Abstract
Devising environmental-friendly processes in biotechnology is a priority in the current economic scenario. We are witnessing a constant and steady push towards finding sustainable solutions to societal challenges by promoting innovation-driven activities minimizing the environmental impact and valorizing natural resources. In bioeconomy, plants are among the most important renewable sources of both fibers (woody and cellulosic) and phytochemicals, which find applications in many industrial sectors, spanning from the textile, to the biocomposite, medical, nutraceutical, and pharma sectors. Given the key role of plants as natural sources of (macro)molecules, we here provide a compendium on the use of plant fibers functionalized/impregnated with phytochemicals (in particular phenolic extracts). The goal is to review the various applications of natural fibers functionalized with plant phenolics and to valorize those plants that are source of both fibers and phytochemicals.
1. Introduction
Plants are bioresources providing valuable raw materials, such as fibers and wood, a major commodity [1], as well as phytochemicals displaying bioactivity and used in the healthcare, pharmaceutical, cosmetic, and nutraceutical industries [2]. Plant fibers have been used since the dawn of time [3]; the increasing environmental concerns draw attention on their exploitation, instead of synthetic ones, in the industrial sector.
Plant fibers can be woody (as those found in the xylem), or rich in crystalline cellulose (as, for example, the bast fibers produced by flax or hemp). Depending on their physicochemical characteristics, plant fibers can, therefore, be used for different purposes. For example, lignocellulosic fibers extracted from the stalk of fiber crops such as hemp are used for the manufacture of bricks (known as “hempcrete”) in buildings: Such fibers (which contain silica [4]) are mixed with lime, resulting in the formation of a lightweight cementitious material with insulating properties. Cellulosic fibers (such as those extracted from the cortex of hemp) find application in the biocomposite sector: They have several advantageous features, namely length (>100 mm in primary bast fibers [5]) and high tensile strength, because of the parallel orientation of the cellulose microfibrils to the fiber axis. Bast fibers are embedded in the stem cortex (Figure 1) and are glued together principally by pectin; therefore, to be used, they need to be separated from the surrounding tissues. This process is known as “retting” and is performed via the intervention of microorganisms (fungi, such as Ascomycota, Basidiomycota, and Zygomycota and bacteria belonging to the phyla Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes [6]) secreting enzymes acting on cell wall polysaccharides [7] (e.g., pectinases degrading the middle lamellas which “glue” together the bundles of bast fibers).
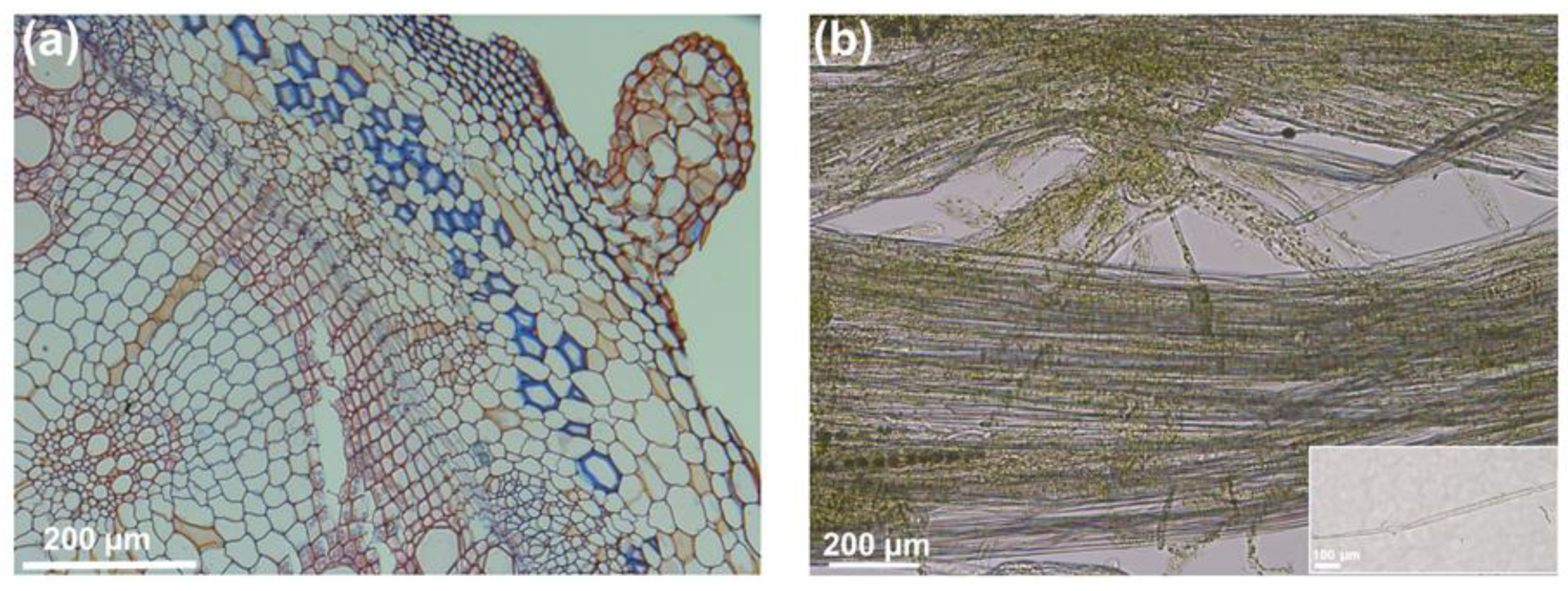
Figure 1.
Cross section of the stem of a representative fiber crop, stinging nettle (Urtica dioica L.), and details of enzyme-treated cortical peels with some separated bast fibers. (a) Transversal cross section stained with Safranin and Alcian blue (FASGA staining), showing in blue the cellulosic bast fibers and in red lignin; (b) cortical peels separated from the stem bottom internodes and treated for 24 h at 50 °C with TEXAZYM BFE (INOTEX Ltd, Czech Republic) showing some separated bast fibers; the inset shows one separated bast fiber.
The use of plant fibers provides several advantages, namely breathability and comfort in case of skin irritations/allergies [8]. Adding to them anti-microbial and anti-oxidant effects greatly widens their spectrum of applications to, for example, the manufacture of technical textiles, such as those used for wound healing. Notably, plants are also rich sources of phytochemicals showing anti-oxidant and bactericidal/fungicidal effects. Some plant species, such as the fiber crops hemp and nettle, are multi-purpose, as they produce both high yields of cellulosic fibers and phytochemicals [2,9].
In this review we will illustrate recent examples of natural fibers functionalized (i.e., with a chemical bond) or impregnated with phytochemicals (more specifically phenolics which show strong antimicrobial activities thanks to their chemical heterogeneity [10]). The goal is to highlight the value of plants as renewable resources of biomass (fibers) on one hand and of molecules with biological effects (e.g., antimicrobial) on the other. More specifically, the review is divided into five parts: One part will describe the synthesis and properties of plant fibers, one section will be devoted to the phenylpropanoid pathway, one paragraph will summarize the current methods used to extract and quantify/identify phenolics from plant matrices, a section will describe the antimicrobial properties of plant phenolics and the last part will report recent data on the combined use of plant fibers and phenolics. We will conclude the survey with an outlook on future prospects concerning the manufacture and use of functionalized plant fibers.
2. Properties of Plant Fibers and Characterization of Cellulose
The chief load-bearing component of plant fibers (both gelatinous and lignocellulosic) is cellulose. This polymer of glucose units linked by β-1,4-linkages is linear, which makes it possible for the glucan chains to pack tightly via hydrogen bonds and van der Waals interactions [11]. It is synthesized by the rosette terminal complex [12], consisting of six particles, each with up to six cellulose synthase (CESA) catalytic subunits.
Cellulose exists in different polymorphs, with cellulose-I being the native form and type Iβ the most abundant in higher plants. It has a crystalline and an amorphous region (Figure 2); it is, hence, interesting to determine its degree of crystallinity for the downstream applications. Cellulose crystallinity varies with the plants’ tissue type and age. An emblematic example is represented by fiber crops: Recently we have shown that, during development, the gelatinous layer in the bast fibers of the hemp hypocotyl undergoes compaction with the transition from a fibrillary texture to a homogeneous layer [13]. As the hypocotyl ages, cellulose crystallinity is a mechanical requirement for the bast fibers which support the stem and, more specifically, the vascular tissue (phloem) of the growing plant.
