Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon
Abstract
:Simple Summary
Abstract
1. Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon
- Define as precisely as possible what the so-called dolomite phenomenon is (dolomite edaphism, dolomitophily)
- Delimit its global extent
- Establish its relationship with other edaphic phenomena in which Ca:Mg ratio is decisive with the aid of:
- 3a
- The edaphic characterization of the substrates in which it occurs and
- 3b
- The ionomic composition of plants that grow on them and the known mechanisms that explain homeostasis and the efficiency of Mg’s use in plants (as a major element in dolomite)
- Raise issues related to the conservation and genetic diversity of this type of flora, very rich in endemisms, many of which are local and threatened.
2. Definition of the Dolomite Phenomenon
- There are patches of exposed dolomite (or dolomitic marble or dolomitic limestone) bedrock, associated with thin and undeveloped soils, on which they become frequently disaggregated rock fragments providing a gravelly or even sandy appearance to their surface [19,20,21]. The pebble or even sandy appearance of these soils results from the fact that they occur in heavily tectonized areas [17]. This geological process is associated with another climate process that also contributes to generating such debris by the mechanical breakdown of the rock, promoting brecciation, disintegration, and the formation of dolomitic sands. Frost shattering [22] and the thermal expansion of these rocks at high temperatures [23] appear to be the dominant local weathering processes. These features lead to strong edaphical stress and prevent the surrounding vegetation, usually conifer forests, from succession and closure.
- As dolomite rocks are relatively slowly weathered, these soils are usually shallower, can thus hold less water and by way of consequence, have a lower capacity for nutrient supply. This feature is accentuated in south-facing, and frequently steep slopes and ridges which, together with the textural characteristics of the soil (from pebbly silt loam to coarse rubble) and high insolation, promotes erosion and drainage. At least in areas with a Mediterranean climate, summer soil moisture levels are extremely low. In general, glades are drought-prone, which offers conditions hostile to not adapted plants; consequently, they represent sharp and obvious discontinuities with the nearby vegetation [24].
- Dolomite soils show a soil exchange complex which is dominated by Ca and Mg, but they differ chemically from their non-carbonate counterparts primarily in that they have a higher pH, and lower Fe, P and K. Moreover, these soils are unlike limestone-derived ones in their highest proportion of Mg [10,17]. In general, these are nutrient-poor soils with low water retention capacity, which makes these communities unproductive in relation to the surrounding vegetation.
- Such a habitat calls for specialized adaptations, promoting endemism [25,26]. In these microclimate-soil areas, there are species which are very rare or absent in other places and, in many cases, have a marked relic character, likely due to a lack of severe competition. This is because they disproportionally contribute to regional plant diversity [27], especially in biodiversity hotspots [17,28,29,30].
- This type of communities is, almost always, easily identifiable due to the physiognomic features and the adaptations shown by the plants composing them. Such adaptations are a consequence of an adaptive convergence process. In most cases, these are open dwarf communities dominated by tough perennial herbs which form flat mats and cushions, frequently silvery white-haired. Some authors have highlighted their convergent adaptive appearance with the dune vegetation [31,32].
