5′-Substituted Indoline Spiropyrans: Synthesis and Applications
Abstract
1. Introduction
- Aza- and thioheterocyclic spiropyran analogs, having benzothiazoline, benzoxazoline, thiazolidine, thiazine, oxazoline, oxazine, pyrrolidine, and piperidine moieties in the indoline fragment, except substituted indoline ring; and heteroanalogs with benzoselenazole, benzoxazole, benzothiazole rings and related spirobenzoxazine, spironaphthoxazine, spirobenzothiopyran derivatives in the benzopyran part; all were excluded from the discussion and analysis.
- Spiropyrans with hetero- and aryl-fused indoline and benzopyrane fragment also were excluded.
- Restrictions were also introduced on the structure of the substituents R1′ at N1′-atom and R3′ at C3′-atom, except for the methyl group.
- They possess a binary set of two different types of analytical signals (photo-induced light absorption in the range 560–600 nm and fluorescence induction in the colored merocyanine form);
- The functional linker fragment is located at the C5′-atom while the EWG group is at C6-postion pyran fragment along one axis (uniaxial orientation).
2. Structure and Spectral Properties of Spiropyrans
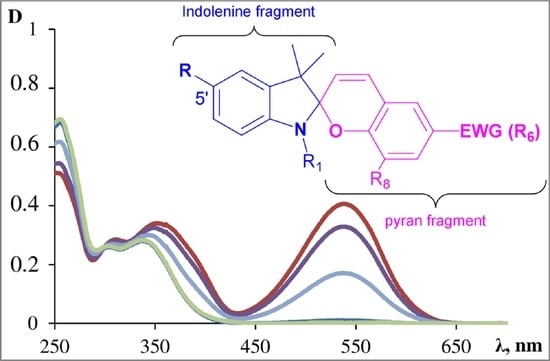
2.1. UV-VIS-Spectra and Fluorescence Spectra of the 5′-Substituted Spiropyran Derivatives
2.2. NMR Spectroscopy
2.3. Solvatochromism
2.4. Acidochromism
3. Chemistry of Spiropyrans
Synthesis of the 5′-Substituted Spiropyran Derivatives. Side Reactions in the Synthesis
4. Chemical Properties
4.1. Classic Methods for the Modification of the Structure and Properties of 5′-Substituted Spiropyrans (Pathways A, B, C)
4.2. New Methods for the Modification of the Structures and Properties of Spiropyrans
- (a).
- For the protein targets: covalent binding of a probe molecule with a target binding site by the self-recognition principle (bacteriorhodopsin). In our works [16,35,45,46,79,80,81,82,83,84,85,86,87,88,89,90,91,92], we have for the first time used photochromic derivatives of series of spiropyrans and dithienylethenes as photochromically labeled analogs of chromophore groups of a photosensitive retinal protein: the light-dependent proton translocase bacteriorhodopsin from Halobacterium salinarum.
- (b).
- For the target proteins: non-covalent affine binding of a probe molecule with the target via the “molecular address” introduced into the probe molecule (photoactive thromboxane A2 receptor inhibitors). We have previously discovered a new class of platelet aggregation inhibitors (5-substituted 3-pyridylisoxazoles) and developed new methods of their synthesis. A library of more than 120 compounds of classes of 3,5-substituted isoxazoles and their 4,5-dihydroderivatives containing 2-, 3-, and 4-pyridine moieties at the C3-position and substituents of different nature at the C5-position of the isoxazole ring was produced. To study the action mechanism of this class of human platelet aggregation inhibitors, three compounds containing the molecular address in a different spatial orientation to a fragment of a photochromic label from the series of spiropyrans were synthesized, and the process of their binding with human platelet membrane receptors was explored [85,93,94].
- (c).
- Covalent binding of a label molecule with an inorganic nanosized target via a selective terminal reactive group. For specific binding with a target (CdSe quantum dots) various derivatives of terminal mono- and dithiols were used and different linkers for their introduction into the molecules of target photochromes were studied [35,91,95].
- (d).
- Covalent binding of a label molecule with a target via a selective terminal reactive group. For specific binding with a target, namely, sulfhydryl groups of Cys-protein residues, a series of photochromic spiropyrans with a maleimide moiety in the molecule was synthesized [81];
- (e).
- Model labeling by the Sonogashira coupling of 5-iodo-1,3-dimethyluracil by terminal acetylene SP12 with formation of target SP236 [80].
- Catalytic cycloaddition of diazocompound to carbon clusters in SP-methanofullerene SP28 synthesis [102].
- Synthesis of bis-SP-functionalized spiro[fluorene-9,9′-xanthene] derivative (SFX-2SP227). The introduction of two SP moieties to the SFX core included the following steps: 1. a Suzuki reaction between the di-Br-SFX and indol derivative, 2. quaternization of product by CH3I, 3. condensation reaction of indolium salt with 2-hydroxy-5-nitrobenzaldehyde producing SFX-2SP227 [104].
- Hybrid dyad DHA-SP255. The synthesis of the dyad from the precursors was carried out under Sonogashira coupling conditions. When using Pd+2/CuI as catalyst system, the authors observed high conversions of the precursors, but also substantial amounts of homocoupling of the acetylenic spiropyran into a butadiyne product. Removing this resulting side product via repeated column chromatography reduced the isolated yield of DHA-SP255 below 10%. It was nevertheless possible to suppress the homocoupling by using tris(dibenzylideneacetone)dipalladium(0) and triphenylarsine as catalyst system, and thereby DHA-SP255 was isolated with 42% yield [105].
- Hybrid dyad SP222, containing a dithienylethene group between two spiropyran moieties was synthesized by the Sonogashira cross-coupling reaction between DTE-bis-alkyne and SP61, using Pd(PPh3)4/CuI/Et3N as catalyst system, dissolved in toluene/THF, with yield of 60% [106].
- Suzuki coupling with thiophene-3-boronic acid, NBS bromination and Stille coupling reactions were used for the mono- and poly-thienyl SP conjugates SP165–SP168, SP223 preparation [107].
- The reactions of 6-iodo and 6-bromo-spiropyrans SP44, SP146, SP43 with phenylboronic acid under Suzuki coupling conditions (palladium acetate/Na2CO3 and DMF as solvent, 80 °C to give the coupling product in high isolated yield (87%). The 5′-substituents (chloro or benzoamido) and C3-C4-double bond of spiropyran SP47 remained intact under these conditions. 6-Bromospiropyran SP43 seemed to be less reactive under the conditions and the reaction gave the coupling product SP47 in 63% yield due to incomplete reaction, even in the extended reaction time [108].
- High molecular weight mechanochromic spiropyran main chain copolymer SP356 via microwave-assisted Suzuki-Miyaura polycondensation. MW irradiation of the sample mixture of 5′,6-dibromo-SP SP52, boronate C10-[B(pin)]2 Pd2dba3, SPHOS in toluene with K2CO3 solution in water + Aliquat 336 [109].
- SP197 precursor with two alkoxy-substituted thienyl units—monomer suitable for electropolymerization. SP197 precursor monomer was prepared from the 5′,6-dibromo-SP52 with thiopheneboronic acid via a double Suzuki coupling reaction. SP351 copolymer was also described [110].
- A series of SP256–SP259 was synthesized via [2+2]cycloaddition click reactions (Hagihara-Sonogashira cross-coupling reaction) [111].
- SP-Bodipy hybrids SP274–SP276 have been designed and synthesized by [3+2]cycloaddition reaction as key step. Click chemistry of terminal alkyne with Bodipy-PEGn-N3, and their electrochemical, photophysical, ultrafast transient absorption, and photochromic properties have been studied [103].
5. Applications of Spiropyran Dyes
5.1. Photopharmacology
- To produce labeled conjugates of these photochromes with various biological substrates: polypeptides, proteins, nucleosides and nucleotides, and other physiologically active substances in order to study their behavior and mechanisms of their action in the body.
- In synthesis of a new type of photochromic labeled lipids and other natural compounds of various structures.
- In studies of process of targeted drug delivery to a selected organ.
- To develop new photo-rearrangeable forms of liposomes in studies of pharmacokinetics, metabolism and transport of drugs in vitro and in vivo.
- In the development of methods for modifying the surface of carrier polymers and films and polymer matrices (creation of photocontrolled mechanophores).
- In creating new reusable test systems in immunology and medicine.
- In tests on antitumor activity and antiviral activity assays.
- In the development of new types of photosensitizers for photodynamic therapy of tumors.
5.2. 5′-Substituted Spiropyran Derivatives with “Molecular Address” Designed for the Labeling of the Diverse Targets: Peptides, Proteins (Retinal-Based Proteins, GPCRs), Nucleic Acids and Their Fragments and Lipids
5.3. SP-Dyads, Dimers, bis-SP Derivatives and poly-SP-Targets
5.4. 5′-SP-Dyads with Fluorophores, Dyes and Others
5.5. Artificial Ion-Binding Receptors on the 5′-R-Spiropyran Basis. Photochromic Ligands for the Conjugation with Metal Cations, Nanoparticles and Quantum Dots
- (a).
- SP173, SP174 with cationic quaternized methylpyridinium moiety were synthesized. A molecular magnetic SP174-CrMn(C2O4)3•H2O, whose spiropyran cation contains a quaternized pyridine fragment in the side aliphatic chain was produced. The major effect of introducing a quaternized pyridinium fragment into the benzopyran part of the spiropyran entails a significant decrease in the rate of thermal relaxation processes [143,144];
- (b).
- Photochromic 8-(5-(p-tolyl)-1,3,4-oxadiazol-2-yl)-substituted SP175, SP176, which are able to undergo light-controllable cation-induced isomerizations, have been prepared. Their MC forms contain bidentate chelating core that includes donor sites represented by the phenolate anion and the nitrogen atom of the oxadiazole ring. Introduction of electron withdrawing formyl group into 6-position of the pyran part leads to an increase in spiropyran photocoloration reaction efficiency, but decreases thermodynamical stability of MC form complexes. The addition of Zn2+, Ni2+, Cu2+, Co2+, Cd2+, and Mn2+ salts to colorless or slightly colored solutions of spiropyrans causes accumulation of strongly colored products that have different position of absorption band maxima in the long-wavelength region depending on the metal ion [145,146];
- (c).
- 8-(4,5-diphenyl-1,3-oxazol-2-yl)-substituted SP177–SP179 were synthesized. They display photochromic properties in solutions. It was found that in contrast to naphthopyran analogs, the synthesized spiropyrans are characterized by significantly higher thermal stability of the MC isomers [147].
- (d).
- Photochromic SP181–SP183 derivatives, containing 8-(1-benzyl-4,5-diphenyl-1H- imidazol-2-yl)-group at the position 8 of the benzopyran fragment were synthesized [148].
- (e).
- 8-benzoxazolyl-substituted spiropyrans SP184, SP186–SP191 with different acceptor groups in the 5′-position of the indoline moiety have been synthesized. Novel spiropyrans exhibit photochromic properties in acetone solution at room temperature and form intensely colored complexes with heavy metal cation [149,150,151,152];
- (f).
5.6. 5′-Spiropyran Derivatives in Polymers and in Related Materials
5.7. Mechanochromism
6. Conclusions
| No | 5′-R | R6 | R8 | Synthetic Method (Yield, %) | Spectral-Kinetic Parameters | Notes and Applications | References |
|---|---|---|---|---|---|---|---|
The substituents are numbered according to the structure: | In Table 1, Table 2, Table 3, Table 4 and Table 5, fragment of 5′-substituted spiropyran is depicted as  | ||||||
| SP1 | H- | -H | -H | A | EtOH:H2O (1:1): λAmax 280, 310, 400, 550 nm, EtOH: λAmax 295 nm, λBmax 550 nm, kBAdb 0.36 s−1 | [32,196] | |
| SP2 | H- | -NO2 | -H | A (92%, in i-Pr-OH) A (89%) | EtOH: λBmax 532 nm, Toluene: λBmax 595 nm, λBmaxBH+ 415, 450 nm, THF: λAmax 269, 344 nm, λBmax 274, 309, 371, 387, 574 nm, λBfl 651 nm, EtOH:H2O (1:1): λAmax 340, 510 nm | Treatment of SP2 with TFA generated corresponding MC form, protonated at phenolate O−atom; neutralization of TFA with an equimolar amount of Et3N gives the starting SP2. Tests on antitumor activity and antiviral activity assays. | [167,196,197,198] |
| SP3 | H- | -H | -NO2 | A | EtOH: λBmax 542 nm, Toluene: λBmax 598 nm | ||
| 5′-R-SP photochrome derivatives with alkyl substituents | |||||||
| SP4 | CH3- | -NO2 | -H | A (83%) | λBfl 610 nm | Light-triggered switch based on SP4/layered double hydroxide ultrathin films. | [199] |
| SP5 | CH3- | -CHO | -CH3 | A (63%) | [200] | ||
| SP6 | CH3- | -CHO | -OCH3 | A (58%) | [200] | ||
| SP7 | CH3- | -CH3 | -CHO | A (57%) | [200] | ||
| SP8 | CH3- | -NO2 | -COOCH3 | A (35%) | [201] | ||
| SP9 | CH3- | -NO2 | -COOEt | A (31%) | [201] | ||
| SP10 | C6H13- | R6 = -NO2 R8 = -CH2CO2C21H43 | A (93%) | λBmax 541 nm, λBmax 468 nm | H-aggregate formation of SP10 in the bilayer. | [202] | |
| 5′-R-SP photochrome derivatives with unsaturated substituents | |||||||
| SP11 | H2C=CH- | -NO2 | -H | B (75%) | EtOH: λAmax 277, 323sh nm, λBmax 547 nm, ΔDBphot 0.36, kBAdb 0.001 s−1, τ1/2 * s, Toluene: λBmax 575sh, 615 nm, ΔDBphot 1.0, kBAdb 0.081 s−1, τ1/2 30 s | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [79,83] |
| SP12 | HCC- | -NO2 | -H | B (50%) B (91%) B (87%) B (89%) | CH3CN: λAmax 275, 331sh nm, λBmax 275, 574 nm, λBmaxBH+ 308, 409 nm, t1/2BAdb 31 s, λAfl 460 nm, λBfl 650 nm THF: λAmax 275 nm, λBmax 275, 598 nm | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) Precursor for functional 5′-R-6-NO2-SP series via [2+2]cycloaddition click reactions. | [80,105,111,203] |
| SP13 | (CH3)3SiCC- | -NO2 | -H | A (88%) A (77%) A (97%) | EtOH: λAmax 329 nm, λBmax 545 nm | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [105,111] |
| SP14 | HCC- | -CCH | -H | A (68%) | When SP14 was irradiated with UV light, there is no detectable MC optical absorption (ca. 600 nm). Only limited switching to the MCH+-14 (420–500 nm) was observed upon the addition of acid. | Precursor for SP14-functionalized Au surface electrode synthesis via a click alkyne−azide copper-catalyzed cycloaddition reaction or Sonogashira coupling | [204] |
| SP15 |  | -NO2 | -H | B (84%) | CH3CN: kBAdb 7.4 10−4 s−1 | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [103] |
| SP16 |  | -NO2 | -H | B (38%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [80] | |
| SP17 |  | -NO2 | -H | B (44–46%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [80] | |
| SP18 |  | -NO2 | -H | B (41%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [80] | |
| SP19 |  | -NO2 | -H | B (38%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [80] | |
| 5′-R-SP photochrome derivatives with aryl(heteroaryl) substituents | |||||||
| SP20 | C6H5- | -NO2 | -OCH3 | A | EtOH: λBmax 557 nm, Toluene: λBmax 609 nm, kBAdb 1.52 102 s−1, Dioxane: kBAdb 1.15 102 s−1 | [67,68] | |
| SP21 | C6H5- | -NO2 | -H | A | EtOH: λBmax 534 nm, Toluene: λBmax 592 nm, kBAdb 3.25 102 s−1, Dioxane: kBAdb 2.4 102 s−1 | [67,68] | |
| SP22 | C6H5- | -NO2 | -Br | A | EtOH: λBmax 531 nm, Toluene: λBmax 598 nm, kBAdb 3.25 102 s−1, Dioxane: kBAdb 2.4 102 s−1 | [67,68] | |
| SP23 | 4-CH3OC6H4- | -NO2 | -OCH3 | A (12%) | EtOH: λBmax 563 nm, Toluene: λBmax 612 nm, kBAdb 1.65 102 s−1, Dioxane: kBAdb 1.38 102 s−1 | [67,68] | |
| SP24 | 4-CH3OC6H4- | -NO2 | -H | A (8%) | EtOH: λBmax 537 nm, Toluene: λBmax 597 nm, kBAdb 5.75 102 s−1, Dioxane: kBAdb 2.11 102 s−1 | [67,68] | |
| SP25 | 4-CH3OC6H4- | -NO2 | -Br | A (22%) | EtOH: λBmax 530 nm, Toluene: λBmax 597 nm, kBAdb 13.65 102 s−1, Dioxane: kBAdb 1.38 102 s−1 | [67,68] | |
| SP26 | C6H5CH=CH- | -NO2 | -OCH3 | A,C (2%) | EtOH: λBmax 580 nm, Toluene: λBmax 617 nm | [66] | |
| SP27 | C6H5CH=CH- | -NO2 | -Br | A,C (2%) | EtOH: λBmax 548 nm, Toluene: λBmax 608 nm | [66] | |
| SP28 |  | -NO2 | -H | A (55%) | Toluene: λAmax 330, 431 nm, λBmax 615 nm, ΔDBphot 0.4, τ1/2 185 s | [102] | |
| SP29 |  | -NO2 | -H | A (60%) | Toluene: λAmax 277, 323sh nm, λBmax 320, 380sh, 580, 620 nm, λBmaxBH+ 415 nm, CHCl3: λAmax 256, 326, 427 nm, Toluene: λAmax 325 nm, λBmax 610 nm, ΔDBphot 0.4, kBAdb 0.04 s−1, τ1/2 143 s, CHCl3: λAmax 325 nm, λBmax 590 nm, ΔDBphot 0.2, kBAdb 0.03 s−1, τ1/2 57 s | [101,197] | |
| 5′-R-SP photochrome derivatives with halogenated substituents | |||||||
| SP30 | CF3- | -NO2 | -H | A (65%) A (33%) | EtOH: λBmax 552 nm, Toluene: λBmax 600 nm | [205,206] | |
| SP31 | CF3- | -H | -OCH3 | A (68%) | [162,206] | ||
| SP32 | CF3- | -SO2CF3 | -H | A (54%) | EtOH: λBmax 535 nm, Toluene: λBmax 575 nm | [205] | |
| SP33 | CF3- | -SO2CF3 | -NO2 | A (58%) | EtOH: λBmax 500 nm, Toluene: λBmax 570 nm | [205] | |
| SP34 | F- | -NO2 | -Br | A (41%) | EtOH: λAmax 380 nm, λBmax 527 nm, Toluene: λAmax 368 nm, λBmax 598 nm, Dioxane: λAmax 377 nm, λBmax 580 nm, λBmax 595 nm, kBAdb 1.12 102 s−1 | [207,208] | |
| SP35 | F- | -NO2 | -COOH | A (71%) | [201] | ||
| SP36 | F- | -NO2 | -COOCH3 | A (53%) | [201] | ||
| SP37 | F- | -NO2 | -COOEt | A (49%) | [201] | ||