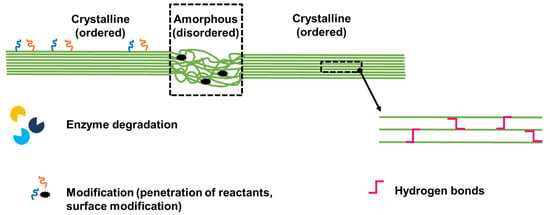
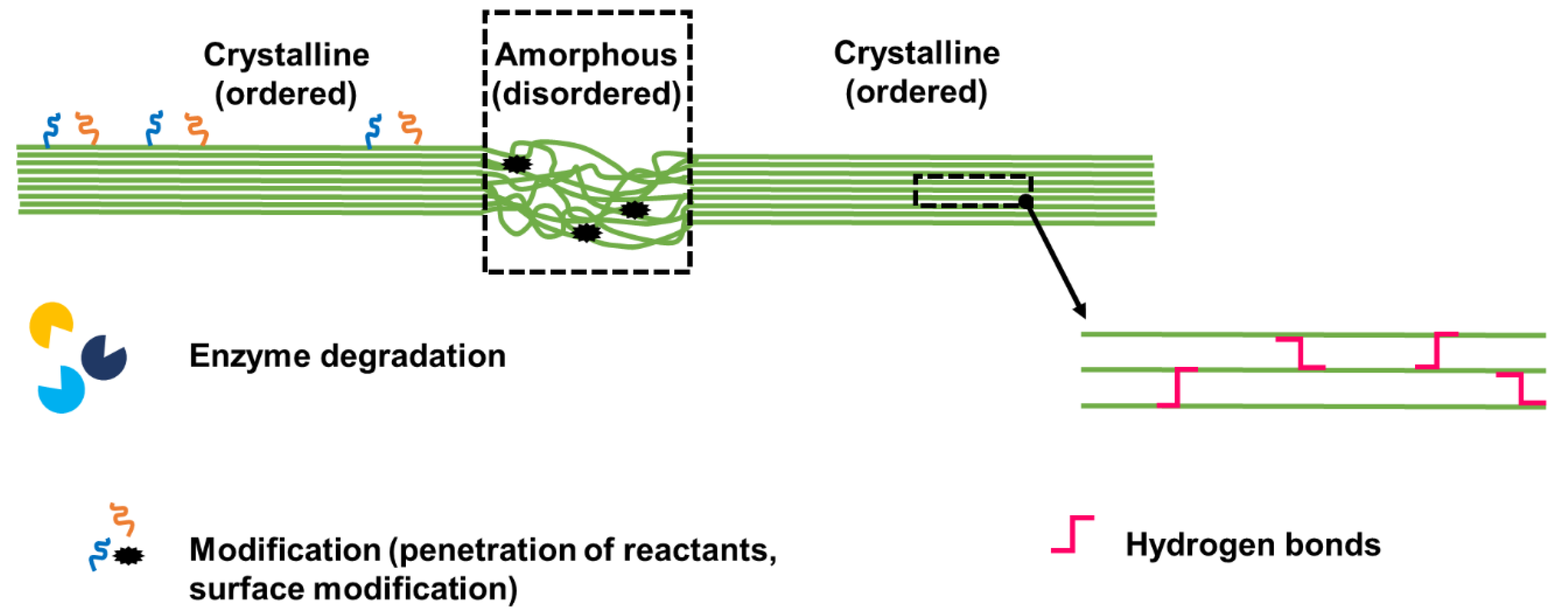
Figure 2.
Cartoon showing the crystalline and amorphous regions of cellulose, with schematic details showing that the amorphous region can be attacked by enzymes and is susceptible to modifications (via the easier penetration of reactants). Only the surface of the crystalline region can be modified, as the core is tightly packed and characterized by hydrogen bonds.
The amorphous region is less ordered and as such it can be attacked by enzymes (endocellulases for example) and is more easily penetrated by reactants. Only the surface of the crystalline region, which is the most exposed, can be modified, as the core is tightly packed and characterized by hydrogen bonds (Figure 2).
Several reviews have focused on the biosynthesis of cellulose (e.g., [14,15,16,17]). Therefore, we will here describe the methods to characterize the degree of crystallinity of cellulose from extracted plant fibers, more specifically we will focus on XRD (X-ray diffraction), NMR (nuclear magnetic resonance) spectroscopy and FTIR (Fourier-transform infrared spectroscopy).
XRD is based on Bragg’s law and determines the d-spacing (the distance in Ångström) between the planes of atoms using a collimated X-ray beam. Diffraction peaks are thus generated which constitute a “fingerprint” of the specimen being examined. XRD was very useful to study the cellulose allomorphs Iα and Iβ and to determine that the former has a triclinic lattice, while the latter a monoclinic one [18].
NMR spectroscopy provides information on the atoms of a specimen and their chemical environment [18] and is based on the principle that nuclei aligned in a strong constant magnetic field are perturbed by a radio-frequency pulse. NMR spectroscopy has been applied to textile blends to quantify cellulose and its crystallinity; this method was not affected by dyes nor finishing agents [19].
FTIR is a vibrational spectroscopy method that is based on the absorption of light in the infra-red (IR) region of the electromagnetic spectrum. The absorption is dependent on the chemical bonds present in the molecule being analyzed. If a molecule has side chains, or functional groups, its vibrational frequency in the IR will be distinguishing. The wavelength is measured in wavenumbers (cm−1); the most useful IR range is between 4000 to 600 cm−1. This technique is non-destructive and has been used to monitor the healthy growth of tomato leaves by following cellulose alterations during development: an increased absorbance at 1125, 1107, and 1018 cm−1 was indeed observed which indicates secondary cell wall formation and cell wall expansion [20].
Despite being green substitutes of glass fibers, natural fibers have a hygroscopic nature, which can affect the bonding with hydrophobic resins. Additionally, this can lead to moisture absorption with consequent swelling and creation of cracks affecting the properties of the material [11].
The presence of an amorphous matrix composed of hemicelluloses and pectins also affects bonding with resins; therefore, both chemical and physical treatments are needed to expose the crystalline region of the fibers [12].
Plasma treatment of ramie fibers was used to increase the microroughness of the surface, hence increasing the surface area available for bonding [12]: After only one minute of treatment, nanostructures caused by the exposed cellulose microfibrils were visible and an increased wettability (using a mixture of phenolic resin and its catalyst as test liquid) was recorded. Among the chemical treatments, the use of alkali (i.e., mercerization) is one of the most common: NaOH is used to remove pectins and non-cellulosic polysaccharides, thereby disassembling the bundles and making the fiber surface rougher and cleaner [13]. The alkali treatment affects the tensile properties of the fibers by affecting the cellulose crystalline lattice (cellulose-I polymorph): Swelling is induced, as Na+ penetrates the pores of the lattice, thereby favoring the entry of water molecules which will increase the distance between the cellulose fibrils [21]. The –OH groups of cellulose become O–Na (Na-cellulose-I form) and, after rinsing with water, Na+ is removed with the subsequent transition to cellulose-II [21].
Cellulose nanocrystals have been treated with tannic acid to make them hydrophobic: Tannic acid works as a primer favoring the attachment of primary amines with long alkyl tails [22].
3. Plant Secondary Metabolism: The Phenylpropanoid Pathway as a Central “Hub”
Plants are sessile organisms and, in order to protect themselves against exogenous (a)biotic constraints, they synthesize a rich panel of secondary metabolites which have important physiological and ecological effects [2].
Among the secondary compounds produced by plants, polyphenols certainly deserve attention, in virtue of their role in controlling plant development and of their use in pharmaceutics/nutraceutics [23,24]. They are produced via the phenylpropanoid pathway, a central metabolic “hub” providing precursors for important structural molecules, such as lignin, as well as molecules responsible for pigments, aroma and flavor of fruits/flowers [25,26].