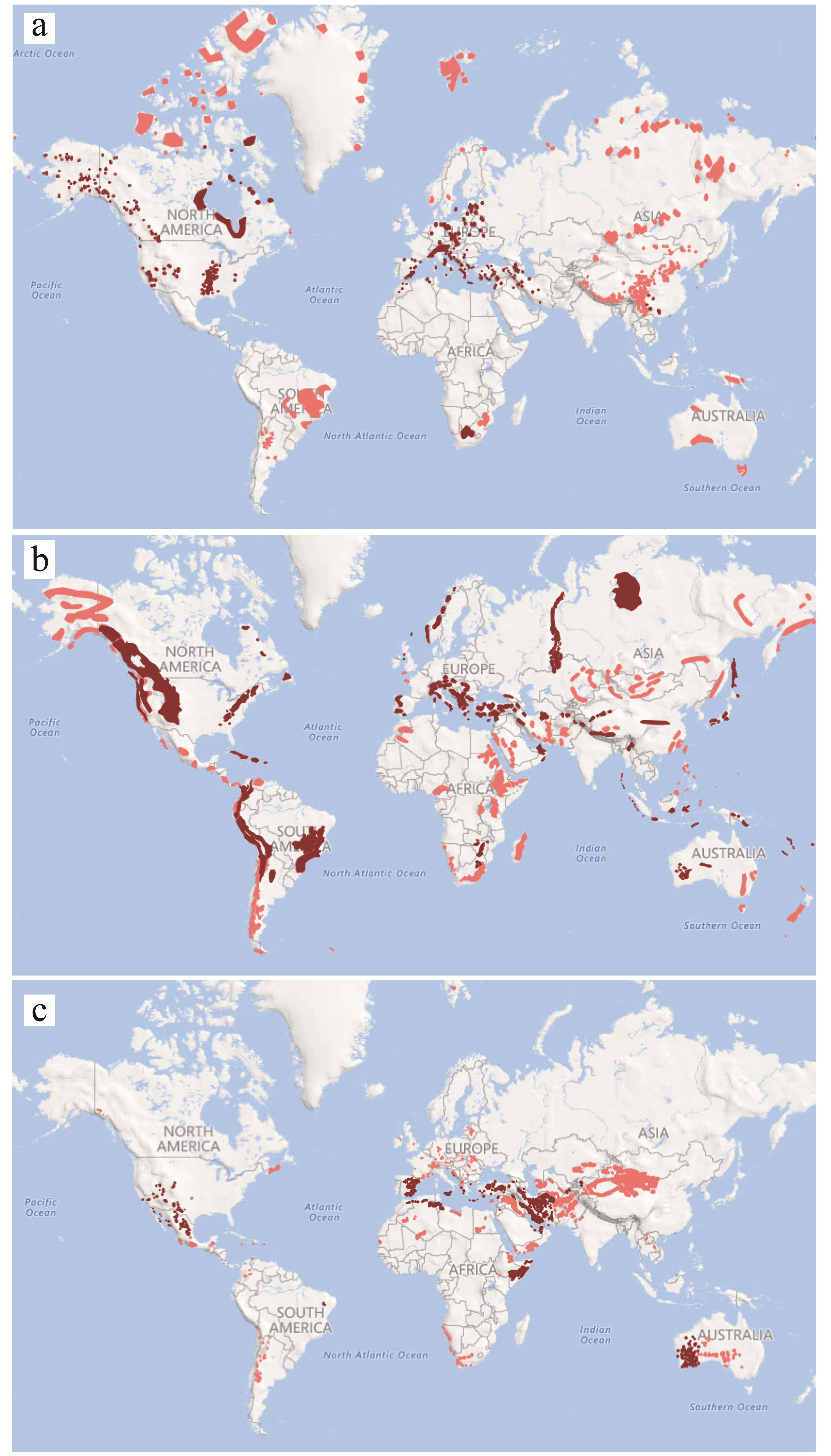
3. The Extent of the Dolomite Phenomenon
4. Dolomitophily and Other Ca:Mg Edaphisms
4.1. Dolomitic Soils
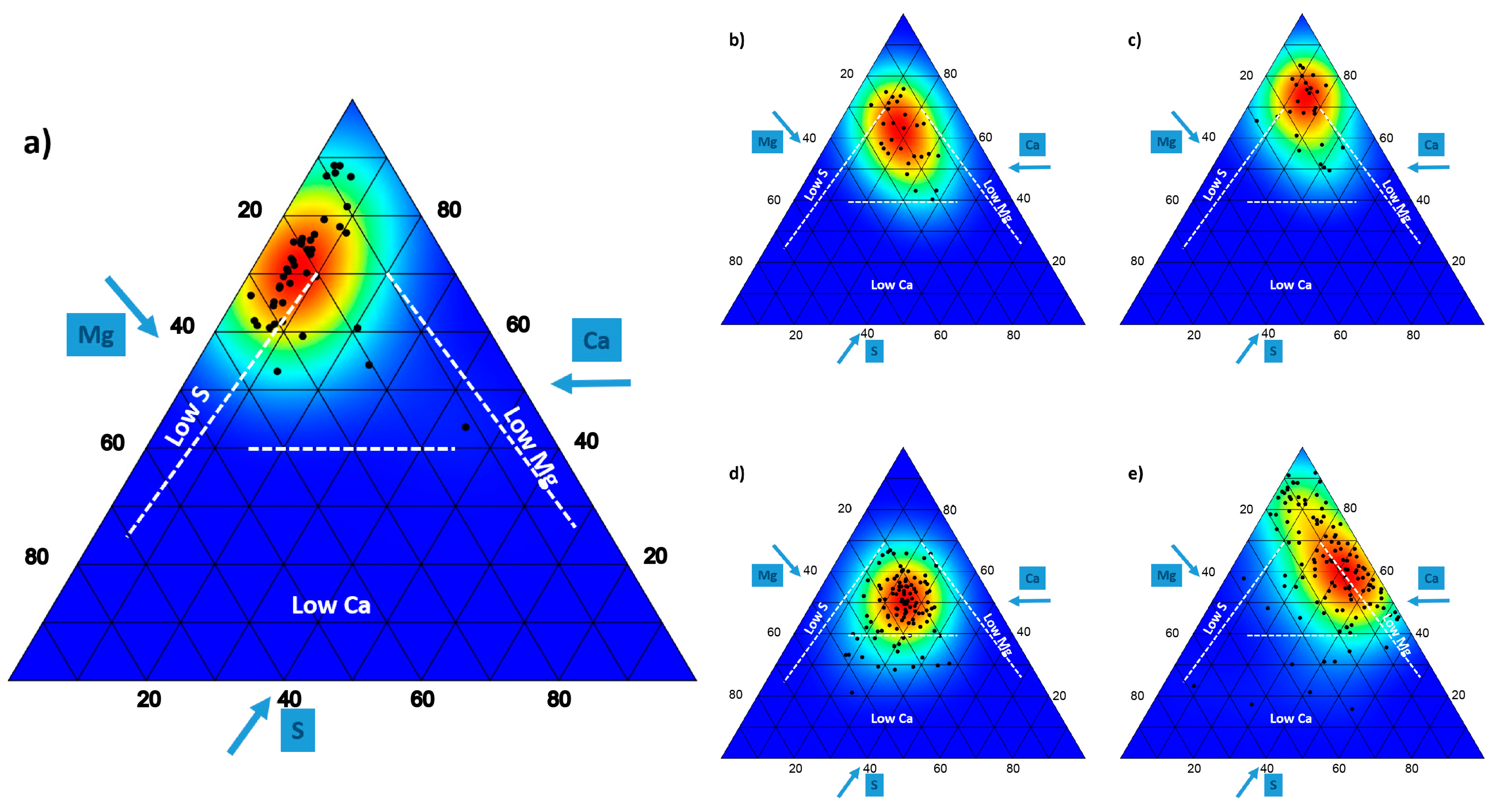
4.2. Ionomic Aspects
5. Conservation and Genetic Diversity
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, A.; Etherington, J.R. The vegetation of carboniferous limestone soils in south Wales: I. dolomitization, soil magnesium status and plant growth. J. Ecol. 1974, 62, 179–190. [Google Scholar] [CrossRef]
- Loew, O.; May, D.W. The Relation of Lime and Magnesia to Plant Growth: I. Liming of Soils from a Physiological Standpoint (No. 1); US Government Printing Office: Washington, DC, USA, 1901. [CrossRef] [Green Version]
- Chilingar, G.V.; Bissell, H.J.; Wolf, K.H. Diagenesis of carbonate rocks. In Developments in Sedimentology; Larsen, G., Cjilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; Volume 8, pp. 179–322. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth–Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Amiotte Suchet, P.; Probst, J.L.; Ludwig, W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob. Biogeochem. Cycles 2003, 17, 1038. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, C.; Rubinato, M.; Guo, K.; Zhou, J.; Cui, M. An Assessment of Soil’s Nutrient Deficiencies and Their Influence on the Restoration of Degraded Karst Vegetation in Southwest China. Forests 2020, 11, 797. [Google Scholar] [CrossRef]
- van Staden, N.; Siebert, S.J.; Cilliers, D.P.; Wilsenach, D.; Frisby, A.W. Floristic analysis of semi–arid mountain ecosystems of the Griqualand West centre of plant endemism, Northern Cape, South Africa. Biodiversitas 2020, 21, 1989–2002. [Google Scholar] [CrossRef]
- Anderson, R.C.; Fralish, J.S.; Baskin, J.M. Savannas, Barrens, and Rock Outcrop Plant Communities of North America; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar] [CrossRef]
- Zólyomi, B. A középdunai flóraválasztó és a dolomitjelenség (Die Mitteldonau–Florenscheide und das Dolomitphänomen). Bot. Közlem. 1942, 39, 209–231. [Google Scholar]
- Fekete, G.; Tölgyesi, G.; Horánszky, A. Dolomite versus limestone habitats: A study of ionic accumulation on a broader floristic basis. Flora 1989, 183, 337–348. [Google Scholar] [CrossRef]
- Kun, A.; Tóth, T.; Szabó, B.; Koncz, J. A dolomitjelenség: Közettani, talajtani és növényzeti összefüggések. (The dolomite phenomenon: Relations among rocks, soils and vegetation). Bot. Közlem. 2005, 92, 1–25. [Google Scholar]
