Dye Adsorption and Electrical Property of Oxide-Loaded Carbon Fiber Made by Electrospinning and Hydrothermal Treatment
Abstract
:1. Introduction
2. Materials, Instrumentation, and Experimental Methods
3. Results and Discussion
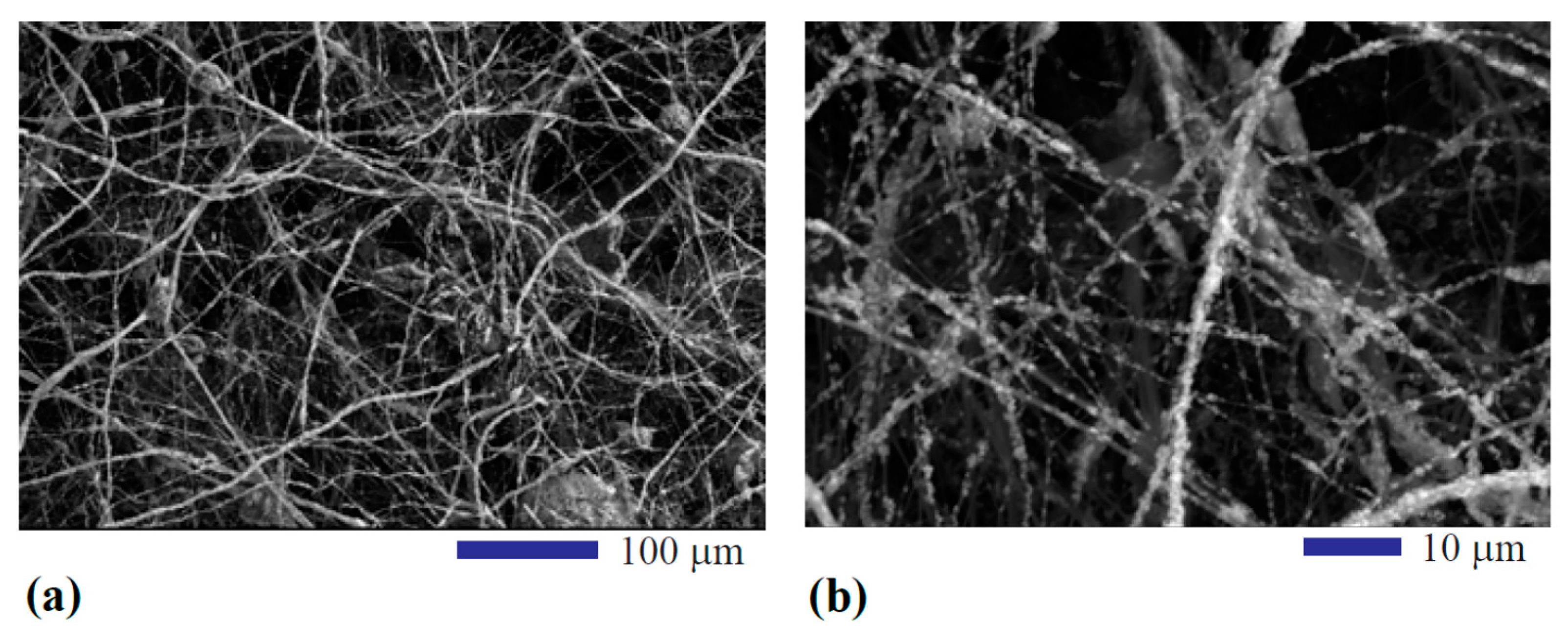
3.1. Morphology of the Processed Fiber
3.2. Effect of Hydrothermal Carbonization
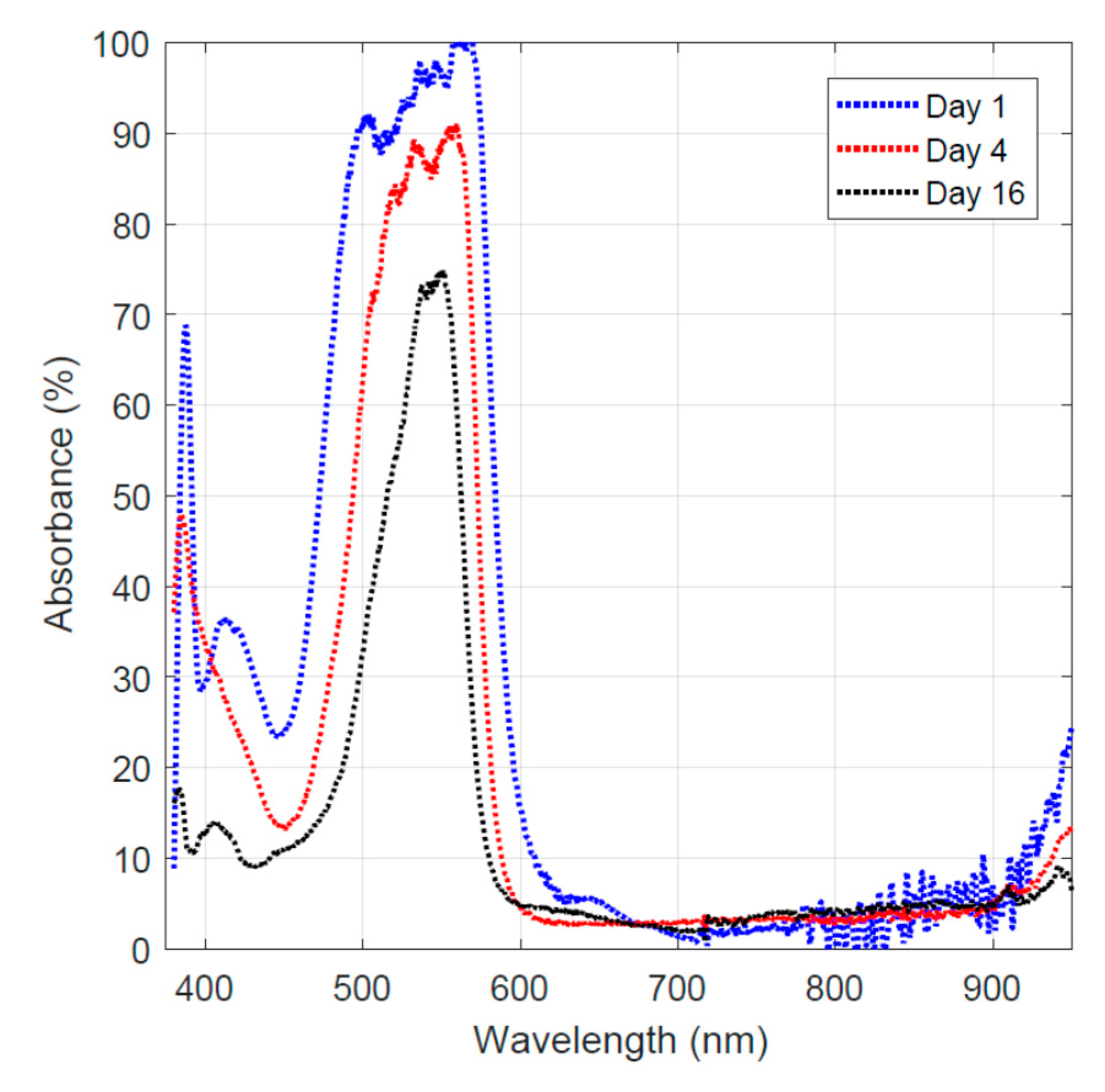
3.3. Dye Adsorption Behavior
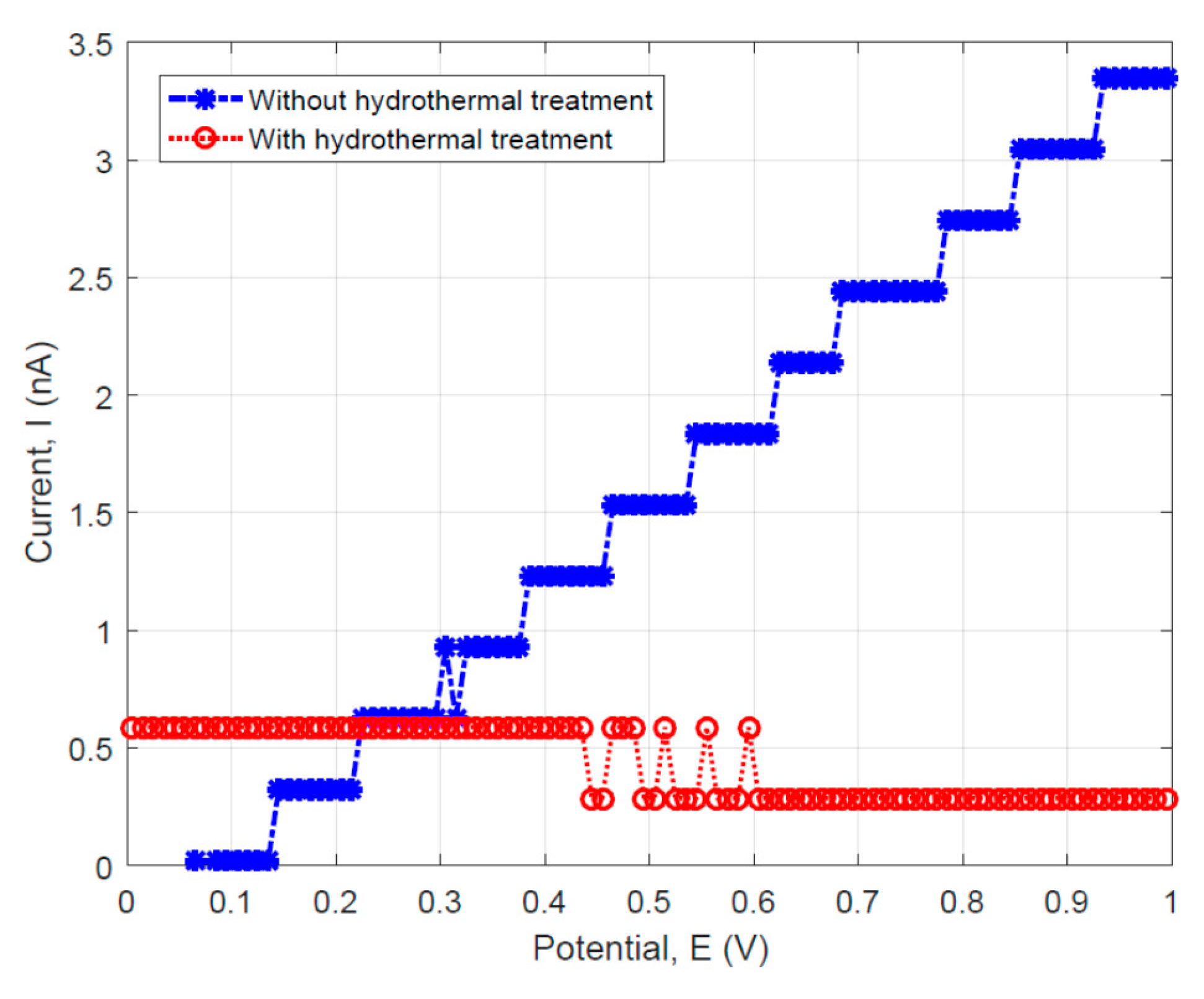
3.4. Electrical Property
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, K.Y.; Lee, K.; Kim, D. Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization. Bioresour. Technol. 2018, 258, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Su, Y.; Xu, D.; Zhu, S.; Liu, X. Assessment of hydrothermal carbonization and coupling washing with torrefaction of bamboo sawdust for biofuels production. Bioresour. Technol. 2018, 258, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, K.; Liu, H.; Li, Y.; Yi, L.; Yao, H. Correlations between hydrochar properties and chemical constitution of orange peel waste during hydrothermal carbonization. Bioresour. Technol. 2018, 265, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, S.; Hao, N.; Wells, T.; Ragauskas, A.J. Characterization of products from hydrothermal carbonization of pine. Bioresour. Technol. 2107, 244, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Ma, X. Characteristics of co-hydrothermal carbonization on polyvinyl chloride wastes with bamboo. Bioresour. Technol. 2018, 247, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, E. Treatment of urban sludge by hydrothermal carbonization. Bioresour. Technol. 2017, 238, 182–187. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Polprasert, C. Faecal sludge treatment and utilization by hydrothermal carbonization. J. Environ. Manag. 2018, 216, 421–426. [Google Scholar] [CrossRef]