Over 8000 phenolic molecules have been identified and classified based on their chemical structure, biological function and origin [27]. From a biochemical point of view, polyphenols are characterized by aromatic rings linked to different chemical groups and they display specific functions depending on the elements bound. An initial classification was made by Manach and co-authors, who distinguished phenolic acids, flavonoids, stilbenes and lignans [28]. Tsao and colleagues extended this classification by further ranking polyphenols in 8 groups (i.e., 1. phenolic acids; 2. flavonoids; 3. isoflavones, neoflavonoids, and chalcones; 4. flavones, flavonols, flavanones, and flavanonols; 5. flavanols and proanthocyanidins; 6. anthocyanidins; 7. polyphenolic amides; 8. other polyphenols), based on their aglycone structures [29]. Thanks to these particular chemical features, polyphenols are able to scavenge reactive oxygen species (ROS), via the so-called hydrogen atom transfer (HAT) mechanism, whereby the –OH groups act as donors [30].
The phenylpropanoid pathway starts with the amino acid phenylalanine (Phe), derived from the shikimate pathway, which in plants is located in the chloroplasts [31].
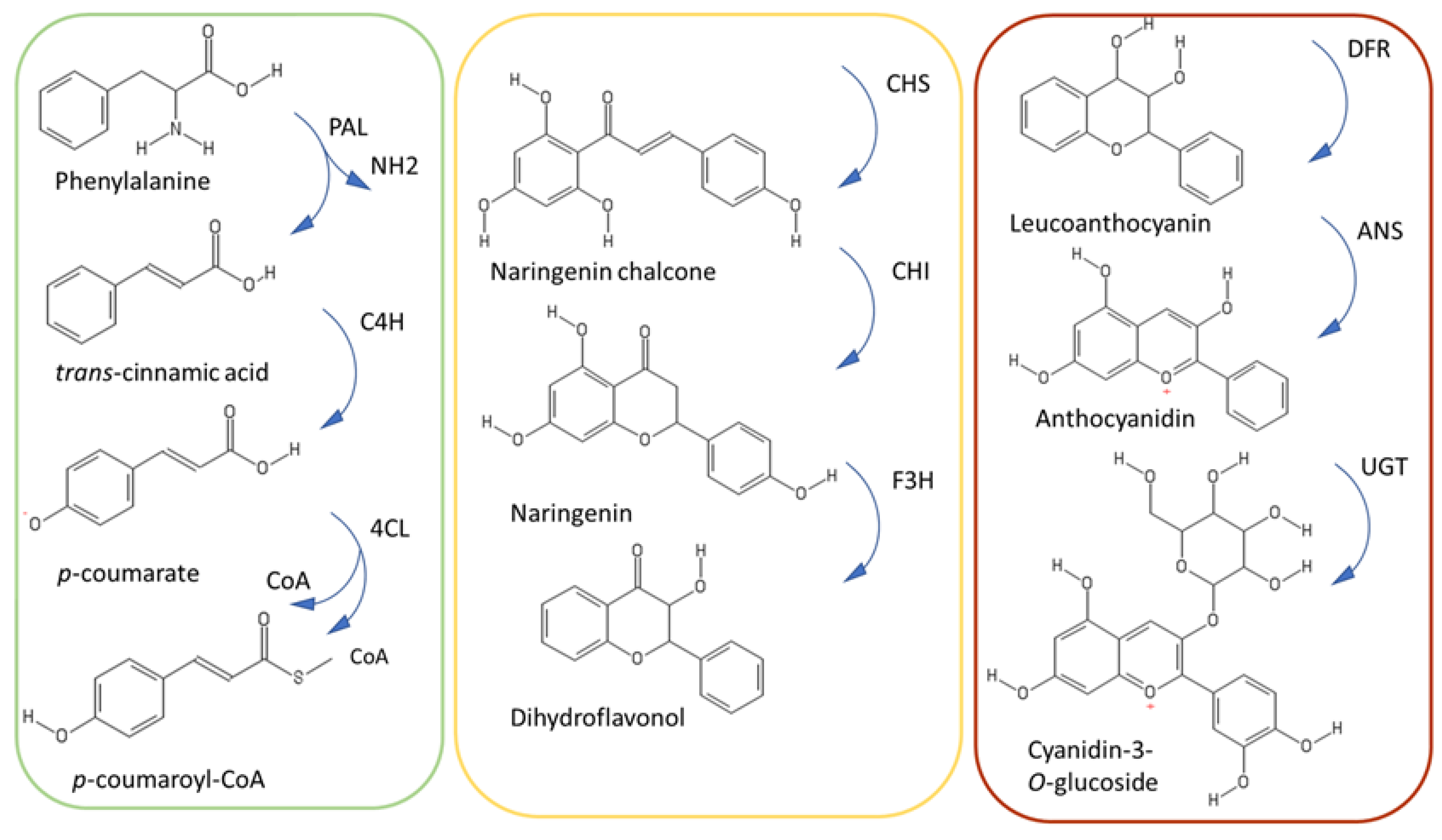
The phenylpropanoid pathway is divided into three main parts, each comprising three different enzymes (Figure 3). The upper part of the pathway constitutes the general phenylpropanoid pathway and is characterized by the reactions of the enzymes phenylalanine ammonia lyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumarate-coenzyme A ligase (4CL).
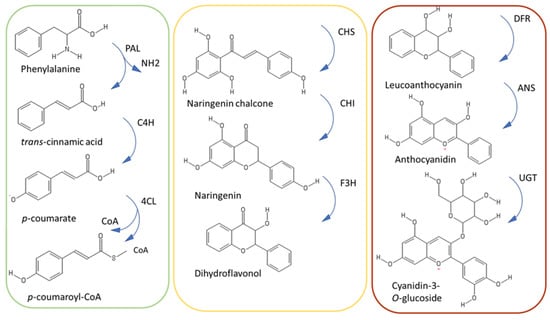
Figure 3.
Simplified scheme of the phenylpropanoid pathway, with the upper, central and late steps in green, yellow and brown, respectively. UGT refers to UDP-glycosyltransferase.
PAL is a tetrameric enzyme with different isoforms encoded by a multigene family in plants [32,33,34,35]. For instance, four different PAL isoforms were found in the model plant Arabidopsis thaliana and at least 20 genes have been described in Lycopersicon esculentum [36]. Such a large number of genes witnesses the importance of PAL in plant physiology. For example, in thale cress, the analysis of pal1 and pal2 mutants revealed a more important role of PAL1 in the biosynthesis of phenylpropanoids. Indeed, genes related to phenylpropanoid biosynthesis had altered expression in pal1 mutants and less extractable phenolics were recovered [37]. PAL is regulated by ubiquitination: PAL1 and PAL2 interact with 3 KFB (Kelch repeat F-box) proteins (namely KFB0-1, -20, -50), which control the proteolytic turnover through the ubiquitin-26S proteasome system [38].
4CL is the last enzyme intervening in the three shared steps of the general phenylpropanoid pathway and as such it channels activated thioesters of hydroxycinnamic acids to the synthesis of different phenylpropanoids [39]. Several 4CL genes are present in plants, they respond to environmental cues and phytohormones and are classified in type I and II: The first ones are related to lignin biosynthesis, while the latter are related to the synthesis of phenylpropanoids other than lignin [39]. In thale cress, different 4CL isoforms are implicated in the shunting of the metabolic flux: AtCL1 is co-expressed with lignin biosynthetic genes, while AtCL3 with flavonoid biosynthetic ones [39].
The central steps of the phenylpropanoid pathway lead to the synthesis of flavonoids through the reactions of chalcone synthase, chalcone isomerase and flavanone 3-hydroxylase (CHS, CHI, F3H). CHS is considered the gatekeeper of flavonoid biosynthesis [40]: It belongs to the plant polyketide synthase superfamily and it catalyzes a condensation reaction of one activated thioester (e.g., p-coumaroyl-CoA,) to three molecules of malonyl-CoA. Studies demonstrate that CHS is under the control of the circadian rhythm [40] and proteolytic regulation. More specifically, CHS is negatively regulated by a KFB protein mediating degradation and whose expression is developmentally controlled in Arabidopsis [41].