| SP38 | Cl- | -NO2 | -H | A (28%) B (81%, Cl2/CHCl3) (83%, CuCl2/CH3CN) | CH3OH: λAmax 334 nm, Solid state film: kBAdb 5.1 10−5 s−1 | 3D-optical random access memory (3D-ORAM) material and readout system for monitoring energetic neutrons. SP-based dosimeter. 3D memory prototype. Tests on antitumor activity and antiviral activity assays | [77,112,198,209,210,211] |
| SP39 | Cl- | -NO2 | -Br | A (45%) | λBmax 616 nm, kBAdb 1.63 102 s−1 | [208] | |
| SP40 | Cl- | -NO2 | -COOH | A (66%) | [201] | ||
| SP41 | Cl- | -NO2 | -COOCH3 | A (41%) | [201] | ||
| SP42 | Cl- | -NO2 | -COOEt | A (40%) | [201] | ||
| SP43 | Cl- | -Br | -H | A | [108] | ||
| SP44 | Cl- | -I | -H | A (89%) | Precursor for functional bis-SP | [108,124,126,212] | |
| SP45 | Cl- | -CH3 | -CHO | A (34%) | CH3CN: λAmax 249, 272, 304, 361 nm, λBmax 627 nm, kBAdb 0.064 s−1 | [52] | |
| SP46 | Cl- | -CCH | -H | A (95%) | Precursor for functional bis-SP | [126] | |
| SP47 | Cl- | -C6H5 | -H | B (87%) B (63%) | SP51 was prepared by the Suzuki coupling. | [108] | |
| SP48 | Cl- | R6 = -CCC6H5 | B (78%) | [212] | |||
| SP49 | Cl- | R6 = -CHCHC6H5 | B (80%) | [212] | |||
| SP50 | Cl- | -C(CH3)3 | -C(CH3)3 | A (31%) | CH3CN (−40 °C): λAmax 260, 320 nm, λAmax (CF3SO3H) 370, 400sh nm, λAmax (NaOAc) 550sh, 590, 640sh nm | SP50 does not show significant photochromism in solution at room temperature. | [213] |
| SP51 | Cl- | R6 = -CH2OCOCH2Cl | A | [102] | |||
| SP52 | Br- | -Br | -H | A (63%) B (87%) B (93%) | EtOH: λAmax 223, 257, 307 nm | Precursor of SP copolymers. | [75] |
| SP53 | Br- | -H | -Br | A | Precursor of SP copolymers. | ||
| SP54 | Br- | -NO2 | -H | A (22%) A (80%) B (77%) B (84%, Br2/CHCl3) B (95%, Br2/AlBr3) B (95%, NBS/CCl4) (80%, NBS/CHCl3) B (87%, CuBr2/CH3CN) B (93%, Br2/BF3 Et2O) | EtOH: λAmax 260, 312, 340 nm, λBmax 545 nm, CH3OH: λBmax 310, 360, 530 nm, Toluene: λBmax 380, 580sh, 605 nm | Precursor of PhotoPAF- (photoresponsive porous aromatic framework) | [75,76,77] |
| SP55 | Br- | -NO2 | -Br | A (78%) | λBmax 595 nm, kBAdb 2.18 102 s−1, CH3CN: λAmax 315 nm, λBmax 556 nm | [61,208] | |
| SP56 | Br- | -NO2 | -I | A (50%) | CH3CN: λAmax 306 nm, λBmax 559 nm | [61] | |
| SP57 | Br- | -NO2 | -COOH | A (67%) | [201] | ||
| SP58 | Br- | -NO2 | -COOCH3 | A (41%) | [201] | ||
| SP59 | Br- | -NO2 | -COOEt | A (40%) | [201] | ||
| SP60 | Br- | -NO2 | -OH | A (88%) | CH3CN: λAmax 355 nm, λBmax 568 nm | [61] | |
| SP61 | Br- | -NO2 | -OSO2CF3 | B (74%) | CH3CN: λAmax 308 nm, λBmax 536 nm | [61] | |
| SP62 | I- | -NO2 | -H | A (37%) | Precursor for functional 5′-R-6-NO2-SP series via Pd-catalyzed Sonogashira coupling (by pathway C) | [106] | |
| SP63 | I- | -NO2 | -Br | A (79%) | CH3CN: λAmax 308 nm, λBmax 559 nm | [61] | |
| SP64 | I- | -NO2 | -I | A (62%) | CH3CN: λAmax 314 nm, λBmax 561 nm | [61] | |
| SP65 | I- | R6 = -NO2 R7 = -I | A,C (62%) | CH3CN: λAmax 314 nm, λBmax 561 nm | [180] | ||
| SP66 | I- | -NO2 | -OH | A (75%) | CH3CN: λAmax 355 nm, λBmax 570 nm | [61] | |
| SP67 | I- | -NO2 | -OSO2CF3 | B (56%) | CH3CN: λAmax 311 nm, λBmax 538 nm | [61] | |
| 5′-R-SP photochrome derivatives with oxygenated substituents | |||||||
| SP68 | HO- | -H | -H | A (49%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP69 | HO- | -Br | -H | A (83%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP70 | HO- | -CN | -H | A (69%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP71 | HO- | -CF3 | -H | A (74%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP72 | HO- | -NO2 | -H | A (68%) A (94%) A (78%) A (62%) | Precursor for functional of LC SP series. | [103,132,155,185,186,214,215] | |
| SP73 | HO- | -NO2 | -Br | A (90%) | CH3CN: λAmax 320 nm, λBmax 547 nm | [61] | |
| SP74 | HO- | -NO2 | -I | A (83%) | CH3CN: λAmax 320 nm, λBmax 549 nm | [61,216] | |
| SP75 | HO- | -NO2 | -OH | A (98%) | [61] | ||
| SP76 | CH3O- | -H | -H | A (44%) A (100%) | EtOH: λBmax 450 nm EtOH:H2O (1:1): λAmax 310, 450 nm, kBAdb 1.83 10−1 s−1 | Photoswitch to gadolinium chelates. | [59,196,217] |
| SP77 | CH3O- | -NO2 | -H | A (77%) A (27%) A (54%) A (69%) | THF: λAmax 320 nm, λBmax 537 nm, λBmax (SP+Fe+3) 424 nm, THF: λAmax 250, 280, 320, 350sh nm, λBmax 580 nm, λBmax (SP+Fe+3) 424 nm, THF: λAmax 315, 340sh nm, λBmax 320, 350sh, 580 nm, λAmax (SP+Fe+3) 320, 480 nm, λBmax (SP+Fe+3) 310, 420 nm, CH3CN: λAmax 230, 250, 270, 300, 320, 350sh nm, λBmax 560 nm, EtOH: λBmax 540 nm, EtOH:H2O (1:1): λAmax 310 340, 520 nm | Reference compound for ion-binding receptors with ionophoric fragment for Fe+3. Photoswitch to gadolinium chelates. | [132,154,155,196,216,217,218] |
| SP78 | CH3O- | -OCH3 | -H | A (93%) | EtOH: λAmax 250, 318 nm, λBmax 250, 318 nm, EtOH:H2O (1:1): λAmax 320, 580 nm, EtOH: λBmax 480, 600 nm, kBAdb 2.9 10−1 s−1, 2.3 10−2 s−1 | Photoswitch to gadolinium chelates. | [196,217] |
| SP79 | CH3O- | -OCH3 | -CH2OH | C (87%) | Precursor for preparation of (SP)-based magnetic resonance imaging (MRI) contrast agents | [156] | |
| SP80 | CH3O- | -OCH3 | -CH2I | C (66%) | Precursor for preparation of (SP)-based magnetic resonance imaging (MRI) contrast agents | [156] | |
| SP81 | CH3O- | -CF3 | -H | A (67%) | EtOH: λBmax 550 nm | Photoswitch to gadolinium chelates. | [217] |
| SP82 | CH3O- | -CN | -H | A (60%) | EtOH: λBmax 550 nm | Photoswitch to gadolinium chelates. | [217] |
| SP83 | CH3O- | -CHO | -OCH3 | A (84%) | Acetone:H2O (1:1): λAmax 400 nm, λBmax 300, 370, 546 nm | [219] | |
| SP84 | C12H25O- | -NO2 | -H | B (28%) | CH2Cl2: λAmax 320 nm, λBmax 320, 550 nm | Organic thin-film transistor (OTFT) | [136,137] |
| SP85 | HOCH2- | -NO2 | -H | B (46%) | EtOH: λAmax 336 nm, λBmax 537 nm, ΔDBphot 1.94, kBAdb 1.44 10−4 s−1, 8.01 10−5 s−1, τ1/2 2700 s, λfl 650 nm Toluene: λAmax 333 nm, λBmax 604 nm, ΔDBphot 4.45, kBAdb 8.74 10−4 s−1, τ1/2 16 s, λfl 675, 505 nm | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [80,81,95] |
| SP86 |  | -NO2 | -H | A,C (16%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [214] | |
| SP87 |  | -NO2 | -H | A,C (46%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [182,214] | |
| SP88 |  | -NO2 | -H | A,C (59%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [214] | |
| SP89 |  | -NO2 | -H | A,C (60%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [214] | |
| SP90 |  | -NO2 | -H | B (25%) | Precursor for functional 5′-R-6-NO2-SP series (by pathway C) | [216] | |
| SP91 | C15H31COO- | -NO2 | -H | Amphiphilic SP91 | [220] | ||
| SP92 |  | -NO2 | -H | A (88%) | SP92 Langmuir Blodgett monolayers | [221] | |
| SP93 |  | -NO2 | -H | A | Hexane: λAmax 316 nm, λBmax 316, 580, 615 nm, | SP93 Langmuir Blodgett monolayers | [221] |
| SP94 | OHC- | -NO2 | -H | B (86%) | EtOH: λAmax 328 nm, λBmax 567 nm, ΔDBphot 0.77, kBAdb 0.069 s−1, τ1/2 44 s, Toluene: λAmax 320 nm, λBmax 590sh, 625 nm, ΔDBphot 1.19, kBAdb 0.139 s−1, τ1/2 31 s, Toluene: λAmax 315 nm, λBmax 580sh, 620 nm, ΔDBphot 0.6, kBAdb 0.1 s−1, τ1/2 52 s, CHCl3: λAmax 320 nm, λBmax 595 nm, ΔDBphot 0.3, kBAdb 0.007 s−1, τ1/2 21 s | Precursor for functional 5′-R-6-NO2-SP series (by pathway C). Photo controlled organic field effect transistors (OFET): mixed-type or multilayer transistor from SP94 and fullerene C60. | [35,45,83,84,86,87,90,100,101,222] |
| SP95 | OHC- | -H | -NO2 | B (79%) | EtOH: λAmax 325 nm, λBmax 580 nm, ΔDBphot 0.7, kBAdb 0.48 s−1, τ1/2 50 s, Toluene: λAmax 317 nm, λBmax 600sh, 640 nm, ΔDBphot 0.45, kBAdb 0.84 s−1, τ1/2 5 s | Precursor for functional 5′-R-SP series (by pathway C) | [90,100] |
| SP96 | OHC- | -Cl | -H | B | Toluene: λAmax 320 nm, λBmax 600 nm, ΔDBphot 0.03, kBAdb 2.15 s−1, τ1/2 40 s, CHCl3: λAmax 325, 382 nm, λBmax 505 nm, ΔDBphot 0.4, kBAdb 0.002 s−1, τ1/2 325 s | Precursor for functional 5′-R-SP series | [101] |
| SP97 | OHC- | -F | -H | B | Toluene: λAmax 315, 390, 580 nm, λBmax 490 nm, ΔDBphot 0.1, kBAdb 0.7 s−1, τ1/2 75 s, CHCl3: λAmax 330, 390, 575 nm, λBmax 540 nm, ΔDBphot 0.2, kBAdb 0.27 s−1, τ1/2 120 s | Precursor for functional 5′-R-SP series | [101] |
| SP98 | OHC- | OHC- | -H | B (77%) | EtOH: λAmax 325 nm, λBmax 570 nm, ΔDBphot 0.9, kBAdb 0.39 s−1, τ1/2 60 s, Toluene: λAmax 317 nm, λBmax 580sh, 620 nm, ΔDBphot 0.23, kBAdb 1.64 s−1, τ1/2 2 s | Precursor for functional 5′-R-SP series (by pathway C) | [90,100] |
| SP99 |  | -NO2 | -H | A (23%) | EtOH: λAmax 255, 270, 301, 340 nm, λBmax 535 nm, Toluene: λBmax 595 nm | [60] | |
| SP100 |  | -NO2 | -OCH3 | A (38%) | EtOH: λAmax 255, 281, 301, 357 nm, λBmax 565 nm, Toluene: λBmax 610 nm | [60] | |
| SP101 |  | -NO2 | -Br | A (33%) | EtOH: λBmax 535 nm, Toluene: λBmax 595 nm | [60] | |
| SP102 |  | -NO2 | -NO2 | A (53%) | EtOH: λBmax 522 nm | [60] | |
| SP103 | HOOC- | -H | -H | A (51%) A,C (40% SPS) A (77% in solution) A (39%) | CH2Cl2: λAmax 299 nm, λAfl 358 nm, CH3OH: λAmax 296 nm, λAfl 367 nm, CH3CN: λAmax 298 nm, λAfl 359 nm, DMSO: λAmax 298 nm, λAfl 359 nm | Precursor for functional 5′-R-SP series. SP103 Bovine serum albumin interaction in PBS investigation. Divergent synthesis of SP derivatives by solid-phase approach or in solution methods. | [186,223,224] |
| SP104 | HOOC- | -NO2 | -H | A (45–50%) A (70%) A (72%) A (60%) A (42%) A (63%) A (99%) A (69%) A,C (45% SPS) A (76% in solution) | CH2Cl2: λAmax 290, 311 nm, λBmax 604 nm, λAfl 480 nm, EtOH: λBmax 522 nm | Precursor for functional 5′-R-6-NO2-SP series. Divergent synthesis of SP derivatives by solid-phase approach or in solution methods. SP104 does not show photochromism in the solid state even after a very long irradiation time. | [64,82,133,138,186,216,224,225] |
| SP105 | HOOC- | -NO2 | -OH | A (58%) | Precursor for functional 5′-R-SP series. | ||
| SP106 | HOOC- | R6 = -NO2 R8 = -O2C(C=CH2)-CH3 | B (33%) | Precursor for functional 5′-R-SP series. | [225] | ||
| SP107 | HOOC- | -NH2 | -H | A,C (28% SPS) A (59% in solution) | Divergent synthesis of SP derivatives by solid-phase approach or in solution methods. | [224] | |
| SP108 | HOOC- | -CN | -H | A (53%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP109 | HOOC- | -Br | -H | A (46%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP110 | HOOC- | -CF3 | -H | A (45%) | Precursor for functional 5′-R-SP series. | [186] | |
| SP111 | HOOC- | -CH3 | -H | A,C (40% SPS) A (71% in solution) | Divergent synthesis of SP derivatives by solid-phase approach or in solution methods. | [224] | |
| SP112 | HOOC- | -CH3 | -CHO | A (62%) | CH3CN: λAmax 228, 243, 272, 299, 357 nm, λBmax 630 nm, kBAdb 0.185 s−1 | [226] | |
| SP113 | HOOC- | -OCH3 | -CHO | A (78%) | CH3CN: λAmax 230, 246, 281, 299, 379 nm, λBmax 660 nm, kBAdb 0.667 s−1 | [226] | |
| SP114 | HOOC- | -CHO | -CH3 | A (36%) | CH3CN: λAmax 255, 303, 333sh nm, λBmax 582 nm, kBAdb 0.047 s−1 | [227] | |
| SP115 | HOOC- | -CHO | -OCH3 | A (74%) | CH3CN: λAmax 230, 273, 301, 338sh nm, λBmax 581 nm, kBAdb 0.051 s−1 | [227] | |
| SP116 | HOOC- | -CO2CH3 | -CHO | A (32%) | CH3CN: λAmax 235, 300, 343 nm, λBmax 579 nm, kBAdb 0.022 s−1 | [226] | |
| SP117 | CH3OOC- | -NO2 | -H | B (60%) B (85%) | CH2Cl2: λAmax 290, 311 nm, λBmax 604 nm, λAfl 480 nm, CH3CN: λAmax 220, 280sh, 300, 345sh nm, λBmax 579 nm | SP117 does not show photochromism in the solid state even after a very long irradiation time. | [133,216] |
| SP118 | EtOOC- | -NO2 | -H | Component of solid polymer electrolyte. | [228] | ||
| SP119 |  | -NO2 | -H | B (85%) | DMSO H2O (5:1): λAmax 340 nm, λBmax 544 nm EtOH: λBmax 564 nm | DNA modification method via transamination with 1,6-diaminohexane and functional substituted SP119 | [64,65] |
| SP120 | C6H5COC6H4OOC- | -NO2 | -H | B (47%) | CH3CN: λAmax 230, 250, 270, 300sh, 340sh nm, λBmax 570sh nm | [216] | |
| SP121 | C6H5COC6H4O(CH2)4O- | -NO2 | -H | B (25%) | CH3CN: λAmax 260, 315 nm, λBmax 415sh nm | [216] | |
| SP122 | OHCCH=CH- | -NO2 | -H | B (60%) | EtOH: λAmax 364 nm, λBmax 567 nm, ΔDBphot 1.94, kBAdb 0.02 s−1, τ1/2 218 s, Toluene: λAmax 357 nm, λBmax 590sh, 628 nm, ΔDBphot 4.45, kBAdb 0.06 s−1, τ1/2 33 s | [45] | |
| SP123 | EtOOC-CH=CH- | -NO2 | -H | C (92%) | EtOH: λAmax 348 nm, λBmax 564 nm, ΔDBphot 0.54, kBAdb 0.011 s−1, τ1/2 580 s, Toluene: λAmax 344 nm, λBmax 624, 587sh nm, ΔDBphot 0.86, kBAdb 0.037 s−1, τ1/2 36 s | [82,84] | |
| SP124 |  | -NO2 | -H | B (45%) | EtOH: λAmax 430 nm, λBmax 575 nm, ΔDBphot 0.04, kBAdb 0.015 s−1, τ1/2 * s, Toluene: λAmax 391 nm, λBmax 637, 603sh nm, ΔDBphot 0.02, kBAdb 0.385 s−1, τ1/2 50 s | functional substituted 5′-vinyl-6-NO2- SP series | [79,83] |
| 5′-R-SP photochrome derivatives with nitrogen-containing fragments | |||||||
| SP125 | O2N- | -H | -H | A (30%) A (63%) | CH3OH: λAmax 372 nm, EtOH: λAmax 230, 260, 320sh, 385 nm, λBmax 230, 260, 320sh, 385 nm, EtOH:H2O (1:1): λAmax 310, 390 nm | Tests on antitumor activity and antiviral activity assays. Photoswitch to gadolinium chelates. | [196,198,217] |
| SP126 | O2N- | -H | -NO2 | A (72%) | λAmax 350 nm, λBmax 350 nm | [229] | |
| SP127 | O2N- | -NO2 | -H | A (67%) A (82%) A (33%) A (75%) A (77%) A (36%) B (43%, HNO3/Ac2O) B (87%, NaNO2/AcOH) B (60%, HNO3/H2SO4) | Toluene: λBmax 630 nm, λAmax 350 nm, λBmax 350, 540 nm, EtOH: λAmax 360 nm, λBmax 360 nm, EtOH:H2O (1:1): λAmax 380 nm | Tests on antitumor activity and antiviral activity assays. Photoswitch to gadolinium chelates. | [77,196,198,206,217,229,230,231] |
| SP128 | O2N- | -NO2 | -OCH3 | A (70%) | CH2Cl2: λAmax 362 nm, λBmax 610 nm, λBmax (SP+CF3CO2H) 420 nm | SP128 is resistant to the TFA acid-induced spiroform C-O bond cleavage. | [231] |
| SP129 | O2N- | -NO2 | -CH2Cl | A (49%) | Precursor for functional ion-binding receptors. | [157] | |
| SP130 | O2N- | -NO2 | -CH2I | B (96%) | Precursor for functional ion-binding receptors. | [157] | |
| SP131 | O2N- | -NO2 | -CHO | A (55%) | λAmax 360 nm, λBmax 370, 540 nm | [229] | |
| SP132 | O2N- | -NO2 | -COOCH3 | A (43%) | [201] | ||
| SP133 | O2N- | -CHO | -NO2 | A (53%) | λAmax 350 nm, λBmax 320, 370, 540 nm | [229] | |
| SP134 | O2N- | -OCH3 | -H | A (56%) | EtOH: λAmax 230, 260, 390 nm, λBmax 230, 260, 390 nm, EtOH:H2O (1:1): λAmax 400 nm | Photoswitch to gadolinium chelates. | [196] |
| SP135 | O2N- | -OCH3 | -CH2OH | A (57%) | Precursor for preparation of (SP)-based magnetic resonance imaging (MRI) contrast agents | [156] | |
| SP136 | O2N- | -OCH3 | -CH2I | C (77%) | Precursor for preparation of (SP)-based magnetic resonance imaging (MRI) contrast agents | [156] | |
| SP137 | O2N- | R6 = -CH2OCOCH2Cl | A (60%) | [102] | |||
| SP138 | O2N- | R6 = -CH2OCOCH2CO2Et | A (65%) | [102] | |||
| SP139 | H2N- | -NO2 | -H | A (43%) | Precursor for functional 5′-R-6-NO2-of LC SP series | [78] | |
| SP140 | HON=CH- | -NO2 | -H | B (77%) | Mix of syn- and anti-isomers | [85,94] | |
| SP141 | NC- | -NO2 | -H | B (22%) | [77] | ||
| SP142 | NC- | -CN | -H | A (43%) | SP142 is resistant to the UV-irradiation or TFA acid-induce spiroform C-O bond cleavage. | [231] | |
| SP143 | 4-O2N-C6H4-N=N- | -NO2 | -H | B (89%) | [77] | ||
| SP144 | ClCH2CONH- | -NO2 | -H | A (47%) | EtOH: λBmax 546 nm | [64] | |
| SP145 | NH2N=CH- | -NO2 | -H | B (89%) | [102] | ||
| SP146 | C6H5-CONH- | -I | -H | A (65%) | Precursor for functional bis-SPs | [108,124,126] | |
| SP147 | HOOC-(CH2)3-CONH- | -NO2 | -H | C | Precursor for SP-self-assembled monolayers (SAMs). | [232] | |
| SP148 |  | -NO2 | -H | C | Toluene: λBmax 611 nm | SP-self-assembled monolayers (SAMs). | [232] |
| SP149 | C21H43CONHCH2- | -NO2 | -H | A | SP149 Langmuir–Blodgett (LB) films | [233,234] | |
| SP150 |  | -NO2 | -H | B (65%) | EtOH: λAmax 404 nm, λBmax 568 nm, ΔDBphot 0.35, kBAdb 0.043 s−1, τ1/2 200 s, Toluene: λAmax 390 nm, λBmax 628, 590sh nm, ΔDBphot 0.13, kBAdb 0.152 s−1, τ1/2 18 s | Functional substituted 5′-vinyl-6-NO2-SP series | [79,83] |
| SP151 | NCCH=CH- | -NO2 | -H | B (90%) | EtOH: λAmax 346 nm, λBmax 567 nm, kBAdb 0.003 s−1, τ1/2 127 s, Toluene: λAmax 347 nm, λBmax 628, 590sh nm, kBAdb 0.07 s−1, τ1/2 40 s | [45] | |
| SP152 |  | -NO2 | -H | B (65%) | EtOH: λAmax 398 nm, λBmax 576 nm, ΔDBphot 0.1, kBAdb 0.143 s−1, τ1/2 120 s, Toluene: λAmax 391 nm, λBmax 637, 603sh nm, ΔDBphot 0.05, kBAdb 0.348 s−1, τ1/2 30 s | Functional substituted 5′-vinyl-6-NO2-SP series | [79,83] |
| SP153 |  | -NO2 | -H | B (80%) | EtOH: λAmax 430 nm, λBmax 575 nm, ΔDBphot 0.06, kBAdb 0.194 s−1, τ1/2 * s, Toluene: λAmax 391 nm, λBmax 637, 603sh nm, ΔDBphot 0.01, kBAdb 0.83 s−1, τ1/2 45 s | Functional substituted 5′-vinyl-6-NO2-SP series | [79,83] |
| SP154 |  | different combinations of R6 = -H, -Br, -F, -Cl, -NO2, -I, -OCH3, -CH3, -OH, -C(CH3)3, -OC2H5 and R8 = -H, -Br, -Cl, -I, -OCH3, -CHO, -CH3, -OH, -C(CH3)3 | C,A (89–100%, SPS) | Solid-phase synthesis SP library with bound solid-supported indoline on the high-loading Wang resin. | [70] | ||
| SP155 |  | -I | -I | B (74%, SPS) | Solid-phase synthesis SP library with bound solid-supported indoline on the high-loading Wang resin. | [70] | |
