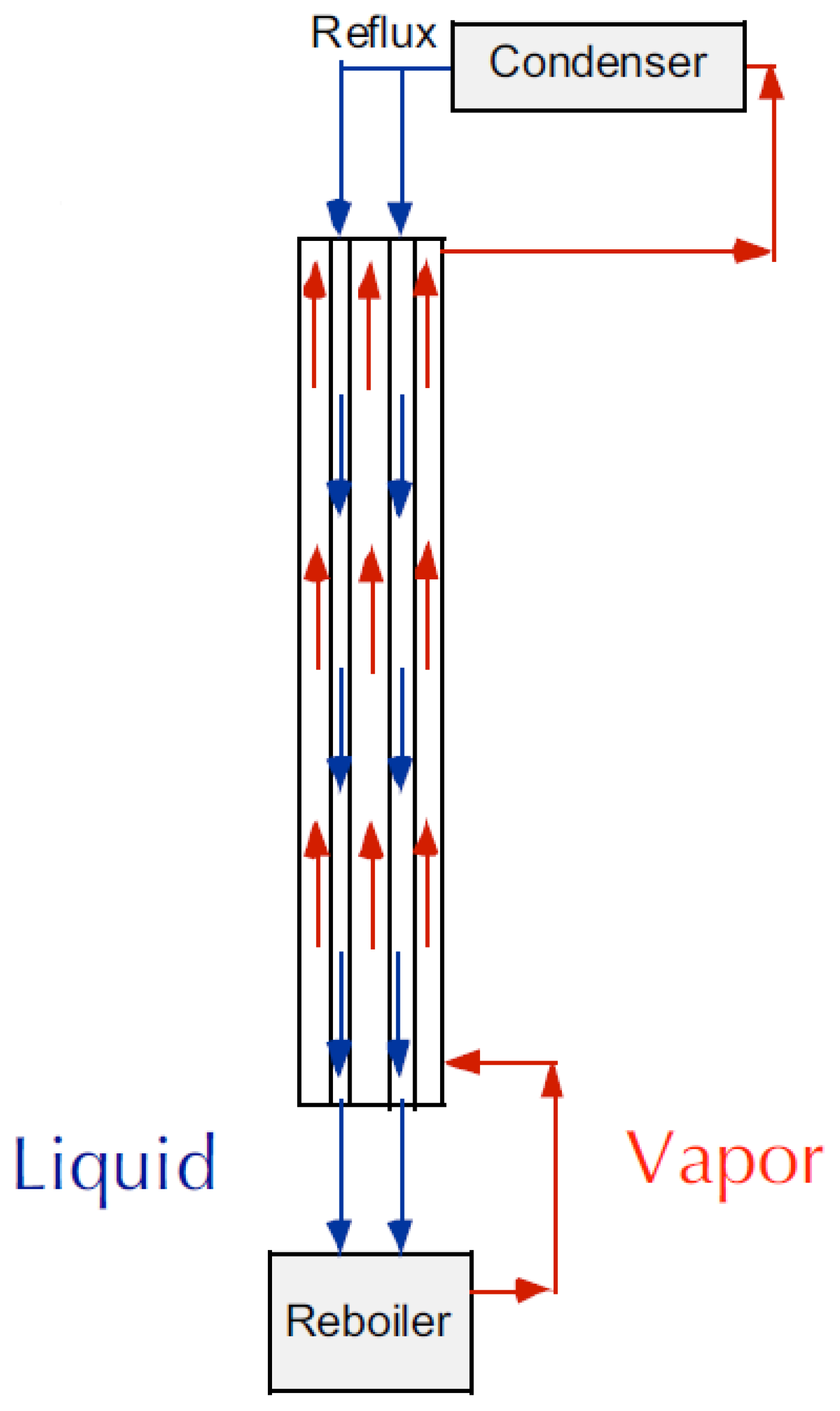
Membrane contactors may be employed not only as chemical absorbers for unsaturated hydrocarbons but also as structured packings in the conventional distillation columns of olefin/paraffin mixtures—i.e., hollow fiber distillation columns. In this case, the gas or vapors flow up the outside of the fiber (shell) while the liquid condensate counter-currently flows down inside the lumen of the hollow fiber (see Figure 10). Using this approach, one can decouple the liquid and vapor flows, allowing for operations above the normal flooding limit.
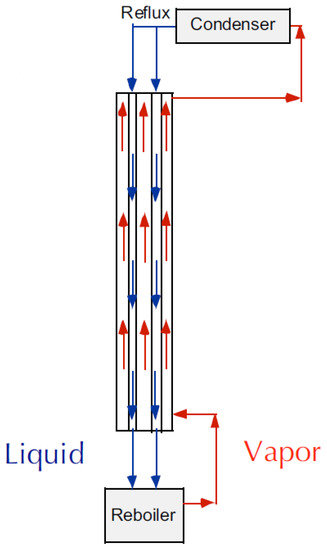
Figure 10.
Distillation column with hollow fiber membranes as structured packings at total reflux condition (adapted from [159]).
The authors succeeded to realize a proof-of-concept of the technology [159] and to select commercially available hollow fiber membranes (PP, Celgard X30) with optimal properties [160]. The work [161] should also be noticed, since the authors implemented pilot-scale technology using membrane modules with different packing density and number of PP fibers up to 2000.
6. Concluding Remarks
Membrane contactors represent an outstanding example of highly efficient contact devices that are provided by rapidly growing membrane technologies. Hollow fiber membrane contactors possess increased efficiency due to the large surface area per unit volume of the module, which is attributed to the hollow fiber membrane configuration. This advantage resulted in rapid growth of membrane contactor applications, which comprise diverse fields of science and chemical technology. To date, the growth continues, resulting in a number of pilot-scale and semi-industrial contactor processes. In the present review, we have made an attempt to explore a great variety of membrane contactor applications and to highlight the key trends in modern research, with a particular focus on the hollow fibers used.