CHI acts as an isomerase, by transforming chalcone in flavanone. A recent study has revealed that CHI evolved from a non-catalytic ancestor related to fatty acid binding proteins and a non-catalytic protein, CHIL (chalcone isomerase-like) [42]. Small changes in the positioning of the substrates, as well as modifications in the positioning and flexibility of the catalytic residues with respect to the substrate, favored the emergence of catalysis in CHI [42].
F3H catalyzes the last step of the central portion of the pathway leading to the formation of dihydroflavonols. These are substrates of the last part of the phenylpropanoid pathway catalyzed by dihydroflavonol 4-reductase (DFR). DFR reduces dihydroflavonols to leucoanthocyanidins, which are substrates of the next enzymatic reactions leading to the synthesis of anthocyanidins [43].
Anthocyanidin synthase (ANS) belongs to the 2–oxoglutarate dependent dioxygenases family (OGD) and catalyzes the conversion of colorless leucoanthocyanidins to colored anthocyanidins. Mutations in ANS cause yellow pigmentation, as observed in yellow raspberry fruits, where a 5 bp insertion in the coding region was associated to the observed phenotype [44].
The complexity of the phenylpropanoid pathway is not only due to the great diversity of phenolic compounds produced, the redundant gene families coding for its enzymes and the transcriptional/post-transcriptional regulation, but also to the association of some of the enzymes in complexes known as “metabolons”.
A strong body of evidence associates these particular structures to the phenylpropanoid pathway [45]. In quaking aspen, several PAL isoforms were identified and assigned to different functions, together with the downstream enzyme 4CL. For example, PAL1 was found to be partially soluble and membrane-bound, while PAL2 was exclusively cytosolic. However, upon overexpression of C4H, both isoforms were membrane-bound, suggesting a potential role of C4H in nucleating the formation of the complex [46]. The metabolon is anchored to the ER surface via P450 proteins: In soybean, the P450s C4H and F3H were shown to anchor the isoflavonoid metabolon [47].
The metabolon can be considered as a metabolic “highway” for plant cells that forms restricted reactive areas where enzymes and substrates are close to each other. This proximity increases the speed of the reactions, limiting energy dispersion and possible toxic intermediates. Understanding more about the mechanisms underlying the formation of metabolons in plants can inspire strategies in biotechnology aimed at boosting the production of specific secondary metabolites of industrial interest.
4. Analytical Methods to Extract and Quantify Phenolics in Plant Tissues
Phenolic compounds have been the object of intense studies in the last decades because of their chemical heterogeneity and their beneficial effects on human health [48,49]. Indeed these molecules show important health benefits, namely the prevention and treatment of diseases [50,51]; their bioactivity inspires studies developing novel formulations for use in food, pharmaceutical and medical fields [23,52]. Moreover, phenolic compounds deriving from natural resources, such as plant biomass, are sought-after because they are used in the thermoset polymer chemistry and provide good thermo-mechanical properties, as well as chemical resistance [53].
Plant phenolics are important secondary metabolites mediating the response to several environmental cues, both biotic and abiotic [2]. Their increased synthesis in condition of stress is known: Indeed, exogenous stresses are used to improve the antioxidant potential of plants for their subsequent use in nutraceutics [54]. Phytohormones such as methyl jasmonate and salicylic acid are key players in the synthesis of phenolics under conditions of stress [55]. Their use for the production of phenolics in plant cell cultures is a common practice on an industrial scale [56]. Besides these inducible systems, a plant matrix rich in polyphenols is also wood, whose cell walls pose a non-negligible challenge to an effective extraction of phenolics. The use of swelling agents, such as formic acid, DMSO, or pyridine, helps in the removal of wood extractives, composed of phenolic molecules, terpenes, resins. A study has shown that the removal of extractives positively impacts digestibility of softwood barks [57]: This is interesting in the framework of a circular economy, since wood extractives display antimicrobial properties [58].
Given the importance of plant phenolics in several industrial fields such as pharmaceutics, nutraceutics, cosmetics, we will here provide an overview of the different methods to extract and quantify phenolics in plant tissues.
4.1. The Soxhlet Method
The Soxhlet technique was initially developed by Franz Ritter von Soxhlet in 1879, to extract fatty acids from milk. Thanks to the low cost of the equipment and the possibility to perform several extractions simultaneously, this method was adapted to various biomolecule extractions across the years, comprising the secondary metabolites, such as polyphenols. Basically, the sample is situated in an extraction flask and the solvent is continuously filled allowing a solute gradual extraction. When the flask reaches the overflow, the upper phase of the liquid solution is aspirated by a siphon and collected in a distillation flask. Then the solute remains in the flask and the solvent comes out.
4.2. Liquid–Liquid Extraction
There are several methods to extract phenolics, the choice depending on the chemical structure of the analyte. For phenolics, the most common method is the liquid–liquid extraction (LLE), which uses different solvents, such as ethanol, acetone, or methanol at varying percentages (between 60% and 80%) [59,60]. Variations of this procedure include the use of acidified solvents (with e.g., HCl 1%) and high temperatures to realize the hydrolysis of the sugar moiety and obtain the aglycone [61].
Differently from the other polyphenol compounds, such as phenolic acids or flavonoids, anthocyanins are usually extracted by acidified organic solvents, most commonly methanol. An acid extraction allows the efficient disruption of the organelles with the simultaneous stabilization of the anthocyanins at low pH. An emblematic example of this has been published by Blackhall and colleagues, who described methanol/ethanol acidified with 0.1% HCl as the best method for the extraction of anthocyanins in Prunus avium [62].
In LLE some factors are fundamental, for instance the time of sample maceration, centrifugation; most importantly, light, air and temperature should be strictly controlled to avoid unwanted effects on the final results.
4.3. Solid-Phase Extraction and Ultrasonic Extraction
Solid-phase (SPE) and ultrasonic extraction (USE) are considered useful techniques to improve sample preparation. Frequently, these two methods are associated with LLE to help disrupt the plant material, allowing the maximum release of molecules in the extraction solution [61].
The use of SPE enables a better separation of the analytes, while avoiding large use of organic solvents; however, it is more expensive than LLE and recommended for low volumes.
SPE is useful for liquid samples with semi-volatile and non-volatile features, but its use is also extended to samples pre-extracted with LLE. The most commonly used column for SPE applied to plant phenolics is C18, which allows an efficient separation from different plant matrices [63,64].
USE is considered the simplest method to perform analyte extraction. The ultrasonic waves are able to improve the lysis of tissues and cells, thereby facilitating sample preparation. This technique is also used individually to disrupt some plant matrices and obtain extracts in short times, at the expense of extraction efficiency. However, it was demonstrated that USE, combined with solvent or water extraction, improves the efficiency by 35%, as compared to single methods [65]. Such an approach was particularly efficient when applied to samples rich in polyphenols, such as strawberries, where a minimal analyte degradation was also proven [66].
4.4. Microwave-Assisted Extraction
Microwave-assisted extraction (MAE) exploits the microwave energy to heat mixtures of solid samples and polar solvents, while reducing the amount of solvent used and the extraction time [67]. Recently MAE was used to compare its extraction efficiency to that of other more conventional methods, such as LLE, SPE, and USE. Nayak and colleagues have demonstrated the capacity of this method in Citrus sinensis peels and showed a more efficient extraction as compared to LLE and UAE [68]. The great advantage of MAE is the total absence of light during the process and its rapidity [61]. Temperature is the only limit of this technique; indeed, it has been demonstrated that phenolic compounds rich in hydroxyl groups are more susceptible to degradation [69].
4.5. Supercritical Fluid Extraction
Supercritical fluid extraction (SFE) is a recent technique that is gaining popularity because it reduces energy consumption and uses moderate critical conditions [61]. However, this technique is limited by the efficiency that decreases with compounds having low or medium polarity. Nevertheless, the use of ethanol as co-solvent increases the yield of the phenolic fraction [61]. Naziri and colleagues used this method to recover nutraceutical compounds, such as squalene, from wine waste derived from industrial macerations [70].