| SP156 |  | -Br | -H | A (82%) | Toluene: λAmax 390 nm, λfl 540 nm | SP156 does not exhibit the photochromic properties. | [235,236] |
| SP157 |  | -NO2 | -H | A (80%) | EtOH: λBmax 550 nm, Toluene: λAmax 375, 385 nm, λBmax 580, 630 nm, λfl 530 nm | [235,236,237] | |
| SP158 |  | -NO2 | -H | A (91%) | CH3OH: λAmax 254, 268, 316 nm, λBmax 267, 315, 537 nm | [238] | |
| SP159 | HO3S- | -NO2 | -H | B | CH3OH: λAmax 260, 296, 334 nm, λBmax 416 nm, λfl 529 nm | [62] | |
| SP160 | KO3S- | -NO2 | -H | A (37%) | CH3OH: λBfl 620 nm, CH3OH: KO3S-SP λAmax 261, 295, 333 nm, λBmax 537 nm, kBAdb 8.1 10−4 s−1, λfl 623 nm | Precursor for organic–inorganic hybrid photomagnet by intercalation of sulfonate-substituted SP160 anions into layered cobalt hydroxides (CoLH) | [62,63] |
| SP161 | S=C=N- | -NO2 | -H | A (44%) | DMSO H2O (5:1): λAmax 305 nm, λBmax 542 nm, EtOH: λBmax 562 nm | DNA modification method via transamination with 1,6-diaminohexane and functional-substituted SP161 | [64,65] |
| SP162 | CF3SO2O- | -NO2 | -Br | B (43%) | CH3CN: λAmax 331 nm, λBmax 561 nm | [61] | |
| SP163 | CF3SO2O- | -NO2 | -I | B (43%) | CH3CN: λAmax 335 nm, λBmax 557 nm | [61] | |
| SP164 | CF3SO2O- | -NO2 | -OSO2CF3 | B (89%) | CH3CN: λAmax 304 nm, λBmax 537 nm | [61] | |
| SP165 |  | -NO2 | -H | C,A (21%) | CH3OH: λBmax 280, 360sh, 540 nm, Toluene: λBmax 390, 580sh, 616 nm | Suzuki coupling with thiophene-3-boronic acid and Stille coupling reactions were used for the SP-T conjugates preparation. | [107] |
| SP166 |  | -NO2 | -H | B (99%) | CH3OH: λBmax 280, 310, 360, 540 nm, Toluene: λBmax 390, 580sh, 616 nm | Suzuki coupling with thiophene-3-boronic acid and Stille coupling reactions were used for the SP-T conjugates preparation. | [107] |
| SP167 |  | -NO2 | -H | B (97%) | CH3OH: λBmax 280, 310, 360, 540 nm, Toluene: λBmax 390, 580sh, 616 nm | Suzuki coupling with thiophene-3-boronic acid and Stille coupling reactions were used for the SP-T conjugates preparation. | [107] |
| SP168 |  | -NO2 | -H | B (58%) | CH3OH: λBmax 280, 310, 360, 540 nm, Toluene: λBmax 390, 580sh, 616 nm | Suzuki coupling with thiophene-3-boronic acid and Stille coupling reactions were used for the SP-T conjugates preparation. | [107] |
| No | Structure of Photochrome Derivatives | Synthetic Method (Yield, %) | Spectral-Kinetic Parameters | Notes and Applications | References |
|---|---|---|---|---|---|
| SP169 |  Where (a) R6 = -NO2, R8=-H, (b) R6 = -H, R8=-H, (c) R6 = -H, R8=-OCH3, (d) R6 = -H, R8=-OC2H5, | A (65–78%) | 5% DMSO/PBS buffer: λAmax 272–296, 323–351 nm, λBmax 480–520 nm | SPs were tested in vitro tubulin polymerization assay. | [239] |
| SP170 |  | A (18%) | CH3OH: λBmax 548 nm, kBAdb 1.58 10−3 s−1 Benzene: λBmax 616 nm, kBAdb 2.47 10−2 s−1 | SP170- monomer with two polymerizable groups. | [240] |
| SP171 |  | A (62%) | CH3CN: λAmax 234, 253, 259, 300, 345 nm, λBmax 560 nm | [241] | |
| SP172 |  | A (29%) | CH3CN: λAmax 253, 299sh, 341sh nm, λBmax 402, 536 nm, λBfl 611 nm | Regioselectivity of condensation process. | [242] |
| SP173 |  | A (55%) | CH3CN: λAmax 259, 317, 345sh nm, λBmax 540 nm, kBAdb 0.8 10−5 s−1, λBfl 618 nm | SP173 cationic SPs | [143] |
| SP174 |  | A (57%) | CH3CN: λAmax 257, 311, 340sh nm, λBmax 544 nm, kBAdb 8.9 10−5 s−1, λBfl 625 nm | A molecular magnetic SP174 CrMn(C2O4)3•H2O whose spiropyran cation contains a quaternized pyridine fragment in the side aliphatic chain was synthesized for the first time. | [143,144] |
| SP175 |  | A (52%) | Acetone: λAmax 334, 351 nm, λBmax 583 nm, kBAdb 3.97 10−2 s−1 | Light-controllable cation binding | [145,146] |
| SP176 |  | A (42%) | Acetone: λAmax 334, 352 nm, λBmax 580 nm, kBAdb 0.99 10−2 s−1 | Light-controllable cation binding | [145,146] |
| SP177 |  | A (52%) | Toluene: λAmax 309, 358 nm, λBmax 470, 650 nm | [147] | |
| SP178 |  | A (46%) | Toluene: λAmax 309, 358 nm, λBmax 470, 650 nm | [147] | |
| SP179 |  | A (42%) | Toluene: λAmax 312, 358 nm, λBmax 440, 655 nm | [147] | |
| SP180 |  | A (48%) | EtOH: λfl 450 nm or 520 nm, Toluene: λfl 500 nm | SP180-triarylimidazole hybrid compound. | [243] |
| SP181 |  | A (47%) | Toluene: λAmax 293sh, 344sh nm, λBmax 634 nm, kBAdb 0.37 s−1 | [148] | |
| SP182 |  | A (43%) | Toluene: λAmax 289sh, 339sh nm, λBmax 628 nm, kBAdb 0.33 s−1 | [148] | |
| SP183 |  | A (50%) | Toluene: λAmax 296sh, 338sh nm, λBmax 627 nm, kBAdb 0.36 s−1 | [148] | |
| SP184 |  | A (59%) | Acetone: λAmax 351, 367sh nm, λBmax 640 nm, kBAdb 0.02 s−1, λAmax (Zn2+) 380, 523 nm, Toluene: λAmax 298, 312, 353, 370sh nm, λBmax 642 nm, kBAdb 0.27 s−1 | Quantitative comparative study of the complexation of a series of SP, the merocyanine form of which contains bidentate chelate site. | [149,150,151] |
| SP185 |  | A (57%) | Acetone: λAmax 355, 370 nm, λBmax 648 nm, λAmax (Zn2+) 380, 525 nm, λAfl (Zn2+) 640 nm | [151] | |
| SP186 |  | A (53%) | Acetone: λAmax 352, 367sh nm, λBmax 640 nm, kBAdb 0.02 s−1, Toluene: λAmax 298, 312, 353, 370sh nm, λBmax 644 nm, kBAdb 0.29 s−1 | Quantitative comparative study of the complexation of a series of SP, the merocyanine form of which contains bidentate chelate site. | [149,150] |
| SP187 |  | A (46%) | Toluene: λAmax 291, 341, 358 nm, λBmax 628 nm, kBAdb 10.1 10−2 s−1, Acetone: λAmax 339, 355 nm, λBmax 593 nm, kBAdb 3.7 10−2 s−1 | [152,244] | |
| SP188 |  | A (41%) | Toluene: λAmax 289, 339, 357 nm, λBmax 634 nm, kBAdb 21.3 10−2 s−1, Acetone: λAmax 339, 355 nm, λBmax 600 nm, kBAdb 3.9 10−2 s−1 | [152,244] | |
| SP189 |  | A (39%) | Toluene: λAmax 296, 344, 357 nm, λBmax 640 nm, kBAdb 58.8 10−2 s−1, Acetone: λAmax 341, 357, 371 nm, λBmax 610 nm, kBAdb 4.8 10−2 s−1 | [152,244] | |
| SP190 |  | A | Toluene: λAmax 297, 342, 358 nm, λBmax 631 nm, kBAdb 4.4 10−2 s−1, Acetone: λAmax 341, 357 nm, λBmax 588 nm, kBAdb 1.8 10−2 s−1 | [244] | |
| SP191 |  | A | Toluene: λAmax 297, 342, 359 nm, λBmax 632 nm, kBAdb 6.3 10−2 s−1, Acetone: λAmax 341, 357 nm, λBmax 586 nm, kBAdb 1.6 10−2 s−1 | [244] | |
| SP192 |  | A (45%) | Toluene: λAmax 294, 321, 345, 363 nm, λBmax 448, 648 nm, Acetone: λAmax 345, 362 nm, λBmax 403, 595 nm | Benzothiazole-substituted SPs demonstrate ion driving photochromic transformations. | [37] |
| SP193 |  | A (46%) | Toluene: λAmax 299, 321, 345, 363 nm, λBmax 447, 647 nm, Acetone: λAmax 345, 362 nm, λBmax 404, 595 nm | Benzothiazole-substituted SPs demonstrate ion driving photochromic transformations. | [37] |
| SP194 |  | A (48%) | Toluene: λAmax 298, 320, 344, 362 nm, λBmax 449, 644 nm, Acetone: λAmax 344, 361 nm, λBmax 407, 600 nm | Benzothiazole-substituted SPs demonstrate ion driving photochromic transformations. | [37] |
| SP195 |  | A (39%) | Toluene: λAmax 297, 305, 322, 344, 361, 373sh nm, λBmax 466, 655 nm, Acetone: λAmax 344, 360, 381sh nm, λBmax 415, 617 nm | Benzothiazole-substituted SPs demonstrate ion driving photochromic transformations. | [37] |
| SP196 |  | A (29%) | CH3CN: λAmax 255, 306, 397, 459sh nm λBmax 255, 306, 397, 459sh nm | [52,245,246] | |
| SP197 |  | B (47%) | CH3OH: CH2Cl2 (1:1): λAmax 215, 315 nm, λBmax 215, 315, 490 nm, λBmax (SP+Co2+) 215, 315, 490 nm | SP197 precursor with two alkoxy-substituted thienyl units—monomer suitable for electropolymerization. SP197 precursor monomer was prepared from the 5′,6-dibromo-SP52 with thiopheneboronic acid via a double Suzuki coupling reaction. | [110] |
| No | Structure of Photochrome Derivatives | Synthetic Method (Yield, %) | Spectral-Kinetic Parameters | Notes and Applications | References | |
|---|---|---|---|---|---|---|
| SP198 |  | A (75%) | EtOAc: λBmax 600 nm | [106] | ||
| SP199 |  | A (87%) | [122,123] | |||
| SP200 |  | A (82%) | [122] | |||
| SP201 |  | A (75%) A (25%) B (77%) | CH3CN (−30 °C): λAmax 270, 294 nm, λBmax 306, 395, 408sh, 619, 660sh nm, λAmax (SP+CF3CO2H) 294, 341, 385 nm | [122,123,124] | ||
| SP202 |  | A (63%) | [122] | |||
| SP203 |  | A (79%) | [122] | |||
| SP204 |  | B (83%) | [124] | |||
| SP205 |  | A | [125] | |||
| SP206 |  | A,B (91%) | λAmax 305 nm | Symmetric and non-symmetric bis-sp via palladium-catalyzed reaction | [126] | |
| SP207 |  | A (73%) | λAmax 305 nm | Symmetric and non-symmetric bis-sp via palladium-catalyzed reaction | [126] | |
| SP208 |  | A,B (97%) | λAmax 310 nm | Symmetric and non-symmetric bis-sp via palladium-catalyzed reaction | [126] | |
| SP209 |  | A,B (96%) | λAmax 308 nm | Symmetric and non-symmetric bis-sp via palladium-catalyzed reaction | [126] | |
| SP210 |  | A (22%) | λAmax 310 nm | Symmetric and non-symmetric bis-sp via palladium-catalyzed reaction | [126] | |
| SP211 |  | A (82%) | [127] | |||
| SP212 |  | A (91%) | EtOH: λBmax 563 nm, Toluene: λBmax 625 nm | [128] | ||
| SP213 |  | A (91%) | EtOH: λBmax 583 nm | [127] | ||
| SP214 |  | A (76%) | EtOH: λBmax 575 nm, Toluene: λBmax 598 nm | [128] | ||
| SP215 |  | A (76%) | EtOH: λBmax 587 nm | [127] | ||
| SP216 |  | (a) n = 3 | A (70%) | EtOH: λBmax 547 nm, CH2Cl2: λBmax 589 nm, Acetone: λBmax 578 nm | [129,130] | |
| (b) n = 5 | A (88%) | EtOH: λBmax 547 nm, CH2Cl2: λBmax 586 nm, Acetone: λBmax 579 nm | ||||
| (c) n = 7 | A (71%) | EtOH: λBmax 548 nm, CH2Cl2: λBmax 588 nm, Acetone: λBmax 579 nm | ||||
| SP217 |  | A (71%) | Toluene: λBmax 657 nm, Acetone: λBmax 623 nm, CH3CN: λBmax 600 nm | [131] | ||
| SP218 |  | A (82%) | Toluene: λBmax 606 nm, Acetone: λBmax 576 nm, CH3CN: λBmax 569 nm | [131] | ||
| SP219 |  | A (61%) | Toluene: λBmax 555 nm, Acetone: λBmax 540 nm, CH3CN: λBmax 529 nm | [131] | ||
| SP220 |  | A (64%) | Toluene: λBmax 607 nm, Acetone: λBmax 577 nm, CH3CN: λBmax 569 nm | [131] | ||
| SP221 |  | (a) Y = -OCH3 | A (43%) | CH3CN: λmax (SP+Me(ClO4)2 520–550 nm | Bis-5′R-SP podands | [153] |
| (b) Y = -Cl | A (31%) | CH3CN: λmax (SP+Me(ClO4)2) 530–557 nm | ||||
| (c) Y = -Br | A (46%) | CH3CN: λmax (SP+Me(ClO4)2) 533–558 nm | ||||
| (d) Y = -CH(CH3)2 | A (84%) | CH3CN: λmax (SP+Me(ClO4)2) 519–552 nm | ||||
| (e) Y = -C(CH3)3 | A (67%) | CH3CN: λmax (SP+Me(ClO4)2) 519–546 nm | ||||
| SP222 |  | B (60%) | EtOAc: λBmax 644 nm | Sonogashira cross-coupling reaction was used. | [106] | |
| SP223 |  | B (19%) | CH3OH: λBmax 280, 310, 360, 545 nm, Toluene: λBmax 390, 580sh, 616 nm | Suzuki coupling with thiophene-3-boronic acid and Stille coupling reactions were used for the SP-T conjugates preparation. | [107] | |
| SP224 |  | B (68%) | THF: λAmax 512, 550 nm, λBmax 550, 602 nm, λBmax (SP+Fe+3) 489, 522 nm, λBfl (SP+Fe+3+ CF3COOH) 560 nm | Light-driven ion-binding receptor with ionophoric fragment for Fe+3. | [154] | |
| SP225 |  X =  | B (42%) | THF: λAmax 325, 340sh, 430, 450, 485 nm, λBmax 325, 340sh, 430, 450, 485, 580 nm, λAfl 530, 550 nm, λBfl 530, 550, 620, 660 nm, λBfl (SP+CF3COOH) 530, 550 nm | Fluorescein (Flu-2 SP225) derivative flanked by two SP units was examined for fluorescence modulation in response to UV and visible-light irradiations and addition of acid. Combinational logic circuit | [132] | |
| SP226 |  | A (58%) | CH3CN: λBmax 560 nm | BINOL-based SP265 molecules. | [139] | |
| SP227 |  | C,A (30%) | EtOH: λAmax 350 nm, λBmax 350, 557 nm, λAfl 435 nm, λBfl 435, 640 nm | SP-functionalized spiro[fluorene-9,9′-xanthene] derivative (SFX-2 SP227) was synthesized. The introduction of two SP227 moieties to the SFX core included the following steps: 1. Suzuki reaction between the di-Br-SFX and indol derivative, 2. quaternization of product by CH3I, 3. condensation reaction of indolium salt with 2-hydroxy-5-nitrobenzaldehyde afforded SFX-2 SP227. | [104] | |
| SP228 |  | B (70%) | CH2Cl2: λAmax 311 nm, λBmax 604 nm, λAfl 480 nm, solid state: λBmax 604 nm, λAfl 435 nm, λBfl 680 nm | Photoswitching characteristics of SP228−TPE−SP228 were studied in the CH2Cl2 and in solid state | [133] | |
| SP229 |  | B (28%) | CH2Cl2: λAmax 311 nm, λBmax 604 nm, λAfl 480 nm, solid state: λBmax 604 nm, λAfl 435 nm, λBfl 680 nm | [133] | ||
| SP230 |  | B (50%) | EtOH: λAmax 385 nm, λBmax 560 nm, ΔDBphot 0.1, kBAdb 0.05 s−1, τ1/2 * s, Toluene: λAmax 365 nm, λBmax 620, 585sh nm, ΔDBphot 0.5, kBAdb 0.08 s−1, τ1/2 15 s | [96] | ||
| No | 5′-R or SP Photochrome Structure | R8 | Synthetic Method (Yield, %) | Spectral-Kinetic Parameters | Notes and Applications | References | |
|---|---|---|---|---|---|---|---|
 5′-R-6-NO2-SP photochrome derivatives with “molecular address” for the labeling of peptides, proteins (retinal-based proteins, GPCRs), nucleic acids and their fragments | |||||||
| SP231 |  | -H | B (50%) | EtOH: λAmax 385 nm, λBmax 563 nm, ΔDBphot 0.3, kBAdb 0.004 s−1, τ1/2 * s, Toluene: λAmax 377 nm, λBmax 630, 590sh nm, ΔDBphot 0.45, kBAdb 0.039 s−1, τ1/2 35 s | Labeling of light-driven translocase bacteriorhodopsin. | [86,88,89] | |
| SP232 |  | -H | B (45%) | EtOH: λAmax 330, 433 nm, λBmax 563 nm, ΔDBphot 0.03, kBAdb 0.002 s−1, τ1/2 * s, Toluene: λAmax 425 nm, λBmax 630, 590sh nm, ΔDBphot 0.03, kBAdb 0.031 s−1, τ1/2 90 s | Labeling of light-driven translocase bacteriorhodopsin | [86,88,89] | |
| SP233 |  | -H | C (56%) | EtOH: λAmax 277, 345sh nm, λBmax 555 nm, ΔDBphot 0.78, kBAdb 9.5 10−3 s−1, τ1/2 73 s, Toluene: λAmax 320 nm, λBmax 617, 575sh nm, ΔDBphot 0.45, kBAdb 0.063 s−1, τ1/2 11 s, water: DMSO 20:1: λAmax 340sh nm, λBmax 537 nm, ΔDBphot 0.31, τ1/2 * s | Labeling of TxA2 receptor in platelets | [85,94] | |
| SP234 |  | -H | C (63%) | EtOH: λAmax 273, 324sh nm, λBmax 556 nm, ΔDBphot 0.67, kBAdb 7.65 10−3 s−1, τ1/2 91 s, Toluene: λAmax 320 nm, λBmax 617, 577sh nm, ΔDBphot 0.5, kBAdb 0.06 s−1, τ1/2 12 s, water: DMSO 20:1: λAmax 340sh nm, λBmax 570 nm, ΔDBphot 0.48, kBAdb 0.06 10−3 s−1, τ1/2 11,200 s | Labeling of TxA2 receptor in platelets | [85,94] | |
| SP235 |  | -H | C (70%) | EtOH: λAmax 265, 338sh nm, λBmax 545 nm, ΔDBphot 0.12, kBAdb 2.46 10−3 s−1, τ1/2 282 s, Toluene: λAmax 320sh nm, λBmax 610, 572sh nm, ΔDBphot 1.1, kBAdb 0.074 s−1, τ1/2 9 s, water: DMSO 20:1: λAmax 345 nm, λBmax 550 nm, ΔDBphot 0.41, kBAdb 0.36 10−3 s−1, τ1/2 1910 s | Labeling of TxA2 receptor in platelets | [85,94] | |
| SP236 |  | -H | B (39%) | Model Sonogashira coupling reaction with 5-iodo-1,3-dimethyluracil gave a gateway to a new procedure of nucleic acid marking with photochromic labels and probes. | [80] | ||
| SP237 |  | -H | B (29%) | DMSO: λAmax 260, 347 nm, λBmax 551 nm | 5′-Maleimidomethyl SP237 derivative was synthesized from a hydroxymethyl precursor by Mitsunobu reaction. Potential photochromic markers for sulfhydryl groups in proteins with Cys residues. | [81] | |
| SP238 |  | -H | B (9%) | DMSO: λAmax 277, 342 nm, λBmax 569 nm | SP238 derivative was synthesized from 5′-carboxy-SP. Potential photochromic markers for sulfhydryl groups in proteins with Cys residues. | [81] | |
| SP239 |  | -H | B (76%) | PBS, (80 °C): λAmax 280, 350sh nm, λBmax 380, 500 nm | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | |
| SP240 |  | -H | A,B (76%) | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | ||
| SP241 |  | -H | A,B (76%) | PBS, (80 °C): λAmax 280, 350sh nm, λBmax 380, 502 nm | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | |
| SP242 |  Where Peptide = tri- hepta- peptide residues | -H | C | PBS, (80 °C): λAmax 350sh nm, λBmax 380, 502 nm | All spiropyran conjugated N-terminal oligopeptides were synthesized through standard solid phase peptide synthesis protocol and purified with preparative HPLC. MC–RGD hydrogel can be employed as an erasable photolithographic material. | [119] | |
| SP243 |  | -H | A,C (66%) | Gel: λAmax 350 nm, λBmax 370, 510 nm | Photo-sensitive hydrogelator SP243 with dipeptide D-Ala–D-Ala. D-Ala–D-Ala was linked to the amino group on SP via succinic acid. | [120] | |
| SP244 |  | -H | C | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | ||
| SP245 |  | -H | C | All spiropyran conjugated N-terminal oligopeptides were synthesized through standard solid phase peptide synthesis protocol and purified with preparative HPLC. | [119] | ||
| SP246 |  | -H | C | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | ||
| SP247 |  | -H | C | All spiropyran conjugated N-terminal oligopeptides were synthesized through standard solid phase peptide synthesis protocol and purified with preparative HPLC. | [119] | ||
| SP248 |  | -H | C | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | ||
| SP249 |  | -H | C | All spiropyran conjugated N-terminal oligopeptides were synthesized through standard solid phase peptide synthesis protocol and purified with preparative HPLC. | [119] | ||
| SP250 |  | -H | C | Precursor of supramolecular hydrogels based on merocyanine-peptide conjugates. | [119] | ||
| SP251 |  | -H | B | All spiropyran conjugated N-terminal oligopeptides were synthesized through standard solid phase peptide synthesis protocol and purified with preparative HPLC. | [119] | ||
| SP252 | Fmoc-Lys-Lys(X)-Lys-Phe-NH2  | C | Peptide synthesis was performed by FMOC protocol on the Rink amide solid-phase resin. | [121] | |||
