Structural Features, Modification, and Functionalities of Beta-Glucan
Abstract
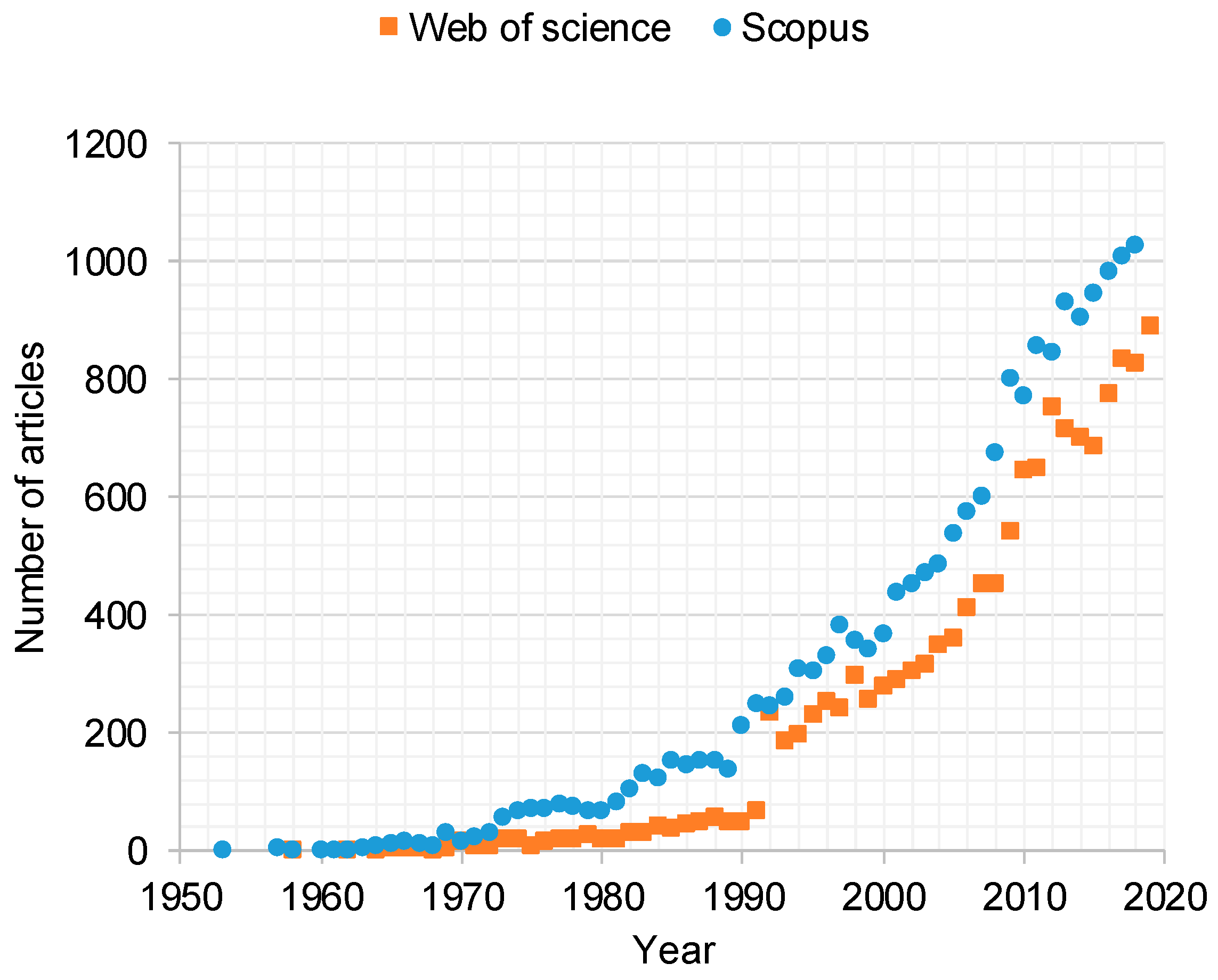
1. Introduction
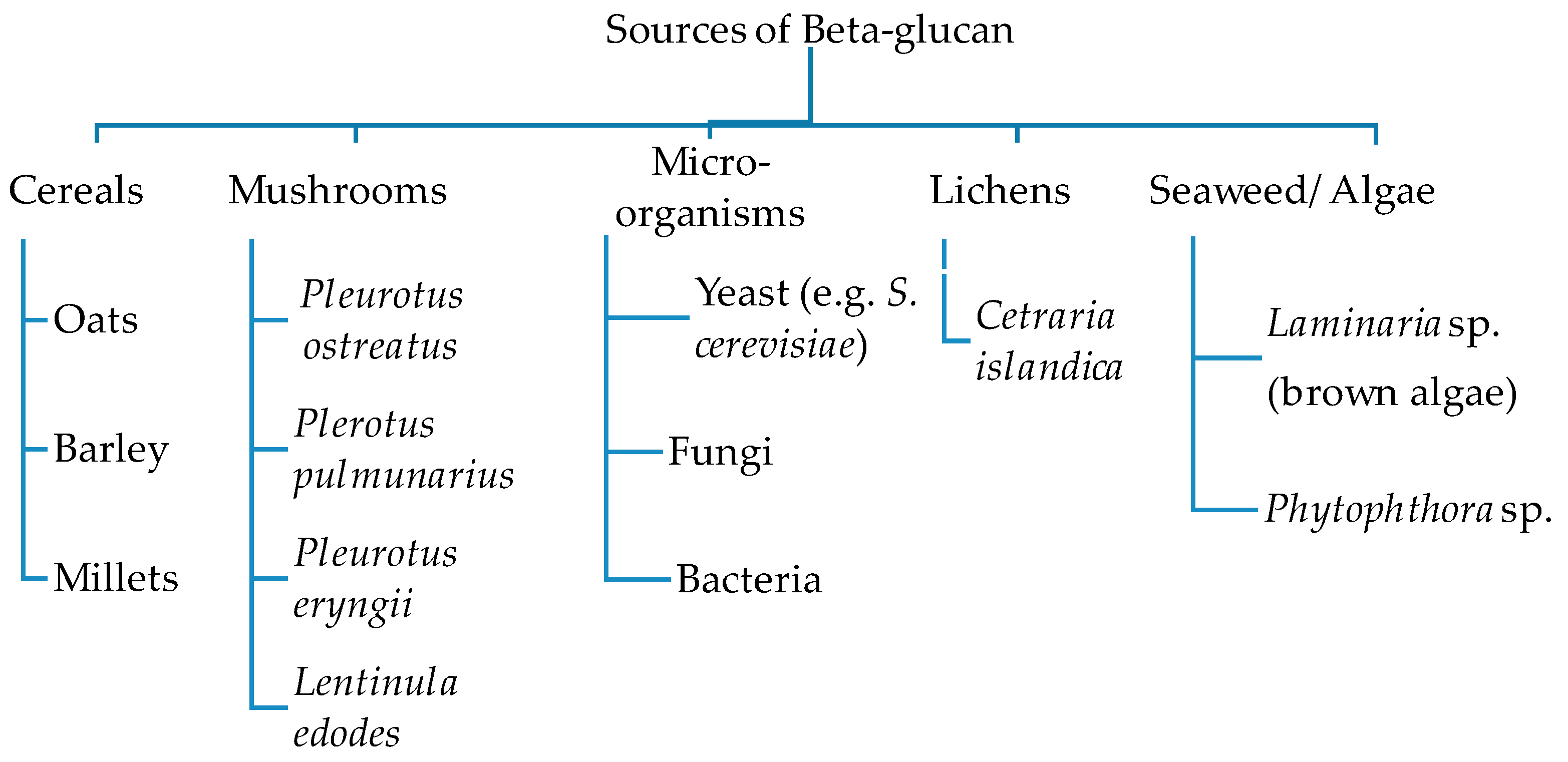
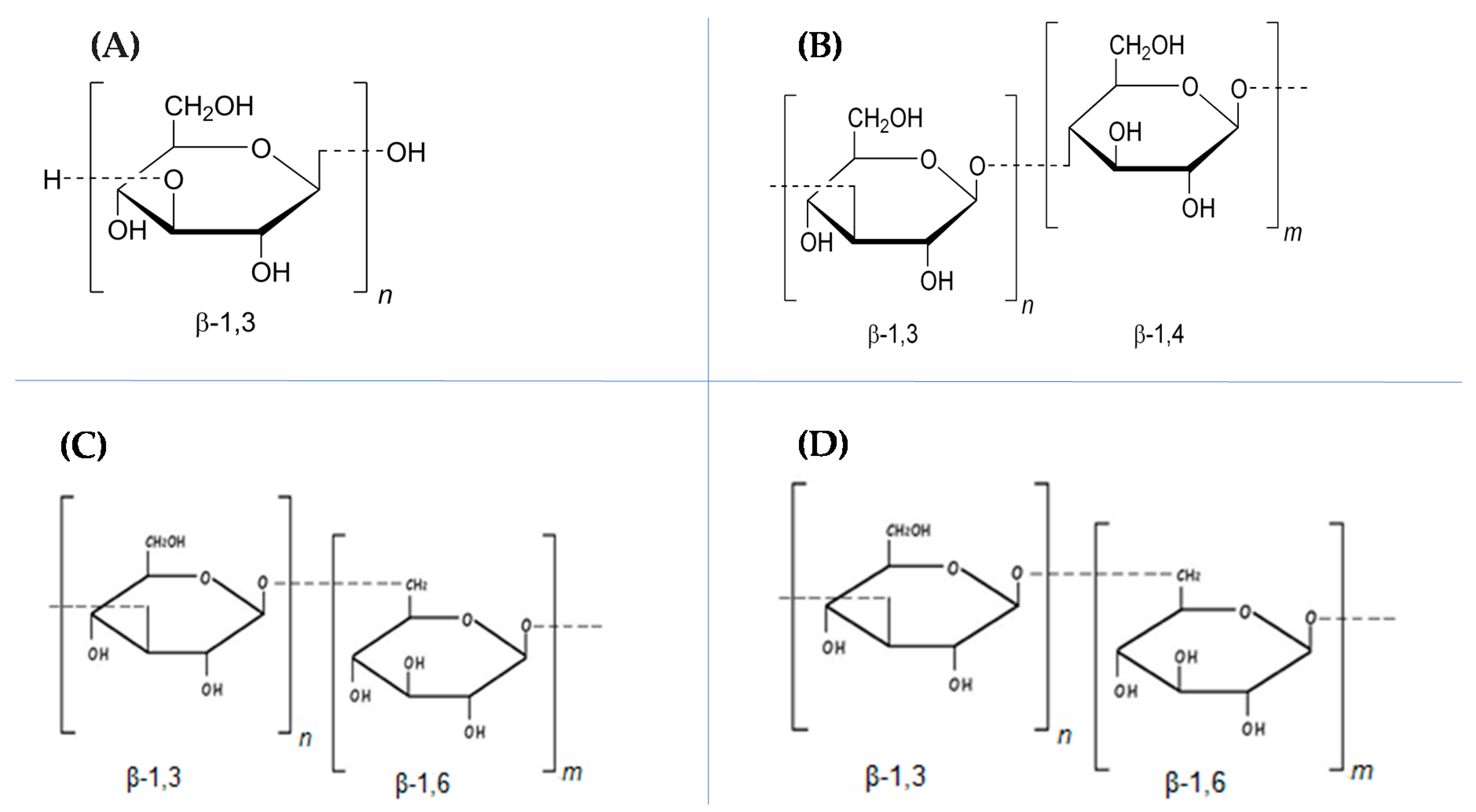
2. Sources, Chemical Structures, and Functionalities of β-Glucan
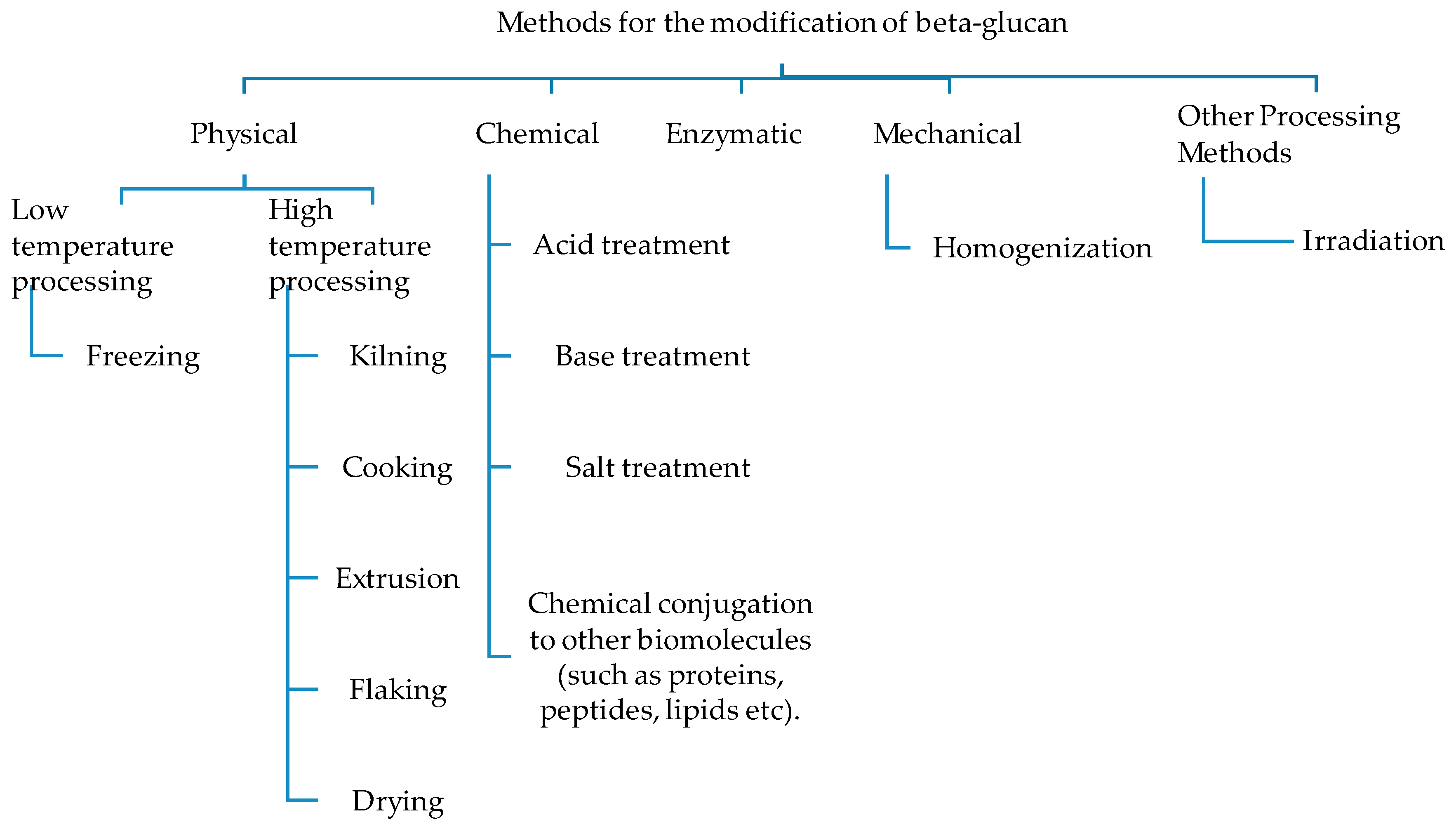
3. Extraction, Modification, and Their Effects on Structure and Properties of β-Glucan
3.1. Physical Treatments
3.1.1. High-Temperature Processing
Kilning
Drying
Flaking
Extrusion
Cooking
3.1.2. Low Temperature Processing
Freezing and Refrigeration
3.2. Chemical Modification
3.2.1. Acid Treatment
Modification by Ascorbic Acid Treatment
Modification by Carboxymethylation
Modification by Sulfation
Modification by Phosphorylation
3.2.2. Conjugation to Other Biomolecules
3.3. Enzymatic Modification
3.4. Mechanical Processing
3.5. Other Processing Methods
3.5.1. Irradiation
3.5.2. Microwave Energy
4. Applications of Modified β-Glucan in Various Food Systems
5. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal β-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, L.M.; Lê, K.-A.; Samra, R.A.; Roger, O.; Green, H.; Macé, K. The scientific basis for healthful carbohydrate profile. Crit. Rev. Food Sci. Nutr. 2019, 59, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Mariani, M. Simple vs. Complex Carbohydrate Dietary Patterns and the Global Overweight and Obesity Pandemic. Int. J. Environ. Res. Public Health 2017, 14, 1174. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Liu, Y.K.; Sun, Y.H.; Wang, X.D.; Chen, K.; Li, T.Y.; Li, E.C.; Zhang, M.L. Influence of different dietary carbohydrate sources on the growth and intestinal microbiota of Litopenaeus vannamei at low salinity. Aquac. Nutr. 2017, 23, 444–452. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M. β-Glucan as a Food Ingredient. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 351–381. [Google Scholar] [CrossRef]
- De Souza Bonfim-Mendonça, P.; Capoci, I.R.G.; Tobaldini-Valerio, F.K.; Negri, M.; Svidzinski, T.I.E. Overview of β Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models. Int. J. Mol. Sci. 2017, 18, 1629. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Editorial (Hot Topic: Recent Advance in the Studies of Beta-glucans for Cancer Therapy). Anti-Cancer Agents Med. Chem. 2013, 13, 679–680. [Google Scholar] [CrossRef]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Izydorczyk, M.S. Cereal β-glucans: Structures, physical properties, and physiological functions. In Functional Food Carbohydrates; CRC Press: Bocaraton, FL, USA, 2006; pp. 15–86. [Google Scholar]
- Liatis, S.; Chala, P.; Dimosthenopoulos, E.; Kyriakopoulos, C.; Kapantais, K.; Katsilambros, E. The consumption of bread enriched with beta-glucanreducesLDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Teas, J. The dietary intake of laminaria, a brown seaweed, and breast cancer prevention. Nutr. Cancer 1982, 4, 217–222. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between modification, conformation and functional activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- e-CFR. Electronic Code of Federal Regulations: Title 21: Food and Drugs; PART 101—Food Labeling; Subpart A—General Provisions; 101.9 Nutrition Labeling of Food. Available online: https://gov.ecfr.io/cgi-bin/text-idx?SID=e81b3ec77d443f8e87ef5f991a582467&mc=true&node=pt21.2.101&rgn=div5 (accessed on 14 January 2019).