4.6. Enzyme-Assisted Extraction (EAE)
The enzyme-assisted extraction (EAE) exploits the catalytic properties of the enzymes to allow the extraction of analytes from different matrix. The success of this technique is determined by the right enzyme selection; for instance: cellulases, pectinases and hemicellulases derived mostly from bacteria or fungi, are commonly used to release bioactive compounds from plant cells [71].
Puri and colleagues [71] commented on the significant increase of bioactive compounds extracted from plants, in particular flavonoid, anthocyanin, and carotenoid, compared to other techniques. The authors also propose the use of EAE in the industrial processes, such as vinification and food processes, reporting their potential.
4.7. Accelerated Solvent Extraction (ASE)
The accelerated solvent extraction (ASE) was first described by Richter and colleagues to recover polycyclic aromatic hydrocarbons, polychlorinated biphenyls and total petroleum hydrocarbons from solid and semi-solid matrices [72]. This method exploits solvents at high temperatures (50–200 °C) and pressure for brief periods (500–3000 psi for 5–10 min) to allow an optimal solute extraction in a short time. The authors described the high temperatures and the pressure as the main efficient features to increase the extraction performance through a better mass solubility and surface disruption. Herrero and colleagues, in 2004, focused their efforts on optimizing the ASE to extract antioxidant molecules from algae (Spirulina platensis) [73]. The authors tested different solvents, hexane, ethanol, petroleum ether and water, stating that ethanol was the best solvent with respect to the total amount of antioxidant extracted. The possible use of ethanol gives a noteworthy value to this technique, indeed this solvent is considered as GRAS (generally recognized as safe) and may allow the use of ASE in food industry [73].
4.8. Different Methods to Determine Phenolic Compounds in Plants
The quantification of phenolic compounds is mainly performed by means of spectrophotometric analyses. These methods ensure good efficiency and rapid results and they are used for the rapid screening of different classes of phenolic compounds. These methods are, for example, frequently used in food research to quantify the nutraceutical potential of different fruits [63,74,75,76].
The most common methodology used to quantify the total phenolic compounds in plants is the Folin–Ciocalteu method. This technique is based on the chemical reduction by a mixture of tungsten and molybdenum oxides, in addition to lithium salts, which prevent the solution turbidity. The reaction with phenols changes to a blue color, which absorbs at 765 nm [77] and whose intensity is proportional to the concentration of phenols. Usually, the absorbance of the sample is compared to a calibration curve composed of a gallic acid (GA) standard solution and reported as equivalents of gallic acid per gram of fresh weight (GAE/g FW) [74,78].
The total flavonoid content in plant tissues is routinely estimated using the aluminum chloride method [79]. The plant extract is mixed with a methanolic solution of aluminum chloride at a specific concentration (usually 1 M). The absorbance is measured after a brief period of incubation (approximately 30 min) and compared to a flavonoid standard curve (e.g., catechin, quercetin, or rutin) [74,78]. The disadvantage of this methodology is that a better sensitivity to a specific group of flavonoids, such as flavones and flavonols (rather than flavanones and flavanonols), has been estimated [80].
To quantify anthocyanins, the most common method is represented by the pH differential method (PD). This methodology is officially adopted by the Association of Official Agricultural Chemists (AOAC) and conventionally used to measure anthocyanins in fruits and vegetables [81]. PD takes advantage of the chemical features of the plant pigments that are able to reversibly change color in solution at different pH. The samples are diluted in two different buffers at pH 1.0 and 4.5 and the absorbance is determined at 520 and 700 nm after 15 min of equilibration. Absorbance values are then used in the following formula [82].
Asp = (A510 − A700) pH 1.0 − (A510 − A700) pH 4.5
Total anthocyanins (TA) = (Asp × M × DF × 1000)/(ε × λ × m)
Asp = spectrophotometrically measured absorption values, M = molecular weight of cyanidin 3-O-glucoside, DF = dilution factor, ε = molar extinction coefficient, λ = cuvette optical path-length (1 cm), m = weight of the sample (g).
The results are expressed as cyanidin-3-O-glucoside equivalents per grams of fresh weight (CyE/g FW).
4.9. Quantitative Techniques for Phenolic Determinations
The spectrophotometric methods are useful for a preliminary screening, but the limits in low accuracy and the impossibility of determining single compounds in a complex mixture require more selective methods to bring the analysis to the next level. Hereafter is a description of methods for the identification and quantification of single phenolic compounds.
4.9.1. High Pressure Liquid Chromatography
High pressure liquid chromatography (HPLC) uses pressure to separate single molecules in the sample via their interaction with the stationary phase. The most commonly used HPLC separation method for phenolics is reverse-phase (RP) HPLC. It exploits a stationary phase, generally packed in C18 silica bonded columns, less polar than the mobile phase. Thanks to this method, polar phenolics (such as gallic acid, epigallocatechin, hydroxycinnamic acids) elute at short retention time, while less polar ones (myricetin, quercetin, kaempferol) elute after these. The elution is typically generated using a gradient mixture of acidified water and a polar solvent (i.e., acetonitrile or methanol), because of the molecules’ chemical structure [76].
4.9.2. Gas Chromatography
In gas chromatography (GC), the stationary phase is represented by a liquid layer on an inert solid support placed in a column. The mobile phase consists of an inert gas, such as helium or nitrogen that passes through the column under high pressure. The sample is vaporized and enters into the mobile phase. The components contained in the sample are dispersed between the mobile phase and the stationary phase on the solid support. However, in some cases, the use of GC to quantify plant phenolics requires prior derivatization (e.g., methylation, trifluoroacetylation, conversion to trimethylsilyl derivatives, or derivatization with N-(tert-butyldimethylsilyl)-N-methyl trifluoroacetamide), to supply optimal chemical parameters (e.g., improve volatility), thermostability and for non-volatile samples [83].
4.9.3. Mass Spectrometry
The mass spectrometer is used to obtain molecular mass and structural features of the analytes with very high sensitivity. The principle of mass spectrometry (MS) is the measurement of the mass-to-charge ratio (m/z) of the ions deriving from fragmented molecules. Once formed, the ions are directed into the mass analyzer where they are separated and detected according to their m/z. MS is typically associated to GC or HPLC, thereby improving the resolution power exponentially and giving more accurate results. Structural details are further obtained with tandem mass spectrometry (MS/MS) in combination with methods of ion fragmentation, such as collision-induced dissociation (CID). HPLC- or GC-MS/MS are also used to quantitatively analyze or identify labile compounds in solution, such as acylated flavonoids [84].
5. Mechanisms of Action of Phenolics on Bacteria and Fungi
Different mechanisms have been invoked to explain the antimicrobial effect of plant phenolics. Hereafter is a description of the key processes reported in the literature.
5.1. Effects on the Peptidoglycan and Fungal Cell Walls
Studies have demonstrated the effects of natural polyphenols on the bacterial peptidoglycan. In the Gram-positive bacterium Staphylococcus aureus, the flavonoids apigenin and quercetin target the enzyme D-Alanine:D-alanine ligase (Ddl), which is responsible for the synthesis of the peptidoglycan and is a target of drugs [85]. The two flavonoids are reversible inhibitors and are competitive with respect to ATP and non-competitive to D-Ala [85]. They recognize the ATP-binding pocket of the enzyme, a finding supporting the effect on other ATP-dependent enzymes, such as gyrase (see Section 5.3). Epicatechin gallate provokes aggregation of S. aureus, an increase in the peptidoglycan thickness, a structural modification of the wall teichoic acid, thereby sensitizing methicillin-resistant bacteria to β-lactam antibiotics [86]. In particular, lipoteichoic acid is released from the membrane, with consequent alterations in the wall teichoic acid architecture and modifications of the cell surface [86].
The different thickness of the peptidoglycan in Gram-positive bacteria and the presence of an outer membrane in Gram-negative ones may result in different effect of flavonoids. However, it was shown that no clear difference existed between Gram-positive or -negative bacteria, the main effect being dependent on the species [87]. Interestingly, the presence of pyrogallol rings was correlated with higher antibacterial properties than catechol or resorcinol, due to the generation of higher H2O2 contents in the culture medium [87].