| SP253 |  | -H | C (12%) | SP253-DNA conjugate | [215] | ||
| Hybrid dyads with dyes | |||||||
| SP254 |  | A (54%) | CH3CN: λAmax 380 nm, λBmax 390, 588 nm | [98] | |||
| SP255 |  DHA-SP | B (42%) | CH3CN: DHA-SP λAmax 274, 392 nm, λAfl 660 nm, DHA-MC λBmax 371, 547 nm, DHA-MCH+ λBmaxBH+ 309, 410 nm, VHF-SP λAmax 268, 317, 473 nm, t1/2BAdb 138 min 40 s, VHF-MC λBmax 318, 437, 580 nm, t1/2BAdb 30 s, VHF-MCH+ λBmaxBH+ 297, 437 nm | Dyad DHA- SP255 was synthesized under Sonogashira coupling conditions. | [105] | ||
| SP256 |  | C (63%) | CH2Cl2: λAmax 264, 348 nm | Precursor for functional 5′-R-6-NO2-SP series via [2+2]cycloaddition click reactions (Hagihara-Sonogashira cross-coupling reaction). | [111] | ||
| SP257 |  | B (92%) | CH2Cl2: λAmax 264, 472 nm | Series of 5′-R-6-NO2-SP was synthesized via [2+2]cycloaddition click reactions (Hagihara-Sonogashira cross-coupling reaction). The third-order nonlinear optical (NLO) properties were investigated. | [111] | ||
| SP258 |  | B (90%) | CH2Cl2: λAmax 420, 690 nm | Series of 5′-R-6-NO2-SP was synthesized via [2+2]cycloaddition click reactions (Hagihara-Sonogashira cross-coupling reaction). The third-order nonlinear optical (NLO) properties were investigated. | [111] | ||
| SP259 |  | B (88%) | CH2Cl2: λAmax 420, 848 nm | Series of 5′-R-6-NO2-SP was synthesized via [2+2]cycloaddition click reactions (Hagihara-Sonogashira cross-coupling reaction). The third-order nonlinear optical (NLO) properties were investigated. | [111] | ||
| SP260 |  | -H | A (67%) | Amide-linked SP260-anthraquinone (SP-AQ) conjugates were investigated in PC vesicles. | [134] | ||
| SP261 |  | -H | A (40%) | Amide-linked SP261-anthraquinone (SP-AQ) conjugates were investigated in PC vesicles. | [134] | ||
| SP262 |  | -H | B (73%) | THF: λAmax 250, 280, 315, 330 nm, λBmax 325, 340sh, 430, 450, 485, 580 nm, λAmax (SP+Fe+3) 610 nm, λBmax (SP+Fe+3) 424 nm | Spectral studies of dyad SP262-TTF, containing an electroactive unit (tetrathiafulvalene), and a photochromic unit SP, in the presence of ferric ions were conducted | [155] | |
| Hybrid dyads with fluorophores | |||||||
| SP263 |  Z-isomer/E-isomer/Z- + E-isomers mix | -H | A,C (3%) B (62% mix E- + Z- isomers) | Z-isomer EtOH: λAmax 315, 335sh nm, λBmax 400sh, 557 nm, λBH+max 320sh, 338, 426 nm, ΔDBphot 0.28, kBAdb 7.02 10−4 s−1, 6.03 10−3 s−1, τ1/2 550 s, λfl 455sh, 478, 645 nm, Toluene: λAmax 319, 340sh, nm, λBmax 390sh, 590sh, 622 nm, ΔDBphot 1.33, kBAdb 6.87 10−2 s−1, 5.08 10−1 s−1, τ1/2 30 s, λfl 510, 685 nm, Acetone: λAmax 440sh nm, λBmax 405sh, 555sh, 585 nm, λBH+max 445 nm, kBAdb 8.76 10−3 s−1, DMSO: λAmax 435sh nm, λBmax 580 nm, E-isomer EtOH: λAmax 320sh, 342 nm, λBmax 341, 395, 556 nm, ΔDBphot 0.16, kBAdb 4.21 10−2 s−1, 7.73 10−4 s−1, τ1/2 1500 s, λfl 485, 646 nm | Wittig olefination followed by HPLC. Z-/E-ratio 39/61 | [16,50,66] | |
| SP264 |  Z-isomer/E-isomer | -H | B (55% mix E- + Z-isomers) | E- + Z-isomer mix: EtOH: λAmax 320sh, 342 nm, λBmax 571 nm, λmaxBH+ 478 nm, Toluene: λAmax 325 nm, λBmax 345, ~385sh, 585sh, 622 nm | Wittig olefination followed by HPLC. Z-/E-ratio 64/36 | [16,50] | |
| SP265 |  Z-isomer/E-isomer | -H | B (72% mix E- + Z- isomers) | Wittig olefination followed by HPLC. Z-/E-ratio 35/65 | [16,50] | ||
| SP266 |  Z-isomer/E-isomer | -H | B (63% mix E- + Z- isomers) | Wittig olefination followed by HPLC. Z-/E-ratio 45/55 | [16,50] | ||
| SP267 |  Z-isomer/E-isomer | -H | B (67% mix E- + Z- isomers) | Wittig olefination followed by HPLC. Z-/E-ratio 49/51 | [16,50] | ||
| SP268 |  Z-isomer/E-isomer | -H | B (58% mix E- + Z- isomers) | Wittig olefination followed by HPLC. Z-/E-ratio 45/55 | [16,50] | ||
| SP269 |  Z-isomer/E-isomer | -H | B (66% mix E- + Z- isomers) | E-isomer EtOH: λAmax 265, 315sh, 339 nm, λBmax 265, 315sh, 339, 400sh, 558 nm, ΔDBphot 0.14, | Wittig olefination followed by HPLC. Z-/E-ratio 59/41 | [16,50] | |
| SP270 |  Z-isomer/E-isomer | -H | B (60% mix E- + Z- isomers) | E- + Z-isomer mix: EtOH: λAmax 267, 364 nm, λBmax 267, 367, 460sh nm, ΔDBphot 0.04, Toluene: λAmax 362, 460sh nm, λBmax 370, 590sh, 625 nm, ΔDBphot 0.46 | Wittig olefination followed by HPLC. Z-/E-ratio 21/79 | [16,50] | |
| SP271 |  Z-isomer/E-isomer | -H | B 50% mix E- + Z- isomers) | Z-isomer EtOH: λAmax 400, 294sh nm, λBmax 563, 405 nm, kBAdb 3.44 10−2 s−1, 1.68 10−3 s−1, τ1/2 7200 s, λfl 430, 470, 654 nm Toluene: λAmax 395 nm, λBmax 629, 595sh nm, kBAdb 3.92 10−2 s−1, τ1/2 26 s, λfl 546 nm, E-isomer EtOH: λAmax 409, 294sh nm, λBmax 563, 405 nm, ΔDBphot 0.16, kBAdb 1.32 10−2 s−1, 2.34 10−3 s−1, τ1/2 7200 s, λfl 654 nm, Toluene: λAmax 407 nm, λBmax 629, 595sh nm, ΔDBphot 0.56, kBAdb 5.47 10−2 s−1, τ1/2 28 s, λfl 546 nm | Wittig olefination followed by HPLC. Z-/E-ratio 55/45 | [16,50] | |
| SP272 |  Z-isomer/E-isomer | -H | B (18% mix E- + Z- isomers) | Wittig olefination followed by HPLC. Z-/E-ratio 9/91 | [16,50] | ||
| SP273 |  Z-isomer/E-isomer | -H | B (90% mix E- + Z- isomers) | E-isomer EtOH: λAmax 260, 353 nm, λBmax 265, 353, 560 nm, ΔDBphot 0.23, kBAdb 1.94 10−2 s−1, 2.31 10−3 s−1, τ1/2 830 s, λfl 647 nm, Toluene: λAmax 354 nm, λBmax 405sh, 585sh, 622 nm, ΔDBphot 0.58, kBAdb 4.72 10−2 s−1, τ1/2 42 s, λfl 558 nm | Wittig olefination followed by HPLC. Z-/E-ratio 3/97 | [16,50] | |
| SP274 |  | -H | C (75%) | CH2Cl2: λAmax 266, 357, 484, 511 nm, λfl 526 nm, φfl 0.11, CH3CN: kBAdb 5.8 10−4 s−1 | SP274-containing Bodipy derivatives have been designed and synthesized by CA reaction click chemistry of terminal alkyne with Bodipy-EOn-N3. | [103] | |
| SP275 |  | -H | C (61%) | CH2Cl2: λAmax 265, 357, 484, 510 nm, λfl 526 nm, φfl 0.14, CH3CN: kBAdb 6.1 10−4 s−1 | SP275-containing Bodipy derivatives have been designed and synthesized by CA reaction click chemistry of terminal alkyne with Bodipy-EOn-N3 | [103] | |
| SP276 |  | -H | C (68%) | CH2Cl2: λAmax 265, 357, 484, 510 nm, λfl 526 nm, φfl 0.16, CH3CN: kBAdb 6.4 10−4 s−1 | SP276-containing Bodipy derivatives have been designed and synthesized by CA reaction click chemistry of terminal alkyne with Bodipy-EOn-N3. | [103] | |
| SP277 |  | -H | C | λfl 620 nm | BG-PEG-NitroBIPS-GFP-AGT fusion protein. OLID-FRET sensor using two-photon excitation of SP (720 nm) to trigger the SP-to-MC transition and 543 nm to trigger the MC-to-SP transition. | [247] | |
| SP278 |  | -H | A,C | λAmax 340, 432 nm, λBmax 350, 432 548 nm, λfl 650, 662 nm | SP278 bonded 1,8-naphthalimide compound is useful as photochromic and photoluminescent material. | [135] | |
| SP279 |  | -H | B (19%) | [136] | |||
| SP280 |  | B | The switching performance of different fluorophore–SP conjugates was studied. It was shown that the fluorescence of the fluorophores can be modulated by switching the SP. | [138] | |||
| SP281 |  | B | The switching performance of different fluorophore–SP conjugates was studied. It was shown that the fluorescence of the fluorophores can be modulated by switching the SP. | [138] | |||
| SP282 |  | B | The switching performance of different fluorophore–SP conjugates was studied. It was shown that the fluorescence of the fluorophores can be modulated by switching the SP. | [138] | |||
| SP283 |  | B | The switching performance of different fluorophore–SP conjugates was studied. It was shown that the fluorescence of the fluorophores can be modulated by switching the SP. | [138] | |||
| SP284 |  | B (34%) | Acetone: λAmax 333, 420 nm, λAmax (+Me+n) 518–555 nm | SP284 conjugate with Rhodamine B aminoethylamide. Irradiation of solutions of the spiropyrans with UV light (365 nm) did not lead to any spectral changes. | [140] | ||
| SP285 |  | B (29%) | Acetone: λAmax 362 nm, λBmax 555 nm (weak), kBAdb 0.021 s−1, Toluene: λAmax 315, 369 nm, λBmax 560 nm (weak), kBAdb 0.127 s−1 | SP285 conjugate with rhodamine B hydrazide. | [141] | ||
| SP286 |  | B (27%) | Acetone: λAmax 362 nm, λBmax 555 nm (weak), kBAdb 0.024 s−1, Toluene: λAmax 315, 369 nm, λBmax 560 nm (weak), kBAdb 0.078 s−1 | SP286 conjugate with rhodamine B hydrazide. | [141] | ||
| SP287 |  | B (26%) | Acetone: λAmax 362 nm, λBmax 555 nm (weak), kBAdb 0.031 s−1, Toluene: λAmax 315, 369 nm, λBmax 560 nm (weak), kBAdb 0.06 s−1 | SP287 conjugate with rhodamine B hydrazide | [141] | ||
| Ion-binding receptors with ionophoric fragment | |||||||
| SP288 |  | -H | B (42%) | EtOH: λAmax 337 nm, λBmax 538 nm, ΔDBphot 0.66, kBAdb 8.74 10−4 s−1, τ1/2 4000 s, λfl 636 nm, Toluene: λAmax 334 nm, λBmax 605 nm, ΔDBphot 3.53, kBAdb 0.123 s−1, τ1/2 28 s, λfl 666 nm CH3CN: λAmax 336 nm, λBmax 561 nm, ΔDBphot 1.47, kBAdb 1.29 10−3 s−1, τ1/2 28 s, λfl 650 nm | SP288 ion-binding receptor with ionophoric fragment for metal cations | [44] | |
| SP289 |  | -H | B (46%) | EtOH: λAmax 338 nm, λBmax 538 nm, ΔDBphot 0.61, kBAdb 1.64 10−3 s−1, λfl 642 nm, Toluene: λAmax 334 nm, λBmax 607, 575sh nm, ΔDBphot 2.62, kBAdb 1.58 10−2 s−1, λfl 672, 530 nm | SP289 ion-binding receptor with ionophoric fragment for metal cations | ||
| SP290 |  | -H | B (82%) | EtOH: λAmax 342 nm, λBmax 538 nm, ΔDBphot 2.04, kBAdb 1.94 10−4 s−1, τ1/2 2140 s, λfl 640 nm, Toluene: λAmax 334 nm, λBmax 606, 575sh nm, ΔDBphot 4.64, kBAdb 7.84 10−2 s−1, τ1/2 20 s, λfl 680 nm | SP290 ion-binding receptor with ionophoric fragment for the metals cations ion-binding receptor with ionophoric fragment for metal cations | ||
| SP291 |  | -H | B (58%) | EtOH: λAmax 321 nm, λBmax 540 nm, ΔDBphot 0.72, kBAdb 4.14 10−2 s−1, τ1/2 1540 s, Toluene: λAmax 350 nm, λBmax 605, 575sh nm, ΔDBphot 2.08, kBAdb 0.212 s−1, τ1/2 14 s | SP291 ion-binding receptor with ionophoric fragment for the metals cations ion-binding receptor with ionophoric fragment for metal cations | ||
| SP292 |  | C (91%) | water (pH = 7.4): complex SP342 might not be responsive to light; furthermore, there was a minimal absorbance difference above 400 nm | (SP292)-based magnetic resonance imaging (MRI) contrast agents | [156] | ||
| SP293 |  | C (75%) | water (pH = 7.4): λAmax 440 nm (without Gd+3), λAmax 530 nm; λAfl 664 nm, after visible light irradiation of sample SP343 fluorescence and absorbance peaks decreases. After visible light irradiation of sample SP343 a new stable absorbance peak appeared at 440 nm. | (SP293)-based magnetic resonance imaging (MRI) contrast agents | [156] | ||
| SP294 |  Where (a) Y = -CF3, (b) Y = -NO2, (c) Y = -COOH | A (90%) A (14%) C,A (18%) | CH3CN: λBmax 550 nm CH3CN: λBmax 550 nm CH3CN: λAmax 360, 400 nm, λBmax 545 nm, λfl 627 nm | Receptor for the cations Li+, Na+, Ca2+, Ba2+ and Mg2+. Receptor for the cations Li+, Na+, Ca2+, Ba2+ and Mg2+. SP294 with tethered aza-12-crown-4 unit was synthesized. | [40,158,159,163] | ||
| SP295 |  | C (62% before comple xation Gd+3) B (79%) | H2O: λAmax 502 nm, λBmax 502 nm↓, λfl 603 nm | [157] | |||
| SP296 |  | A (85%) | CH3CN: λAmax 360, 400 nm, λBmax 545 nm, λfl 627 nm | SP296 with tethered aza-15-crown-5 unit was synthesized. Spectral changes induced by cations binding with (perchlorates: Li+, Na+ and K+ and Cs2SO4) were investigated. | [159,160,248] | ||
| SP297 |  | B (70%) B (36%) | CH3CN: λfl 640 nm | SP297 Li+ ion sensor the molecular switch was developed. It was based on covalently attached SP to the internal surface of the microstructured optical fiber (MOF). | [160] | ||
| SP298 |  | C,A (20%) | CH3CN: λAmax 360 nm, λBmax 545 nm, λfl 632 nm | SP298 with tethered aza-18-crown-6 unit was synthesized. Spectral changes induced by cations binding with (perchlorates: Li+, Na+ and K+ and Cs2SO4) were investigated. | [159] | ||
| SP299 |  | (a) Y = -CF3 | A (38%) | Reversible photochemical ion chelation. | [161] | ||
| (b) Y = -NO2 | A (8%) | ||||||
| SP300 |  | A (47%) | EtOH: λAmax 250, 320, 360 nm, λBmax 250, 320, 360 nm | SP300 was synthesized. The formation of a metal complex between SP300 and Cu2+ was associated with a color change. Sensor for Cu+2 ions. | [164] | ||
| SP301 |  | A,C (75%) | 20% CH3CN in water: λfl 620 nm CH3CN: λfl 640 nm, DMSO: λfl 640 nm, | Light-driven ion-binding receptor with ionophoric fragment for Zn+2. | [165,166] | ||
| SP302 |  | A (70%) | THF: λAmax 231, 273, 295, 328 nm λBmax 231, 277, 381, 590 nm, φ334 0.078, λBfl 660 nm | Precursor for ion-binding receptor with ionophoric fragment for Ru, Os | [38,167,249] | ||
| SP303 |  | A (68%) | Precursor for ion-binding receptor with ionophoric fragment for Ru, Os | [39,249] | |||
| SP304 |  | B (54%) | THF: λAmax 291, 365, 459 nm, λBmax 291, 391, 461, 603 nm, φ334 0.0065, λBfl 634 nm, λBfl 655 nm | [Ru(bpy)2(SP)] (PF6)2, ion-binding receptor with ionophoric fragment for Ru | [38,167] | ||
| SP305 |  | B (45%) | THF: λAmax 294, 373, 490, 591 nm, λBmax 294, 386, 490, 605 nm, φ334 0.0049, λAfl 765 nm, λBfl 765 nm | [Os(bpy)2(SP)] (PF6)2, ion-binding receptor with ionophoric fragment for Os | [38,167] | ||
| SP306 |  | A (59%) | Precursor for ion-binding receptor with ionophoric fragment for Ru, Os | [38] | |||
| SP307 |  | B (80%) | Precursor heterobinuclear SP metal complex [Ru(bpy)2-4bpy-Sp- PhenIm-Os (bpy)2](PF6)4 | [38] | |||
| SP308 |  | B (64%) | Precursor heterobinuclear SP metal complex [Ru(bpy)2-4bpy-Sp- PhenIm-Os (bpy)2](PF6)4 | [38] | |||
| SP309 |  | (a) Me+2 = Os+2 | B (35%) | CH3CN: λAmax 288, 359, 461, 620 nm, λAfl 619, 742 nm | SP309(a,b) metal complexes Ru, Os [Ru(bpy)2-4bpy-Sp- PhenIm-Me+2(bpy)2] (PF6)4. were synthesized via Suzuki coupling. Closed form of the SP309(a) metal complex is inactive and cannot be converted to the open form either by UV light or irradiation at 450 nm. | [38] | |
| (b) Me+2 = Ru+2 | B (10%) | CH3CN: λAmax 287, 339, 458 nm, λAfl 618 nm | |||||
| SP310 |  | B (31%) | [39,249] | ||||
| SP311 |  | (a) Me+2 = Ru+2 | B (21%) | CH3CN: λAmax 288, 338, 458 nm λAfl 619 nm | Ion-binding receptor with ionophoric fragment for Ru, Os | [39] | |
| (b) Me+2 = Os+2 | B (15%) | CH3CN: λAmax 291, 374, 449, 620, 825 nm λAfl 741 nm | |||||
| SP312 |  | -H | B (74%) | THF: λAmax 270 nm, λBmax 270, 633 nm, kBAdb 1.61 10−3 s−1 | [203] | ||
| SP313 | CoLH-O3SP | -H | B | CoLH-O3S-SP λBmax 564 nm | Organic–inorganic hybrid photomagnet, the intercalation of sulfonate-substituted SP anions into layered cobalt hydroxides (CoLH) was performed. | [63] | |
| SP314 |  | (a) n = 1 | -H | C (68%) | 90% CH3CN in water: λAmax 342 nm/ λBmax 340, 550 nm, λfl 530 nm | SP314 light-gated artificial transducers. Zn complex. | [168] |
| (b) n = 2 | C (63%) | ||||||
| (c) n = 3 | C (81%) | ||||||
| (d) n = 4 | C (20%) | ||||||
| (e) n = 6 | C (23%) | ||||||
| Photochromic ligands for the conjugation with metal cations, nanoparticles, and quantum dots | |||||||
| SP315 | HOOC-CH=CH- | -H | B, C (35%/ 54%) | EtOH: λAmax 340 nm, λBmax 555 nm, ΔDBphot 0.44, kBAdb 0.004 s−1, τ1/2 * s, Toluene: λAmax 346 nm, λBmax 622, 585 nm, ΔDBphot 0.48, kBAdb 0.027 s−1, τ1/2 46 s | Two-step procedure for the preparation of SP315 by the Horner olefination with C2-phosphonate followed by the saponification of intermediate ester turned out to be more effective. One-step synthesis consisted in the Knoevenagel reaction with a yield of 35%. | [35,82,84] | |
| SP316 |  | -H | B (72–42%) | EtOH: λAmax 336 nm, λBmax 541 nm, ΔDBphot 5.3, kBAdb 1.94 10−2 s−1, 6.82 10−4 s−1, τ1/2 326 s, λfl 642 nm, Toluene: λAmax 334 nm, λBmax 606, 580sh nm, ΔDBphot 3.63, kBAdb 0.141 s−1, 6.59 10−2 s−1, τ1/2 85 s, λfl 686 nm, CHCl3: λAmax 342 nm, λBmax 586 nm, ΔDBphot 0.95, kBAdb 1.21 s−1, 4.96 10−2 s−1, τ1/2 4 s, λfl 663 nm, THF: λAmax 336 nm, λBmax 587 nm, ΔDBphot 5.3, kBAdb 1.21 s−1, 3.82 10−2 s−1, τ1/2 70 s, λfl 672 nm | [95] | ||
| SP317 |  | -H | B (50–55%) | EtOH: λAmax 335 nm, λBmax 542 nm, ΔDBphot 1.63, λfl 642 nm, Toluene: λAmax 333 nm, λBmax 604, 575sh nm, ΔDBphot 3.91, λfl 677 nm, CHCl3: λAmax 342 nm, λBmax 586 nm, ΔDBphot 2.21, λfl 670 nm, THF: λAmax 338 nm, λBmax 588 nm, ΔDBphot 5.05 | [95] | ||
| SP318 |  | -H | C (31%) | EtOH: λAmax 336 nm, λBmax 390sh, 538 nm, ΔDBphot 1.94, λfl 638 nm | [95] | ||
| SP319 |  | -H | B (43%) | EtOH: λAmax 302, 335 nm, λBmax 362, 540 nm, ΔDBphot 1.78, λfl 640 nm | [95] | ||
| SP320 |  | -H | B (51%) | EtOH: λAmax 330sh, 390sh nm, λBmax 538, 390sh nm, ΔDBphot 0.82, λfl 635 nm | [95] | ||
| SP321 |  | -H | C (42%) | Toluene: λAmax 341 nm, λBmax 620, 580sh nm, ΔDBphot 0.4, λfl 677 nm, kBAdb 0.055 s−1, τ1/2 16 s, CHCl3: λAmax 348 nm, λBmax 603, 562sh nm, ΔDBphot 0.25, λfl 670 nm, kBAdb 0.089 s−1, τ1/2 2.5 s, λfl 670 nm, THF: λAmax 340 nm, λBmax 604, 560sh nm, ΔDBphot 0.65, kBAdb 0.078 s−1, τ1/2 60 s, CH3CN:H2O: λAmax 224, 266, 348 nm, λBmax 542 nm, λBmax (+ graphene oxide) 432 nm, DMSO: λAmax 342 nm, λBmax 570 nm, ΔDBphot 0.1, kBAdb 0.036 s−1, τ1/2 19 s | SP321-functionalized CdSe QDs | [35,91,250] | |
| SP322 |  | B (78%) | SP322 was synthesized through the palladium-catalyzed coupling reaction. | [251] | |||