- Catalkopru, A.K.; Kantarli, I.C.; Yanik, J. Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 2017, 226, 89–93. [Google Scholar] [CrossRef]
- Tradler, S.B.; Mayr, S.; Himmelsbach, M.; Priewasser, R.; Stadler, A.T. Hydrothermal carbonization as an all-inclusive process for food-waste conversion. Bioresour. Technol. Rep. 2018, 2, 77–83. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Nelles, M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour. Technol. 2018, 260, 404–412. [Google Scholar] [CrossRef]
- Idowu, I.; Li, L.; Flora, J.R.V.; Pellechia, P.J.; Berge, N.D. Hydrothermal carbonization of food waste for nutrient recovery and reuse. Waste Manag. 2017, 69, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Saqib, U.N.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Zheng, C. Effects of aqueous phase recirculation in hydrothermal carbonization of sweet potato waste. Bioresour. Technol. 2018, 267, 167–174. [Google Scholar] [CrossRef]
- Cruz, O.F.; Silvestre-Albero, J.; Casco, M.E.; Hotza, D.; Rambo, C.R. Activated nanocarbons produced by microwave-assisted hydrothermal carbonization of Amazonian fruit waste for methane storage. Mater. Chem. Phys. 2018, 216, 42–46. [Google Scholar] [CrossRef]
- Yeoh, K.H.; Shafie, S.A.; Al-attab, K.A.; Zainal, Z.A. Upgrading agricultural wastes using three different carbonization methods: Thermal, hydrothermal and vapothermal. Bioresour. Technol. 2018, 265, 365–371. [Google Scholar] [CrossRef]
- Seyedsadr, S.; Al Afif, R.; Pfeifer, C. Hydrothermal carbonization of agricultural residues: A case study of the farm residues-based biogas plants. Carbon Resour. Convers. 2018, 1, 81–85. [Google Scholar] [CrossRef]
