Targeting Penicillium expansum GMC Oxidoreductase with High Affinity Small Molecules for Reducing Patulin Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Protein Sequences and Crystallized Structures
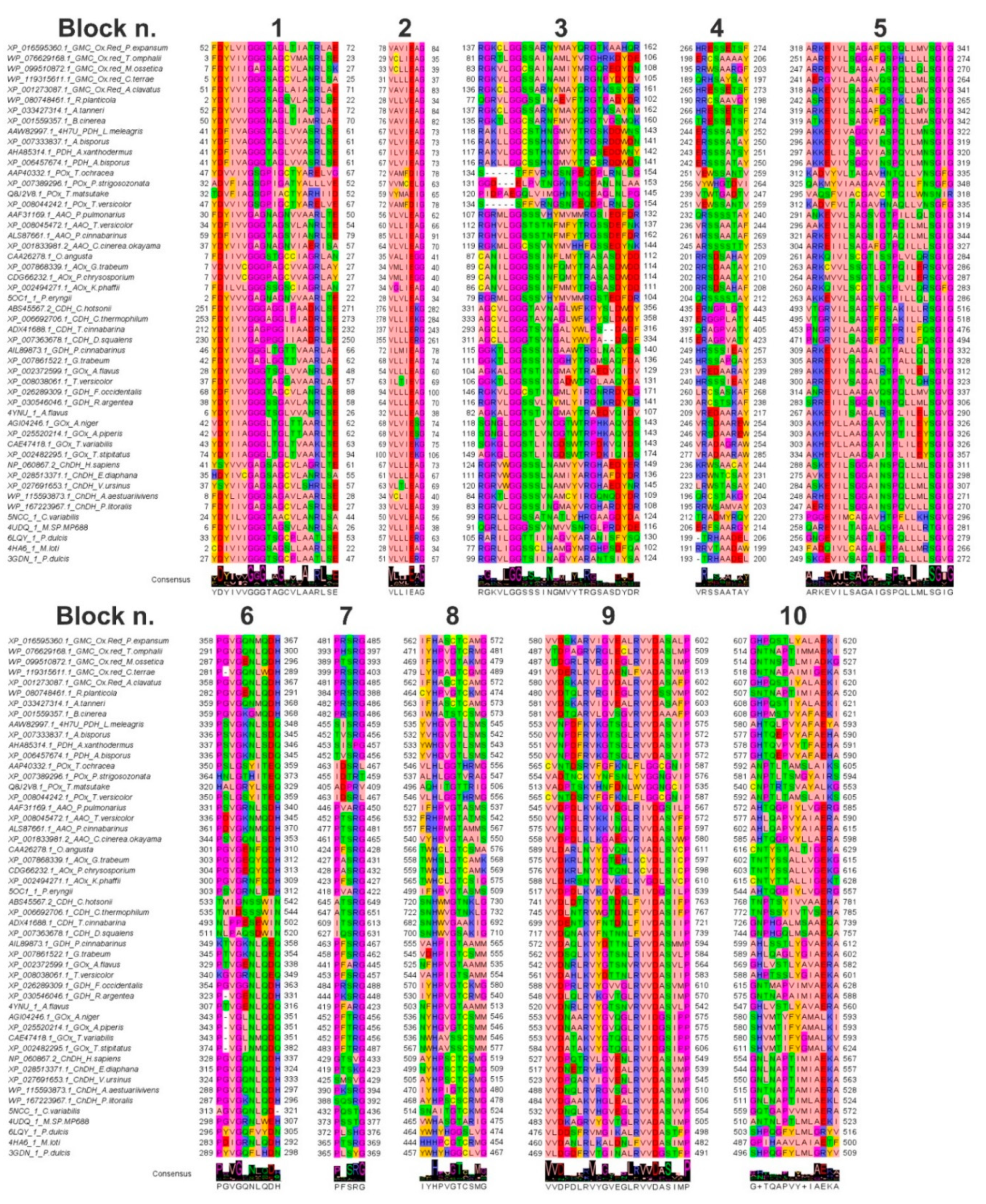
2.2. Multiple Sequence Alignment (MSA) and Phylogenetic Analyses
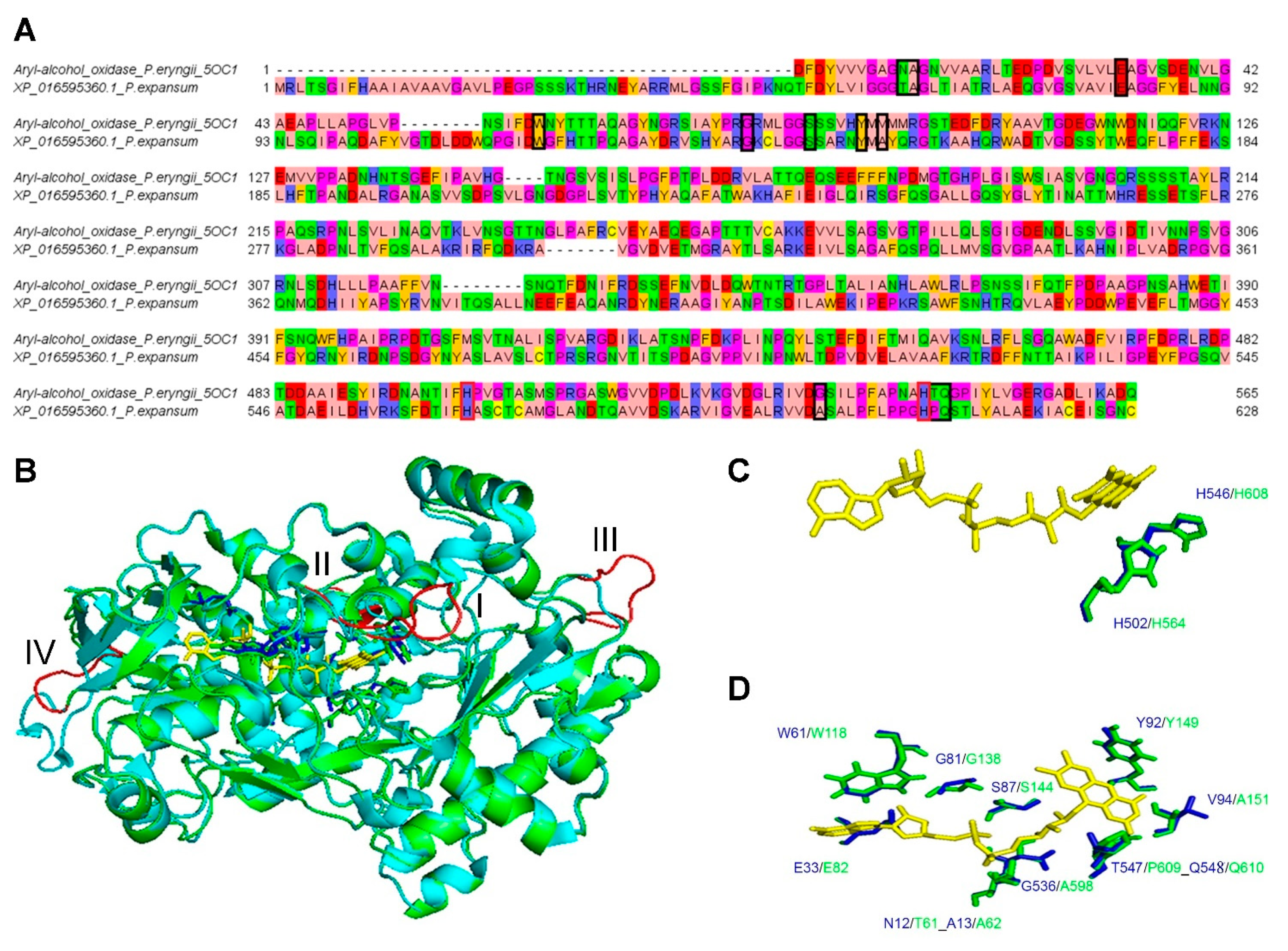
2.3. Comparative 3D Modelling
2.4. Virtual Screening of a Chemical Library
2.5. Quantum Mechanical Calculations
2.6. Preparation of the Solutions of the Predicted Highly Affinity Ligands
2.7. Preparation of Patulin Solution
2.8. Preparation of Penicillium Expansum Conidial Suspension
2.9. In Vitro Assays for Monitoring the Effects of the Predicted GMC Oxidoreductase High Affinity Ligands on Fungal Growth and Patulin Production
2.10. In Vivo Assays for Monitoring the Effects of the Predicted GMC Oxidoreductase High Affinity Ligands on Disease Incidence/Severity and Patulin Production
2.10.1. Sample Preparation
2.10.2. In Vivo Assays
2.11. Patulin Analysis
2.11.1. In Vitro Assays
2.11.2. In Vivo Assays
2.11.3. Analytical Conditions
2.12. Data Statistical Analysis
3. Results
3.1. Evolutionary Relationships among the Sampled GMC Oxidoreductase Homologous Sequences
3.2. Features of the Sampled GMC Oxidoreductase Homologous Protein Sequences
3.3. Comparative 3D Modelling of P. expansum GMC Oxidoreductase
3.4. Virtual Screening
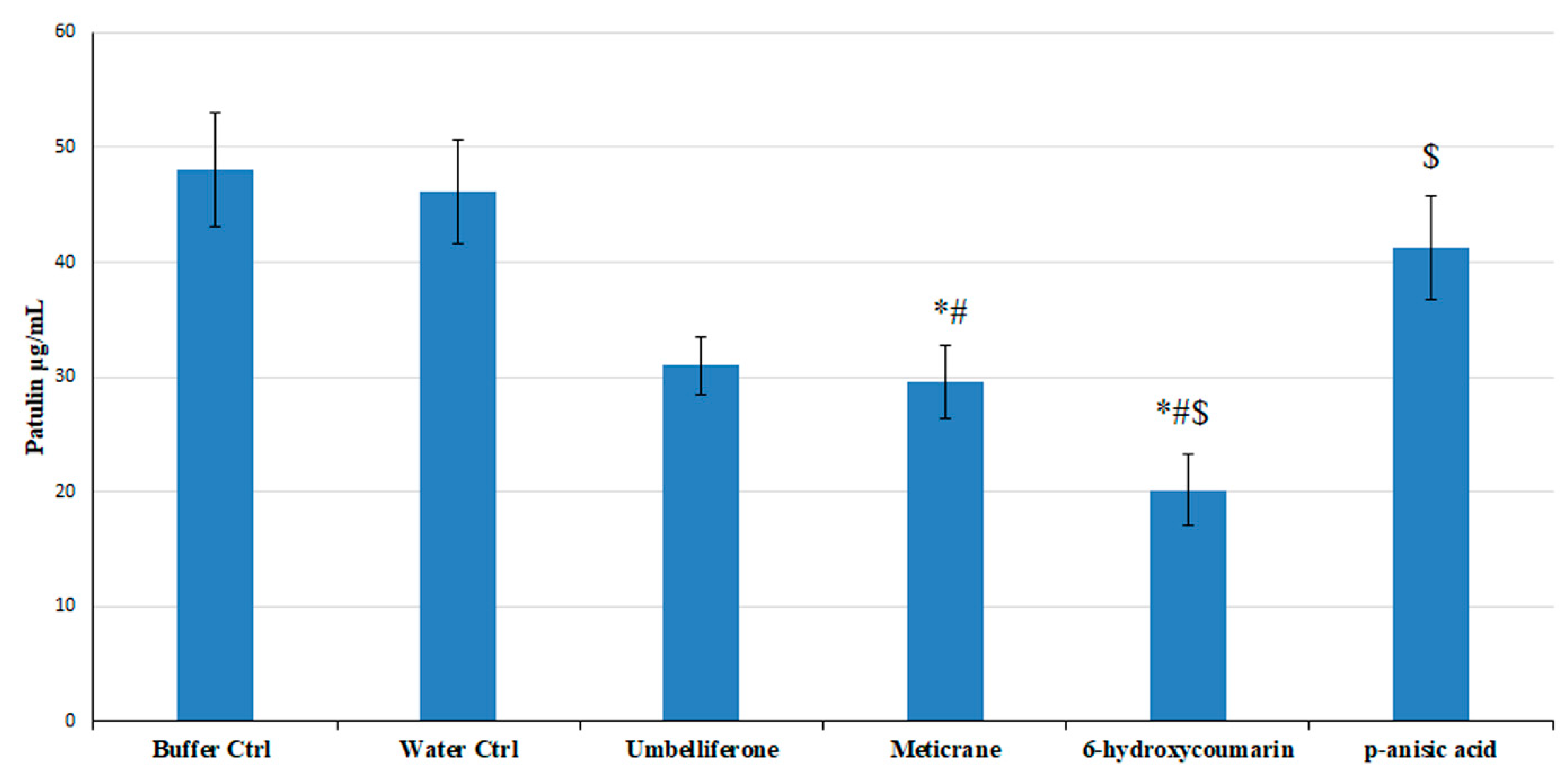
3.5. Effect of the Predicted High Affinity Ligands on Colony Growth and Patulin Accumulation in In Vitro Assays
3.6. The Effect of the Predicted High Affinity Ligands in In Vivo Assays
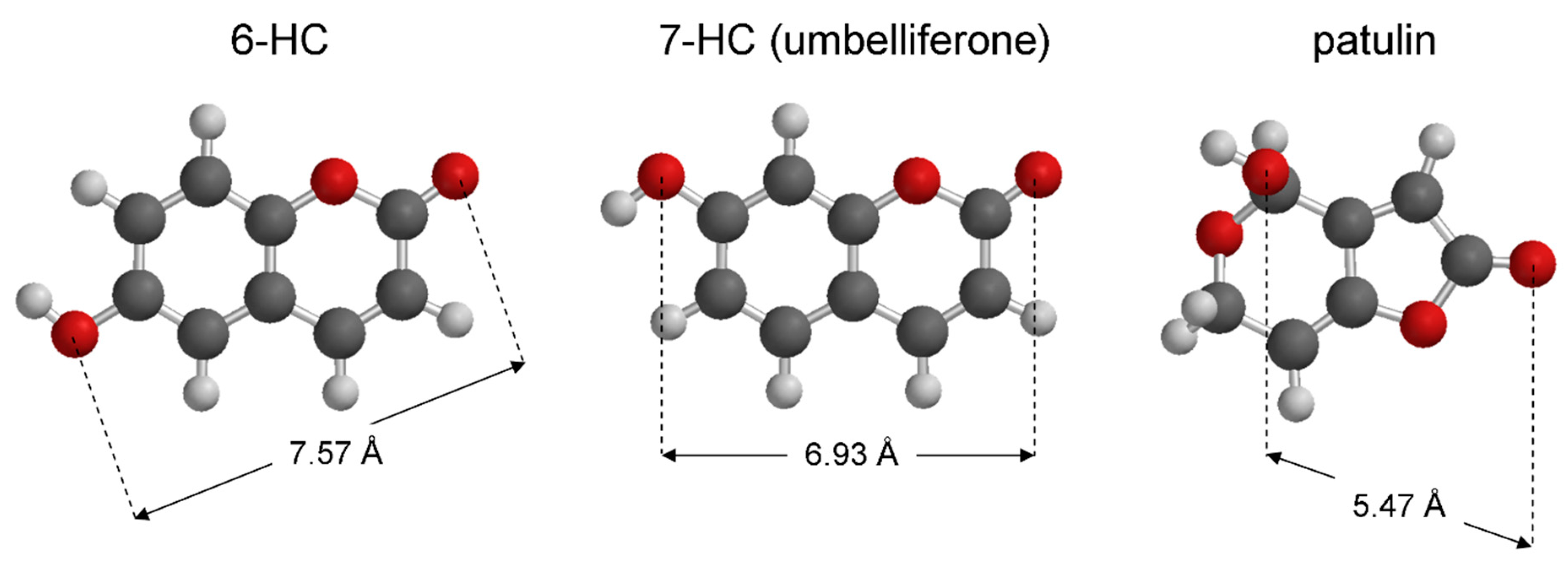
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallberg, B.M.; Bergfors, T.; Bäckbro, K.; Pettersson, G.; Henriksson, G.; Divne, C. A new scaffold for binding haem in the cytochrome domain of the extracellular flavocytochrome cellobiose dehydrogenase. Structure 2000, 8, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Bottoms, C.A.; Smith, P.E.; Tanner, J.J. A structurally conserved water molecule in Rossmann dinucleotide-binding domains. Protein Sci. 2002, 11, 2125–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sützl, L.; Foley, G.; Gillam, E.M.J.; Bodén, M.; Haltrich, D. The GMC superfamily of oxidoreductases revisited: Analysis and evolution of fungal GMC oxidoreductases. Biotechnol. Biofuels 2019, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Tragni, V.; Cotugno, P.; De Grassi, A.; Massari, F.; Di Ronzo, F.; Aresta, A.M.; Zambonin, C.; Sanzani, S.M.; Ippolito, A.; Pierri, C.L. Targeting mitochondrial metabolite transporters in Penicillium expansum for reducing patulin production. Plant Physiol. Biochem. 2020, 158, 158–181. [Google Scholar] [CrossRef]