We presume that further research should focus on novel membrane materials and highly efficient hollow fibers with increased resistivity towards harsh conditions in different processes (e.g., petrochemical processes): aggressive media (organic mixtures and solvents, acids and alkalies), increased temperatures, etc. Another direction is the development and employment of novel composite membranes with thin dense non-porous layers able to operate under high transmembrane pressure drops without a decline in productivity caused by wetting. Experimental and theoretical studies in this field will provide a way for the further extension of application fields for highly efficient hollow fiber membrane contactors.
Author Contributions
Conceptualization, writing—original draft preparation, S.D.B.; writing—review and editing, A.V.B. and A.V.V.
Funding
This work was carried out in A.V. Topchiev Institute of Petrochemical Synthesis (Russian Academy of Sciences) and was funded by Russian Science Foundation, grant number 14-49-00101.
Acknowledgments
Stepan D. Bazhenov and Alexandr V. Bildyukevich acknowledge the financial support of Russian Science Foundation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mahon, H.I. Permeability Separatory Apparatus, Permeability Separatory Membrane Element, Method of Making the Same and Process Utilizing the Same. US Patent 3,228,876, 11 January 1966. [Google Scholar]
- Chung, T.S. Fabrication of hollow-fiber membranes by phase inversion. In Advanced Membrane Technology and Applications, 1st ed.; Li, N., Fane, A., Winston, W., Matsuura, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 821–839. ISBN 9780471731672. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 148–149. ISBN 9780470743720. [Google Scholar]
- Qi, Z.; Cussler, E.L. Microporous hollow fibers for gas absorption: I. Mass transfer in the liquid. J. Membr. Sci. 1985, 23, 321–332. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E.L. Microporous hollow fibers for gas absorption: II. Mass transfer across the membrane. J. Membr. Sci. 1985, 23, 333–345. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.T. Hollow fiber membrane contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Dibrov, G.A.; Volkov, V.V.; Vasilevsky, V.P.; Shutova, A.A.; Bazhenov, S.D.; Khotimsky, V.S.; van de Runstraat, A.; Goetheer, E.L.V.; Volkov, A.V. Robust high-permeance PTMSP composite membranes for CO2 membrane gas desorption at elevated temperatures and pressures. J. Membr. Sci. 2014, 470, 439–450. [Google Scholar] [CrossRef]
- Karoor, S.; Sirkar, K.K. Gas absorption studies in microporous hollow fiber membrane modules. Ind. Eng. Chem. Res. 1993, 32, 674–684. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.; Versteeg, G.F. CO2 absorption at elevated pressures using a hollow fiber membrane contactor. J. Membr. Sci. 2004, 235, 99–109. [Google Scholar] [CrossRef]
- Rongwong, W.; Jiraratananon, R.; Atchariyawut, S. Experimental study on membrane wetting in gas–liquid membrane contacting process for CO2 absorption by single and mixed absorbents. Sep. Purif. Technol. 2009, 69, 118–125. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.Y.; Feron, P.H.M.; Liang, D.T. Influence of membrane wetting on CO2 capture in microporous hollow fiber membrane contactors. Sep. Purif. Technol. 2005, 46, 33–40. [Google Scholar] [CrossRef]
- Kreulen, H.; Smolders, C.A.; Versteeg, G.F.; Van Swaaij, W.P.M. Determination og mass transfer rates in wetted and non-wetted microporous membranes. Chem. Eng. Sci. 1993, 48, 2093–2102. [Google Scholar] [CrossRef]
- Rangwala, H.A. Absorption of carbon dioxide into aqueous solutions using hollow fiber membrane contactors. J. Membr. Sci. 1996, 112, 229–240. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wang, R.; Liang, D.T.; Tay, J.H. Theoretical and experimental studies of membrane wetting in the membrane gas–liquid contacting process for CO2 absorption. J. Membr. Sci. 2008, 308, 162–170. [Google Scholar] [CrossRef]
- Malek, A.; Li, K.; Teo, W.K. Modeling of Microporous Hollow Fiber Membrane Modules Operated under Partially Wetted Conditions. Ind. Eng. Chem. Res. 1997, 36, 784–793. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. Hollow fiber gas–liquid membrane contactors for acid gas capture: A review. J. Hazard. Mater. 2009, 171, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Teo, W.K. An Ultrathin Skinned Hollow Fibre Module for Gas Absorption at Elevated Pressures. Chem. Eng. Res. Des. 1996, 74, 856–862. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Lasseuguette, E.; Medina-Gonzalez, Y.; Remigy, J.C.; Roizard, D.; Favre, E. A dense membrane contactor for intensified CO2 gas/liquid absorption in post-combustion capture. J. Membr. Sci. 2011, 377, 261–272. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W.; deMontigny, D. Comparison of thin film composite and microporous membrane contactors for CO2 absorption into monoethanolamine. Int. J. Greenh. Gas Control 2015, 42, 66–74. [Google Scholar] [CrossRef]
- Falk-Pedersen, O.; Dannström, H. Separation of carbon dioxide from offshore gas turbine exhaust. Energy Conver. Manag. 1997, 38, 81–86. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Jansen, A.E. Capture of carbon dioxide using membrane gas absorption and reuse in the horticultural industry. Energy Conver. Manag. 1995, 36, 411–414. [Google Scholar] [CrossRef]
- deMontigny, D.; Tontiwachwuthikul, P.; Chakma, A. Comparing the Absorption Performance of Packed Columns and Membrane Contactors. Ind. Eng. Chem. Res. 2005, 44, 5726–5732. [Google Scholar] [CrossRef]
- Cui, Z.; deMontigny, D. Part 7: A review of CO2 capture using hollow fiber membrane contactors. Carbon Manag. 2013, 4, 69–89. [Google Scholar] [CrossRef]
- 3MTM Liqui-CelTM Data Sheets. Available online: https://www.3m.com/3M/en_US/liquicel-us/resources/data-sheets/ (accessed on 29 September 2018).