- Mota, J.F. Vegetación de escarpes, gleras y rocas. In Proyecto Andalucía Botánica V; Publicaciones Comunitarias: Sevilla, Spain, 2007; Volume 24, pp. 139–162. [Google Scholar]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Mota, J.F.; Peñas, J.; Cabello, J. Scree and ruderal weed vegetation of Andalusian highlands (south Spain). Fitosociologia 1997, 32, 229–237. [Google Scholar]
- Baskin, J.M.; Baskin, C.C.; Chester, E.W. The Big Barrens Region of Kentucky and Tennessee: Further observations and considerations. Castanea 1994, 59, 226–254. [Google Scholar]
- Migoń, P.; Duszyński, F.; Goudie, A. Rock cities and ruiniform relief: Forms–processes–terminology. Earth–Sci. Rev. 2017, 171, 78–104. [Google Scholar] [CrossRef]
- Mota, J.F.; Medina-Cazorla, J.M.; Navarro, F.B.; Pérez-García, F.J.; Pérez-Latorre, A.; Sánchez-Gómez, P.; Torres, J.A.; Benavente, A.; Blanca, G.; Gil, C.; et al. Dolomite flora of the Baetic Ranges glades (South Spain): A review. Flora 2008, 203, 359–375. [Google Scholar] [CrossRef]
- Mota, J.F.; Garrido-Becerra, J.A.; Merlo, M.E.; Medina-Cazorla, J.M.; Sánchez-Gómez, P. The Edaphism: Gypsum, Dolomite and Serpentine Flora and Vegetation. In The Vegetation of the Iberian Peninsula; Plant and Vegetation series; Loidi, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 3, pp. 277–354. [Google Scholar] [CrossRef]
- Molero-Mesa, J.; García-Martínez, E. Resumen fitosociológico de la vegetación de Sierra Nevada. Cuad. Gec. 1983, 11, 215–266. [Google Scholar]
- Mota, J.F.; Valle, F.; Cabello, J. Dolomitic vegetation of South Spain. Vegetatio 1993, 109, 29–45. [Google Scholar] [CrossRef]
- Taleb, M.S.; Fennane, M. Vascular Plant Communities of Morocco; Geobotany Studies Series; Springer International Publishing: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- LaMarche, V.C. Rates of Slope Degradation as Determined from Botanical Evidence, White Mountains, California; US Government Printing Office: Washington, DC, USA, 1968. [CrossRef]
- Luque, A.; Leiss, B.; Alvarez-Lloret, P.; Cultrone, G.; Siegesmund, S.; Sebastian, E.; Cardell, C. Potential thermal expansion of calcitic and dolomitic marbles from Andalusia (Spain). J. Appl. Crystallogr. 2011, 44, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Lawless, P.J.; Baskin, J.M.; Baskin, C.C. Xeric limestone prairies of eastern United States: Review and synthesis. Bot. Rev. 2006, 72, 235–272. [Google Scholar] [CrossRef]