- Tannous, J.; Snini, S.P.; El Khoury, R.; Canlet, C.; Pinton, P.; Lippi, Y.; Alassane-Kpembi, I.; Gauthier, T.; El Khoury, A.; Atoui, A.; et al. Patulin transformation products and last intermediates in its biosynthetic pathway, E- and Z-ascladiol, are not toxic to human cells. Arch. Toxicol. 2017, 91, 2455–2467. [Google Scholar] [CrossRef]
- Maidana, L.; Gerez, J.R.; El Khoury, R.; Pinho, F.; Puel, O.; Oswald, I.P.; Bracarense, A.P.F.R.L. Effects of patulin and ascladiol on porcine intestinal mucosa: An ex vivo approach. Food Chem. Toxicol. 2016, 98, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Chen, Y.; Zong, Y.; Shang, Y.; Zhang, Z.; Xu, X.; Wang, X.; Long, M.; Tian, S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef]
- Ballester, A.R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lázaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; González-Candelas, L.; Gabaldón, T. Genome, transcriptome, and functional analyses of Penicillium expansum provide new insights into secondary metabolism and pathogenicity. Mol. Plant Microbe Interact. 2015, 28, 232–248. [Google Scholar] [CrossRef] [Green Version]
- Trisolini, L.; Gambacorta, N.; Gorgoglione, R.; Montaruli, M.; Laera, L.; Colella, F.; Volpicella, M.; De Grassi, A.; Pierri, C.L. FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. J. Clin. Med. 2019, 8, 2117. [Google Scholar] [CrossRef] [Green Version]
- Bossis, F.; De Grassi, A.; Palese, L.L.; Pierri, C.L. Prediction of high- and low-affinity quinol-analogue-binding sites in the aa3 and bo3 terminal oxidases from Bacillus subtilis and Escherichia coli. Biochem. J. 2014, 461, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Regalado, A.; Pierri, C.L.; Bitetto, M.; Laera, V.L.; Pimentel, C.; Francisco, R.; Passarinho, J.; Chaves, M.M.; Agrimi, G. Characterization of mitochondrial dicarboxylate/tricarboxylate transporters from grape berries. Planta 2013, 237, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; De Leonardis, F.; Paradies, E.; De Grassi, A.; Pierri, C.L.; Parisi, G.; Marobbio, C.M.T.; Lasorsa, F.M.; Muto, L.; Capobianco, L.; et al. Biochemical characterization of a new mitochondrial transporter of dephosphocoenzyme A in Drosophila melanogaster. Biochim Biophys Acta 2017, 1858, 137–146. [Google Scholar] [CrossRef]
- Lunetti, P.; Cappello, A.R.; Marsano, R.M.; Pierri, C.L.; Carrisi, C.; Martello, E.; Caggese, C.; Dolce, V.; Capobianco, L. Mitochondrial glutamate carriers from Drosophila melanogaster: Biochemical, evolutionary and modeling studies. Biochim Biophys Acta 2013, 1827, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Pierri, C.L.; Parisi, G.; Porcelli, V. Computational approaches for protein function prediction: A combined strategy from multiple sequence alignment to molecular docking-based virtual screening. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1695–1712. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onofrio, A.; Parisi, G.; Punzi, G.; Todisco, S.; Di Noia, M.A.; Bossis, F.; Turi, A.; De Grassi, A.; Pierri, C.L. Distance-dependent hydrophobic-hydrophobic contacts in protein folding simulations. Phys Chem Chem Phys 2014, 16, 18907–18917. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED protein structure prediction server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Pierri, C.L.; De Grassi, A.; Turi, A. Lattices for ab initio protein structure prediction. Proteins 2008, 73, 351–361. [Google Scholar] [CrossRef]
- Carro, J.; Martínez-Júlvez, M.; Medina, M.; Martínez, A.T.; Ferreira, P. Protein dynamics promote hydride tunnelling in substrate oxidation by aryl-alcohol oxidase. Phys. Chem. Chem. Phys. 2017, 19, 28666–28675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobley, A.; Sadowski, M.I.; Jones, D.T. pGenTHREADER and pDomTHREADER: New methods for improved protein fold recognition and superfamily discrimination. Bioinformatics 2009, 25, 1761–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guex, N.; Diemand, A.; Peitsch, M.C. Protein modelling for all. Trends Biochem. Sci 1999, 24, 364–367. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Ordog, R. PyDeT, a PyMOL plug-in for visualizing geometric concepts around proteins. Bioinformation 2008, 2, 346–347. [Google Scholar] [CrossRef] [Green Version]
- Cavener, D.R. GMC oxidoreductases. A newly defined family of homologous proteins with diverse catalytic activities. J. Mol. Biol. 1992, 223, 811–814. [Google Scholar] [CrossRef]
- Kiess, M.; Hecht, H.-J.; Kalisz, H.M. Glucose oxidase from Penicillium amagasakiense Primary structure and comparison. Eur. J. Biochem. 1998, 252, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Sorigué, D.; Légeret, B.; Cuiné, S.; Blangy, S.; Moulin, S.; Billon, E.; Richaud, P.; Brugière, S.; Couté, Y.; Nurizzo, D.; et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 2017, 357, 903–907. [Google Scholar] [CrossRef] [Green Version]
- Carro, J.; Amengual-Rigo, P.; Sancho, F.; Medina, M.; Guallar, V.; Ferreira, P.; Martínez, A.T. Multiple implications of an active site phenylalanine in the catalysis of aryl-alcohol oxidase. Sci. Rep. 2018, 25, 8121–8132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugo, A.N.; Kobayashi, J.; Yamasaki, T.; Mikami, B.; Ohnishi, K.; Yoshikane, Y.; Yagi, T. Crystal structure of pyridoxine 4-oxidase from Mesorhizobium loti. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 953–963. [Google Scholar] [CrossRef]
- Dreveny, I.; Andryushkova, A.S.; Glieder, A.; Gruber, K.; Kratky, C. Substrate binding in the FAD-dependent hydroxynitrile lyase from almond provides insight into the mechanism of cyanohydrin formation and explains the absence of dehydrogenation activity. Biochemistry 2009, 48, 3370–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.C.; Li, F.L.; Lin, Z.; Lin, G.Q.; Hong, R.; Yu, H.L.; Xu, J.H. Structure-Guided Tuning of a Hydroxynitrile Lyase to Accept Rigid Pharmaco Aldehydes. ACS Catal. 2020, 10, 5757–5763. [Google Scholar] [CrossRef]