- Gallifuoco, A.; Giacomo, G.D. Novel kinetic studies on biomass hydrothermal carbonization. Bioresour. Technol. 2018, 266, 189–193. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Jung, D.; Kruse, A. Evaluation of Arrhenius-type overall kinetic equations for hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2017, 127, 286–291. [Google Scholar] [CrossRef]
- Liu, F.; Yu, R.; Ji, X.; Guo, M. Hydrothermal carbonization of holocellulose into hydrochar: Structural, chemical characteristics, and combustion behavior. Bioresour. Technol. 2018, 263, 508–516. [Google Scholar] [CrossRef]
- Wettayavong, S.; Sangnoi, S.; Kaewtrakulchai, N.; Eiad-Ua, A. Characterization of carbon fibers from Thai horse manure via hydrothermal carbonization. Mater. Today Proc. 2018, 5, 10940–10945. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Li, G. Process characteristics for microwave assisted hydrothermal carbonization of cellulose. Bioresour. Technol. 2018, 259, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Guo, X.; Li, J.; Sun, H.; Zhang, H.; Wang, W. Electrospinning preparation and dye adsorption capacity of TiO2@Carbon flexible fiber. Ceram. Int. 2019, 45, 1156–1160. [Google Scholar] [CrossRef]
- Yuan, L.; Wei, X.; Martinez, J.P.; Yu, C.; Panahi, N.; Gan, J.B.; Zhang, Y.P.; Gan, Y.X. Reaction spinning titanium dioxide particle-coated carbon fiber for photoelectric energy conversion. Fibers 2019, 7, 49. [Google Scholar] [CrossRef]
- Mousavi, S.; Deuber, F.; Petrozzi, S.; Federer, L.; Aliabadi, M.; Shahraki, F.; Adlhart, C. Efficient dye adsorption by highly porous nanofiber aerogels. Colloids Surf. A 2018, 547, 117–125. [Google Scholar] [CrossRef]