An inhibition of the sortase enzyme by plant polyphenols has been reported in the Gram-positive bacterium Streptococcus mutans: this enzyme mediates crucial transpeptidase reactions implicated in surface protein attachment to the cell wall, biofilm formation, adherence to hosts [88]. Trans-chalcone covalently modifies sortase A by forming a Michael addition adduct to Cys205 [88].
Plant polyphenols also display antifungal activity (e.g., towards Candida; recently reviewed by [89]). In fungi, derivatives of caffeic acid and quinic acid with an H2N-orn-4-(octyloxy) aniline group displayed high inhibition of Candida albicans β-1,3-glucan synthase, an enzyme involved in the synthesis of an important component of the fungal cell wall matrix [90]. Additionally, cardanol azo derivatives showed chitin binding activity, with consequent damages at the cell wall level (chitin is, with glucan, one the major constituents of the fungal wall) and leaking of cytoplasmic content of the cells [91].
5.2. Effects on the Bacterial and Fungal Membrane
Phytochemicals, notably flavonoids, interact with the membrane and affect its fluidity, by rigidifying it [92]. In particular, the number and position of hydroxyl groups (C-3 in the C ring decreases membrane fluidity), as well as glycosylation (which decreases the interaction with membranes) are important for the antimicrobial effect [92,93]. Kaempferol for example interacts deep in the hydrophobic core of the membrane and causes rigidification [93]. Rigidification affects the partitioning of membrane protein. Flavonoids can also exert their antimicrobial effect by causing an oxidative burst that generates reactive oxygen species (ROS). ROS alter the bacterial membrane permeability [94]. Additionally, the bacterial fatty acid synthases (FAS) are regulated by flavonoids via an acyl carrier protein transacylases responsible for the synthesis of phospholipid components [95].
In fungi, such as C. albicans, phenolic acids bind to ergosterol and distort the membrane [89]. A connection between dihydrofolate reductase (inhibited by flavonoids) and ergosterol biosynthesis was demonstrated: This mechanism implies a depletion of S-adenosylmethionine (SAM) with an effect on the enzyme C-24 methyltransferase [96]. Isoquercitrin induces membrane damage in C. albicans by causing pore formation and disturbance of the lipid bilayer which was estimated to be between 2.3 to 3.3 nm, as calculated from assays based on the release of dextrans coupled to fluorescein isothiocyanate [97].
5.3. Other Effects
Flavonoids are inhibitors of topoisomerases: For example, quercetin binds to a 24 kDa fragment of the B subunit of Escherichia coli gyrase, it inhibits supercoiling activity and induces DNA cleavage [98]. Quercetin’s binding site overlaps with the ATP binding pocket and its mode of action also implies binding to DNA through hydrophobic interactions [98]. Flavonoids also display an inhibitory effect on E. coli F1F0 ATPases: The binding site is in the F1 sector, at the interface of the α, β, and γ-subunits, with consequent hindering of clockwise/anticlockwise rotation of the γ-subunit [99].
In fungi, caffeic and rosmarinic acid, as well as apigenin inhibited the activity of isocitrate lyase 1, an enzyme involved in the glyoxylate cycle, in C. albicans grown on glucose-depleted medium [100].
6. Combining Phytochemicals and Cellulosic Fibers: Methods and Uses
Combining plant polyphenols with plant fibers provides anti-microbial properties to, for example, biosourced packaging material and textiles. These properties are due to the effects described in Section 5.
In this paragraph we will report recent literature data on the functionalization of plant fibers with plant phenolics.
Cotton fibers functionalized with mango leaf polyphenols were recently prepared using supercritical solvent impregnation, a technique enabling the adsorption of the molecules to the matrix and the use of moderate temperatures which; therefore, do not destroy thermolabile molecules [101]. By using CO2 (which improves the penetration of the bioactives in the matrix) together with 6% ethanol as co-solvent under conditions of 300 bar of pressure and 45 °C of temperature, functionalization of cotton fibers was achieved [101]. The antioxidant activity increased with higher contact times (which enhanced also the impregnation).
The antimicrobial activity of textiles functionalized with plant extracts can either take place by contact or by diffusion: In the first case, the molecules are deposited on the fiber surface, while in the latter, they leach over time and are, therefore, useful in case of textiles for wound dressing.
An interesting technology to functionalize the surface of plant fibers is the use of enzymes, such as laccases and peroxidases. Laccases and peroxidases have been used to modify the surface of plant fibers by acting on the encrusting lignin and favor grafting of plant phenolics. Lignin is present in lignocellulosic, as well as in primary and secondary bast fibers (but in very small amounts) of fiber crops like hemp [102]. Laccases and peroxidases are oxidative enzymes that can transform phenols to their phenoxy radicals and are widely used as biocatalysts for different biotechnological applications [103]. One example is the creation of a stable reactive surface to prepare the grafting of plant phenolics. By creating a radical, several inter-unit linkages can be generated with reacting molecules, depending on the position of the radical [104]. For example, jute fibers pre-treated to remove waxes have been functionalized with gallate esters using horseradish peroxidase. The procedure resulted in fibers with increased hydrophobicity, as revealed by the higher water contact angle [105]. Laccases have been used to biograft antimicrobial phenolics on fibers for use as packaging material: both phenolic acids and components of essential oils were tested and showed the desired antimicrobial effect [106]. Laccases were used to functionalize in situ the surface of cotton fibers with polymerized polyphenols (caffeic acid and morin), after deposition of cationic polyelectrolytes [107]. The cationic layer interacts with the negatively charged cellulose fibers and, in its turn, it anchors polymerized polyphenols via electrostatic interactions. The polymerized polyphenols offer protection against UV [107].
Laccases have been also used on unbleached flax bast fibers to confer antimicrobial properties by biografting phenolics, notably syringaldehyde, acetosyringone, and p-coumaric acid [108].
Fibers with antibacterial properties also occur naturally; therefore, they can be used without the need to functionalize them with extracted plant phytochemicals. For example, naturally brown-colored cotton shows high antimicrobial properties (as tested against S. aureus and Klebsiella pneumoniae) due to the presence of pigments related to condensed tannins [109]. Interestingly, naturally colored cottons also show high antioxidant activity which are durable even after laundering and scouring [110]. Antioxidant activities were also proven in hemp and flax fibers, two examples of multi-purpose crops. More specifically, the properties were shown to be affected not only by the varieties, but also by the processing. Decortication was the procedure ensuring the highest amount of syringic acid and, hence, antioxidant activity [8].
7. Conclusions and Future Perspectives
The use of biomaterials deriving from renewable resources such as plants reduces the environmental impact. Plants are sources of both phytochemicals (molecules chiefly deriving from the secondary metabolism and possessing bioactivity) and lignocellulosic biomass and, therefore, attract much interest for exploitation in sectors such as technical textiles for medical application. Combining natural fibers with functionalization using plant extract (prepared, for example, via greener techniques which replace hazardous solvents, e.g., supercritical fluid extraction-SFE) offers the possibility of coupling antimicrobial properties to the advantages of natural fibers, namely breathability and skin comfort. Researches aimed at devising improved methods of impregnation and grafting, as well as studying the antioxidant and antimicrobial potential of natural fibers will be important for the design of functionalized plant-sourced textiles. In this perspective, the use of enzymes (like laccases) immobilized on magnetic nanoparticles will offer advantages in terms of reusability and improvement of catalytic properties, as compared to free enzymes [111].
Author Contributions
G.G. conceived the idea of writing the Review. G.G. wrote the part on plant fibers’ properties, cellulose characterization, antimicrobial effects. R.B. wrote the part on the phenylpropanoid pathway and phenolic extraction/quantification. J.-F.H. and G.C. provided inputs on all the sections of the review. All the authors have read and approved the final version of this manuscript.
Funding
This research received no external funding.