- Pierri, C.L.C.L.; Bossis, F.; Punzi, G.; De Grassi, A.; Cetrone, M.; Parisi, G.; Tricarico, D. Molecular modeling of antibodies for the treatment of TNFα-related immunological diseases. Pharmacol. Res. Perspect. 2016, 4, e00197. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, I.; Tragni, V.; Busto, F.; De Grassi, A.; Pierri, C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: From conformational changes to novel neutralizing antibodies. Cell. Mol. Life Sci. 2020, 1–22. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Pierri, C.L.; Palmieri, F.; Klingenberg, M. The switching mechanism of the mitochondrial ADP/ATP carrier explored by free-energy landscapes. Biochim Biophys Acta. 2016, 1857, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of AutoDock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 20, 2781–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todisco, S.; Di Noia, M.A.; Onofrio, A.; Parisi, G.; Punzi, G.; Redavid, G.; De Grassi, A.; Pierri, C.L. Identification of new highly selective inhibitors of the human ADP/ATP carriers by molecular docking and in vitro transport assays. Biochem. Pharmacol. 2016, 100, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Dard, L.; Sarlak, S.; Mahfouf, W.; Blanchard, W.; Rousseau, B.; Izotte, J.; Claverol, S.; Lacombe, D.; Rezvani, H.R.; et al. Targeting human lung adenocarcinoma with a suppressor of mitochondrial superoxide production. Antioxid. Redox Signal. 2020, 33. [Google Scholar] [CrossRef]
- Tavani, C.; Bianchi, L.; De Palma, A.; Passeri, G.I.; Punzi, G.; Pierri, C.L.; Lovece, A.; Cavalluzzi, M.M.; Franchini, C.; Lentini, G.; et al. Nitro-substituted tetrahydroindolizines and homologs: Design, kinetics, and mechanism of α-glucosidase inhibition. Bioorg. Med. Chem. Lett. 2017, 27, 3980–3986. [Google Scholar] [CrossRef]
- Carocci, A.; Roselli, M.; Budriesi, R.; Micucci, M.; Desaphy, J.F.; Altamura, C.; Cavalluzzi, M.M.; Toma, M.; Passeri, G.I.; Milani, G.; et al. Synthesis and Evaluation of Voltage-Gated Sodium Channel Blocking Pyrroline Derivatives Endowed with Both Antiarrhythmic and Antioxidant Activities; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; ISBN 0805442731. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 36, 3098. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.R. Basis Set Selection for Molecular Calculations. Chem. Rev. 1988, 86, 681–696. [Google Scholar] [CrossRef]
- Sanzani, S.M.; De Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res. Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittl, R.; Sygmund, C.; Halada, P.; Volc, J.; Divne, C.; Haltrich, D.; Peterbauer, C.K. Molecular cloning of three pyranose dehydrogenase-encoding genes from Agaricus meleagris and analysis of their expression by real-time RT-PCR. Curr. Genet. 2008, 53, 117–127. [Google Scholar] [CrossRef]