- Xu, S.; Xu, X.; Zhang, L. Effect of Heating on Chain Conformation of Branched β-Glucan in Water. J. Phys. Chem. B 2013, 117, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Cui, S.W.; Xie, M. Beta-Glucans and Their Derivatives. In Bioactive Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–141. [Google Scholar] [CrossRef]
- Bernstein, A.M.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M.F. Major Cereal Grain Fibers and Psyllium in Relation to Cardiovascular Health. Nutrients 2013, 5, 1471–1487. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. Beta-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Boil. FG B 2016, 90, 53–60. [Google Scholar] [CrossRef]
- Cho, K.C.; White, P.J. Enzymatic analysis of β-glucan content in different oat genotypes. Cereal Chem. 1993, 70, 539–542. [Google Scholar]
- Ragaee, S.; Campbell, G.; Scoles, G.; McLeod, J.; Tyler, R. Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 1. Composition, molecular weight distribution of water extracts, and biochemical characteristics of purified water-extractable arabinoxylan. J. Agric. Food Chem. 2001, 49, 2437–2445. [Google Scholar] [CrossRef]
- Barsanti, L.; Vismara, R.; Passarelli, V.; Gualtieri, P. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J. Appl. Phycol. 2001, 13, 59–65. [Google Scholar] [CrossRef]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1, 6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Kim, Y.-S. Water-solubility of β-glucans in various edible mushrooms-research note. Cereal Chem. 2005, 10, 294–297. [Google Scholar] [CrossRef]
- Park, H.-G.; Shim, Y.Y.; Choi, S.-O.; Park, W.-M. New method development for nanoparticle extraction of water-soluble β-(1→3)-D-glucan from edible mushrooms, Sparassis crispa and Phellinus linteus. J. Agric. Food Chem. 2009, 57, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of its By-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef]
- Johansson, L.; Virkki, L.; Maunu, S.; Lehto, M.; Ekholm, P.; Varo, P. Structural characterization of water soluble β-glucan of oat bran. Carbohydr. Polym. 2000, 42, 143–148. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef]
- Manners, D.J.; Masson, A.J.; Patterson, J.C. The structure of a β-(1→3)-D-glucan from yeast cell walls. Biochem. J. 1973, 135, 19–30. [Google Scholar] [CrossRef]
- Han, M.D.; Han, Y.S.; Hyun, S.H.; Shin, H.W. Solubilization of water-insoluble beta-glucan isolated from Ganoderma lucidum. J. Environ. Boil. 2008, 29, 237–242. [Google Scholar] [CrossRef]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1→3)-β-d-glucans. Appl. Microbiol. Biotechnol. 2005, 68, 163–173. [Google Scholar] [CrossRef]
- Li, W.; Cui, S.W.; Wang, Q. Solution and Conformational Properties of Wheat β-d-Glucans Studied by Light Scattering and Viscometry. Biomacromolecules 2006, 7, 446–452. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, F.; Xu, X.; Zhang, L. Extended chain conformation of β-glucan and its effect on antitumor activity. J. Mater. Chem. B 2017, 5, 5623–5631. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y.; Wang, S.; Xu, X.; Zhang, L. Determination of the Triple Helical Chain Conformation of β-Glucan by Facile and Reliable Triple-Detector Size Exclusion Chromatography. J. Phys. Chem. B 2014, 118, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Q.; Cui, S.W.; Burchard, W.; Yada, R. Carbanilation of cereal β-glucans for molecular weight determination and conformational studies. Carbohydr. Res. 2007, 342, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and Solubility, Solubility of Polysaccharides. In Solubility of Polysaccharides; Zhenbo, X., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Kojima, T.; Tabata, K.; Itoh, W.; Yanaki, T. Molecular weight dependence of the antitumor activity of schizophyllan. Agric. Biol. Chem. 1986, 50, 231–232. [Google Scholar]
- Doublier, J.-L.; Wood, P.J. Rheological Properties of Aqueous Solutions of (1-3) (1-4)-beta-D-Glucan from Oats (Avena sativa L.). Cereal Chem. 1995, 72, 335–340. [Google Scholar]
- Tejinder, S.; Bhupinder, K.; Harinder, K. Flow behavior and functional properties of barley and oat water-soluble β-D-glucan rich extractions. Int. J. Food Prop. 2000, 3, 259–274. [Google Scholar] [CrossRef]
- Zielke, C.; Stradner, A.; Nilsson, L. Characterization of cereal β-glucan extracts: Conformation and structural aspects. Food Hydrocoll. 2018, 79, 218–227. [Google Scholar] [CrossRef]
- Temelli, F.; Bansema, C.; Stobbe, K. Development of an Orange-Flavored Barley β-Glucan Beverage. Cereal Chem. J. 2004, 81, 499–503. [Google Scholar] [CrossRef]