- Allison, J.R.; Stevens, T.E. Vascular flora of Ketona dolomite outcrops in Bibb County, Alabama. Castanea 2001, 66, 154–205. [Google Scholar]
- Frisby, A.W.; Siebert, S.J.; Struwig, M.; Cilliers, D.P. Plant endemism in Griqualand West, South Africa. S. Afr. J. Bot. 2019, 124, 127–137. [Google Scholar] [CrossRef]
- Cacho, N.I.; Strauss, S.Y. Occupation of bare habitats, an evolutionary precursor to soil specialization in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 15132–15137. [Google Scholar] [CrossRef] [Green Version]
- Medina-Cazorla, J.M.; Gil de Carrasco, C.; Merlo, M.E.; Martínez-Hernández, F.; Garrido-Becerra, J.A.; Salmerón-Sánchez, E.; Mendoza-Fernández, A.; Pérez-García, F.J.; Mota, J.F. The dolomite shrublands of the Convolvuletalia boissieri order and their preservation by means of the Habitats Directive. Acta Bot. Gall. 2010, 157, 611–625. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Spasojevic, M.J. The edaphic control of plant diversity. Glob. Ecol. Biogeogr. 2020, 29, 1634–1650. [Google Scholar] [CrossRef]
- Buira, A.; Fernández-Mazuecos, M.; Aedo, C.; Molina-Venegas, R. The contribution of the edaphic factor as a driver of recent plant diversification in a Mediterranean biodiversity hotspot. J. Ecol. 2020. [Google Scholar] [CrossRef]
- Reid, J.B.; Hill, R.S.; Brown, M.J.; Hovenden, M.J. Vegetation of Tasmania; CSIRO Publishing: Clayton, Victoria, Australia, 1999. [Google Scholar]
- Merlo, M.E.; Mota, J.F.; Sánchez-Gómez, P. Ecofisiología y adaptaciones de las plantas vasculares a las características físicas y químicas de sustratos especiales. In Diversidad Vegetal de las Yeseras Ibéricas; Mota, J.F., Sánchez-Gómez, P., Guirado, J.S., Eds.; ADIF–Mediterráneo Asesores Consultores: Almería, Spain, 2011; pp. 51–74. [Google Scholar]
- Billings, W.D. Alpine phytogeography across the Great Basin. Great Basin Nat. 1978, 2, 105–117. [Google Scholar]
- Ritter-Studnička, H. Reliktgesellschaften auf Dolomitböden in Bosnien und der Hercegovina. Vegetatio 1967, 15, 190–212. [Google Scholar] [CrossRef]