- Varela, E.; Böckle, B.; Romero, A.; Martínez, A.T.; Martínez, M.J. Biochemical characterization, cDNA cloning and protein crystallization of aryl-alcohol oxidase from Pleurotus pulmonarius. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1476, 129–138. [Google Scholar] [CrossRef]
- Linke, D.; Lehnert, N.; Nimtz, M.; Berger, R.G. An alcohol oxidase of Phanerochaete chrysosporium with a distinct glycerol oxidase activity. Enzyme Microb. Technol. 2014, 61–62, 7–12. [Google Scholar] [CrossRef]
- Pulci, V.; D’Ovidio, R.; Petruccioli, M.; Federici, F. The glucose oxidase of Penicillium variabile P16: Gene cloning, sequencing and expression. Lett. Appl. Microbiol. 2004, 38, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Rosenstein, R.; Futter-Bryniok, D.; Götz, F. The choline-converting pathway in Staphylococcus xylosus C2A: Genetic and physiological characterization. J. Bacteriol. 1999, 181, 2273–2278. [Google Scholar] [CrossRef] [Green Version]
- Piumi, F.; Levasseur, A.; Navarro, D.; Zhou, S.; Mathieu, Y.; Ropartz, D.; Ludwig, R.; Faulds, C.B.; Record, E. A novel glucose dehydrogenase from the white-rot fungus Pycnoporus cinnabarinus: Production in Aspergillus niger and physicochemical characterization of the recombinant enzyme. Appl. Microbiol. Biotechnol. 2014, 98, 10105–10118. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, N.D.; Khaldi, N.; Joardar, V.S.; Maiti, R.; Amedeo, P.; Anderson, M.J.; Crabtree, J.; Silva, J.C.; Badger, J.H.; Albarraq, A.; et al. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 2008, 4, e1000046. [Google Scholar] [CrossRef] [PubMed]
- Bey, M.; Berrin, J.G.; Poidevin, L.; Sigoillot, J.C. Heterologous expression of Pycnoporus cinnabarinus cellobiose dehydrogenase in Pichia pastoris and involvement in saccharification processes. Microb. Cell Fact. 2011, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takakura, Y.; Kuwata, S. Purification, characterization, and molecular cloning of a pyranose oxidase from the fruit body of the basidiomycete, Tricholoma matsutake. Biosci. Biotechnol. Biochem. 2003, 67, 2598–2607. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Sakai, G.; Mori, K.; Kojima, K.; Kamitori, S.; Sode, K. Structural analysis of fungus-derived FAD glucose dehydrogenase. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dijkman, W.P.; Binda, C.; Fraaije, M.W.; Mattevi, A. Structure-based enzyme tailoring of 5-hydroxymethylfurfural oxidase. ACS Catal. 2015, 5, 1833–1839. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxins in the Food Chain and Human Health Implications. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444639523. [Google Scholar]
- European Union Off J Eur Union, L364/5-25. Eur. Community Brussels 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 5 December 2020).