Acknowledgments
G.G. acknowledges the Fonds National de la Recherche, Luxembourg, (Project CABERNET C16/SR/11289002) for financial support. R.B. acknowledges the region Tuscany for the financial support through the PhD fellowship “Pegaso”. The authors thank Laurent Solinhac for providing the image shown in Figure 1a.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guerriero, G.; Sergeant, K.; Hausman, J.-F. Wood biosynthesis and typologies: A molecular rhapsody. Tree Physiol. 2014, 34, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Bergfjord, C.; Mannering, U.; Frei, K.M.; Gleba, M.; Scharff, A.B.; Skals, I.; Heinemeier, J.; Nosch, M.-L.; Holst, B. Nettle as a distinct Bronze Age textile plant. Sci. Rep. 2012, 2, 664. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Impact of Silicon in Plant Biomass Production: Focus on Bast Fibres, Hypotheses, and Perspectives. Plants 2017, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Sergeant, K.; Hausman, J.-F. Integrated -omics: A powerful approach to understanding the heterogeneous lignification of fibre crops. Int. J. Mol. Sci. 2013, 14, 10958–10978. [Google Scholar] [CrossRef] [PubMed]
- Djemiel, C.; Grec, S.; Hawkins, S. Characterization of Bacterial and Fungal Community Dynamics by High-Throughput Sequencing (HTS) Metabarcoding during Flax Dew-Retting. Front. Microbiol. 2017, 8, 2052. [Google Scholar] [CrossRef] [PubMed]
- Antonov, V.; Marek, J.; Bjelkova, M.; Smirous, P.; Fischer, H. Easily available enzymes as natural retting agents. Biotechnol. J. 2007, 2, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Zimniewska, M.; Rozańska, W.; Gryszczynska, A.; Romanowska, B.; Kicinska-Jakubowska, A. Antioxidant Potential of Hemp and Flax Fibers Depending on Their Chemical Composition. Molecules 2018, 23, 1993. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N.J. The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Kimura, S.; Laosinchai, W.; Itoh, T.; Cui, X.; Linder, C.R.; Brown, R.M. Immunogold Labeling of Rosette Terminal Cellulose-Synthesizing Complexes in the Vascular Plant Vigna angularis. Plant Cell 1999, 11, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Faleri, C.; Hausman, J.-F.; Planchon, S.; Renaut, J.; Cai, G.; Guerriero, G. Distribution of cell-wall polysaccharides and proteins during growth of the hemp hypocotyl. Planta 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mutwil, M.; Debolt, S.; Persson, S. Cellulose synthesis: A complex complex. Curr. Opin. Plant Biol. 2008, 11, 252–257. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.E.; Döring, A.; Persson, S. The cell biology of cellulose synthesis. Annu. Rev. Plant Biol. 2014, 65, 69–94. [Google Scholar] [CrossRef]
- Endler, A.; Persson, S. Cellulose synthases and synthesis in Arabidopsis. Mol. Plant 2011, 4, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Fugelstad, J.; Bulone, V. What Do We Really Know about Cellulose Biosynthesis in Higher Plants? J. Integr. Plant Biol. 2010, 52, 161–175. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose–An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef]
- Haslinger, S.; Hietala, S.; Hummel, M.; Maunu, S.L.; Sixta, H. Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydr. Polym. 2019, 207, 11–16. [Google Scholar] [CrossRef]
- Butler, H.; McAinsh, R.M.; Adams, S.; Martin, F.L. Application of vibrational spectroscopy techniques to non-destructively monitor plant health and development. Anal. Methods 2015, 7, 4059–4070. [Google Scholar] [CrossRef]
- Hashim, M.Y.; Roslan, M.N.; Amin, A.M.; Zaidi, A.M.A.; Ariffin, S. Mercerization Treatment Parameter Effect on Natural Fiber Reinforced Polymer Matrix Composite: A Brief Review. World Acad. Sci. Eng. Technol. 2012, 6, 1382–1388. [Google Scholar]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nanocrystals Using Plant Polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar]
- Del Rio, D.; Bresciani, L. Phenolic compounds as functional ingredients and nutraceuticals: The case of Juice PLUS+. FASEB J. 2017, 31, 646.3. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Peled-Zehavi, H.; Oliva, M.; Xie, Q.; Tzin, V.; Oren-Shamir, M.; Aharoni, A.; Galili, G. Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers. Bioengineering 2015, 2, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Bioactive Natural Products (Part I); Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 257–312. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. Plant Physiol. Asp. Phenolic Compd. 2019. [Google Scholar] [CrossRef]
- Reichert, A.I.; He, X.-Z.; Dixon, R.A. Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): Characterization of the four tobacco PAL genes and active heterotetrameric enzymes. Biochem. J. 2009, 424, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, F.C.; Davin, L.B.; Lewis, N.G. The Arabidopsis phenylalanine ammonia lyase gene family: Kinetic characterization of the four PAL isoforms. Phytochemistry 2004, 65, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, B.; Ellis, M.; Friedmann, M.; de Azevedo Souza, C.; Barbazuk, B.; Douglas, C.J. Genome-wide analyses of phenylpropanoid-related genes in Populus trichocarpa, Arabidopsis thaliana, and Oryza sativa: The Populus lignin toolbox and conservation and diversification of angiosperm gene families. Can. J. Bot. 2007, 85, 1182–1201. [Google Scholar] [CrossRef]
- Chang, A.; Lim, M.-H.; Lee, S.-W.; Robb, E.J.; Nazar, R.N. Tomato phenylalanine ammonia-lyase gene family, highly redundant but strongly underutilized. J. Biol. Chem. 2008, 283, 33591–33601. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.-P.; Vuylsteke, M.; et al. Molecular Phenotyping of the pal1 and pal2 Mutants of Arabidopsis thaliana Reveals Far-Reaching Consequences on Phenylpropanoid, Amino Acid, and Carbohydrate Metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef]
- Zhang, X.; Gou, M.; Liu, C.-J. Arabidopsis Kelch repeat F-box proteins regulate phenylpropanoid biosynthesis via controlling the turnover of phenylalanine ammonia-lyase. Plant Cell 2013, 25, 4994–5010. [Google Scholar] [CrossRef]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef]
- Zhang, X.; Abrahan, C.; Colquhoun, T.A.; Liu, C.-J. A Proteolytic Regulator Controlling Chalcone Synthase Stability and Flavonoid Biosynthesis in Arabidopsis. Plant Cell 2017, 29, 1157–1174. [Google Scholar] [CrossRef]
- Kaltenbach, M.; Burke, J.R.; Dindo, M.; Pabis, A.; Munsberg, F.S.; Rabin, A.; Kamerlin, S.C.L.; Noel, J.P.; Tawfik, D.S. Evolution of chalcone isomerase from a noncatalytic ancestor. Nat. Chem. Biol. 2018, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Wilmouth, R.C.; Turnbull, J.J.; Welford, R.W.D.; Clifton, I.J.; Prescott, A.G.; Schofield, C.J. Structure and Mechanism of Anthocyanidin Synthase from Arabidopsis thaliana. Structure 2002, 10, 93–103. [Google Scholar] [CrossRef]
- Rafique, M.Z.; Carvalho, E.; Stracke, R.; Palmieri, L.; Herrera, L.; Feller, A.; Malnoy, M.; Martens, S. Nonsense Mutation Inside Anthocyanidin Synthase Gene Controls Pigmentation in Yellow Raspberry (Rubus idaeus L.). Front. Plant Sci. 2016, 7, 1892. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.; Rasmussen, A.V.; Morant, M.; Nielsen, A.H.; Bjarnholt, N.; Zagrobelny, M.; Bak, S.; Møller, B.L. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr. Opin. Plant Biol. 2005, 8, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Achnine, L.; Blancaflor, E.B.; Rasmussen, S.; Dixon, R.A. Colocalization of l-Phenylalanine Ammonia-Lyase and Cinnamate 4-Hydroxylase for Metabolic Channeling in Phenylpropanoid Biosynthesis. Plant Cell 2004, 16, 3098–3109. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Bernards, M.A.; Dhaubhadel, S. Twin anchors of the soybean isoflavonoid metabolon: Evidence for tethering of the complex to the endoplasmic reticulum by IFS and C4H. Plant J. Cell Mol. Biol. 2016, 85, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N. Phenolic Compounds as Potential Antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149–150. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Aguilera, Y.; Martin-Cabrejas, M.A.; González de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Thitilertdecha, P.; Rowan, M.G.; Guy, R.H. Topical formulation and dermal delivery of active phenolic compounds in the Thai medicinal plant-Clerodendrum petasites S. Moore. Int. J. Pharm. 2015, 478, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Decostanzi, M.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased phenol and furan derivative coupling for the synthesis of functional monomers. Green Chem. 2019, 21, 724–747. [Google Scholar] [CrossRef]