- Parolly, G.; Hein, P. Arabis lycia (Cruciferae), a new chasmophyte from the Taurus Mts, Turkey, and notes on related species. Willdenowia 2014, 30, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Parolly, G. The high–mountain flora and vegetation of the western and central Taurus Mts.(Turkey) in the times of climate change. In Climate Change Impacts on High–Altitude Ecosystems; Öztürk, M., Hakeem, K., Faridah-Hanum, I., Efe, R., Eds.; Springer: Cham, Switzerland, 2015; pp. 99–133. [Google Scholar] [CrossRef]
- Ritter-Studnyka, H. Flora i vegetacija na dolomitima Bosne i Hercegovine, I, Konjic. God. Biol. Inst. U Sarajevu 1956, 9, 73–122. [Google Scholar]
- Ritter-Studnyka, H. Flora i vegetacija na dolomitima Bosne i Hercegovine, II i III, Dalja okolina Konjica, kompleks Drvara i dva manja natazigta u Bosni. God. Biol. Inst. U Sarajevu 1957, 10, 129–161. [Google Scholar]
- Ritter-Studnyka, H. Flora i vegetacija na dolomitima Bosne i Hercegovine, IV, Lastva kod Trebinja. God. Biol. Inst. 1959, 12, 137–186. [Google Scholar]
- Vogiatzakis, I.N.; Griffiths, G.H. A GIS–based empirical model for vegetation prediction in Lefka Ori, Crete. Plant Ecol. 2006, 184, 311–323. [Google Scholar] [CrossRef]
- El Abidine, A.Z.; Lamhamedi, M.S.; Taoufik, A. Relations hydriques des arbres sains et dépérissants de Cedrus atlantica M. au Moyen Atlas Tabulaire au Maroc. Geo–Eco–Trop 2013, 37, 157–176. [Google Scholar]
- Brullo, C.; Brullo, S.; Giusso, G. Considerations on the endemic flora of Sicily. In Islands and Plants: Preservation and Understanding of Flora on Mediterranean Islands. Proceedings of the 2nd Botanical Conference in Menorca. Proceedings and Abstracts; Cardona Pons, E., Estaún Clarisó, I., Comas Casademont, M., Fraga i Arguimbau, P., Eds.; Institut Menorquí d’Estudis: Menorca, Spain, 2013; pp. 177–199. [Google Scholar]
- Pignatti, E.; Pignatti, S. Plant life of the Dolomites; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Sanz de Galdeano, C.; López-Garrido, A.C. Las fallas tortonienses a cuaternarias entre Granada y la Costa: El límite occidental del Nevado–Filábride y de las unidades alpujárrides inferiores. Rev. Soc. Geol. Esp. 2000, 13, 519–528. [Google Scholar]
- Papadopoulos, G.A.; Gràcia, E.; Urgeles, R.; Sallares, V.; De Martini, P.M.; Pantosti, D.; González, M.; Yalciner, A.C.; Mascle, J.; Sakellariou, D.; et al. Historical and pre–historical tsunamis in the Mediterranean and its connected seas: Geological signatures, generation mechanisms and coastal impacts. Mar. Geol. 2014, 354, 81–109. [Google Scholar] [CrossRef]