- Mosadegh-Sedghi, S.; Rodrigue, D.; Brisson, J.; Iliuta, M.C. Wetting phenomenon in membrane contactors—Causes and prevention. J. Membr. Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Trusov, A.; Legkov, S.; van den Broeke, L.J.; Goetheer, E.; Khotimsky, V.; Volkov, A. Gas/liquid membrane contactors based on disubstituted polyacetylene for CO2 absorption liquid regeneration at high pressure and temperature. J. Membr. Sci. 2011, 383, 241–249. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, R.; Sha, Y.; Wang, X.; Yang, L. Membrane gas absorption for CO2 capture from flue gas containing fine particles and gaseous contaminants. Int. J. Greenh. Gas Control 2015, 33, 10–17. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.-T.; Dahlquist, E. Effects of SO2 on CO2 capture using a hollow fiber membrane contactor. Appl. Energy 2013, 112, 755–764. [Google Scholar] [CrossRef]
- Franco, J.A.; deMontigny, D.; Kentish, S.E.; Perera, J.M.; Stevens, G.W. Effect of amine degradation products on the membrane gas absorption process. Chem. Eng. Sci. 2009, 64, 4016–4023. [Google Scholar] [CrossRef]
- Wickramasinghe, S.R.; Semmens, M.J.; Cussler, E.L. Better hollow fiber contactors. J. Membr. Sci. 1991, 62, 371–388. [Google Scholar] [CrossRef]
- Wang, K.L.; Cussler, E.L. Baffled membrane modules made with hollow fiber fabric. J. Membr. Sci. 1993, 85, 265–268. [Google Scholar] [CrossRef]
- Tai, M.S.L.; Chua, I.; Li, K.; Ng, W.J.; Teo, W.K. Removal of dissolved oxygen in ultrapure water production using microporous membrane modules. J. Membr. Sci. 1994, 87, 99–105. [Google Scholar] [CrossRef]
- Bhaumik, D.; Majumdar, S.; Sirkar, K.K. Absorption of CO2 in a transverse flow hollow fiber membrane module having a few wraps of the fiber mat. J. Membr. Sci. 1998, 138, 77–82. [Google Scholar] [CrossRef]
- Muelen, B.P.T. Transfer Device for the Transfer of Matter and/or Heat from One Medium Flow to Another Medium Flow. Patent US 5,230,796, 27 July 1993. [Google Scholar]
- Feron, P.H.M.; Jansen, A.E. CO2 separation with polyolefin membrane contactors and dedicated absorption liquids: Performances and prospects. Sep. Purif. Technol. 2002, 27, 231–242. [Google Scholar] [CrossRef]
- Jansen, A.E.; Feron, P.H.M.; Hanemaaijer, J.H.; Huisies, P. Apparatus and Method for Performing Membrane Gas/Liquid Absorption at Elevated Pressure. Patent US 6,355,092, 2002. [Google Scholar]
- 3MTM Liqui-CelTM Publications and Case Studies. Optimized Deaeration System for Paulaner Brewery. Available online: https://multimedia.3m.com/mws/media/1412652O/3m-liqui-cel-membrane-contactors-optimized-deaeration-system.pdf (accessed on 7 September 2018).
- 3MTM Liqui-CelTM Publications and Case Studies. Available online: https://www.3m.com/3M/en_US/liquicel-us/resources/publications-and-case-studies/ (accessed on 7 September 2018).
- Vladisavljevic, G.T. Use of polysulfone hollow fibers for bubbleless membrane oxygenation/deoxygenation of water. Sep. Purif. Technol. 1999, 17. [Google Scholar] [CrossRef]
- Bakeri, G.; Naeimifard, S.; Matsuura, T.; Ismail, A.F. A porous polyethersulfone hollow fiber membrane in a gas humidification process. RSC Adv. 2015, 5, 14448–14457. [Google Scholar] [CrossRef]
- Nymeijer, K.; Visser, T.; Assen, R.; Wessling, M. Super selective membranes in gas–liquid membrane contactors for olefin/paraffin separation. J. Membr. Sci. 2004, 232, 107–114. [Google Scholar] [CrossRef]
- Kneifel, K.; Nowak, S.; Albrecht, W.; Hilke, R.; Just, R.; Peinemann, K.V. Hollow fiber membrane contactor for air humidity control: Modules and membranes. J. Membr. Sci. 2006, 276, 241–251. [Google Scholar] [CrossRef]
- Leikness, T.O.; Semmens, M.J. Vacuum degassing using microporous hollow fiber membranes. Sep. Purif. Technol. 2000, 22–23, 287–294. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Experimental studies on simultaneous removal of CO2 and SO2 in a polypropylene hollow fiber membrane contactor. Appl. Energy 2012, 97, 283–288. [Google Scholar] [CrossRef]
- Su, J.; Wei, Y. Novel tri-bore PVDF hollow fiber membranes for the control of dissolved oxygen in aquaculture water. J. Water Process Eng. 2018. [Google Scholar] [CrossRef]
- Merle, T.; Pronk, W.; Von Gunten, U. MEMBRO3X, a novel combination of a membrane contactor with advanced oxidation (O3/H2O2) for simultaneous micropollutant abatement and bromate minimization. Environ. Sci. Technol. Lett. 2017, 4, 180–185. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Zheng, Z.; Fan, W.; Mao, C.; Shi, J.; Li, L. Surface monofunctionalized polymethyl pentene hollow fiber membranes by plasma treatment and hemocompatibility modification for membrane oxygenators. Appl. Surf. Sci. 2016, 362, 355–363. [Google Scholar] [CrossRef]
- Tilahun, E.; Bayrakdar, A.; Sahinkaya, E.; Çalli, B. Performance of polydimethylsiloxane membrane contactor process for selective hydrogen sulfide removal from biogas. Waste Manag. 2017, 61, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, B.H. Review of CO2 absorption using chemical solvents in hollow fiber membrane contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon capture: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Lyubimova, E.S. Gas–liquid membrane contactors for carbon dioxide capture from gaseous streams. Petrol. Chem. 2016, 56, 889–914. [Google Scholar] [CrossRef]