- Świeca, M. Elicitation with abiotic stresses improves pro-health constituents, antioxidant potential and nutritional quality of lentil sprouts. Saudi J. Biol. Sci. 2015, 22, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Frankó, B.; Carlqvist, K.; Galbe, M.; Lidén, G.; Wallberg, O. Removal of Water-Soluble Extractives Improves the Enzymatic Digestibility of Steam-Pretreated Softwood Barks. Appl. Biochem. Biotechnol. 2018, 184, 599–615. [Google Scholar] [CrossRef]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M. Antifungal activities of wood extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Montella, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D. Characterization of polyphenolic and oligosaccharidic fractions extracted from grape seeds followed by the evaluation of prebiotic activity related to oligosaccharides. Int. J. Food Sci. Technol. 2019, 54, 1283–1291. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Dobiáš, P.; Pavlíková, P.; Adam, M.; Eisner, A.; Beňová, B.; Ventura, K. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent. Eur. J. Chem. 2010, 8, 87–95. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Cheng, H.N.; Willett, J.L.; Lesch, W.C.; Tangsrud, R.R.; Biswas, A. Microwave-assisted extraction of phenolics from bean (Phaseolus vulgaris L.). Food Res. Int. 2010, 43, 516–519. [Google Scholar] [CrossRef]
- Naziri, E.; Glisic, S.B.; Mantzouridou, F.T.; Tsimidou, M.Z.; Nedovic, V.; Bugarski, B. Advantages of supercritical fluid extraction for recovery of squalene from wine lees. J. Supercrit. Fluids 2016, 107, 560–565. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Guarnieri, M.; Nepi, M.; Hausman, J.-F.; Guerriero, G.; Romi, M.; Cai, G. Nutraceutical Characteristics of Ancient Malus x domestica Borkh. Fruits Recovered across Siena in Tuscany. Medicines 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Haminiuk, C.; Maciel, G.; Plata-Oviedo, M.; Peralta, R. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.-F.; Guerriero, G.; Cai, G. Functional Molecules in Locally-Adapted Crops: The Case Study of Tomatoes, Onions, and Sweet Cherry Fruits From Tuscany in Italy. Front. Plant Sci. 2019, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Maciel, L.G.; Nunes, D.S. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. TrAC Trends Anal. Chem. 2016, 80, 266–279. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Lee, S.G.; Vance, T.M.; Nam, T.-G.; Kim, D.-O.; Koo, S.I.; Chun, O.K. Evaluation of pH differential and HPLC methods expressed as cyanidin-3-glucoside equivalent for measuring the total anthocyanin contents of berries. J. Food Meas. Charact. 2016, 3, 562–568. [Google Scholar] [CrossRef]
- Tõnutare, T.; Moor, U.; Szajdak, L.W. Strawberry anthocyanin determination by pH differential spectroscopic method-how to get true results? Acta Sci. Pol. Hortorum Cultus 2014, 13, 35–47. [Google Scholar]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Keskes, H.; Belhadj, S.; Jlail, L.; Feki, A.E.; Damak, M.; Sayadi, S.; Allouche, N. LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharm. Biol. 2017, 55, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The β-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Wallock-Richards, D.J.; Marles-Wright, J.; Clarke, D.J.; Maitra, A.; Dodds, M.; Hanley, B.; Campopiano, D.J. Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. Chem. Commun. 2015, 51, 10483–10485. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef]
- Ma, C.-M.; Abe, T.; Komiyama, T.; Wang, W.; Hattori, M.; Daneshtalab, M. Synthesis, anti-fungal and 1,3-β-d-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7009–7014. [Google Scholar] [CrossRef]
- Mahata, D.; Mandal, S.M.; Bharti, R.; Gupta, V.K.; Mandal, M.; Nag, A.; Nando, G.B. Self-assembled cardanol azo derivatives as antifungal agent with chitin-binding ability. Int. J. Biol. Macromol. 2014, 69, 5–11. [Google Scholar] [CrossRef]
- Tsuchiya, H. Membrane Interactions of Phytochemicals as Their Molecular Mechanism Applicable to the Discovery of Drug Leads from Plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta BBA -Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Elmasri, W.A.; Zhu, R.; Peng, W.; Al-Hariri, M.; Kobeissy, F.; Tran, P.; Hamood, A.N.; Hegazy, M.F.; Paré, P.W.; Mechref, Y. Multitargeted Flavonoid Inhibition of the Pathogenic Bacterium Staphylococcus aureus: A Proteomic Characterization. J. Proteome Res. 2017, 16, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martínez, M.D.; García-Cánovas, F.; Rodríguez-López, J.N. Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J. Antimicrob. Chemother. 2006, 57, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.-R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta BBA-Biomembr. 2015, 1848, 695–701. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.W.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef]
- Cheah, H.-L.; Lim, V.; Sandai, D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 2014, 9, e95951. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Medina-Ruiz, E.; Casas, L.; Mantell, C.; Martínez de la Ossa-Fernández, E.J. Development of cotton fabric impregnated with antioxidant mango polyphenols by means of supercritical fluids. J. Supercrit. Fluid 2018, 140, 310–319. [Google Scholar] [CrossRef]
- Behr, M.; Sergeant, K.; Leclercq, C.C.; Planchon, S.; Guignard, C.; Lenouvel, A.; Renaut, J.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Insights into the molecular regulation of monolignol-derived product biosynthesis in the growing hemp hypocotyl. BMC Plant Biol. 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M.; et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Nyanhongo, G.; Kudanga, T.; Prasetyo, E.; Guebitz, G. Mechanistic insights into laccase-mediated functionalisation of lignocellulose material. Biotechnol. Genet. Eng. Rev. 2010, 27, 305–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dong, A.; Fan, X.; Yu, Y.; Yuan, J.; Wang, P.; Wang, Q.; Cavaco-Paulo, A. Enzymatic Hydrophobic Modification of Jute Fibers via Grafting to Reinforce Composites. Appl. Biochem. Biotechnol. 2016, 178, 1612–1629. [Google Scholar] [CrossRef] [PubMed]
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzyme Microb. Technol. 2008, 43, 84–92. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Kim, J.; Oliveira, F.; Souto, P.; Kim, H.; Nakamatsu, J. Laccase-mediated grafting of polyphenols onto cationized cotton fibers to impart UV protection and antioxidant activities. J. Appl. Polym. Sci. 2018, 135, 45801. [Google Scholar] [CrossRef]
- Fillat, A.; Gallardo, O.; Vidal, T.; Pastor, F.I.J.; Díaz, P.; Roncero, M.B. Enzymatic grafting of natural phenols to flax fibres: Development of antimicrobial properties. Carbohydr. Polym. 2012, 87, 146–152. [Google Scholar] [CrossRef]
- Ma, M.; Li, R.; Du, Y.; Tang, Z.; Zhou, W. Analysis of antibacterial properties of naturally colored cottons. Text. Res. J. 2013, 83, 462–470. [Google Scholar] [CrossRef]
- Ma, M.; Luo, S.; Hu, Z.; Tang, Z.; Zhou, W. Antioxidant properties of naturally brown-colored cotton fibers. Text. Res. J. 2016, 86, 256–263. [Google Scholar] [CrossRef]
- Shemsi, A.M.; Khanday, F.A.; Qurashi, A.; Khalil, A.; Guerriero, G.; Siddiqui, K.S. Site-directed chemically-modified magnetic enzymes: Fabrication, improvements, biotechnological applications and future prospects. Biotechnol. Adv. 2019, 37, 357–381. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).