- Kaur, R.; Riar, C.S. Sensory, rheological and chemical characteristics during storage of set type full fat yoghurt fortified with barley β-glucan. J. Food Sci. Technol. 2019, 1–11. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M. Cereal polysaccharides as sources of functional ingredient for reformulation of meat products: A review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- Vaikousi, H.; Biliaderis, C.G.; Izydorczyk, M.S. Solution flow behavior and gelling properties of water-soluble barley (1→3,1→4)-β-glucans varying in molecular size. J. Cereal Sci. 2004, 39, 119–137. [Google Scholar] [CrossRef]
- Wood, P.J. Cereal β-glucans in diet and health. J. Cereal Sci. 2007, 46, 230–238. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Sello, C.T.; Qin, G.-X.; Che, D.; Han, R. Does Dietary Fiber Affect the Levels of Nutritional Components after Feed Formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef]
- Santos, A.; Marquina, D.; Leal, J.; Peinado, J. (1→6)-β-D-glucan as cell wall receptor for Pichia membranifaciens killer toxin. Appl. Environ. Microbiol. 2000, 66, 1809–1813. [Google Scholar] [CrossRef]
- Bhatty, R. Laboratory and pilot plant extraction and purification of β-glucans from hull-less barley and oat brans. J. Cereal Sci. 1995, 22, 163–170. [Google Scholar] [CrossRef]
- Immerstrand, T.; Bergenståhl, B.; Trägårdh, C.; Nyman, M.; Cui, S.; Öste, R. Extraction of β-Glucan from Oat Bran in Laboratory Scale. Cereal Chem. 2009, 86, 601–608. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, N.N.; Adachi, Y.; Tamura, H.; Tanaka, S.; Ohno, N. The solubilization and biological activities of Aspergillus β-(1,3)-d-glucan. FEMS Immunol. Med. Microbiol. 2004, 42, 155–166. [Google Scholar] [CrossRef]
- Du, B.; Zhu, F.; Xu, B. β-Glucan extraction from bran of hull-less barley by accelerated solvent extraction combined with response surface methodology. J. Cereal Sci. 2014, 59, 95–100. [Google Scholar] [CrossRef]
- Ookushi, Y.; Sakamoto, M.; Azuma, J.-I. Optimization of Microwave-assisted Extraction of Polysaccharides from the Fruiting Body of Mushrooms. J. Appl. Glycosci. 2006, 53, 267–272. [Google Scholar] [CrossRef][Green Version]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. Cancer 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Methacanon, P.; Weerawatsophon, U.; Tanjak, P.; Rachtawee, P.; Prathumpai, W. Interleukin-8 stimulating activity of low molecular weight β-glucan depolymerized by γ-irradiation. Carbohydr. Polym. 2011, 86, 574–580. [Google Scholar] [CrossRef]
- Zhang, M. Heating-induced conformational change of a novel β-(1→3)-D-glucan from Pleurotus geestanus. Biopolymers 2010, 93, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Knuckles, B.E.; Chiu, M.C.M. β-Glucanase activity and molecular weight of β-glucans in barley after various treatments. Cereal Chem. 1999, 76, 92–95. [Google Scholar] [CrossRef]
- Oliveira, L.D.C.; Oliveira, M.; Meneghetti, V.L.; Mazzutti, S.; Colla, L.M.; Elias, M.C.; Gutkoski, L.C. Effect of drying temperature on quality of β-glucan in white oat grains. Food Sci. Technol. 2012, 32, 775–783. [Google Scholar] [CrossRef][Green Version]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Galović, P.; Galović, V.; Filipović-Grčić, J.; Srečec, S. Application of different drying methods on β-glucan isolated from spent brewer’s yeast using alkaline procedure. Agric. Conspec. Sci. 2010, 75, 45–50. [Google Scholar]
- Upadhyay, T.K.; Fatima, N.; Sharma, D.; Saravanakumar, V.; Sharma, R. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. EXCLI J. 2017, 16, 210. [Google Scholar] [CrossRef]
- Liu, Y. Beta-glucan effects on pasting properties and potential health benefits of flours from different oat lines. Grad. Theses Diss. 2010. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Miller, S.S.; Regand, A.; Defelice, C.; Duss, R.; Wolever, T.M.; Wood, P.J. Processing affects the physicochemical properties of β-glucan in oat bran cereal. J. Agric. Food Chem. 2010, 58, 7723–7730. [Google Scholar] [CrossRef]
- Lan-Pidhainy, X.; Brummer, Y.; Tosh, S.M.; Wolever, T.M.; Wood, P.J. Reducing beta-glucan solubility in oat bran muffins by freeze-thaw treatment attenuates its hypoglycemic effect. Cereal Chem. 2007, 84, 512–517. [Google Scholar] [CrossRef]
- Ames, N.; Storsley, J.; Tosh, S. Effects of processing on physicochemical properties and efficacy of β-glucan from oat and barley. Cereal Foods World 2015, 60, 4–8. [Google Scholar] [CrossRef]
- Ames, N.; Tosh, S. Validating the health benefits of barley foods: Effect of processing on physiological properties of beta-glucan in test foods. In Proceedings of the 2011 AACCI Annual Meeting Abstracts, Cereal Foods World (Supplement Online), Palm Springs, CA, USA, 16–19 October 2011; p. A28. [Google Scholar]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.H.; Knuckles, B.E.; Wood, D.; Inglett, G.E. Food processing reduces size of soluble cereal β-glucan polymers without loss of cholesterol-reducing properties. In Inglett Bioactive Compounds in Foods; American Chemical Society: Washington, DC, USA, 2002; pp. 105–116. [Google Scholar] [CrossRef]