| SP323 |  | C (86%) | When SP382 was irradiated with UV light, there is no detectable MC optical absorption (ca. 600 nm). λBmax 415 nm, MCH+ form | SP323-functionalized Au surface electrode synthesis via Sonogashira coupling | [204] | ||
| SP324 |  | -H | A (37%) | CH3OH: λAmax 345 nm, λBmax 530 nm, CH2Cl2: λBmax 578 nm | 5′-ferrocenylspiropyran (Fc-SP324) was synthesized. | [252] | |
| SP325 |  | -H | A (87%) | EtOH: λBmax 565 nm, CH3CN: λBmax 583 nm | [253] | ||
| SP326 |  | A,C (6 steps, 17.5%) | Precursor for 5′-R-6-NO2-SP series synthesis | [98] | |||
| SP327 |  | C (63%) | CH2Cl2: λAmax 334, 456 nm λBmax 334, 590 nm, CH3CN: λAmax 350 nm, λBmax 334, 585 nm | 5′-ferrocenylvinylSP was synthesized. | [98,254] | ||
| SP328 |  | SP328-functionalized Au surface electrode synthesis via a click alkyne−azide copper-catalyzed cycloaddition reaction | [204] | ||||
| SP329 |  | A | Acetone: λBmax 554 nm | Metal complexes were synthesized | [255] | ||
| SP330 |  | A (68%) | Acetone: λBmax 557 nm | Metal complexes were synthesized | [255] | ||
| SP331 |  | -H | B (81%) | Reversible modulation of conductance in silicon-based metal-oxide-semiconductor field-effect transistor via UV/Visible-light irradiation | [256] | ||
| SP332 | X—(CH2)12—S—S—(CH2)12—X X =  | B (31%) | THF/water (9:1): λBmax 556 nm, λBmax (SP+Zn+2) 486 nm | SP-modified Au electrode could be reversibly modulated by UV/visible light irradiation in the presence of Zn2+. A new molecular switch and an ‘‘AND’’ logic gates | [257] | ||
| SP333 |  | B (88%) | H2O, pH 7.0: λBmax 380, 540 nm H2O, pH <7.0: λBH+max 432 nm | pH- and light-responsive Spiropyran-based surfactant | [258] | ||
| No | 5′-R or SP Photochrome Structure | R8 | Synthetic Method (Yield, %) | Spectral-Kinetic Parameters | Notes and Applications | References | |
|---|---|---|---|---|---|---|---|
| SP334 | Cl- | -H | in PMMA: λAmax 260, 325 nm, λBmax 575 nm, λBfl 650 nm | SP334 in PMMA. Prototype of 3D volume memory. 3D optical random access memories (3D ORAM). | |||
| SP335 | EtOOC- | -H | SP335 solid polymer electrolyte LiClO4, poly[(ω-hydroxy) oligo(oxyethyene) methacrylate] | ||||
| SP336 |  | B (50%) | Toluene: λAmax 310 nm, λBmax 608, 570sh nm, λBfl 667 nm | Synthesis of spiropyran SP336-functionalized dendron and organogel are reported. | [182] | ||
| SP337 |  | A (37%) | Benzene: λAmax 370 nm | SP337 practically not photochromic. Precursor for synthesis. | [78] | ||
| SP338 |  | A (45%) | Benzene: λAmax 375 nm | SP338 practically not photochromic. Precursor for synthesis. | [78] | ||
| SP339 |  | A (48%) | Benzene: λAmax 370 nm | SP339 practically not photochromic. Precursor for synthesis. | [78] | ||
| SP340 |  | -H | B (65%) | THF: λAmax 355 nm, λBmax 370, 585 nm, λBmax (SP+CH3SO3H) 420 nm | New family of SP liquid crystal materials. | [187] | |
| SP341 |  | -H | B (81%) | THF: λAmax 355 nm, λBmax 370, 585 nm, λBmax (SP+CH3SO3H) 420 nm | New family of SP liquid crystal materials. | [187] | |
| SP342 |  | -H | B (95%) | THF: λAmax 355 nm, λBmax 370, 585 nm, λBmax (SP+CH3SO3H) 420 nm | New family of SP liquid crystal materials. | [187] | |
| SP343 |  | (a) Y = -H | B (77%) | CH2Cl2: λAmax 230, 270, 310 nm, λBmax 230, 270, 310, 390, 490 nm | Photochromic SP-based liquid crystals. | [186] | |
| (b) Y = -Br | B (80%) | CH2Cl2: λAmax 230, 270, 310 nm, λBmax 230, 270, 310, 380, 500 nm | |||||
| (c) Y = -CF3 | B (77%) | CH2Cl2: λAmax 230, 270, 310 nm | |||||
| (d) Y = -CN | B (45%) | CH2Cl2: λAmax 230, 270, 310 nm, λBmax 230, 270, 310, 470 nm | |||||
| (e) Y = -NO2 | B (41%) | CH2Cl2: λAmax 230, 270, 310, 365sh nm, λBmax 230, 270, 310, 480, 600 nm | |||||
| SP344 |  | (a) Y = -H | B (59%) | CH2Cl2: λAmax 230, 270, 310 nm | Photochromic SP-based liquid crystals. | [186] | |
| (b) Y = -Br | B (82%) | CH2Cl2: λAmax 230, 270, 310 nm | |||||
| (c) Y = -CF3 | B (28%) | CH2Cl2: λAmax 230, 270, 310 nm, λBmax 230, 270, 310, 440 nm | |||||
| (d) Y = -CN | B (65%) | CH2Cl2: λAmax 230, 270, 310 nm | |||||
| (e) Y = -NO2 | B (44%) | CH2Cl2: λAmax 230, 270, 310, 365sh nm, λBmax 230, 270, 310, 350sh, 440 nm | |||||
| SP345 |  | B | SP345 QLCs | [185] | |||
| SP346 |  | B | SP346 QLCs | [185] | |||
| SP347 |  | B | SP347 QLCs | [185] | |||
| SP348 |  | B | SP348 QLCs | [185] | |||
| SP349 |  | B (55%) | SP349 QLCs | [185] | |||
| SP350 |  | (a) n = 2 | B (37%) | Precursors for synthesis of photochromic polyacrylates and polysiloxanes. | [183,184] | ||
| (b) n = 5 | B (71%) | ||||||
| (c) n =11 | B (43%) | ||||||
| SP351 |  | electropolymerization | CH3OH: CH2Cl2 (1:1) polyTMC4: λAmax 425, 490 nm, λBmax (SP+Co2+) 425, 517, 597, 655 nm | SP351 with two alkoxy-substituted thienyl units furnishing a monomer suitable for electropolymerization | [110] | ||
| SP352 |  | B | CHCl3: λAmax 315, 397 nm, λBmax 309, 388, 500 nm | SP352 containing polyphenyleneethynylene copolymer. SP-copolymer was prepared by palladium-catalyzed polymerization of monomer by Pd(PPh3)2Cl2 and CuI in a mixture of toluene and triethylamine. | [180] | ||
| SP353 |  | Copolymer of bis-SP with phenylene. | [192] | ||||
| SP354 |  | Copolymer of SP354-sulfone with phenylene | [192] | ||||
| SP355 |  | SP main chain copolymers prepared by Suzuki polycondensation. | [192] | ||||
| SP356 |  | SP main chain copolymers prepared by MW-assisted Suzuki–Miyaura polycondensation. | [109,177] | ||||
| SP357 |  | SP main chain copolymers based on alternating spiropyran (SP) and 9,9-dioctylfluorene (F8) units were synthesized via Suzuki polycondensation (SPC). | [176] | ||||
| SP358 |  | D | Synthesis of photochromic SP-polyacrylates and SP-polysiloxanes. | [184,259] | |||
| SP359 |   or/and framework extension or/and framework extension | C | Toluene: λAmax 320, 340sh nm, λBmax 590sh, 610 nm, λBfl 660 nm | PhotoPAF- (photoresponsive porous aromatic framework). 3D rigid and porous SP networks. | [76] | ||
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barachevsky, V.A.; Lashkov, G.I.; Tsekhomskii, V.A. Fotokhromism i Ego Primenenie (Photochromism and Its Application); Khimiya: Moscow, Russia, 1977. [Google Scholar]
- Dürr, H.; Bouas-Laurent, H. (Eds.) Photochromism: Molecules and Systems; Elsevier B.V.: Amsterdam, The Netherlands, 2003; ISBN 978-0-444-51322-9. [Google Scholar]
- Crano, J.C.; Guglielmetti, R.J. (Eds.) Organic Photochromic and Thermochromic Compounds; Topics in Applied Chemistry; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; ISBN 978-0-306-45882-8. [Google Scholar]
- Kozlenko, A.S.; Ozhogin, I.V.; Pugachev, A.D.; Lukyanova, M.B.; El-Sewify, I.M.; Lukyanov, B.S. A Modern Look at Spiropyrans: From Single Molecules to Smart Materials. Top. Curr. Chem. 2023, 381, 8. [Google Scholar] [CrossRef] [PubMed]
- Towns, A. Spiropyran Dyes. Phys. Sci. Rev. 2021, 6, 341–368. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, J.; He, T. (Eds.) Photochromic Materials: Preparation, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2016; ISBN 978-3-527-68370-3. [Google Scholar]
- Feringa, B.L.; Browne, W.R. (Eds.) Molecular Switches, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-31365-5. [Google Scholar]
- Coudret, C.; Chernyshev, A.V.; Metelitsa, A.V.; Micheau, J.C. New Trends in Spiro-Compounds Photochromic Metals Sensors: Quantitative Aspects. In Photon-Working Switches; Yokoyama, Y., Nakatani, K., Eds.; Springer: Tokyo, Japan, 2017; pp. 3–35. ISBN 978-4-431-56542-0. [Google Scholar]
- Kortekaas, L.; Browne, W.R. Spiropyran—Multifaceted Chromic Compounds. In Molecular Photoswitches; Pianowski, Z.L., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 131–149. ISBN 978-3-527-35104-6. [Google Scholar]
- Kortekaas, L.; Browne, W.R. The Evolution of Spiropyran: Fundamentals and Progress of an Extraordinarily Versatile Photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef]
- Barachevsky, V.A. Photochromic Spirocompounds and Chromenes for Sensing Metal Ions. Rev. J. Chem. 2013, 3, 52–94. [Google Scholar] [CrossRef]
- Barachevsky, V.A. Negative Photochromism in Organic Systems. Rev. J. Chem. 2017, 7, 334–371. [Google Scholar] [CrossRef]
- Paramonov, S.V.; Lokshin, V.; Fedorova, O.A. Spiropyran, Chromene or Spirooxazine Ligands: Insights into Mutual Relations between Complexing and Photochromic Properties. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 209–236. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-Based Advanced Photoswitchable Materials: A Fascinating Pathway to the Future Stimuli-Responsive Devices. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100487. [Google Scholar] [CrossRef]
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and Biosensing Applications of Spiropyran and Its Derivatives—A Review. Anal. Chim. Acta 2020, 1110, 199–223. [Google Scholar] [CrossRef]
- Demina, O.V.; Belikov, N.E.; Melnikova, I.A.; Lukin, A.Y.; Varfolomeev, S.D.; Khodonov, A.A. New Labels and Probes for the Application in Bionanophotonics. Russ. J. Phys. Chem. B 2019, 13, 938–941. [Google Scholar] [CrossRef]
- Bertelson, R.C. Reminiscences about Organic Photochromics. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. Mol. Cryst. Liq. Cryst. 1994, 246, 1–8. [Google Scholar] [CrossRef]
- Pianowski, Z.L. (Ed.) Molecular Photoswitches: Chemistry, Properties, and Applications, 2 Volume Set; Wiley: Hoboken, NJ, USA, 2022; ISBN 978-3-527-35104-6. [Google Scholar]
- Dzaparidze, K.G. Spirochromenes; Mezniereba: Tbilisi, Georgia, 1979. [Google Scholar]
- Fischer, E.; Hirshberg, Y. Formation of Coloured Forms of Spirans by Low-Temperature Irradiation. J. Chem. Soc. 1952, 4522–4524. [Google Scholar]
- Bertelson, R.C. Chapter 3 “Photochromic Process Involving Heterolytic Cleavage”. In Photochromism; Brown, G.H., Ed.; Techniques of Chemistry; Wiley: London, UK, 1971; Volume III, pp. 45–431. [Google Scholar]
- Bertelson, R.C. Spiropyrans. In Organic Photochromic and Thermochromic Compounds; Crano, J.C., Guglielmetti, R., Eds.; Plenum Press: New York, NY, USA, 1999; Volume I, pp. 11–83. [Google Scholar]
- Zakhs, E.R.; Martynova, V.M.; Efros, L.S. Synthesis and Properties of Spiropyrans That Are Capable of Reversible Opening of the Pyran Ring (Review). Chem. Heterocycl. Compd. 1979, 15, 351–372. [Google Scholar] [CrossRef]
- Lukyanov, B.S.; Lukyanova, M.B. Spiropyrans: Synthesis, Properties, and Application. (Review). Chem. Heterocycl. Compd. 2005, 41, 281–311. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef]
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Kozlenko, A.S.; Pugachev, A.D.; Ozhogin, I.V.; El-Sewify, I.M.; Lukyanov, B.S. Spiropyrans: Molecules in Motion. Chem. Heterocycl. Compd. 2021, 57, 984–989. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Mukhanov, E.L.; Ozhogin, I.V.; Kozlenko, A.S.; Metelitsa, A.V.; Lukyanov, B.S. Isomerization and Changes of the Properties of Spiropyrans by Mechanical Stress: Advances and Outlook. Chem. Heterocycl. Compd. 2021, 57, 122–130. [Google Scholar] [CrossRef]
- Aiken, S.; Edgar, R.J.L.; Gabbutt, C.D.; Heron, B.M.; Hobson, P.A. Negatively Photochromic Organic Compounds: Exploring the Dark Side. Dyes Pigments 2018, 149, 92–121. [Google Scholar] [CrossRef]
- Chibisov, A.K.; Görner, H. Photochromism of Spirobenzopyranindolines and Spironaphthopyranindolines. Phys. Chem. Chem. Phys. 2001, 3, 424–431. [Google Scholar] [CrossRef]
- Görner, H. Photochromism of Nitrospiropyrans: Effects of Structure, Solvent and Temperature. Phys. Chem. Chem. Phys. 2001, 3, 416–423. [Google Scholar] [CrossRef]
- Belikov, N.E.; Lukin, A.Y.; Varfolomeev, S.D.; Levina, I.I.; Petrovskaya, L.E.; Demina, O.V.; Barachevsky, V.A.; Khodonov, A.A. Spectral Study of the Structure and Properties of Complexes of Unsubstituted Indoline Spiropyran with Aluminum Ions. Opt. Spectrosc. 2022, 130, 538–546. [Google Scholar] [CrossRef]
- Kume, S.; Nishihara, H. Photochrome-Coupled Metal Complexes: Molecular Processing of Photon Stimuli. Dalton Trans. 2008, 25, 3260–3271. [Google Scholar] [CrossRef] [PubMed]
- Guerchais, V.; Ordronneau, L.; Le Bozec, H. Recent Developments in the Field of Metal Complexes Containing Photochromic Ligands: Modulation of Linear and Nonlinear Optical Properties. Coord. Chem. Rev. 2010, 254, 2533–2545. [Google Scholar] [CrossRef]
- Zvezdin, K.V.; Belikov, N.E.; Laptev, A.V.; Lukin, A.Y.; Demina, O.V.; Levin, P.P.; Brichkin, S.B.; Spirin, M.G.; Razumov, V.F.; Shvets, V.I.; et al. New Hybrid Photochromic Materials with Switchable Fluorescence. Nanotechnol. Russ. 2012, 7, 308–317. [Google Scholar] [CrossRef]
- Kume, S.; Nishihara, H. Metal-Based Photoswitches Derived from Photoisomerization. In Photofunctional Transition Metal Complexes; Yam, V.W.W., Ed.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2006; Volume 123, pp. 79–112. ISBN 978-3-540-36809-0. [Google Scholar]
- Chernyshev, A.V.; Voloshin, N.A.; Rostovtseva, I.A.; Demidov, O.P.; Shepelenko, K.E.; Solov’eva, E.V.; Gaeva, E.B.; Metelitsa, A.V. Benzothiazolyl Substituted Spiropyrans with Ion-Driven Photochromic Transformation. Dyes Pigments 2020, 178, 108337. [Google Scholar] [CrossRef]
- Belser, P.; De Cola, L.; Hartl, F.; Adamo, V.; Bozic, B.; Chriqui, Y.; Iyer, V.M.; Jukes, R.T.F.; Kühni, J.; Querol, M.; et al. Photochromic Switches Incorporated in Bridging Ligands: A New Tool to Modulate Energy-Transfer Processes. Adv. Funct. Mater. 2006, 16, 195–208. [Google Scholar] [CrossRef]
- Jukes, R.T.F.; Bozic, B.; Belser, P.; De Cola, L.; Hartl, F. Photophysical and Redox Properties of Dinuclear Ru and Os Polypyridyl Complexes with Incorporated Photostable Spiropyran Bridge. Inorg. Chem. 2009, 48, 1711–1721. [Google Scholar] [CrossRef]
- Abdullah, A.; Roxburgh, C.J.; Sammes, P.G. Photochromic Crowned Spirobenzopyrans: Quantitative Metal-Ion Chelation by UV, Competitive Selective Ion-Extraction and Metal-Ion Transportation Demonstration Studies. Dyes Pigments 2008, 76, 319–326. [Google Scholar] [CrossRef]
- Kholmanskii, A.S.; Dyumaev, K.M. The Photochemistry and Photophysics of Spiropyrans. Russ. Chem. Rev. 1987, 56, 136–151. [Google Scholar] [CrossRef]
- Krysanov, S.A.; Alfimov, M.V. Ultrafast Formation of Transients in Spiropyran Photochromism. Chem. Phys. Lett. 1982, 91, 77–80. [Google Scholar] [CrossRef]
- Görner, H.; Atabekyan, L.S.; Chibisov, A.K. Photoprocesses in Spiropyran-Derived Merocyanines: Singlet versus Triplet Pathway. Chem. Phys. Lett. 1996, 260, 59–64. [Google Scholar] [CrossRef]
- Melnikova, I.A.; Belikov, N.E.; Bakholdina, A.G.; Lukin, A.Y.; Varfolomeev, S.D.; Demina, O.V.; Khodonov, A.A. Study of the Interaction of a New Photochromic Podand with Metal Salts in Solutions of Organic Solvents. In Proceedings of the XIX Annual International Youth Conference IBCP RAS-Universities “Biochemical Physics”, Moscow, Russia, 28–30 October 2019; pp. 152–155. [Google Scholar]
- Demina, O.V.; Levin, P.P.; Belikov, N.E.; Laptev, A.V.; Lukin, A.Y.; Barachevsky, V.A.; Shvets, V.I.; Varfolomeev, S.D.; Khodonov, A.A. Synthesis and Photochromic Reaction Kinetics of Unsaturated Spiropyran Derivatives. J. Photochem. Photobiol. Chem. 2013, 270, 60–66. [Google Scholar] [CrossRef]
- Levin, P.P.; Tatikolov, A.S.; Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Demina, O.V.; Khodonov, A.A.; Shvets, V.I.; Varfolomeev, S.D. The Investigation of the Intermediates of Spiropyran Retinal Analogs by Laser Flash Photolysis Techniques with Different Excitation Wavelengths. J. Photochem. Photobiol. Chem. 2012, 231, 41–44. [Google Scholar] [CrossRef]
- Tamai, N.; Miyasaka, H. Ultrafast Dynamics of Photochromic Systems. Chem. Rev. 2000, 100, 1875–1890. [Google Scholar] [CrossRef] [PubMed]
- Murin, V.A.; Mandjikov, V.F.; Barachevskii, V.A. Study of Triplet-Triplet Absorption of Photochromic Spiropyran by Laser Photoexcitation. Opt. Spectrosc. 1976, 40, 1084–1086. [Google Scholar]
- Murin, V.A.; Mandjikov, V.F.; Barachevskii, V.A. Study of Photochromism of Methoxysubstituted Indolenine Spiropyrans by Laser Nanosecond Spectroscopy. Opt. Spectrosc. 1977, 41, 79–81. [Google Scholar]
- Melnikova, I.A.; Lukin, A.Y.; Belikov, N.E.; Demina, O.V.; Levin, P.P.; Varfolomeev, S.D.; Khodonov, A.A. Synthesis and Study of the Spectral Properties of a Series of Stilbenes Containing, as One of the Aryl Fragments, a Molecule of a Photochromic Label of the Spiropyran Series. In Proceedings of the XIV Annual International Youth Conference IBCP RAS-Universities “Biochemical Physics”, Moscow, Russia, 28–30 October 2014; pp. 128–133. [Google Scholar]
- Feuerstein, T.J.; Müller, R.; Barner-Kowollik, C.; Roesky, P.W. Investigating the Photochemistry of Spiropyran Metal Complexes with Online LED-NMR. Inorg. Chem. 2019, 58, 15479–15486. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Ozhogin, I.V.; Lukyanova, M.B.; Lukyanov, B.S.; Kozlenko, A.S.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Aldoshin, S.M.; Metelitsa, A.V. Synthesis, Structure and Photochromic Properties of Indoline Spiropyrans with Electron-Withdrawing Substituents. J. Mol. Struct. 2021, 1229, 129615. [Google Scholar] [CrossRef]