- Hedayat, M.; Soltanieh, M.; Mousavi, S.A. Simultaneous separation of H2S and CO2 from natural gas by hollow fiber membrane contactor using mixture of alkanolamines. J. Membr. Sci. 2011, 377, 191–197. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K.; Al-Marzouqi, M. H2S absorption at high pressure using hollow fibre membrane contactors. Chem. Eng. Process. Process Intensif. 2014, 83, 33–42. [Google Scholar] [CrossRef]
- Al-Marzouqi, M.H.; Marzouk, S.A.; Abdullatif, N. High pressure removal of acid gases using hollow fiber membrane contactors: Further characterization and long-term operational stability. J. Nat. Gas Sci. Eng. 2017, 37, 192–198. [Google Scholar] [CrossRef]
- Marzouk, S.A.; Al-Marzouqi, M.H.; Teramoto, M.; Abdullatif, N.; Ismail, Z.M. Simultaneous removal of CO2 and H2S from pressurized CO2–H2S–CH4 gas mixture using hollow fiber membrane contactors. Sep. Purif. Technol. 2012, 86, 88–97. [Google Scholar] [CrossRef]
- Rongwong, W.; Boributh, S.; Assabumrungrat, S.; Laosiripojana, N.; Jiraratananon, R. Simultaneous absorption of CO2 and H2S from biogas by capillary membrane contactor. J. Membr. Sci. 2012, 392, 38–47. [Google Scholar] [CrossRef]
- Jin, P.; Huang, C.; Shen, Y.; Zhan, X.; Hu, X.; Wang, L.; Wang, L. Simultaneous Separation of H2S and CO2 from Biogas by Gas–Liquid Membrane Contactor Using Single and Mixed Absorbents. Energy Fuels 2017, 31, 11117–11126. [Google Scholar] [CrossRef]
- Tilahun, E.; Sahinkaya, E.; Çalli, B. A hybrid membrane gas absorption and bio-oxidation process for the removal of hydrogen sulfide from biogas. Int. Biodeterior. Biodegrad. 2018, 127, 69–76. [Google Scholar] [CrossRef]
- Wang, D.; Teo, W.K.; Li, K. Removal of H2S to ultra-low concentrations using an asymmetric hollow fibre membrane module. Sep. Purif. Technol. 2002, 27, 33–40. [Google Scholar] [CrossRef]
- Boucif, N.; Favre, E.; Roizard, D.; Belloul, M. Hollow fiber membrane contactor for hydrogen sulfide odor control. Am. Inst. Chem. Eng. J. 2008, 54, 122–131. [Google Scholar] [CrossRef]
- Esquiroz-Molina, A.; Georgaki, S.; Stuetz, R.; Jefferson, B.; McAdam, E.J. Influence of pH on gas phase controlled mass transfer in a membrane contactor for hydrogen sulphide absorption. J. Membr. Sci. 2013, 427, 276–282. [Google Scholar] [CrossRef]
- Lee, H.K.; Jo, H.D.; Choi, W.K.; Park, H.H.; Lim, C.W.; Lee, Y.T. Absorption of SO2 in hollow fiber membrane contactors using various aqueous absorbents. Desalination 2006, 1, 604–605. [Google Scholar] [CrossRef]
- Yu, H.; Tan, Z.; Feng, X. Modeling SO2 absorption into water accompanied with reversible reaction in a hollow fiber membrane contactor. Chem. Eng. Sci. 2016, 156, 136–146. [Google Scholar] [CrossRef]
- Park, H.H.; Deshwal, B.R.; Kim, I.W.; Lee, H.K. Absorption of SO2 from flue gas using PVDF hollow fiber membranes in a gas–liquid contactor. J. Membr. Sci. 2008, 319, 29–37. [Google Scholar] [CrossRef]
- Luis, P.; Garea, A.; Irabien, A. Sulfur dioxide non-dispersive absorption in N, N-dimethylaniline using a ceramic membrane contactor. Journal of Chemical Technology & Biotechnology: International Research in Process. Environ. Clean Technol. 2008, 83, 1570–1577. [Google Scholar] [CrossRef]
- Luis, P.; Garea, A.; Irabien, A. Environmental and economic evaluation of SO2 recovery in a ceramic hollow fibre membrane contactor. Chem. Eng. Process. Process Intensif. 2012, 52, 151–154. [Google Scholar] [CrossRef]
- Luis, P.; Garea, A.; Irabien, A. Zero solvent emission process for sulfur dioxide recovery using a membrane contactor and ionic liquids. J. Membr. Sci. 2009, 330, 80–89. [Google Scholar] [CrossRef]
- Albo, J.; Luis, P.; Irabien, A. Absorption of coal combustion flue gases in ionic liquids using different membrane contactors. Desalin. Water Treat. 2011, 27, 54–59. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Bazhenov, S.; Malakhov, A.; Bakhtin, D.; Khotimskiy, V.; Bondarenko, G.; Volkov, V.; Ramdin, M.; Vlugt, T.; Volkov, A. CO2 stripping from ionic liquid at elevated pressures in gas-liquid membrane contactor. Int. J. Greenh. Gas Control 2018, 71, 293–302. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Precombustion CO2 capture in polymeric hollow fiber membrane contactors using ionic liquids: Porous membrane versus nonporous composite membrane. Ind. Eng. Chem. Res. 2016, 55, 5983–5992. [Google Scholar] [CrossRef]
- Ramdin, M.; Amplianitis, A.; Bazhenov, S.; Volkov, A.; Volkov, V.; Vlugt, T.J.H.; de Loos, T.W. Solubility of CO2 and CH4 in ionic liquids: Ideal CO2/CH4 selectivity. Ind. Eng. Chem. Res. 2014, 53, 15427–15435. [Google Scholar] [CrossRef]