- Kim, J.; Tanhehco, E.; Ng, P. Effect of extrusion conditions on resistant starch formation from pastry wheat flour. Food Chem. 2006, 99, 718–723. [Google Scholar] [CrossRef]
- Yiu, S.; Wood, P.; Weisz, J. Effects of cooking on starch and β-glucan of rolled oats. Cereal Chem. 1987, 64, 373–379. [Google Scholar]
- Ahmad, N.H.; Mustafa, S.; Che Man, Y.B. Microbial polysaccharides and their modification approaches: A review. Int. J. Food Prop. 2015, 18, 332–347. [Google Scholar] [CrossRef]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and modified (1→3)-β-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1β Production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef]
- Kivelä, R.; Gates, F.; Sontag-Strohm, T. Degradation of cereal beta-glucan by ascorbic acid induced oxygen radicals. J. Cereal Sci. 2009, 49, 1–3. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, Y.; Hou, X.; Zeng, F. Correlation of structure to antitumor activities of five derivatives of a β-glucan from Poria cocos sclerotium. Carbohydr. Res. 2004, 339, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheung, P.C.; Zhang, L.; Chiu, C.-M.; Ooi, V.E. Carboxymethylated β-glucans from mushroom sclerotium of Pleurotus tuber-regium as novel water-soluble anti-tumor agent. Carbohydr. Polym. 2004, 57, 319–325. [Google Scholar] [CrossRef]
- Magnani, M.; Calliari, C.M.; de Macedo, F.C., Jr.; Mori, M.P.; de Syllos Cólus, I.M.; Castro-Gomez, R.J. Optimized methodology for extraction of (1→3) (1→6)-β-D-glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L. Structure and chain conformation of five water-soluble derivatives of a β-D-glucan isolated from Ganoderma lucidum. Carbohydr. Res. 2009, 344, 105–112. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Y.; Xiong, S.; Zhao, S.; Huang, Q. Optimised methodology for carboxymethylation of (1→3)-β-d-glucan from Y east (S accharomyces cerevisiae) and promotion of mechanical activation. Int. J. Food Sci. Technol. 2013, 48, 253–259. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.D.; Dekker, R.F.; Barbosa, A.M.; Carbonero, E.R.; Silveira, J.L.; Glauser, B.; Pereira, M.S.; de Lourdes Corradi da Silva, M. Sulfonation and anticoagulant activity of fungal exocellular β-(1→6)-D-glucan (lasiodiplodan). Carbohydr. Polym. 2013, 92, 1908–1914. [Google Scholar] [CrossRef]
- Williams, D.L.; McNamee, R.B.; Jones, E.L.; Pretus, H.A.; Ensley, H.E.; Browder, I.W.; Di Luzio, N.R. A method for the solubilization of a (1→3)-β-D-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991, 219, 203–213. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yao, S.-J.; Guan, Y.-X.; Wu, T.-X.; Kennedy, J. A novel process for preparation of (1→3)-β-D-glucan sulphate by a heterogeneous reaction and its structural elucidation. Carbohydr. Polym. 2005, 59, 93–99. [Google Scholar] [CrossRef]
- Yoshida, T.; Yasuda, Y.; Mimura, T.; Kaneko, Y.; Nakashima, H.; Yamamoto, N.; Uryu, T. Synthesis of curdlan sulfates having inhibitory effects in vitro against AIDS viruses HIV-1 and HIV-2. Carbohydr. Res. 1995, 276, 425–436. [Google Scholar] [CrossRef]
- Sun, T.; Xu, H.; Zhang, H.; Ding, H.; Cui, S.; Xie, J.; Xue, B.; Hua, X. Maillard reaction of oat β-glucan and the rheological property of its amino acid/peptide conjugates. Food Hydrocoll. 2018, 76, 30–34. [Google Scholar] [CrossRef]
- De Souza, N.L.; Bartz, J.; da Rosa Zavareze, E.; de Oliveira, P.D.; da Silva, W.S.V.; Alves, G.H.; Dias, A.R.G. Functional, thermal and rheological properties of oat β-glucan modified by acetylation. Food Chem. 2015, 178, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Lee, S.; Lee, K.Y.; Lee, H.G. Structural and biological characterization of aminated-derivatized oat β-glucan. J. Agric. Food Chem. 2005, 53, 5554–5558. [Google Scholar] [CrossRef] [PubMed]
- De Nooy, A.E.; Rori, V.; Masci, G.; Dentini, M.; Crescenzi, V. Synthesis and preliminary characterisation of charged derivatives and hydrogels from scleroglucan. Carbohydr. Res. 2000, 324, 116–126. [Google Scholar] [CrossRef]
- Kogan, G. (1→3, 1→6)-β-d-Glucans of yeasts and fungi and their biological activity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 23, pp. 107–152. [Google Scholar]
- Chen, C.; Wu, W.; Xu, X.; Zhang, L.; Liu, Y.; Wang, K. Chain conformation and anti-tumor activity of derivatives of polysaccharide from Rhizoma Panacis Japonici. Carbohydr. Polym. 2014, 105, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- McCleary, B.V. Enzymatic modification of plant polysaccharides. Int. J. Biol. Macromol. 1986, 8, 349–354. [Google Scholar] [CrossRef]