- Ren, J.; Tian, H. Thermally Stable Merocyanine Form of Photochromic Spiropyran with Aluminum Ion as a Reversible Photo-Driven Sensor in Aqueous Solution. Sensors 2007, 7, 3166–3178. [Google Scholar] [CrossRef]
- Tian, W.; Tian, J. An Insight into the Solvent Effect on Photo-, Solvato-Chromism of Spiropyran through the Perspective of Intermolecular Interactions. Dyes Pigments 2014, 105, 66–74. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Tang, Y.; Zhao, F.; Song, X.; Li, E. Detailed Investigation on a Negative Photochromic Spiropyran. J. Photochem. Photobiol. Chem. 1995, 90, 117–123. [Google Scholar] [CrossRef]
- Song, X.; Zhou, J.; Li, Y.; Tang, Y. Correlations between Solvatochromism, Lewis Acid-Base Equilibrium and Photochromism of an Indoline Spiropyran. J. Photochem. Photobiol. Chem. 1995, 92, 99–103. [Google Scholar] [CrossRef]
- Seiler, V.K.; Robeyns, K.; Tumanov, N.; Cinčić, D.; Wouters, J.; Champagne, B.; Leyssens, T. A Coloring Tool for Spiropyrans: Solid State Metal–Organic Complexation versus Salification. CrystEngComm 2019, 21, 4925–4933. [Google Scholar] [CrossRef]
- Keum, S.-R.; Ku, B.-S.; Shin, J.-T.; Ko, J.J.; Buncel, E. Stereoselective Formation of Dicondensed Spiropyran Product Obtained from the Reaction of Excess Fischer Base with Salicylaldehydes: First Full Characterization by X-Ray Crystal Structure Analysis of a DC·acetone Crystal. Tetrahedron 2005, 61, 6720–6725. [Google Scholar] [CrossRef]
- Wizinger, R.; Wenning, H. Über Intramolekulare Ionisation. Helv. Chim. Acta 1940, 23, 247–271. [Google Scholar] [CrossRef]
- Gal’bershtam, M.A.; Bondarenko, E.M.; Khrolova, O.R.; Bolyleva, G.K.; Pod’yachev, Y.B.; Przhiyalgovskaya, N.M.; Suvorov, N.N. Synthesis and Photochromic Properties of 5-Acetyl-Substituted Indolinospirochromenes. Chem. Heterocycl. Compd. 1979, 15, 1329–1333. [Google Scholar] [CrossRef]
- Schulz-Senft, M.; Gates, P.J.; Sönnichsen, F.D.; Staubitz, A. Diversely Halogenated Spiropyrans—Useful Synthetic Building Blocks for a Versatile Class of Molecular Switches. Dyes Pigments 2017, 136, 292–301. [Google Scholar] [CrossRef]
- Sugahara, A.; Tanaka, N.; Okazawa, A.; Matsushita, N.; Kojima, N. Photochromic Property of Anionic Spiropyran with Sulfonate-Substituted Indoline Moiety. Chem. Lett. 2014, 43, 281–283. [Google Scholar] [CrossRef]
- Tanaka, N.; Okazawa, A.; Sugahara, A.; Kojima, N. Development of a Photoresponsive Organic–Inorganic Hybrid Magnet: Layered Cobalt Hydroxides Intercalated with Spiropyran Anions. Bull. Chem. Soc. Jpn. 2015, 88, 1150–1155. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, J.; Li, X.; Wang, Y.; Matsuura, T. Synthesis and Photochromism of Photochromic Spiro Compounds Having a Reactive Pendant Group. J. Heterocycl. Chem. 2002, 39, 179–184. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, J.B.; Matsuura, T.; Wang, Y.M. DNA Modification with Photochromic Spiro Compounds. Chin. Chem. Lett. 2002, 13, 299–302. [Google Scholar]
- Gal’bershtam, M.A.; Lazarenko, I.B.; Bobyleva, G.K.; Pod’yachev, Y.B.; Przhiyalgovskaya, N.M.; Suvorov, N.N. Photochromic 5-Styryl-Substituted Indolinospirochromenes. Chem. Heterocycl. Compd. 1984, 20, 1222–1225. [Google Scholar] [CrossRef]
- Gal’bershtam, M.A.; Przhiyalgovskaya, N.M.; Lazarenko, I.B.; Kononova, V.S.; Suvorov, N.N. Synthesis and Spectral Characteristics of Photochromic 5′-Aryl-1′,3′,3′-trimethyl-6-nitro-2H-chromene-2-spiro-2′-indolines. Chem. Heterocycl. Compd. 1976, 12, 417–419. [Google Scholar] [CrossRef]
- Gal’bershtam, M.A.; Przhiyalgovskaya, N.M.; Samoilova, N.P.; Braude, E.V.; Lazarenko, I.B.; Suvorov, N.N. Effect of Aryl Substituents on the Rate of Dark Decolorization of Photochromic Spirochromenes of the Indoline Series. Chem. Heterocycl. Compd. 1977, 13, 67–69. [Google Scholar] [CrossRef]
- Samat, A.; De Keukeleire, D.; Guglielmetti, R. Synthesis and Spectrokinetic Properties of Photochromic Spiropyrans. Bull. Sociétés Chim. Belg. 2010, 100, 679–700. [Google Scholar] [CrossRef]
- Zhao, W.; Carreira, E.M. Solid-Phase Synthesis of Photochromic Spiropyrans. Org. Lett. 2005, 7, 1609–1612. [Google Scholar] [CrossRef]
- Perry, A.; Davis, K.; West, L. Synthesis of Stereochemically-Biased Spiropyrans by Microwave-Promoted, One-Pot Alkylation–Condensation. Org. Biomol. Chem. 2018, 16, 7245–7254. [Google Scholar] [CrossRef]
- Silvia, T.R.; Ana, V.S.L.; González, E.A.S. Novel Syntheses of Spiropyran Photochromatic Compounds Using Ultrasound. Synth. Commun. 1995, 25, 105–110. [Google Scholar] [CrossRef]
- Pargaonkar, J.G.; Patil, S.K.; Vajekar, S.N. Greener Route for the Synthesis of Photo- and Thermochromic Spiropyrans Using a Highly Efficient, Reusable, and Biocompatible Choline Hydroxide in an Aqueous Medium. Synth. Commun. 2018, 48, 208–215. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, S.H.; Bae, J.W.; Pyun, H.-J.; Yoon, C.M. Fischer’s Base as a Protecting Group: Protection and Deprotection of 2-Hydroxybenzaldehydes. Tetrahedron Lett. 2000, 41, 3915–3917. [Google Scholar] [CrossRef]
- Zakhs, E.R.; Zvenigorodskaya, L.A.; Leshenyuk, N.G.; Martynova, V.P. Bromination of Spiropyrans and Reduction of Their Nitro Derivatives. Chem. Heterocycl. Compd. 1977, 13, 1055–1061. [Google Scholar] [CrossRef]
- Kundu, P.K.; Olsen, G.L.; Kiss, V.; Klajn, R. Nanoporous Frameworks Exhibiting Multiple Stimuli Responsiveness. Nat. Commun. 2014, 5, 3588. [Google Scholar] [CrossRef]
- Samoilova, N.P.; Gal’bershtam, M.A. Some Substitution Reactions in a Number of Photochromic Indolinespirochromenes. Chem. Heterocycl. Compd. 1977, 13, 855–858. [Google Scholar] [CrossRef]
- Shvartsman, F.P.; Krongauz, V.A. Quasi-Liquid Crystals of Thermochromic Spiropyrans. A Material Intermediate between Supercooled Liquids and Mesophases. J. Phys. Chem. 1984, 88, 6448–6453. [Google Scholar] [CrossRef]
- Laptev, A.V.; Lukin, A.J.; Belikov, N.E.; Fomin, M.A.; Demina, O.V.; Shvets, V.I.; Khodonov, A.A. Photochromic 5′-Vinyl-6-nitro-spirobenzopyran Derivatives and Production Method Thereof. Patent RU2458927C1, 20 August 2012. [Google Scholar]
- Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Barachevskii, V.A.; Demina, O.V.; Khodonov, A.A.; Varfolomeev, S.D.; Shvets, V.I. Ethynyl-Equipped Spirobenzopyrans as Promising Photochromic Markers for Nucleic Acid Fragments. Mendeleev Commun. 2013, 23, 145–146. [Google Scholar] [CrossRef]
- Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Demina, O.V.; Khodonov, A.A.; Shvets, V.I. New Maleimide Spirobenzopyran Derivatives as Photochromic Labels for Macromolecules with Sulfhydryl Groups. Mendeleev Commun. 2014, 24, 245–246. [Google Scholar] [CrossRef]
- Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Zvezdin, K.V.; Demina, O.V.; Barachevsky, V.A.; Varfolomeev, S.D.; Khodonov, A.A.; Shvets, V.I. Synthesis and Studies of Photochromic Properties of Spirobenzopyran Carboxy Derivatives and Their Model Compounds as Potential Markers. Russ. Chem. Bull. 2014, 63, 2026–2035. [Google Scholar] [CrossRef]
- Laptev, A.V.; Lukin, A.Y.; Belikov, N.V.; Demina, O.V.; Varfolomeev, S.D.; Barachevsky, V.A.; Khodonov, A.A.; Shvets, V.I. Synthesis and Study of the Photochromic Behavior of Substituted 6′-Nitro-1,3,3-trimethyl-5-vinylspiro(indolino-2,2′-[2H]chromenes). Vestn. MITHT 2013, 8, 18–25. [Google Scholar]
- Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Zemtsov, R.V.; Shvets, V.I.; Demina, O.V.; Varfolomeev, S.D.; Barachevskii, V.A.; Khodonov, A.A. Synthesis and Study of the Photochromic Behavior of 3-[6′-Nitro-1,3,3-trimethylspiro(indolino-2,2′-[2H]-chromen-5-yl)]Propenoic Acid and Its Ethyl Ester. High Energy Chem. 2010, 44, 211–215. [Google Scholar] [CrossRef]
- Khodonov, A.A.; Demina, O.V.; Laptev, A.V.; Belikov, N.E.; Lukin, A.Y.; Fomin, M.A.; Shvets, V.I.; Varfolomeev, S.D. Synthesis and Study of Spectral Properties of Labelled Isoxazole Derivatives. In Proceedings of the Materials of Russian-French Joint Symposium on Organic Photochromes “Phenics in Russia”, Chernogolovka, Russia, 6–8 October 2011; p. 50. [Google Scholar]
- Laptev, A.; Lukin, A.; Belikov, N.; Fomin, M.; Zvezdin, K.; Demina, O.; Barachevsky, V.; Varfolomeev, S.; Shvets, V.; Khodonov, A. Polyenic Spirobenzopyrans: Synthesis and Study of Photochromic Properties. J. Photochem. Photobiol. Chem. 2011, 222, 16–24. [Google Scholar] [CrossRef]
- Laptev, A.V.; Lukin, A.J.; Belikov, N.E.; Shvets, V.I.; Demina, O.V.; Barachevskij, V.A.; Khodonov, A.A. 5-Formyl-Substituted Indoline Spirobenzopyrans and Method of Producing Them. Patent RU2358977C1, 20 June 2009. [Google Scholar]
- Laptev, A.V.; Belikov, N.E.; Lukin, A.Y.; Barachevskii, V.A.; Alfimov, M.V.; Demina, O.V.; Varfolomeev, S.D.; Shvets, V.I.; Khodonov, A.A. Spiropyran Analogs of Retinal: Synthesis and Study of Their Photochromic Properties. High Energy Chem. 2008, 42, 601–603. [Google Scholar] [CrossRef]
- Barachevsky, V.A.; Khodonov, A.A.; Belikov, N.E.; Laptev, A.V.; Lukin, A.Y.; Demina, O.V.; Luyksaar, S.I.; Krayushkin, M.M. Properties of Photochromic Retinals. Dyes Pigments 2012, 92, 831–837. [Google Scholar] [CrossRef]
- Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Fomin, M.A.; Demina, O.V.; Shvets, V.I.; Khodonov, A.A. Study of Substituted Spirobenzopyrans Formylation Process. In Proceedings of the Materials of the 17th European Symposium on Organic Chemistry (ESOC-17), Crete, Greece, 10–15 July 2011; p. P2.041. [Google Scholar]
- Barachevskii, V.A.; Kobeleva, O.I.; Valova, T.M.; Ait, A.O.; Kol’tsova, L.S.; Shienok, A.I.; Zaichenko, N.L.; Laptev, A.V.; Khodonov, A.A.; Kuznetsova, O.Y.; et al. Spectral Indications of Interaction of Functionalized Photochromic Compounds with Ag and Au Nanoparticles. Theor. Exp. Chem. 2012, 48, 14–20. [Google Scholar] [CrossRef]
- Belikov, N.; Lukin, A.; Laptev, A.; Shvets, V.; Barachevsky, V.; Strokach, Y.; Valova, T.; Krayushkin, M.; Demina, O.; Varfolomeev, S.; et al. Photochromic Behavior of Retinal Analogs. J. Photochem. Photobiol. Chem. 2008, 196, 262–267. [Google Scholar] [CrossRef]
- Demina, O.V.; Belikov, N.E.; Varfolomeev, S.D.; Khodonov, A.A. 3-Pyridylisoxazoles as Prototypes of Antiaggregatory Agents. Russ. Chem. Bull. 2018, 67, 866–877. [Google Scholar] [CrossRef]
- Demina, O.V.; Belikov, N.E.; Varfolomeev, S.D.; Khodonov, A.A. Synthesis of Isoxazoles with Photochromic Label. In Proceedings of the Book of Abstracts of XV International Conference on Heterocycles in Bioorganic Chemistry (Bio-Heterocycles-2013), Riga, Latvia, 27–30 May 2013; p. 68. [Google Scholar]
- Khodonov, A.A.; Belikov, N.E.; Lukin, A.Y.; Levin, P.P.; Varfolomeev, S.D.; Demina, O.V. Photochromic Derivatives of 5′-Hydroxymethyl-6-nitro-1′,3′,3′-trimethylspiro [2H 1-benzopyran-2,2′-indoline]. Patent RU2694904C1, 18 July 2019. [Google Scholar]
- Laptev, A.V.; Pugachev, D.E.; Lukin, A.Y.; Nechaev, A.V.; Belikov, N.E.; Demina, O.V.; Levin, P.P.; Khodonov, A.A.; Mironov, A.F.; Varfolomeev, S.D.; et al. Synthesis of 5,10,15,20-Tetra [6′-nitro-1,3,3-trimethylspiro-(indolino-2,2′-2H-chromen-5-yl)]porphyrin and Its Metal Complexes. Mendeleev Commun. 2013, 23, 199–201. [Google Scholar] [CrossRef]
- Gal’bershtam, M.A.; Artamonova, N.N.; Samoilova, N.P. Synthesis of 3′-Acyl-Substituted Indoline Spiropyrans. Chem. Heterocycl. Compd. 1975, 11, 167–172. [Google Scholar] [CrossRef]
- Niu, C.; Song, Y.; Yang, L. Synthesis of 5′-Functionalized Indolinospiropyrans with Vinylene Unit as Linker. Chin. J. Chem. 2009, 27, 2001–2006. [Google Scholar] [CrossRef]
- Braude, E.V.; Gal’bershtam, M.A. Styryl-Substituted Spirochromenes of the Indoline Series. Chem. Heterocycl. Compd. 1979, 15, 173–179. [Google Scholar] [CrossRef]
- Demina, O.V.; Laptev, A.V.; Lukin, A.Y.; Belikov, N.E.; Fomin, M.A.; Shvets, V.I.; Khodonov, A.A. Study of Substituted Spirobenzopyran Formylation Process. In Proceedings of the Materials of the 23rd International Congress on Heterocyclic Chemistry, Glasgow, UK, 31 July–4 August 2011; p. 273. [Google Scholar]
- Tuktarov, A.R.; Khuzin, A.A.; Tulyabaev, A.R.; Venidictova, O.V.; Valova, T.M.; Barachevsky, V.A.; Khalilov, L.M.; Dzhemilev, U.M. Synthesis, Structure and Photochromic Properties of Hybrid Molecules Based on Fullerene C60 and Spiropyrans. RSC Adv. 2016, 6, 71151–71155. [Google Scholar] [CrossRef]
- Khuzin, A.A.; Tuktarov, A.R.; Barachevsky, V.A.; Valova, T.M.; Tulyabaev, A.R.; Dzhemilev, U.M. Synthesis, Photo and Acidochromic Properties of Spiropyran-Containing Methanofullerenes. RSC Adv. 2020, 10, 15888–15892. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wong, H.-L.; Tam, A.Y.-Y.; Lam, W.H.; Wu, L.; Yam, V.W.-W. Synthesis, Characterization, and Photophysical Properties of Bodipy-Spirooxazine and -Spiropyran Conjugates: Modulation of Fluorescence Resonance Energy Transfer Behavior via Acidochromic and Photochromic Switching. ACS Appl. Mater. Interfaces 2014, 6, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, W.; Tang, H.; Cao, D. A Multistimuli-Responsive Fluorescent Switch in the Solution and Solid States Based on Spiro[fluorene-9,9′-xanthene]-spiropyran. J. Mater. Chem. C 2019, 7, 9102–9111. [Google Scholar] [CrossRef]
- Dowds, M.; Stenspil, S.G.; de Souza, J.H.; Laursen, B.W.; Cacciarini, M.; Nielsen, M.B. Orthogonal- and Path-Dependent Photo/Acidoswitching in an Eight-State Dihydroazulene-Spiropyran Dyad. ChemPhotoChem 2022, 6, e202200152:1–e202200152:9. [Google Scholar] [CrossRef]
- Lee, S.; Ji, S.; Kang, Y. Synthesis and Characterizations of Bis-Spiropyran Derivatives. Bull. Korean Chem. Soc. 2012, 33, 3740–3744. [Google Scholar] [CrossRef]
- Dissanayake, D.S.; McCandless, G.T.; Stefan, M.C.; Biewer, M.C. Systematic Variation of Thiophene Substituents in Photochromic Spiropyrans. Photochem. Photobiol. Sci. 2017, 16, 1057–1062. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, E.S.; Yoon, C.M. Suzuki Coupling Reaction of 6-Iodo- or 6,8-Diiodospiropyran: Synthesis of Spiropyran Analogs. Tetrahedron Lett. 2001, 42, 8311–8314. [Google Scholar] [CrossRef]
- Metzler, L.; Reichenbach, T.; Brügner, O.; Komber, H.; Lombeck, F.; Müllers, S.; Hanselmann, R.; Hillebrecht, H.; Walter, M.; Sommer, M. High Molecular Weight Mechanochromic Spiropyran Main Chain Copolymers via Reproducible Microwave-Assisted Suzuki Polycondensation. Polym. Chem. 2015, 6, 3694–3707. [Google Scholar] [CrossRef]
- Wagner, K.; Zanoni, M.; Elliott, A.B.S.; Wagner, P.; Byrne, R.; Florea, L.E.; Diamond, D.; Gordon, K.C.; Wallace, G.G.; Officer, D.L. A Merocyanine-Based Conductive Polymer. J. Mater. Chem. C 2013, 1, 3913. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, D.; Gao, H.; Zhang, J.; Xing, Y.; Yang, Z.; Cao, H.; He, W. Third-Order Nonlinear Optical Properties of the “Clicked” Closed-Ring Spiropyrans. Dyes Pigments 2019, 162, 451–458. [Google Scholar] [CrossRef]
- Dvornikov, A.S.; Malkin, J.; Rentzepis, P.M. Spectroscopy and Kinetics of Photochromic Materials for 3D Optical Memory Devices. J. Phys. Chem. 1994, 98, 6746–6752. [Google Scholar] [CrossRef]
- Irie, M. Diarylethenes for Memories and Switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef] [PubMed]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, J.; Zheng, M.; Zheng, L.; Wang, H.; Zhang, Y. Multi-Responsive Supramolecular Hydrogels Based on Merocyanine–Peptide Conjugates. Org. Biomol. Chem. 2015, 13, 11492–11498. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, H.; Li, J.; Wang, Y.; Zhang, Y. Spiropyran-Linked Dipeptide Forms Supramolecular Hydrogel with Dual Responses to Light and to Ligand–Receptor Interaction. Chem. Commun. 2009, 3342–3344. [Google Scholar] [CrossRef]
- Liu, M.; Creemer, C.N.; Reardon, T.J.; Parquette, J.R. Light-Driven Dissipative Self-Assembly of a Peptide Hydrogel. Chem. Commun. 2021, 57, 13776–13779. [Google Scholar] [CrossRef]
- Keum, S.-R.; Choi, Y.-K.; Kim, S.-H.; Yoon, C.-M. Symmetric and Unsymmetric Indolinobenzospiropyran Dimers: Synthesis and Characterization. Dyes Pigments 1999, 41, 41–47. [Google Scholar] [CrossRef]
- Kortekaas, L.; Steen, J.D.; Duijnstee, D.R.; Jacquemin, D.; Browne, W.R. Noncommutative Switching of Double Spiropyrans. J. Phys. Chem. A 2020, 124, 6458–6467. [Google Scholar] [CrossRef]
- Chang, Y.M.; Lee, S.H.; Cho, M.Y.; Yoo, B.W.; Rhee, H.J.; Lee, S.H.; Yoon, C.M. Homocoupling of Aryl Iodides and Bromides Using a Palladium/Indium Bimetallic System. Synth. Commun. 2005, 35, 1851–1857. [Google Scholar] [CrossRef]
- Flerova, A.N.; Prokhoda, A.L.; Zaitseva, E.L.; Krongauz, V.A. Synthesis and Properties of Photochromic Bisindolinospiropyrans. Chem. Heterocycl. Compd. 1973, 9, 1476–1482. [Google Scholar] [CrossRef]