- Bazhenov, S.; Ramdin, M.; Volkov, A.; Volkov, V.; Vlugt, T.J.; de Loos, T.W. CO2 Solubility in biodegradable hydroxylammonium-based ionic liquids. J. Chem. Eng. Data 2014, 59, 702–708. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Rezakazemi, M.; Chen, F.; Luis, P.; Van der Bruggen, B. Effect of flow and module configuration on SO2 absorption by using membrane contactors. Glob. Nest J. 2017, 19, 716–725. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Wood, D.A.; Zhang, W.; Li, L.; Zhang, L.; Van der Bruggen, B. Influence of the membrane module geometry on SO2 removal: A numerical study. Ind. Eng. Chem. Res. 2015, 54, 11619–11627. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Hosseinzadeh, M.; Vatani, A.; Mohammadi, T. Mathematical modeling for the simultaneous absorption of CO2 and SO2 using MEA in hollow fiber membrane contactors. Chem. Eng. Process. Process Intensif. 2017, 111, 35–45. [Google Scholar] [CrossRef]
- Klaassen, R. Achieving flue gas desulphurization with membrane gas absorption. Filtr. Sep. 2003, 40, 26–28. [Google Scholar] [CrossRef]
- Klaassen, R.; Feron, P.H.M.; Jansen, A.E. Membrane contactors in industrial applications. Chem. Eng. Res. Des. 2005, 83, 234–246. [Google Scholar] [CrossRef]
- Sun, X.; Meng, F.; Yang, F. Application of seawater to enhance SO2 removal from simulated flue gas through hollow fiber membrane contactor. J. Membr. Sci. 2008, 312, 6–14. [Google Scholar] [CrossRef]
- Tan, X.; Capar, G.; Li, K. Analysis of dissolved oxygen removal in hollow fibre membrane modules: Effect of water vapour. J. Membr. Sci. 2005, 251, 111–119. [Google Scholar] [CrossRef]
- Sengupta, A.; Peterson, P.A.; Miller, B.D.; Schneider, J.; Fulk, C.W. Large-scale application of membrane contactors for gas transfer from or to ultrapure water. Sep. Purif. Technol. 1998, 14, 189–200. [Google Scholar] [CrossRef]
- Kartohardjono, S.; Chen, V. Mass Transfer and Fluid Hydrodynamics in Sealed End Hydrophobic Hollow Fiber Membrane Gas-liquid Contactors. J. Appl. Membr. Sci. Technol. 2005, 2. [Google Scholar] [CrossRef]
- Peng, Z.-G.; Lee, S.-H.; Zhou, T.; Shieh, J.-J.; Chung, T.-Sh. A study on pilot-scale degassing by polypropylene (PP) hollow fiber membrane contactors. Desalination 2008, 234, 316–322. [Google Scholar] [CrossRef]
- Ito, A.; Yamagiwa, K.; Tamura, M.; Furusawa, M. Removal of dissolved oxygen using non-porous hollow-fiber membranes. J. Membr. Sci. 1998, 145, 111–117. [Google Scholar] [CrossRef]
- Shao, J.; Liu, H.; He, Y. Boiler feed water deoxygenation using hollow fiber membrane contactor. Desalination 2008, 234, 370–377. [Google Scholar] [CrossRef]
- Kattan, O.; Ebbers, K.; Koolaard, A.; Vos, H.; Bargeman, G. Membrane contactors: An alternative for de-aeration of salt solutions? Sep. Purif. Technol. 2018, 205, 231–240. [Google Scholar] [CrossRef]
- Sinha, V.; Li, K. Alternative methods for dissolved oxygen removal from water: A comparative study. Desalination 2000, 127, 155–164. [Google Scholar] [CrossRef]
- Martić, I.; Maslarević, A.; Mladenović, S.; Lukić, U.; Budimir, S. Water deoxygenation using hollow fiber membrane module with nitrogen as inert gas. Desalin. Water Treat. 2015, 54, 1563–1567. [Google Scholar] [CrossRef]
- Liu, L.; Ding, Z.; Chang, L.; Ma, R.; Yang, Z. Ultrasonic enhancement of membrane-based deoxygenation and simultaneous influence on polymeric hollow fiber membrane. Sep. Purif. Technol. 2007, 56, 133–142. [Google Scholar] [CrossRef]
- Bhaumik, D.; Majumdar, S.; Fan, Q.; Sirkar, K.K. Hollow fiber membrane degassing in ultrapure water and microbiocontamination. J. Membr. Sci. 2004, 235, 31–41. [Google Scholar] [CrossRef]
- Volkov, V.V.; Lebedeva, V.I.; Petrova, I.V.; Bobyl, A.V.; Konnikov, S.G.; Roldughin, V.I.; van Erkel, J.; Tereshchenko, G.F. Adlayers of palladium particles and their aggregates on porous polypropylene hollow fiber membranes as hydrogenization contractors/reactors. Adv. Colloid Interface Sci. 2011, 164, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Van der Vaart, R.; Lebedeva, V.I.; Petrova, I.V.; Plyasova, L.M.; Rudina, N.A.; Kochubey, D.I.; Tereshchenko, G.F.; Volkov, V.V.; Van Erkel, J. Preparation and characterisation of palladium-loaded polypropylene porous hollow fibre membranes for hydrogenation of dissolved oxygen in water. J. Membr. Sci. 2007, 299, 38–44. [Google Scholar] [CrossRef]
- Lebedeva, V.I.; Gryaznov, V.M.; Petrova, I.V.; Volkov, V.V.; Tereshchenko, G.F.; Shkol’nikov, E.I.; Plyasova, L.M.; Kochubey, D.I.; van der Vaart, R.; van Soest-Verecammen, E.L.J. Porous Pd-containing polypropylene membranes for catalytic water deoxygenation. Kinet. Catal. 2006, 47, 867–872. [Google Scholar] [CrossRef]
- Su, J. Effects of Vacuum and Flow Rate on Water Deoxygenation through Tri-Bore PVDF Hollow Fiber Membranes. SF J. Mater. Chem. Eng. 2018, 1, 1010. [Google Scholar]
- Mao, L.; Wang, F.; Su, J. Development of Robust Tri-Bore Hollow Fiber Membranes for the Control of Dissolved Oxygen in Water. SF J. Mater. Chem Eng. 2018, 1, 1002. [Google Scholar]