- Fallanaj, F.; Sanzani, S.M.; Youssef, K.; Zavanella, C.; Salerno, M.G.; Ippolito, A. A new perspective in controlling postharvest citrus rots: The use of electrolyzed water. Acta Hortic. 2015, 1065, 1599. [Google Scholar] [CrossRef]
- Gonçalves, A.; Gkrillas, A.; Dorne, J.L.; Dall’Asta, C.; Palumbo, R.; Lima, N.; Battilani, P.; Venâncio, A.; Giorni, P. Pre- and Postharvest Strategies to Minimize Mycotoxin Contamination in the Rice Food Chain. Compr. Rev. Food Sci. Food Saf. 2019, 18, 441–454. [Google Scholar] [CrossRef]
- Khamis, Y.; Hashim, A.F.; Margarita, R.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Fungicidal efficacy of chemically-produced copper nanoparticles against Penicillium digitatum and Fusarium solani on citrus fruit. Philipp. Agric. Sci. 2017, 100, 69–78. [Google Scholar]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive Review of Patulin Control Methods in Foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- Garganese, F.; Schena, L.; Siciliano, I.; Prigigallo, M.I.; Spadaro, D.; De Grassi, A.; Ippolito, A.; Sanzani, S.M. Characterization of citrus-associated Alternaria species in Mediterranean areas. PLoS ONE 2016, 11, e0163255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Singh, N.; Ansari, K.M. Toxicological effects of patulin mycotoxin on the mammalian system: An overview. Toxicol. Res. 2017, 6, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zong, Y.; Du, Z.; Chen, Y.; Zhang, Z.; Qin, G.; Zhao, W.; Tian, S. Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Mol. Plant Microbe Interact. 2015, 28, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Carrozzo, R.; Torraco, A.; Fiermonte, G.; Martinelli, D.; Di Nottia, M.; Rizza, T.; Vozza, A.; Verrigni, D.; Diodato, D.; Parisi, G.; et al. Riboflavin responsive mitochondrial myopathy is a new phenotype of dihydrolipoamide dehydrogenase deficiency. The chaperon-like effect of vitamin B2. Mitochondrion 2014, 18, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Corina Vlot, A.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Sagan, F.; Mitoraj, M.P. Origin of Remarkably Different Acidity of Hydroxycoumarins—Joint Experimental and Theoretical Studies. J. Phys. Chem. B 2017, 121, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.A.I.; de Felice, D.V.; Ianiri, G.; Pinedo-Rivilla, C.; De Curtis, F.; Castoria, R. Two rapid assays for screening of patulin biodegradation. Int. J. Environ. Sci. Technol. 2014, 11, 1387–1398. [Google Scholar] [CrossRef] [Green Version]
- Cavalluzzi, M.M.; Mangiatordi, G.F.; Nicolotti, O.; Lentini, G. Ligand efficiency metrics in drug discovery: The pros and cons from a practical perspective. Expert Opin. Drug Discov. 2017, 12, 1087–1104. [Google Scholar] [CrossRef] [PubMed]


| Rank | Chemical Code | Structure | Name/IUPAC | Binding Energy (kcal/mol) | ChEMBL/ChEBI/Zink | Medical Indication |
|---|---|---|---|---|---|---|