- Cho, Y.J.; Rho, K.Y.; Kim, S.H.; Keum, S.R.; Yoon, C.M. Synthesis and Characterization of Symmetric and Non-Symmetric Bis-Spiropyranylethyne. Dyes Pigments 1999, 44, 19–25. [Google Scholar] [CrossRef]
- Keum, S.-R.; Choi, Y.-K.; Lee, M.-J.; Kim, S.-H. Synthesis and Properties of Thermo- and Photochromic Bisindolinobenzospiropyrans Linked by Thio- and Carbonyl Groups. Dyes Pigments 2001, 50, 171–176. [Google Scholar] [CrossRef]
- Keum, S.-R.; Ku, B.-S.; Kim, S.-E.; Choi, Y.-K.; Kim, S.-H.; Koh, K.-N. Solvatokinetic and Solvatochromic Behavior of Bis(indolinobenzospiropyranyl) Sulfide Derivatives in Various Solvents. Bull. Korean Chem. Soc. 2004, 25, 1361–1365. [Google Scholar] [CrossRef]
- Keum, S.-R.; Lee, J.-H.; Seok, M.-K.; Yoon, C.-M. A Simple and Convenient Synthetic Route to the Bis-Indolinospirobenzopyrans. Bull. Korean Chem. Soc. 1994, 15, 275–277. [Google Scholar] [CrossRef]
- Keum, S.-R.; Lee, J.-H.; Seok, M.-K. Synthesis and Characterization of Bis-Indolinospirobenzopyrans, New Photo- and Thermochromic Dyes. Dyes Pigments 1994, 25, 21–29. [Google Scholar] [CrossRef]
- Keum, S.-R.; Lim, S.-S.; Min, B.-H.; Kazmaier, P.M.; Buncel, E. Synthesis and Characterization of Unsymmetrical Bis-Indolinospirobenzopyrans, a New Class of Thermo- and Photo-Chromic Dyes. Dyes Pigments 1996, 30, 225–234. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, D.; Zhou, Y.; Zhu, D. Synthesis and Spectral Investigations of a New Dyad with Spiropyran and Fluorescein Units: Toward Information Processing at the Single Molecular Level. J. Org. Chem. 2003, 68, 5681–5687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pan, K.; Mo, S.; Wang, B.; Zhao, X.; Yin, M. Tetraphenylethene-Induced Free Volumes for the Isomerization of Spiropyran toward Multifunctional Materials in the Solid State. ACS Appl. Mater. Interfaces 2018, 10, 30879–30886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Khairutdinov, R.F.; Cape, J.L.; Hurst, J.K. Photoregulated Transmembrane Charge Separation by Linked Spiropyran−Anthraquinone Molecules. J. Am. Chem. Soc. 2006, 128, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Lin, X.; Sun, W. Preparation of Spiropyran Bonded 1,8-Naphthalimide Compound Useful as Photochromic and Photoluminescent Material. Patent CN201510863463A, 4 May 2016. [Google Scholar]
- Guo, X.; Huang, L.; O’Brien, S.; Kim, P.; Nuckolls, C. Directing and Sensing Changes in Molecular Conformation on Individual Carbon Nanotube Field Effect Transistors. J. Am. Chem. Soc. 2005, 127, 15045–15047. [Google Scholar] [CrossRef]
- Shen, Q.; Cao, Y.; Liu, S.; Steigerwald, M.L.; Guo, X. Conformation-Induced Electrostatic Gating of the Conduction of Spiropyran-Coated Organic Thin-Film Transistors. J. Phys. Chem. C 2009, 113, 10807–10812. [Google Scholar] [CrossRef]
- Seefeldt, B.; Kasper, R.; Beining, M.; Mattay, J.; Arden-Jacob, J.; Kemnitzer, N.; Drexhage, K.H.; Heilemann, M.; Sauer, M. Spiropyrans as Molecular Optical Switches. Photochem. Photobiol. Sci. 2010, 9, 213–220. [Google Scholar] [CrossRef]
- Qu, L.; Xu, X.; Song, J.; Wu, D.; Wang, L.; Zhou, W.; Zhou, X.; Xiang, H. Solid-State Photochromic Molecular Switches Based on Axially Chiral and Helical Spiropyrans. Dyes Pigments 2020, 181, 108597. [Google Scholar] [CrossRef]
- Solovyova, E.V.; Rostovtseva, I.A.; Shepelenko, K.E.; Voloshin, N.A.; Chernyshev, A.V.; Borodkin, G.S.; Metelitsa, A.V.; Minkin, V.I. Synthesis and Complex Formation of Spirobenzopyranindolines Containing Rhodamine Fragment. Russ. J. Gen. Chem. 2017, 87, 1007–1014. [Google Scholar] [CrossRef]
- Solov’eva, E.V.; Rostovtseva, I.A.; Shepelenko, K.E.; Voloshin, N.A.; Chernyshev, A.V.; Borodkin, G.S.; Trofimova, N.S.; Metelitsa, A.V.; Minkin, V.I. Synthesis and Complex Formation of Rhodamine-Substituted Spirobenzopyranindolines. Russ. J. Gen. Chem. 2018, 88, 968–972. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Prakash, K.; Kumar, S. Light Controlled Receptors for Heavy Metal Ions. Coord. Chem. Rev. 2018, 357, 18–49. [Google Scholar] [CrossRef]
- Voloshin, N.A.; Bezugliy, S.O.; Solov’eva, E.V.; Metelitsa, A.V.; Minkin, V.I. Photo- and Thermochromic Spiranes. 31. Photochromic Cationic Spiropyrans with a Pyridinium Fragment in the Aliphatic Side Chain. Chem. Heterocycl. Compd. 2008, 44, 1229–1237. [Google Scholar] [CrossRef]
- Aldoshin, S.M.; Sanina, N.A.; Nadtochenko, V.A.; Yur’eva, E.A.; Minkin, V.I.; Voloshin, N.A.; Ikorskii, V.N.; Ovcharenko, V.I. Specific Spectral Properties of a Photochromic Ferromagnetic (C25H23N3O3Cl)CrMn(C2O4)3·H2O. Russ. Chem. Bull. 2007, 56, 1095–1102. [Google Scholar] [CrossRef]
- Chernyshev, A.V.; Voloshin, N.A.; Sletova, V.V.; Metelitsa, A.V. Chromogenic Spiroindolinobenzopyrans of the Oxadiazole Series with Photodriven Ionochromic Properties. Dokl. Chem. 2018, 481, 145–149. [Google Scholar] [CrossRef]
- Chernyshev, A.V.; Voloshin, N.A.; Solov’eva, E.V.; Gaeva, E.B.; Zubavichus, Y.V.; Lazarenko, V.A.; Vlasenko, V.G.; Khrustalev, V.N.; Metelitsa, A.V. Ion-Depended Photochromism of Oxadiazole Containing Spiropyrans. J. Photochem. Photobiol. Chem. 2019, 378, 201–210. [Google Scholar] [CrossRef]
- Voloshin, N.A.; Gaeva, E.B.; Chernyshev, A.V.; Metelitsa, A.V.; Minkin, V.I. Spiropyrans and Spirooxazines 5. Synthesis of Photochromic 8-(4,5-Diphenyl-1,3-oxazol-2-yl)-Substituted Spiro[indoline-benzopyrans]. Russ. Chem. Bull. 2009, 58, 156–161. [Google Scholar] [CrossRef]
- Solov’eva, E.V.; Chernyshev, A.V.; Voloshin, N.A.; Metelitsa, A.V.; Minkin, V.I. Photo- and Thermochromic Spirans. 38. New (1-Alkyl-4,5-diphenyl)imidazolyl-Substituted Spirobenzopyrans. Chem. Heterocycl. Compd. 2013, 48, 1533–1538. [Google Scholar] [CrossRef]
- Rostovtseva, I.A.; Solov’eva, E.V.; Chernyshev, A.V.; Voloshin, N.A.; Trofimova, N.S.; Metelitsa, A.V. Photo- and Thermochromic Spiropyrans 43. Spectral Kinetic Study of New Benzoxazolyl-Substituted Spirobenzopyrans. Chem. Heterocycl. Compd. 2015, 51, 223–228. [Google Scholar] [CrossRef]
- Chernyshev, A.V.; Metelitsa, A.V.; Rostovtseva, I.A.; Voloshin, N.A.; Solov’eva, E.V.; Gaeva, E.B.; Minkin, V.I. Chromogenic Systems Based on 8-(1,3-Benzoxazol-2-yl) Substituted Spirobenzopyrans Undergoing Ion Modulated Photochromic Rearrangements. J. Photochem. Photobiol. Chem. 2018, 360, 174–180. [Google Scholar] [CrossRef]
- Chernyshev, A.V.; Guda, A.A.; Cannizzo, A.; Solov’eva, E.V.; Voloshin, N.A.; Rusalev, Y.; Shapovalov, V.V.; Smolentsev, G.; Soldatov, A.V.; Metelitsa, A.V. Operando XAS and UV–Vis Characterization of the Photodynamic Spiropyran–Zinc Complexes. J. Phys. Chem. B 2019, 123, 1324–1331. [Google Scholar] [CrossRef]
- Rostovtseva, I.A.; Voloshin, N.A.; Solov’eva, E.V.; Shepelenko, K.E.; Chernyshev, A.V.; Metelitsa, A.V.; Minkin, V.I. Photo- and Ionochromism of Benzoxazolyl-Substituted Spirobenzopyrans. Dokl. Chem. 2018, 478, 26–30. [Google Scholar] [CrossRef]
- Yagi, S.; Nakamura, S.; Watanabe, D.; Nakazumi, H. Colorimetric Sensing of Metal Ions by Bis(Spiropyran) Podands: Towards Naked-Eye Detection of Alkaline Earth Metal Ions. Dyes Pigments 2009, 80, 98–105. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, D.; Zhu, D. Logic Control of the Fluorescence of a New Dyad, Spiropyran-Perylene Diimide-Spiropyran, with Light, Ferric Ion, and Proton: Construction of a New Three-Input “AND” Logic Gate. Adv. Mater. 2004, 16, 125–130. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, D.; Zhu, D. Photocontrolled Electron Transfer Reaction between a New Dyad, Tetrathiafulvalene−Photochromic Spiropyran, and Ferric Ion. J. Phys. Chem. B 2004, 108, 212–217. [Google Scholar] [CrossRef]
- Gao, M.; Shen, B.; Zhou, J.; Kapre, R.; Louie, A.Y.; Shaw, J.T. Synthesis and Comparative Evaluation of Photoswitchable Magnetic Resonance Imaging Contrast Agents. ACS Omega 2020, 5, 14759–14766. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Osborne, E.A.; Louie, A.Y. Synthesis and Characterization of a Redox- and Light-Sensitive MRI Contrast Agent. Tetrahedron 2009, 65, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.J.; Sammes, P.G. Substituent Tuning of Photoreversible Lithium Chelating Agents. Dyes Pigments 1995, 28, 317–325. [Google Scholar] [CrossRef]
- Stubing, D.B.; Heng, S.; Abell, A.D. Crowned Spiropyran Fluoroionophores with a Carboxyl Moiety for the Selective Detection of Lithium Ions. Org. Biomol. Chem. 2016, 14, 3752–3757. [Google Scholar] [CrossRef]
- Heng, S.; Nguyen, M.-C.; Kostecki, R.; Monro, T.M.; Abell, A.D. Nanoliter-Scale, Regenerable Ion Sensor: Sensing with a Surface Functionalized Microstructured Optical Fibre. RSC Adv. 2013, 3, 8308. [Google Scholar] [CrossRef]
- Roxburgh, C.J.; Sammes, P.G. Synthesis of Some New Substituted Photochromic N,N′-Bis(spiro [1-benzopyran-2,2′-indolyl])diazacrown Systems with Substituent Control over Ion Chelation. Eur. J. Org. Chem. 2006, 2006, 1050–1056. [Google Scholar] [CrossRef]
- Roxburgh, C.J.; Sammes, P.G.; Abdullah, A. Photoreversible Zn2+ Ion Transportation Across an Interface Using Ion-Chelating Substituted Photochromic 3,3′-Indolospirobenzopyrans: Steric and Electronic Controlling Effects. Eur. J. Inorg. Chem. 2008, 2008, 4951–4960. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Chen, L.; Yang, J.; Wang, Z.; Yang, F.; He, L.; Li, X. Engineering Highly Efficient Li+ Responsive Nanochannels via Host–Guest Interaction and Photochemistry Regulation. J. Colloid Interface Sci. 2022, 615, 674–684. [Google Scholar] [CrossRef]
- Trevino, K.M.; Tautges, B.K.; Kapre, R.; Franco, F.C., Jr.; Or, V.W.; Balmond, E.I.; Shaw, J.T.; Garcia, J.; Louie, A.Y. Highly Sensitive and Selective Spiropyran-Based Sensor for Copper(II) Quantification. ACS Omega 2021, 6, 10776–10789. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; McDevitt, C.A.; Stubing, D.B.; Whittall, J.J.; Thompson, J.G.; Engler, T.K.; Abell, A.D.; Monro, T.M. Microstructured Optical Fibers and Live Cells: A Water-Soluble, Photochromic Zinc Sensor. Biomacromolecules 2013, 14, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- Stubing, D.B.; Heng, S.; Monro, T.M.; Abell, A.D. A Comparative Study of the Fluorescence and Photostability of Common Photoswitches in Microstructured Optical Fibre. Sens. Actuators B Chem. 2017, 239, 474–480. [Google Scholar] [CrossRef]
- Jukes, R.T.F.; Bozic, B.; Hartl, F.; Belser, P.; De Cola, L. Synthesis, Photophysical, Photochemical, and Redox Properties of Nitrospiropyrans Substituted with Ru or Os Tris(bipyridine) Complexes. Inorg. Chem. 2006, 45, 8326–8341. [Google Scholar] [CrossRef]
- Yang, H.; Du, S.; Ye, Z.; Wang, X.; Yan, Z.; Lian, C.; Bao, C.; Zhu, L. A System for Artificial Light Signal Transduction via Molecular Translocation in a Lipid Membrane. Chem. Sci. 2022, 13, 2487–2494. [Google Scholar] [CrossRef]
- Colaco, R.; Shree, S.; Siebert, L.; Appiah, C.; Dowds, M.; Schultzke, S.; Adelung, R.; Staubitz, A. Mechanochromic Microfibers Stabilized by Polymer Blending. ACS Appl. Polym. Mater. 2020, 2, 2055–2062. [Google Scholar] [CrossRef]
- Potisek, S.L.; Davis, D.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanophore-Linked Addition Polymers. J. Am. Chem. Soc. 2007, 129, 13808–13809. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R.; et al. Force-Induced Activation of Covalent Bonds in Mechanoresponsive Polymeric Materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef]
- Lee, C.K.; Davis, D.A.; White, S.R.; Moore, J.S.; Sottos, N.R.; Braun, P.V. Force-Induced Redistribution of a Chemical Equilibrium. J. Am. Chem. Soc. 2010, 132, 16107–16111. [Google Scholar] [CrossRef]
- O’Bryan, G.; Wong, B.M.; McElhanon, J.R. Stress Sensing in Polycaprolactone Films via an Embedded Photochromic Compound. ACS Appl. Mater. Interfaces 2010, 2, 1594–1600. [Google Scholar] [CrossRef]
- Gossweiler, G.R.; Kouznetsova, T.B.; Craig, S.L. Force-Rate Characterization of Two Spiropyran-Based Molecular Force Probes. J. Am. Chem. Soc. 2015, 137, 6148–6151. [Google Scholar] [CrossRef]
- Gossweiler, G.R.; Brown, C.L.; Hewage, G.B.; Sapiro-Gheiler, E.; Trautman, W.J.; Welshofer, G.W.; Craig, S.L. Mechanochemically Active Soft Robots. ACS Appl. Mater. Interfaces 2015, 7, 22431–22435. [Google Scholar] [CrossRef]
- Sommer, M.; Komber, H. Spiropyran Main-Chain Conjugated Polymers. Macromol. Rapid Commun. 2013, 34, 57–62. [Google Scholar] [CrossRef]
- Komber, H.; Müllers, S.; Lombeck, F.; Held, A.; Walter, M.; Sommer, M. Soluble and Stable Alternating Main-Chain Merocyanine Copolymers through Quantitative Spiropyran–Merocyanine Conversion. Polym. Chem. 2014, 5, 443–453. [Google Scholar] [CrossRef]
- Kempe, F.; Brügner, O.; Buchheit, H.; Momm, S.N.; Riehle, F.; Hameury, S.; Walter, M.; Sommer, M. A Simply Synthesized, Tough Polyarylene with Transient Mechanochromic Response. Angew. Chem. Int. Ed. 2018, 57, 997–1000. [Google Scholar] [CrossRef]
- Vidavsky, Y.; Yang, S.J.; Abel, B.A.; Agami, I.; Diesendruck, C.E.; Coates, G.W.; Silberstein, M.N. Enabling Room-Temperature Mechanochromic Activation in a Glassy Polymer: Synthesis and Characterization of Spiropyran Polycarbonate. J. Am. Chem. Soc. 2019, 141, 10060–10067. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ng, M.-K. Synthesis of a Photochromic Conjugated Polymer Incorporating Spirobenzopyran in the Backbone. Synthesis 2006, 2006, 3075–3079. [Google Scholar] [CrossRef]
- Kadokawa, J.; Tanaka, Y.; Yamashita, Y.; Yamamoto, K. Synthesis of Poly(Spiropyran)s by Polycondensation and Their Photoisomerization Behaviors. Eur. Polym. J. 2012, 48, 549–559. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, Y.; Zhang, D.; Zhang, G.; Fan, Q.; Sun, S.; Zhu, D. Light-Triggered Self-Assembly of a Spiropyran-Functionalized Dendron into Nano-/Micrometer-Sized Particles and Photoresponsive Organogel with Switchable Fluorescence. Adv. Funct. Mater. 2010, 20, 36–42. [Google Scholar] [CrossRef]
- Cabrera, I.; Krongauz, V. Physical Crosslinking of Mesomorphic Polymers Containing Spiropyran Groups. Macromolecules 1987, 20, 2713–2717. [Google Scholar] [CrossRef]
- Cabrera, I.; Krongauz, V.; Ringsdorf, H. Photo- and Thermo-Chromic Liquid Crystal Polymers with Spiropyran Groups. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1988, 155, 221–230. [Google Scholar] [CrossRef]
- Shragina, L.; Buchholtz, F.; Yitzchaik, S.; Krongauz, V. Searching for Photochromic Liquid Crystals Spironaphthoxazine Substituted with a Mesogenic Group. Liq. Cryst. 1990, 7, 643–655. [Google Scholar] [CrossRef]
- Malinčík, J.; Kohout, M.; Svoboda, J.; Stulov, S.; Pociecha, D.; Böhmová, Z.; Novotná, V. Photochromic Spiropyran-Based Liquid Crystals. J. Mol. Liq. 2022, 346, 117842. [Google Scholar] [CrossRef]
- Tan, B.-H.; Yoshio, M.; Kato, T. Induction of Columnar and Smectic Phases for Spiropyran Derivatives: Effects of Acidichromism and Photochromism. Chem.-Asian J. 2008, 3, 534–541. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B. Stimuli-Chromism of Photoswitches in Smart Polymers: Recent Advances and Applications as Chemosensors. Prog. Polym. Sci. 2019, 98, 101149. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Zhou, Y.-N.; Zhu, S. Let Spiropyran Help Polymers Feel Force! Prog. Polym. Sci. 2018, 79, 26–39. [Google Scholar] [CrossRef]
- Shibaev, V.; Bobrovsky, A.; Boiko, N. Photoactive Liquid Crystalline Polymer Systems with Light-Controllable Structure and Optical Properties. Prog. Polym. Sci. 2003, 28, 729–836. [Google Scholar] [CrossRef]
- Qiu, W.; Scofield, J.M.P.; Gurr, P.A.; Qiao, G.G. Mechanochromophore-Linked Polymeric Materials with Visible Color Changes. Macromol. Rapid Commun. 2022, 43, 2100866. [Google Scholar] [CrossRef]
- Sommer, M. Substituent Effects Control Spiropyran–Merocyanine Equilibria and Mechanochromic Utility. Macromol. Rapid Commun. 2021, 42, 2000597. [Google Scholar] [CrossRef]
- Caruso, M.M.; Davis, D.A.; Shen, Q.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009, 109, 5755–5798. [Google Scholar] [CrossRef]
- Lee, C.K.; Diesendruck, C.E.; Lu, E.; Pickett, A.N.; May, P.A.; Moore, J.S.; Braun, P.V. Solvent Swelling Activation of a Mechanophore in a Polymer Network. Macromolecules 2014, 47, 2690–2694. [Google Scholar] [CrossRef]
- Cao, Z. Highly Stretchable Tough Elastomers Crosslinked by Spiropyran Mechanophores for Strain-Induced Colorimetric Sensing. Macromol. Chem. Phys. 2020, 221, 2000190. [Google Scholar] [CrossRef]
- Garcia, J.; Addison, J.B.; Liu, S.Z.; Lu, S.; Faulkner, A.L.; Hodur, B.M.; Balmond, E.I.; Or, V.W.; Yun, J.H.; Trevino, K.; et al. Antioxidant Sensing by Spiropyrans: Substituent Effects and NMR Spectroscopic Studies. J. Phys. Chem. B 2019, 123, 6799–6809. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Dzhemilev, U.M. Acid-Base Isomerization of Hybrid Molecules Based on Fullerene C60 and Spiropyrans. Mendeleev Commun. 2019, 29, 229–231. [Google Scholar] [CrossRef]