- Todorović, J.; Krstić, D.M.; Vatai, G.N.; Tekić, M.N. Gas absorption in a hollow-fiber membrane contactor with pseudo-plastic liquid as an absorbent. Desalination 2006, 193, 286–290. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Khivantsev, K.; Pyrzynski, T.; Klinghoffer, N.; Shakouri, A.N.; Yu, M.; Li, S. Bio-mimetic Oxygen Separation via Hollow Fiber Membrane Contactor with O2 Carrier Solutions. Chem. Commun. 2018, 54, 9454–9457. [Google Scholar] [CrossRef] [PubMed]
- Banazadeh, H.; Mousavi, S.M. A novel process for oxygen absorption from air using hollow fiber gas-liquid membrane contactor. Sep. Purif. Technol. 2018, 193, 283–288. [Google Scholar] [CrossRef]
- Dutton, R.C.; Mather, F.W.; Walker, S.N.; Lipps, B.J., Jr.; Rudy, L.W.; Severinghaus, J.W.; Edmunds, L.H., Jr. Development and evaluation of a new hollow-fiber membrane oxygenator. ASAIO J. 1971, 17, 331–336. [Google Scholar]
- Iwahashi, H.; Yuri, K.; Nosé, Y. Development of the oxygenator: Past, present, and future. J. Art. Organs. 2004, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Madhani, S.P.; D’aloiso, B.D.; Frankowski, B.; Federspiel, W.J. Darcy permeability of hollow fiber membrane bundles made from Membrana® Polymethylpentene (PMP) fibers used in respiratory assist devices. ASAIO J. 1992, 62, 329. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.R.; Garcia, J.D.; Han, B. Mass and momentum transfer in hollow fibre blood oxygenators. J. Membr. Sci. 2002, 208, 247–256. [Google Scholar] [CrossRef]
- Wickramasinghe, S.R.; Semmens, M.J.; Cussler, E.L. Hollow fiber modules made with hollow fiber fabric. J. Membr. Sci. 1993, 84, 1–14. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Ding, Z.; Ma, R.; Yang, Z. Mass transfer enhancement in coiled hollow fiber membrane modules. J. Membr. Sci. 2005, 264, 113–121. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.P.; Xu, Y.Y.; Zhu, B.K. Oxygen transfer characteristics of hydrophilic treated polypropylene hollow fiber membranes for bubbleless aeration. J. Membr. Sci. 2010, 362, 47–57. [Google Scholar] [CrossRef]
- Haramoto, H.; Kokubo, K.I.; Sakai, K.; Kuwana, K.; Nakanishi, H. An artificial gill system for oxygen uptake from water using perfluorooctylbromide. ASAIO J. 1992, 40, M803–M807. [Google Scholar] [CrossRef]
- Heo, P.W.; Park, I.S. Separation characteristics of dissolved gases from water using a polypropylene hollow fiber membrane module with high surface area. World Acad. Sci. Eng. Technol. 2014, 8, 1266–1269. [Google Scholar]
- Heo, P.W. Increasing separation of dissolved gases using a portable system with hollow fiber membrane modules including two inlets. Int. Res. J. Eng. Technol. 2015, 2, 1457–1460. [Google Scholar]
- Santos, F.R.A.D.; Borges, C.P.; Fonseca, F.V.D. Polymeric materials for membrane contactor devices applied to water treatment by ozonation. Mater. Res. 2015, 18, 1015–1022. [Google Scholar] [CrossRef]
- Wenten, I.G.; Julian, H.; Panjaitan, N.T. Ozonation through ceramic membrane contactor for iodide oxidation during iodine recovery from brine water. Desalination 2012, 306, 29–34. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Sklari, S.D.; Zamboulis, D.; Zaspalis, V.T.; Zouboulis, A.I. Development of bubble-less ozonation and membrane filtration process for the treatment of contaminated water. J. Membr. Sci. 2015, 492, 40–47. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Application of a ceramic membrane contacting process for ozone and peroxone treatment of micropollutant contaminated surface water. J. Hazard. Mater. 2018, 358, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kukuzaki, M.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Baba, Y. Ozone mass transfer in an ozone–water contacting process with Shirasu porous glass (SPG) membranes—A comparative study of hydrophilic and hydrophobic membranes. Sep. Purif. Technol. 2010, 72, 347–356. [Google Scholar] [CrossRef]
- Gottschalk, C.; Beuscher, U.; Hardwick, S.; Kobayashi, M.; Schweckendiek, J.; Wikol, M. Production of high concentrations of bubble-free dissolved ozone in water. In Solid State Phenomena; Heyns, M., Marc Meuris, M., Mertens, P., Eds.; Trans Tech Publications: Zürich, Switzerland, 1999; Volume 65, pp. 59–62. [Google Scholar] [CrossRef]
- Cornelissen, I.; Meuris, M.; Wolke, K.; Wikol, M.; Loewenstein, L.M.; Doumen, G.; Heyns, M.M. Ozonated DI-water for clean chemical oxide growth. In Solid State Phenomena; Heyns, M., Marc Meuris, M., Mertens, P., Eds.; Trans Tech Publications: Zürich, Switzerland, 1999; Volume 65, pp. 77–80. [Google Scholar] [CrossRef]
- Atchariyawut, S.; Phattaranawik, J.; Leiknes, T.; Jiraratananon, R. Application of ozonation membrane contacting system for dye wastewater treatment. Sep. Purif. Technol. 2009, 66, 153–158. [Google Scholar] [CrossRef]
- Bamperng, S.; Suwannachart, T.; Atchariyawut, S.; Jiraratananon, R. Ozonation of dye wastewater by membrane contactor using PVDF and PTFE membranes. Sep. Purif. Technol. 2010, 72, 186–193. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W. Oxidation of ci acid orange 7 with ozone and hydrogen peroxide in a hollow fiber membrane reactor. Chem. Eng. Commun. 2011, 198, 1530–1544. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Zhang, D.; Xia, Q. Decomposition of 4-nitrophenol by ozonation in a hollow fiber membrane reactor. Chem. Eng. Commun. 2009, 197, 377–386. [Google Scholar] [CrossRef]