- Roubroeks, J.; Andersson, R.; Mastromauro, D.; Christensen, B.; Åman, P. Molecular weight, structure and shape of oat (1→3), (1→4)-β-d-glucan fractions obtained by enzymatic degradation with (1→4)-β-d-glucan 4-glucanohydrolase from Trichodermareesei. Carbohydr. Polym. 2001, 46, 275–285. [Google Scholar] [CrossRef]
- Bae, I.Y.; Lee, S.; Kim, S.M.; Lee, H.G. Effect of partially hydrolyzed oat β-glucan on the weight gain and lipid profile of mice. Food Hydrocoll. 2009, 23, 2016–2021. [Google Scholar] [CrossRef]
- Duan, H.; Xiong, S.; Liu, H. Study on Solubility and Properties of Enzymolysates of Yeast β-1,3-Glucan. J. Food Sci. 2008, 1, 185–189. [Google Scholar]
- Bae, I.Y.; Kim, S.M.; Lee, S.; Lee, H.G. Effect of enzymatic hydrolysis on cholesterol-lowering activity of oat β-glucan. New Biotechnol. 2010, 27, 85–88. [Google Scholar] [CrossRef]
- Kivelä, R.; Pitkänen, L.; Laine, P.; Aseyev, V.; Sontag-Strohm, T. Influence of homogenisation on the solution properties of oat β-glucan. Food Hydrocoll. 2010, 24, 611–618. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Shi, F.; Shi, J.; Li, Y. Mechanochemical phosphorylation and solubilisation of β-D-glucan from yeast Saccharomyces cerevisiae and its biological activities. PLoS ONE 2014, 9, e103494. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yi, C.; Wu, H.; Guo, S. Degradation kinetics and molecular structure development of hydroxyethyl cellulose under the solid state mechanochemical treatment. Carbohydr. Polym. 2010, 81, 188–195. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, Z.; Hu, H.; Zhang, Y.; Tan, Y. Microscopic structure and properties changes of cassava stillage residue pretreated by mechanical activation. Bioresour. Technol. 2011, 102, 7953–7958. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, L.; Pang, W.; Wang, T.; Chen, P.; Zhu, B.; Zhang, J. A novel soluble β-1, 3-d-glucan Salecan reduces adiposity and improves glucose tolerance in high-fat diet-fed mice. Br. J. Nutr. 2013, 109, 254–262. [Google Scholar] [CrossRef]
- Shah, A.; Ahmad, M.; Ashwar, B.A.; Gani, A.; Masoodi, F.A.; Wani, I.A.; Wani, S.M.; Gani, A. Effect of γ-irradiation on structure and nutraceutical potential of β-d-glucan from barley (Hordeum vulgare). Int. J. Boil. Macromol. 2015, 72, 1168–1175. [Google Scholar] [CrossRef]
- Byun, E.-H.; Kim, J.-H.; Sung, N.-Y.; Choi, J.-I.; Lim, S.-T.; Kim, K.-H.; Yook, H.-S.; Byun, M.-W.; Lee, J.-W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Byun, E.B.; Park, S.H.; Jang, B.S.; Sung, N.Y.; Byun, E.H. Gamma-irradiated β-glucan induces immunomodulation and anticancer activity through MAPK and NF-κB pathways. J. Sci. Food Agric. 2016, 96, 695–702. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, W. Microwave-assisted solubilization and solution properties of hyperbranched polysaccharide. Carbohydr. Res. 2008, 343, 3071–3078. [Google Scholar] [CrossRef]
- Liu, R.; Wang, N.; Li, Q.; Zhang, M. Comparative studies on physicochemical properties of raw and hydrolyzed oat β-glucan and their application in low-fat meatballs. Food Hydrocoll. 2015, 51, 424–431. [Google Scholar] [CrossRef]
- Klopfenstein, C.F. The role of cereal beta-glucans in nutrition and health. Cereal Foods World 1988, 33, 865–869. [Google Scholar]
- Wood, P.; Anderson, J.; Braaten, J.; Cave, N.; Scott, F.; Vachon, C. Physiological effects of beta-D-glucan rich fractions from oats. Cereal Foods World 1989, 34, 878–882. [Google Scholar]
- Lee, S.; Inglett, G.E. Rheological and physical evaluation of jet-cooked oat bran in low calorie cookies. Int. J. Food Sci. Technol. 2006, 41, 553–559. [Google Scholar] [CrossRef]
- Cleary, L.J.; Andersson, R.; Brennan, C. The behaviour and susceptibility to degradation of high and low molecular weight barley β-glucan in wheat bread during baking and in vitro digestion. Food Chem. 2007, 102, 889–897. [Google Scholar] [CrossRef]
- Cavallero, A.; Empilli, S.; Brighenti, F.; Stanca, A. High (1→3, 1→4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci. 2002, 36, 59–66. [Google Scholar] [CrossRef]



| Food Source | Content | References |
|---|---|---|
| Oats | 4.5%–5.5% | [21] |
| Barley | 4.5% | [21] |
| Whole rye flour | 1.0%–2.5% | [22] |
| Saccharomyces cerevisiae | 5%–7% | [23] |
| Euglena | 90% | [23] |
| Stipes of Durvillaea antarctica | 33% | [24] |
| Holdfast of Durvillaea antarctica | <5% | [24] |
| Sparassis crispa | 43.6% | [26] |
| Inonotus obliquus | 3.1% | [25] |
| Gyrophora esculenta | 22.7% | [25] |
| Coriolus versicolor | 46.5% | [25] |
| Sources of β-Glucan | Modification Method | Modification Condition(s) | Characterization Approach | Outcome of Modification | Reference |
|---|---|---|---|---|---|
| Edible fungus (Pleurotusgeestanus) | Physical | Thermal treatment |
|
| [58] |
| Barley | Physical | Hydrothermal treatment (autoclave) in combination with different chemical treatments (50 mM CaCl2, 70% ethanol, 100 mM HCl, and 2.5 mM NaOH) |
|
| [59] |