| 16 | D01605 |  | Meticrane | −8.5 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL1318341/ | Cardiovascular agent |
| 1454 | C12284 |  | Saccharin sodium anhydrous | −6.99 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL2219743/ | / |
| 3369 | ZINC175734 | 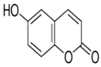 | 6-hydroxycoumarin | −5.88 | https://zinc.docking.org/substances/ZINC000001757340/ | / |
| 4394 | C02519 |  | p-anisic acid | −5.34 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL21932/ | / |
| 4702 | C16748 |  | Patulin | −5.18 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL294018/ | / |
| 5003 | C10788 |  | Ellagic acid | −4.99 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL6246/ | Antiproliferative and antioxidant agent |
| 5686 | C06044 |  | 4-Hydroxyphenylethanol (tyrosol) | −4.58 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL53566/ | / |
| 6154 | C01424 | 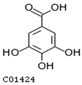 | Gallic acid | −4.29 | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL288114/ | / |
| Colony Growth (mm) ± SE | Patulin Accumulation ± SE (μg/mL) | |||
|---|---|---|---|---|
| 4 dpi | 8 dpi | 12 dpi | 12 dpi | |
| Water ctrl (H2O) | 23 ± 3 a | 54 ± 4 a | 78 ± 5 a | 1130 ± 40 d |
| Buffer ctrl (PBS + NaOH) | 25 ± 1 a | 55 ± 1.5 a | 80 ± 3 a | 1190 ± 70 d |
| Umbelliferone 0.01 mM | 24.5 ± 0.5 a | 54.8 ± 0.3 a | 79.3 ± 2.0 a | 890 ± 28 e |
| Umbelliferone 0.1 mM | 24.3 ± 0.3 a | 54.7 ± 1.0 a | 79.5 ± 2.1 a | 810 ± 30 e |
| Umbelliferone 0.5 mM | 24.6 ± 0.8 a | 54.6 ± 0.8 a | 79.8 ± 1.9 a | 743 ± 40 h |
| Umbelliferone 1 mM | 24.6 ± 0.7 a | 54.8 ± 1.2 a | 79.5 ± 2.2 a | 590 ± 60 g |
| Meticrane 0.01 mM | 22.5 ± 3 a | 53 ± 4 a | 79 ± 6 a | 650 ± 30 g |
| Meticrane 0.1 mM | 23 ± 4 a | 53 ± 5 a | 78 ± 5 a | 612.3 ± 26 g |
| Meticrane 0.5 mM | 23 ± 3 a | 53 ± 4 a | 78 ± 6 a | 640 ± 30 g |
| Meticrane 1 mM | 23 ± 4 a | 54 ± 4 a | 78 ± 6 a | 610 ± 40 g |
| Anisic acid 0.01 mM | 23 ± 3 a | 53 ± 4 a | 79 ± 3 a | 1100 ± 120 d |
| Anisic acid 0.1 mM | 23 ± 3 a | 53 ± 4 a | 79 ± 4 a | 910 ± 80 e |
| Anisic acid 0.5 mM | 24 ± 4 a | 54 ± 4 a | 77 ± 5 a | 840 ± 110 e |
| Anisic acid 1 mM | 22 ± 4 a | 54 ± 4 a | 78 ± 5 a | 760 ± 90 f |
| Saccharin 0.01 mM | 23 ± 4 a | 56 ± 3 a | 80 ± 3 a | 1180 ± 50 d |
| Saccharin 0.1 mM | 24 ± 4 a | 56 ± 3 a | 80 ± 4 a | 1310 ± 70 c |
| Saccharin 0.5 mM | 24.5 ± 2.0 a | 56 ± 4 a | 80 ± 5 a | 1510 ± 80 a |
| Saccharin 1 mM | 24 ± 3 a | 56 ± 3 a | 79 ± 3 a | 1420 ± 30 b |
| 6-hydroxycoumarin 0.01 mM | 21 ± 3 a | 53 ± 3 a | 78 ± 4 | 834 ± 70 e |
| 6-hydroxycoumarin 0.1 mM | 22 ± 3 a | 54 ± 4 a | 79 ± 4 a | 710 ± 50 f |
| 6-hydroxycoumarin 0.5 mM | 22.3 ± 2.0 a | 52 ± 4 a | 76 ± 3 a | 346 ± 25 h |
| 6-hydroxycoumarin 1 mM | 22 ± 3 a | 52 ± 3 a | 77 ± 4 a | 240 ± 30 i |
| Tyrosol 0.01 mM | 24 ± 3 a | 55 ± 4 a | 79 ± 5 a | 1120 ± 60 d |