- Wang, Z.; Xiu, G.; Qiao, T.; Zhao, K.; Zhang, D. Coupling ozone and hollow fibers membrane bioreactor for enhanced treatment of gaseous xylene mixture. Bioresour. Technol. 2013, 130, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.H.S.; De Rijk, J.W.; Zwijnenburg, A.; Mulder, M.H.V.; Wessling, M. Hollow fiber membrane contactors—A means to study the reaction kinetics of humic substance ozonation. J. Membr. Sci. 2005, 257, 48–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Wang, J.; Hou, D.; Liu, H. Ozone mass transfer behaviors on physical and chemical absorption for hollow fiber membrane contactors. Water Sci. Technol. 2017, 76, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Zhang, L.Z.; Tang, K.; Pei, L.X. Turbulent heat and mass transfer across a hollow fiber membrane tube bank in liquid desiccant air dehumidification. J. Heat Transf. 2012, 134, 082001. [Google Scholar] [CrossRef]
- Woods, J. Membrane processes for heating, ventilation, and air conditioning. Renew. Sustain. Energy Rev. 2014, 33, 290–304. [Google Scholar] [CrossRef]
- Pantelic, J.; Teitelbaum, E.; Bozla, M.; Kim, S.; Meggers, F. Development of moisture absorber based on hydrophilic nonporous membrane mass exchanger and alkoxylated siloxane liquid desiccant. Energy Build. 2018, 160, 34–43. [Google Scholar] [CrossRef]
- Zhang, L.Z. Coupled heat and mass transfer in an application-scale cross-flow hollow fiber membrane module for air humidification. Int. J. Heat Mass Transf. 2012, 55, 5861–5869. [Google Scholar] [CrossRef]
- Charles, N.T.; Johnson, D.W. The occurrence and characterization of fouling during membrane evaporative cooling. J. Membr. Sci. 2008, 319, 44–53. [Google Scholar] [CrossRef]
- Johnson, D.W.; Yavuzturk, C.C.; Rangappa, A.S. Formaldehyde removal from air during membrane air humidification evaporative cooling: Effects of contactor design and operating conditions. J. Membr. Sci. 2010, 354, 55–62. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Huang, S.M.; Pei, L.X. Conjugate heat and mass transfer in a cross-flow hollow fiber membrane contactor for liquid desiccant air dehumidification. Int. J. Heat Mass Transf. 2012, 55, 8061–8072. [Google Scholar] [CrossRef]
- Huang, S.M.; Qin, F.G.; Yang, M.; Yang, X.; Zhong, W.F. Heat and mass transfer deteriorations in an elliptical hollow fiber membrane tube bank for liquid desiccant air dehumidification. Appl. Therm. Eng. 2013, 57, 90–98. [Google Scholar] [CrossRef]
- Bettahalli, N.S.; Lefers, R.; Fedoroff, N.; Leiknes, T.; Nunes, S.P. Triple-bore hollow fiber membrane contactor for liquid desiccant based air dehumidification. J. Membr. Sci. 2016, 514, 135–142. [Google Scholar] [CrossRef]
- Lefers, R.; Bettahalli, N.S.; Fedoroff, N.; Nunes, S.P.; Leiknes, T. Vacuum membrane distillation of liquid desiccants utilizing hollow fiber membranes. Sep. Purif. Technol. 2018, 199, 57–63. [Google Scholar] [CrossRef]
- Fakharnezhad, A.; Keshavarz, P. Experimental investigation of gas dehumidification by tri-ethylene glycol in hollow fiber membrane contactors. J. Ind. Eng. Chem. 2016, 34, 390–396. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, W.; He, X.; Ren, K. Air dehumidification by hollow fibre membrane with chilled water for spacecraft applications. Indoor Built Environ. 2016, 25, 758–771. [Google Scholar] [CrossRef]
- He, K.; Chen, S.; Huang, C.C.; Zhang, L.Z. Fluid flow and mass transfer in an industrial-scale hollow fiber membrane contactor scaled up with small elements. Int. J. Heat Mass Transf. 2018, 127, 289–301. [Google Scholar] [CrossRef]
- Bai, H.; Zhu, J.; Chen, Z.; Chu, J. State-of-art in modelling methods of membrane-based liquid desiccant heat and mass exchanger: A comprehensive review. Int. J. Heat Mass Transf. 2018, 125, 445–470. [Google Scholar] [CrossRef]
- Qu, M.; Abdelaziz, O.; Gao, Z.; Yin, H. Isothermal membrane-based air dehumidification: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 82, 4060–4069. [Google Scholar] [CrossRef]
- Abdel-Salam, M.R.; Ge, G.; Fauchoux, M.; Besant, R.W.; Simonson, C.J. State-of-the-art in liquid-to-air membrane energy exchangers (LAMEEs): A comprehensive review. Renew. Sustain. Energy Rev. 2014, 39, 700–728. [Google Scholar] [CrossRef]
- Huang, S.M.; Zhang, L.Z. Researches and trends in membrane-based liquid desiccant air dehumidification. Renew. Sustain. Energy Rev. 2013, 28, 425–440. [Google Scholar] [CrossRef]
- Dalane, K.; Svendsen, H.F.; Hillestad, M.; Deng, L. Membrane contactor for subsea natural gas dehydration: Model development and sensitivity study. J. Membr. Sci. 2018, 556, 263–276. [Google Scholar] [CrossRef]
- Macedonio, F.; Brunetti, A.; Barbieri, G.; Drioli, E. Membrane condenser as a new technology for water recovery from humidified “waste” gaseous streams. Ind. Eng. Chem. Res. 2012, 52, 1160–1167. [Google Scholar] [CrossRef]