| Oats | Physical | Constant drying at air temperatures of 25, 50, 75, and 100 °C |
|
| [60] |
| Oats | Physical | Constant drying at air temperatures of 25, 50, 75, and 100 °C |
|
| [60] |
| Brewer’s yeast (S. cerevisiae) | Physical | Various drying methods (spray drying, air drying, and lyophilization) |
|
| [61] |
| Yeast (S. cerevisiae) | Physical | Spray drying and lyophilization |
|
| [62] |
| Oats | Physical | Steaming and flaking |
|
| [63] |
| Oat bran | Physical | Extrusion |
|
| [64] |
| Oat bran | Physical | Extrusion |
|
| [65] |
| Oats | Physical | Freezing |
|
| [66] |
| Sources of β-Glucan | Modification Method | Modification Condition(s) | Characterization Approach | Outcome of Modification | Reference |
|---|---|---|---|---|---|
| Cereal | Chemical | Addition of ascorbic acid (vitamin C) and dehydroascorbic acid to the pure cereal β-glucan (0.6%) in the presence of iron sulfate |
|
| [77] |
| Cereals | Chemical | Addition of apple juice with high levels of ascorbic acid into barley porridge |
|
| [68] |
| Fungus (Poriacocos) | Chemical | Carboxymethylated β-glucan developed by using chloroacetic acid in either 2-propyl alcohol or water at high pH ≥ 12 |
|
| [78] |
| Mushroom (Pleurotus tuber-regium) | Chemical | Monochloroacetic acid in sodium hydroxide solution |
|
| [79] |
| S. cerevisiae | Chemical | Carboxymethylated by using monochloroacetic acid |
|
| [80] |
| Gonderma lucidum | Chemical | Sulfation, carboxymethylation, hydroxypropylation, hydroxyethylation, and methylation |
|
| [81] |
| S. cerevisie | Chemical | Carboxymethylation (double step alkalization and etherification with the monochloroaetic acid) |
|
| [82] |
| Lasiodiplodia theobromae | Chemical | Sulfation by using solvent formaldehyde, catalytic reagent pyridines, and chlorosulfonic acid in the form of hydroxyl group donating compound |
|
| [83] |
| Baker’s yeast source (S. cerevisiae). | Chemical | Sulfation by using dimethyl sulfoxide (DMSO)-containing urea |
|
| [84] |
| Yeast | Chemical | Sulfation process by utilizing combination of sulfuric acid and n-propanol (1:4 molar ratio) |
|
| [85] |
| Curdlans | Chemical | Sulfation (sulfation of curdlans by various chemicals such as piperidine-N-sulfonic acid (PSA method), SO3− pyridine complex in pyridine (SPC method), and chlorosulfonic acid in pyridine (CSA method)) |
|
| [86] |
| Oats | Chemical | Non-enzymatic reactions (Maillard reaction) |
|
| [87] |
| Oat β-glucan | Chemical | Acetylation (using 4% and 6% acetic anhydride for 10 and 20 min) |
|
| [88] |
| Oats | Chemical | Reductive amination by using dimethyl sulfoxide (DMSO), sodium acetate and sodium cyanoborohydride reagents |
|
| [89] |
| Sources of β-Glucan | Modification Method | Modification Condition(s) | Characterization Approach | Outcome of Modification | Reference |
|---|---|---|---|---|---|
| Oats | Enzymatic | Enzymatic hydrolysis (Lichenase) |
|
| [95] |
| Yeast (Trichoderma strain LE02) | Enzymatic | Enzymatic hydrolysis (β-1,3 Glucanase) | Functional characterization |
| [15] |
| Oats | Enzymatic | Enzymatic hydrolysis (Cellulase) |
|
| [96] |
| Sources of β-Glucan | Modification Method | Modification Condition(s) | Characterization Approach | Outcome of Modification | Reference |
|---|---|---|---|---|---|
| Oat | Mechanical | Homogenization (colloid mill, high-pressure valve homogenizer, microfluidizer) |
|
| [77] |
| Microbial (S. cerevisiae) | Mechanical | Homogenization |
|
| [100] |
| Brewer’s yeast, (S. cerevisiae) | Mechanical | Mechanochemical Method |
|
| [101] |
| Sources of β-Glucan | Modification Method | Modification Condition(s) | Characterization Approach | Outcome of Modification | Reference |
|---|---|---|---|---|---|
| Barley (Hordeum vulgare L.) | Irradiation | Gamma irradiation |
|
| [105] |
| Fungus (Oppiocordyceps dipterigenia) BCC2073 | Irradiation | Gamma rays (various doses 0–100 kGy) |
|
| [57] |
| Black yeast (Aureobasidium Sp) | Irradiation | Gamma radiations (Co-60 source and different doses of irradiations) |
|
| [106] |
| Black yeast (Aureobasidium Sp). | Irradiation | Gamma Irradiation (dose 50 kGy) |
|
| [107] |
| Pleurotus tuber-regium | Irradiation | Microwave (0.02 wt% aqueous sodium azide) |
|
| [108] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. https://doi.org/10.3390/fib8010001
Kaur R, Sharma M, Ji D, Xu M, Agyei D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers. 2020; 8(1):1. https://doi.org/10.3390/fib8010001
Chicago/Turabian StyleKaur, Ramandeep, Minaxi Sharma, Dawei Ji, Min Xu, and Dominic Agyei. 2020. "Structural Features, Modification, and Functionalities of Beta-Glucan" Fibers 8, no. 1: 1. https://doi.org/10.3390/fib8010001
APA StyleKaur, R., Sharma, M., Ji, D., Xu, M., & Agyei, D. (2020). Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers, 8(1), 1. https://doi.org/10.3390/fib8010001