| Tyrosol 0.1 mM | 24 ± 3 a | 56 ± 4 a | 79 ± 4 a | 950 ± 50 e |
| Tyrosol 0.5 mM | 24 ± 3 a | 55 ± 4 a | 78 ± 4 a | 690 ± 30 f |
| Tyrosol 1 mM | 24 ± 3 a | 56 ± 4 a | 78 ± 6 a | 600 ± 40 g |
| Gallic acid 0.01 mM | 21 ± 4 a | 54 ± 5 a | 77 ± 6 a | 900 ± 50 e |
| Gallic acid 0.1 mM | 22 ± 2 a | 55 ± 3 a | 81 ± 5 a | 1020 ± 70 d |
| Gallic acid 0.5 mM | 24 ± 4 a | 60 ± 5 a | 81 ± 5 a | 1390 ± 50 b |
| Gallic acid 1 mM | 24 ± 3 a | 59 ± 5 a | 79 ± 4 a | 1473 ±22 a |
| Ellagic acid 0.01 mM | 23.8 ± 2.7 a | 59.5 ± 4 a | 81 ± 5 a | 1190 ± 80 d |
| Ellagic acid 0.1 mM | 24 ± 3 a | 59.7 ± 2.5 a | 81.4 ± 2.4 a | 1320 ± 40 b |
| Ellagic acid 0.5 mM | 23.7 ± 1.9 a | 60 ± 3 a | 82 ± 3 a | 1280 ± 60 b |
| Ellagic acid 1 mM | 24 ± 3 a | 60 ± 3 a | 82 ± 4 a | 1300 ± 30 b |
| 2 dpi | 4 dpi | 8 dpi | ||||
|---|---|---|---|---|---|---|
| Infected Wounds ± SE (%) | Lesion Diameters ± SE (mm) | Infected Wounds ± SE (%) | Lesion Diameters ± SE (mm) | Infected Wounds ± SE (%) | Lesion Diameters ± SE (mm) | |
| Water ctrl (H2O) | 17 ± 3 a | 11 ± 1 a | 82 ± 2 a | 55 ± 2 a | 100 a | 105 ± 4 a |
| Buffer ctrl (PBS+NaOH) | 16 ± 3 a | 12.2 ± 1.4 a | 80 ± 3 a | 57 ± 3 a | 100 a | 112 ± 5 a |
| Umbelliferone | 9 ± 3 b | 9.1 ± 1.2 a | 75 ± 6 a | 53 ± 4 a | 100 a | 107 ± 4 a |
| Meticrane | 17 ± 2 a | 8 ± 1.5 b | 80.5 ± 2 a | 47 ± 2 b | 100 a | 83 ± 2 b |
| p-anisic acid | 5 ± 3 b | 3.5 ± 0.5 c | 30 ± 3 b | 42 ± 1 c | 78 ± 1 b | 76 ± 3 c |
| 6-hydroxycoumarin | 17 ± 1 a | 10 ± 0.5 a | 83 ± 3 a | 46 ± 3 b | 100 a | 86 ± 4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tragni, V.; Cotugno, P.; De Grassi, A.; Cavalluzzi, M.M.; Mincuzzi, A.; Lentini, G.; Sanzani, S.M.; Ippolito, A.; Pierri, C.L. Targeting Penicillium expansum GMC Oxidoreductase with High Affinity Small Molecules for Reducing Patulin Production. Biology 2021, 10, 21. https://doi.org/10.3390/biology10010021
Tragni V, Cotugno P, De Grassi A, Cavalluzzi MM, Mincuzzi A, Lentini G, Sanzani SM, Ippolito A, Pierri CL. Targeting Penicillium expansum GMC Oxidoreductase with High Affinity Small Molecules for Reducing Patulin Production. Biology. 2021; 10(1):21. https://doi.org/10.3390/biology10010021
Chicago/Turabian StyleTragni, Vincenzo, Pietro Cotugno, Anna De Grassi, Maria Maddalena Cavalluzzi, Annamaria Mincuzzi, Giovanni Lentini, Simona Marianna Sanzani, Antonio Ippolito, and Ciro Leonardo Pierri. 2021. "Targeting Penicillium expansum GMC Oxidoreductase with High Affinity Small Molecules for Reducing Patulin Production" Biology 10, no. 1: 21. https://doi.org/10.3390/biology10010021
APA StyleTragni, V., Cotugno, P., De Grassi, A., Cavalluzzi, M. M., Mincuzzi, A., Lentini, G., Sanzani, S. M., Ippolito, A., & Pierri, C. L. (2021). Targeting Penicillium expansum GMC Oxidoreductase with High Affinity Small Molecules for Reducing Patulin Production. Biology, 10(1), 21. https://doi.org/10.3390/biology10010021