- Brunetti, A.; Santoro, S.; Macedonio, F.; Figoli, A.; Drioli, E.; Barbieri, G. Waste gaseous streams: From environmental issue to source of water by using membrane condensers. Clean Soil Air Water 2014, 42, 1145–1153. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, S.; Wardhaugh, L.; Feron, P.H. Innovative use of membrane contactor as condenser for heat recovery in carbon capture. Environ. Sci. Technol. 2015, 49, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yue, M.; Qi, H.; Feron, P.H.; Zhao, S. Transport membrane condenser for water and heat recovery from gaseous streams: Performance evaluation. J. Membr. Sci. 2015, 484, 10–17. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Huang, S.M. Coupled heat and mass transfer in a counter flow hollow fiber membrane module for air humidification. Int. J. Heat Mass Transf. 2011, 54, 1055–1063. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Aydin, D.; Ding, Y.; Zhang, S.; Reay, D.; Riffat, S. A novel evaporative cooling system with a polymer hollow fibre spindle. Appl. Therm. Eng. 2018, 132, 665–675. [Google Scholar] [CrossRef]
- Safarik, D.J.; Eldridge, R.B. Olefin/paraffin separations by reactive absorption: A review. Ind. Eng. Chem. Res. 1998, 37, 2571–2581. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Supported liquid membrane separation of propylene–propane mixtures using a metal ion carrier. Desalination 2010, 250, 130–135. [Google Scholar] [CrossRef]
- Agel, F.; Pitsch, F.; Krull, F.F.; Schulz, P.; Wessling, M.; Melin, T.; Wassercheid, P. Ionic liquid silver salt complexes for propene/propane separation. Phys. Chem. Chem. Phys. 2011, 13, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Tsou, D.T.; Blachman, M.W.; Davis, J.C. Silver-facilitated olefin/paraffin separation in a liquid membrane contactor system. Ind. Eng. Chem. Res. 1994, 33, 3209–3216. [Google Scholar] [CrossRef]
- Nymeijer, D.C.; Visser, T.; Assen, R.; Wessling, M. Composite hollow fiber gas–liquid membrane contactors for olefin/paraffin separation. Sep. Purif. Technol. 2004, 37, 209–220. [Google Scholar] [CrossRef]
- Nymeijer, K.; Visser, T.; Assen, R.; Wessling, M. Olefin-Selective Membranes in Gas− Liquid Membrane Contactors for Olefin/Paraffin Separation. Ind. Eng. Chem. Res. 2004, 43, 720–727. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Fallanza, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Improving the mass transfer rate in G–L membrane contactors with ionic liquids as absorption medium. Recovery of propylene. J. Membr. Sci. 2011, 385, 217–225. [Google Scholar] [CrossRef]
- Fallanza, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Effect of liquid flow on the separation of propylene/propane mixtures with a gas/liquid membrane contactor using Ag+-RTIL solutions. Desalin. Water Treat. 2011, 27, 123–129. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Ortiz, I.; Li, K. Separation of olefin/paraffin gas mixtures using ceramic hollow fiber membrane contactors. Ind. Eng. Chem. Res. 2013, 52, 7918–7929. [Google Scholar] [CrossRef]
- Yang, D.; Barbero, R.S.; Devlin, D.J.; Cussler, E.L.; Colling, C.W.; Carrera, M.E. Hollow fibers as structured packing for olefin/paraffin separations. J. Membr. Sci. 2006, 279, 61–69. [Google Scholar] [CrossRef]
- Yang, D.; Devlin, D.J.; Barbero, R.S. Effect of hollow fiber morphology and compatibility on propane/propylene separation. J. Membr. Sci. 2007, 304, 88–101. [Google Scholar] [CrossRef]
- Yang, D.; Le, L.; Martinez, R.; Morrison, M. Hollow fibers structured packings in olefin/paraffin distillation: Apparatus scale-up and long-term stability. Ind. Eng. Chem. Res. 2013, 52, 9165–9179. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Ismail, Z. Gas–liquid membrane contactor for ethylene/ethane separation by aqueous silver nitrate solution. Sep. Purif. Technol. 2014, 127, 140–148. [Google Scholar] [CrossRef]
- Kirsch, V.A.; Roldugin, V.I.; Ovcharova, A.A.; Bildyukevich, A.V. Modeling of Ethylene Absorption from an Ethylene–Ethane Mixture by Silver Nitrate Aqueous Solution in a Hollow-Fiber Membrane Contactor. Petrol. Chem. 2017, 57, 1242–1249. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Teramoto, M.; Al-Marzouqi, M.H.; Kamio, E.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Experimental and theoretical study on propylene absorption by using PVDF hollow fiber membrane contactors with various membrane structures. J. Membr. Sci. 2010, 346, 86–97. [Google Scholar] [CrossRef]
- Ortiz, A.; Gorri, D.; Irabien, Á.; Ortiz, I. Separation of propylene/propane mixtures using Ag+–RTIL solutions. Evaluation and comparison of the performance of gas–liquid contactors. J. Membr. Sci. 2010, 360, 130–141. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Boributh, S.; Jiraratananon, R.; Ortiz, I.; Li, K. Long term stability of PTFE and PVDF membrane contactors in the application of propylene/propane separation using AgNO3 solution. Chem. Eng. Sci. 2013, 94, 108–119. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).