- Raic-Malic, S.; Tomaskovic, L.; Mrvos-Sermek, D.; Prugovecki, B.; Cetina, M.; Grdiša, M.; Pavelić, K.; Mannschreck, A.; Balzarini, J.; De Clercq, E.; et al. Spirobipyridopyrans, Spirobinaphthopyrans, Indolinospiropyridopyrans, Indolinospironaphthopyrans and Indolinospironaphtho-1,4-oxazines: Synthesis, Study of X-Ray Crystal Structure, Antitumoral and Antiviral Evaluation. Bioorg. Med. Chem. 2004, 12, 1037–1045. [Google Scholar] [CrossRef]
- Li, Z.; Wan, S.; Shi, W.; Wei, M.; Yin, M.; Yang, W.; Evans, D.G.; Duan, X. A Light-Triggered Switch Based on Spiropyran/Layered Double Hydroxide Ultrathin Films. J. Phys. Chem. C 2015, 119, 7428–7435. [Google Scholar] [CrossRef]
- Voloshin, N.A.; Volbushko, N.V.; Voloshina, E.N.; Shelepin, N.E.; Minkin, V.I. New Formyl-Substituted Spiropyrans of the Indoline Series. Chem. Heterocycl. Compd. 1996, 32, 1427–1428. [Google Scholar] [CrossRef]
- Pantsyrnyi, V.I.; Gal’bershtam, M.A. Preparation of Spiropyrans from 3-Formylsalicylic Acid Derivatives. Chem. Heterocycl. Compd. 1971, 7, 134–135. [Google Scholar] [CrossRef]
- Seki, T.; Ichimura, K. Formation of Head-to-Tail and Side-by-Side Aggregates of Photochromic Spiropyrans in Bilayer Membrane. J. Phys. Chem. 1990, 94, 3769–3775. [Google Scholar] [CrossRef]
- Walkey, M.C.; Byrne, L.T.; Piggott, M.J.; Low, P.J.; Koutsantonis, G.A. Enhanced Bi-Stability in a Ruthenium Alkynyl Spiropyran Complex. Dalton Trans. 2015, 44, 8812–8815. [Google Scholar] [CrossRef] [PubMed]
- Walkey, M.C.; Peiris, C.R.; Ciampi, S.; Aragonès, A.; Domínguez-Espíndola, R.B.; Jago, D.; Pulbrook, T.; Skelton, B.W.; Sobolev, A.N.; Díez Pérez, I.; et al. Chemically and Mechanically Controlled Single-Molecule Switches Using Spiropyrans. ACS Appl. Mater. Interfaces 2019, 11, 36886–36894. [Google Scholar] [CrossRef] [PubMed]
- Yagupolskii, L.M.; Pasenok, S.V.; Gal’bershtam, M.A.; Bobyleva, G.K.; Popov, V.I.; Kondratenko, N.V. Synthesis and Photochromic Properties of Perfluoroalkyl and Trifluoromethylsulphonyl Substituted Indoline Spirochromenes. Dyes Pigments 1981, 2, 205–213. [Google Scholar] [CrossRef]
- Roxburgh, C.J.; Sammes, P.G.; Abdullah, A. Steric and Electronically Biasing Substituent Effects on the Photoreversibility of Novel, 3′-, 5′- and 3-Substituted Indolospirobenzopyrans. Thermal Evaluation Using 1H NMR Spectroscopy and Overhauser Enhancement Studies. Dyes Pigments 2009, 83, 31–50. [Google Scholar] [CrossRef]
- Gal’bershtam, M.A.; Mikheeva, L.M.; Samoilova, N.P. Electronic Absorption Spectra of Merocyanine Forms of Spiropyrans. Chem. Heterocycl. Compd. 1972, 8, 1386–1389. [Google Scholar] [CrossRef]
- Gordin, M.B.; Gal’bershtam, M.A. Existence of a Photodecolorization Reaction of the Colored Form of Spiropyrans under the Influence of Initiating UV Irradiation. Chem. Heterocycl. Compd. 1971, 7, 1089–1091. [Google Scholar] [CrossRef]
- Cullum, B.M.; Mobley, J.; Bogard, J.S.; Moscovitch, M.; Phillips, G.W.; Vo-Dinh, T. Three-Dimensional Optical Random Access Memory Materials for Use as Radiation Dosimeters. Anal. Chem. 2000, 72, 5612–5617. [Google Scholar] [CrossRef]
- Parthenopoulos, D.A.; Rentzepis, P.M. Three-Dimensional Optical Storage Memory. Science 1989, 245, 843–845. [Google Scholar] [CrossRef]
- Moscovitch, M.; Phillips, G.W. Radiation Dosimetry Using Three-Dimensional Optical Random Access Memories. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 184, 207–218. [Google Scholar] [CrossRef]
- Cho, Y.J.; Rho, K.Y.; Rok Keum, S.; Kim, S.H.; Yoon, C.M. Synthesis of Conjugated Spiropyran Dyes via Palladium-Catalyzed Coupling Reaction. Synth. Commun. 1999, 29, 2061–2068. [Google Scholar] [CrossRef]
- Steen, J.D.; Duijnstee, D.R.; Sardjan, A.S.; Martinelli, J.; Kortekaas, L.; Jacquemin, D.; Browne, W.R. Electrochemical Ring-Opening and -Closing of a Spiropyran. J. Phys. Chem. A 2021, 125, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Deng, Y.; Tang, Z.; Zheng, N.; Zhang, C.; Xie, C.; Wu, Z. One-Pot Synthesis of Spiropyrans. Asian J. Org. Chem. 2019, 8, 1866–1869. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Yang, Y.; Wang, W.; Qu, L.; Chen, C.; Liu, D.; Zhang, D.; Zhu, D. Photo-pH Dually Modulated Fluorescence Switch Based on DNA Spatial Nanodevice. J. Phys. Chem. B 2008, 112, 6893–6896. [Google Scholar] [CrossRef] [PubMed]
- Tomasulo, M.; Kaanumal, S.L.; Sortino, S.; Raymo, F.M. Synthesis and Properties of Benzophenone−Spiropyran and Naphthalene−Spiropyran Conjugates. J. Org. Chem. 2007, 72, 595–605. [Google Scholar] [CrossRef]
- Balmond, E.I.; Tautges, B.K.; Faulkner, A.L.; Or, V.W.; Hodur, B.M.; Shaw, J.T.; Louie, A.Y. Comparative Evaluation of Substituent Effect on the Photochromic Properties of Spiropyrans and Spirooxazines. J. Org. Chem. 2016, 81, 8744–8758. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, D.; Zhang, G.; Zhu, D. Monomolecular Logic: “Half-Adder” Based on Multistate/Multifunctional Photochromic Spiropyrans. J. Phys. Chem. B 2004, 108, 11942–11945. [Google Scholar] [CrossRef]
- Chernyshev, A.V.; Chernov’yants, M.S.; Voloshina, E.N.; Voloshin, N.A. To Estimation of PKa for Spiropyrans of the Indoline Series. Russ. J. Gen. Chem. 2002, 72, 1468–1472. [Google Scholar] [CrossRef]
- Khairutdinov, R.F.; Hurst, J.K. Photocontrol of Ion Permeation through Bilayer Membranes Using an Amphiphilic Spiropyran. Langmuir 2001, 17, 6881–6886. [Google Scholar] [CrossRef]
- Collier, C.P.; Ma, B.; Wong, E.W.; Heath, J.R.; Wudl, F. Photochemical Response of Electronically Reconfigurable Molecule-Based Switching Tunnel Junctions. ChemPhysChem 2002, 3, 458. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Salikhov, R.B.; Khuzin, A.A.; Safargalin, I.N.; Mullagaliev, I.N.; Venidiktova, O.V.; Valova, T.M.; Barachevsky, V.A.; Dzhemilev, U.M. Optically Controlled Field Effect Transistors Based on Photochromic Spiropyran and Fullerene C60 Films. Mendeleev Commun. 2019, 29, 160–162. [Google Scholar] [CrossRef]
- da Costa Duarte, R.; da Silveira Santos, F.; Bercini de Araújo, B.; Cercena, R.; Brondani, D.; Zapp, E.; Fernando Bruno Gonçalves, P.; Severo Rodembusch, F.; Gonçalves Dal-Bó, A. Synthesis of a 5-Carboxy Indole-Based Spiropyran Fluorophore: Thermal, Electrochemical, Photophysical and Bovine Serum Albumin Interaction Investigations. Chemosensors 2020, 8, 31. [Google Scholar] [CrossRef]
- Kollarigowda, R.H.; Braun, P.V. Direct and Divergent Solid-Phase Synthesis of Azobenzene and Spiropyran Derivatives. J. Org. Chem. 2021, 86, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Grady, M.E.; Birrenkott, C.M.; May, P.A.; White, S.R.; Moore, J.S.; Sottos, N.R. Localization of Spiropyran Activation. Langmuir 2020, 36, 5847–5854. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Chernyavina, V.V.; Lukyanov, B.S.; Malay, V.I.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Lukyanova, M.B.; Metelitsa, A.V.; Aldoshin, S.M. Synthesis and Study of New Photochromic Spiropyrans Modified with Carboxylic and Aldehyde Substituents. J. Mol. Struct. 2019, 1196, 409–416. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Chernyshev, A.V.; Butova, V.V.; Lukyanov, B.S.; Radchenko, E.A.; Mukhanov, E.L.; Soldatov, A.V. Structure and Photochromic Properties of New Spiropyrans of Indoline Series Containing Free Carboxylic Groups. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2020, 14, 534–539. [Google Scholar] [CrossRef]
- Kubo, N.; Yoshii, T.; Kobayashi, N.; Ikeda, K.; Hirohashi, R. Photoresponse in Ionic Conductivity for Poly[(ω-hydroxy)oligo(oxyethylene) methacrylate-co-butyl methacrylate] Doped with Spiropyran Derivative and LiClO4. Polym. Adv. Technol. 1993, 4, 119–123. [Google Scholar] [CrossRef]
- Sepehr, Z.; Nasr-Isfahani, H.; Mahdavian, A.R.; Amin, A.H. Synthesis, Characterization, and UV–Visible Study of Some New Photochromic Formyl-Containing 1′,3′,3′-Trimethylspiro[chromene-2,2′-indoline] Derivatives. J. Iran. Chem. Soc. 2021, 18, 3061–3067. [Google Scholar] [CrossRef]
- Arsenov, V.D.; Manakova, I.V.; Przhiyalgovskaya, N.M.; Suvorov, N.N. Thermal and Photostability of 5′,7′-Dialkoxy-5,6′-dinitro-1,3,3-trimethylspiro(indolin-2,2′-[2H]chromenes). Chem. Heterocycl. Compd. 1987, 23, 961–964. [Google Scholar] [CrossRef]
- Darwish, N.; Aragonès, A.C.; Darwish, T.; Ciampi, S.; Díez-Pérez, I. Multi-Responsive Photo- and Chemo-Electrical Single-Molecule Switches. Nano Lett. 2014, 14, 7064–7070. [Google Scholar] [CrossRef]
- Patel, K.; Castillo-Muzquiz, A.; Biewer, M.C. Studying Monolayer/Solvent Interactions with a Photochromic Compound in a Self-Assembled Monolayer. Tetrahedron Lett. 2002, 43, 5933–5935. [Google Scholar] [CrossRef]
- McArdle, C.B.; Blair, H.; Barraud, A.; Ruaudel-Teixier, A. Positive and Negative Photochromism in Thin Organic Langmuir-Blodgett Films. Thin Solid Films 1983, 99, 181–188. [Google Scholar] [CrossRef]
- Blair, H.S.; McArdle, C.B. Photoresponsive Polymeric Systems: 1. Mixed Monomolecular Films of Some Synthetic Polymers and Indolinospiropyrans. Polymer 1984, 25, 999–1005. [Google Scholar] [CrossRef]
- Mardaleishvili, I.R.; Kol’tsova, L.S.; Zaichenko, N.L.; Sister, V.G.; Shienok, A.I.; Levin, P.P.; Tatikolov, A.S. Spectral and Luminescent Properties of Hydroxyazomethines of Indoline Spiropyrans. High Energy Chem. 2012, 46, 160–165. [Google Scholar] [CrossRef]
- Shienok, A.I.; Ivashina, N.A.; Kol’tsova, L.S.; Zaichenko, N.L. One-Step Synthesis of Novel Photochemically Bifunctional Compounds of the Spiropyran Class. Russ. Chem. Bull. 2008, 57, 2437–2439. [Google Scholar] [CrossRef]
- Mardaleishvili, I.R.; Kol’tsova, L.S.; Zaichenko, N.L.; Shienok, A.I.; Levin, P.P.; Tatikolov, A.S. Peculiarities of Photochromism and Luminescence of Dinitrosubstituted Hydroxyazomethinespiropyran. High Energy Chem. 2015, 49, 30–35. [Google Scholar] [CrossRef]
- Andrews, M.C.; Peng, P.; Rajput, A.; Cozzolino, A.F. Modulation of the Carboxamidine Redox Potential through Photoinduced Spiropyran or Fulgimide Isomerisation. Photochem. Photobiol. Sci. 2018, 17, 432–441. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Zhao, Z.; Gildner, M.B.; Shoulders, B.A.; Velasquez, T.L.; Blumenthal, M.O.; Wang, L.; Li, X.; Hudnall, T.W.; Betancourt, T.; et al. Synthesis, Optical Properties and in Vitro Cell Viability of Novel Spiropyrans and Their Photostationary States. Tetrahedron 2021, 80, 131854. [Google Scholar] [CrossRef]
- Lyubimov, A.V.; Zaichenko, N.L.; Marevtsev, V.S.; Cherkashin, M.I. Indolinospiropyrans with Two Polymerizable Groups. Bull. Acad. Sci. USSR Div. Chem. Sci. 1982, 31, 585–587. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Tkachev, V.V.; Mukhanov, E.L.; Lukyanov, B.S.; Chernyshev, A.V.; Lukyanova, M.B.; Aldoshin, S.M. Studies of Structure and Photochromic Properties of Spiropyrans Based on 4,6-Diformyl-2-methylresorcinol. Russ. Chem. Bull. 2015, 64, 672–676. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Pugachev, A.D.; Kozlenko, A.S.; Rostovtseva, I.A.; Makarova, N.I.; Borodkin, G.S.; El-Sewify, I.M.; Metelitsa, A.V.; Lukyanov, B.S. Methyl 5′-Chloro-8-formyl-5-hydroxy-1′,3′,3′-trimethyl-spiro-[chromene-2,2′-indoline]-6-carboxylate. Molbank 2023, 2023, M1549. [Google Scholar] [CrossRef]
- Zaichenko, N.L.; Shienok, A.I.; Kol’tsova, L.S.; Lyubimov, A.V.; Mardaleishvili, I.R.; Retivov, V.M.; Belus’, S.K.; Ait, A.O. Synthesis of Triarylimidazole Hybrid Compound with Switchable Luminescence. Russ. J. Gen. Chem. 2016, 86, 1022–1027. [Google Scholar] [CrossRef]
- Chernyshev, A.V.; Voloshin, N.A.; Rostovtseva, I.A.; Solov’eva, E.V.; Gaeva, E.B.; Metelitsa, A.V. Polychromogenic Molecular Systems Based on Photo- and Ionochromic Spiropyrans. Dyes Pigments 2018, 158, 506–516. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Lukyanova, M.B.; Lukyanov, B.S.; Ozhogin, I.V.; Kozlenko, A.S.; Tkachev, V.V.; Chepurnoi, P.B.; Shilov, G.V.; Minkin, V.I.; Aldosin, S.M. Replacement of the Hetarene Moiety of Molecule in the Synthesis of Indoline Spiropyran with Cationic Fragment. Dokl. Chem. 2020, 492, 76–83. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Kozlenko, A.S.; Luk’yanova, M.B.; Luk’yanov, B.S.; Tkachev, V.V.; Shilov, G.V.; Demidov, O.P.; Minkin, V.I.; Aldoshin, S.M. One-Pot Synthesis and Structure Study of a New Indoline Spiropyran with Cationic Substituent. Dokl. Chem. 2019, 488, 252–256. [Google Scholar] [CrossRef]
- Mao, S.; Benninger, R.K.P.; Yan, Y.; Petchprayoon, C.; Jackson, D.; Easley, C.J.; Piston, D.W.; Marriott, G. Optical Lock-In Detection of FRET Using Synthetic and Genetically Encoded Optical Switches. Biophys. J. 2008, 94, 4515–4524. [Google Scholar] [CrossRef]
- Heng, S.; Nguyen, M.-C.; Kostecki, R.; Monro, T.M.; Abell, A.D. Nanoliter-Scale, Regenerable Ion Sensor: Sensing with Surface Functionalized Microstructured Optical Fiber. Proc. SPIE 2013, 8774, 877403. [Google Scholar] [CrossRef]
- Querol, M.; Bozic, B.; Salluce, N.; Belser, P. Synthesis, Metal Complex Formation, and Switching Properties of Spiropyrans Linked to Chelating Sites. Polyhedron 2003, 22, 655–664. [Google Scholar] [CrossRef]
- Venidiktova, O.V.; Gorelik, A.M.; Barachevsky, V.A.; Khuzin, A.A.; Tuktarov, A.R. Study of the Possibility of Functionalization of Graphene Oxide Nanoplatelets with Photochromic Chromene and Spiropyrans in Solutions. Russ. J. Gen. Chem. 2021, 91, 2640–2646. [Google Scholar] [CrossRef]
- Tamaki, T.; Minode, K.; Numai, Y.; Ohto, T.; Yamada, R.; Masai, H.; Tada, H.; Terao, J. Mechanical Switching of Current–Voltage Characteristics in Spiropyran Single-Molecule Junctions. Nanoscale 2020, 12, 7527–7531. [Google Scholar] [CrossRef]
- Nagashima, S.; Murata, M.; Nishihara, H. A Ferrocenylspiropyran That Functions as a Molecular Photomemory with Controllable Depth. Angew. Chem. Int. Ed. 2006, 45, 4298–4301. [Google Scholar] [CrossRef]
- Takase, M.; Inouye, M. Synthesis and Photochromic Properties of Ferrocene-Modified Bis(spirobenzopyran)s. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. Mol. Cryst. Liq. Cryst. 2000, 344, 313–318. [Google Scholar] [CrossRef]
- Ma, Y.; Niu, C.; Wen, Y.; Li, G.; Wang, J.; Li, H.; Du, S.; Yang, L.; Gao, H.; Song, Y. Stable and Reversible Optoelectrical Dual-Mode Data Storage Based on a Ferrocenylspiropyran Molecule. Appl. Phys. Lett. 2009, 95, 183307. [Google Scholar] [CrossRef]
- Miyashita, A.; Iwamoto, A.; Kuwayama, T.; Shitara, H.; Aoki, Y.; Hirano, M.; Nohira, H. Synthesis and Diastereoselective Structural Change of a Photochromic Transition Metal Complex, (H6-Spirobenzopyran)(tricarbonyl)chromium. Chem. Lett. 1997, 26, 965–966. [Google Scholar] [CrossRef]
- He, T.; Lu, M.; Yao, J.; He, J.; Chen, B.; Di Spigna, N.H.; Nackashi, D.P.; Franzon, P.D.; Tour, J.M. Reversible Modulation of Conductance in Silicon Devices via UV/Visible-Light Irradiation. Adv. Mater. 2008, 20, 4541–4546. [Google Scholar] [CrossRef]
- Wen, G.; Yan, J.; Zhou, Y.; Zhang, D.; Mao, L.; Zhu, D. Photomodulation of the Electrode Potential of a Photochromic Spiropyran-Modified Au Electrode in the Presence of Zn2+: A New Molecular Switch Based on the Electronic Transduction of the Optical Signals. Chem. Commun. 2006, 3016. [Google Scholar] [CrossRef]
- Reifarth, M.; Bekir, M.; Bapolisi, A.M.; Titov, E.; Nußhardt, F.; Nowaczyk, J.; Grigoriev, D.; Sharma, A.; Saalfrank, P.; Santer, S.; et al. A Dual PH- and Light-Responsive Spiropyran-Based Surfactant: Investigations on Its Switching Behavior and Remote Control over Emulsion Stability. Angew. Chem. Int. Ed. 2022, 61, e202114687:1–e202114687:9. [Google Scholar] [CrossRef]
- Cabrera, I.; Krongauz, V.; Ringsdorf, H. Photo- and Thermochromic Liquid Crystal Polysiloxanes. Angew. Chem. Int. Ed. Engl. 1987, 26, 1178–1180. [Google Scholar] [CrossRef]























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodonov, A.A.; Belikov, N.E.; Lukin, A.Y.; Laptev, A.V.; Barachevsky, V.A.; Varfolomeev, S.D.; Demina, O.V. 5′-Substituted Indoline Spiropyrans: Synthesis and Applications. Colorants 2023, 2, 264-404. https://doi.org/10.3390/colorants2020017
Khodonov AA, Belikov NE, Lukin AY, Laptev AV, Barachevsky VA, Varfolomeev SD, Demina OV. 5′-Substituted Indoline Spiropyrans: Synthesis and Applications. Colorants. 2023; 2(2):264-404. https://doi.org/10.3390/colorants2020017
Chicago/Turabian StyleKhodonov, Andrey A., Nikolay E. Belikov, Alexey Yu. Lukin, Alexey V. Laptev, Valery A. Barachevsky, Sergey D. Varfolomeev, and Olga V. Demina. 2023. "5′-Substituted Indoline Spiropyrans: Synthesis and Applications" Colorants 2, no. 2: 264-404. https://doi.org/10.3390/colorants2020017
APA StyleKhodonov, A. A., Belikov, N. E., Lukin, A. Y., Laptev, A. V., Barachevsky, V. A., Varfolomeev, S. D., & Demina, O. V. (2023). 5′-Substituted Indoline Spiropyrans: Synthesis and Applications. Colorants, 2(2), 264-404. https://doi.org/10.3390/colorants2020017