- Deuber, F.; Mousavi, S.; Hofer, M.; Adlhart, C. Tailoring pore structures of ultralight electrospun sponges by solid templating. ChemistrySelect 2016, 1, 5595–5598. [Google Scholar] [CrossRef]
- Deuber, F.; Adlhart, C. From short electrospun nanofibers to ultralight aerogels with tunable pore structure. Chimia 2017, 71, 236–240. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Su, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243. [Google Scholar] [CrossRef]







| Element | C | O | Ti | Fe | Ni |
|---|---|---|---|---|---|
| Untreated 1 | 86.84 | 7.98 | 0.42 | 2.82 | 1.15 |
| Treated 2 | 98.49 | 0.19 | 0.02 | 0.98 | 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kansara, S.; Patel, S.; Gan, Y.X.; Jaimes, G.; Gan, J.B. Dye Adsorption and Electrical Property of Oxide-Loaded Carbon Fiber Made by Electrospinning and Hydrothermal Treatment. Fibers 2019, 7, 74. https://doi.org/10.3390/fib7080074
Kansara S, Patel S, Gan YX, Jaimes G, Gan JB. Dye Adsorption and Electrical Property of Oxide-Loaded Carbon Fiber Made by Electrospinning and Hydrothermal Treatment. Fibers. 2019; 7(8):74. https://doi.org/10.3390/fib7080074
Chicago/Turabian StyleKansara, Saurabh, Shivani Patel, Yong X. Gan, Gabriela Jaimes, and Jeremy B. Gan. 2019. "Dye Adsorption and Electrical Property of Oxide-Loaded Carbon Fiber Made by Electrospinning and Hydrothermal Treatment" Fibers 7, no. 8: 74. https://doi.org/10.3390/fib7080074
APA StyleKansara, S., Patel, S., Gan, Y. X., Jaimes, G., & Gan, J. B. (2019). Dye Adsorption and Electrical Property of Oxide-Loaded Carbon Fiber Made by Electrospinning and Hydrothermal Treatment. Fibers, 7(8), 74. https://doi.org/10.3390/fib7080074