- Matthews, W.S.; Van Wyk, A.E.; Bredenkamp, G.J. Endemic flora of the north–eastern Transvaal escarpment, South Africa. Biol. Conserv. 1993, 63, 83–94. [Google Scholar] [CrossRef]
- Cantero, J.J.; Sfragulla, J.; Nuñez, C.; Mulko, J.; Bonalumi, A.; Amuchastegui, A.; Barzoza, G.E.; Chiarini, F.; Ariza Espinar, L. Vegetación de afloramientos carbonáticos de montañas del centro de Argentina. Bol. Soc. Argent. Bot. 2014, 49, 559–580. [Google Scholar] [CrossRef] [Green Version]
- Naqinezhad, A.; Esmailpoor, A. Flora and vegetation of rocky outcrops/cliffs near the Hyrcanian forest timberline in the Mazandaran mountains, northern Iran. Nord. J. Bot. 2017, 35, 449–466. [Google Scholar] [CrossRef]
- Dickoré, W.B.; Nüsser, M. Flora of Nanga Parbat (NW Himalaya, Pakistan): An annotated inventory of vascular plants with remarks on vegetation dynamics. Englera 2000, 19, 3–253. [Google Scholar] [CrossRef]
- Shrestha, M.R.; Rokaya, M.B.; Ghimire, S.K. Vegetation Pattern of Trans–Himalayan Zone in the North–West Nepal. Nepal J. Plant Sci. 2005, 1, 129–135. [Google Scholar]
- Nowak, A.; Nowak, S.; Nobis, M.; Nobis, A. Vegetation of rock clefts and ledges in the Pamir Alai Mts, Tajikistan (Middle Asia). Cent. Eur. J. Biol. 2014, 9, 444–460. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, R.; Hamel, C.; Friesen, C. Manitoba’s endangered alvars: An initial description of their extent and status. Can. Field Nat. 2019, 132, 238–253. [Google Scholar] [CrossRef] [Green Version]
- Belcher, J.W.; Keddy, P.A.; Catling, P.A. Alvar vegetation in Canada: A multivariate description at two scales. Can. J. Bot. 1992, 70, 1279–1291. [Google Scholar] [CrossRef]
- Catling, P.K.; Catling, P.M.; Cayouette, J.; Oldham, M.; Ford, B.; Hamel, C.; Friesen, C. Canadian alvars and limestone barrens: Areas of “Special Conservation Concern” for plants. Can. Bot. Assoc. Bull. 2014, 47, 9–11. [Google Scholar]
- Damsholt, K. Liverworts collected during the Norwegian east Greenland expeditions 1929—1933. Lindbergia 2010, 33, 92–113. [Google Scholar]
- Hartmann, J.; Moosdorf, N. The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochem. Geophys. 2012, 13. [Google Scholar] [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Pereira, P.; Wu, X.; Zhou, J.; Cao, J.; Zhang, W. Global karst vegetation regime and its response to climate change and human activities. Ecol. Indic. 2020, 113, 106208. [Google Scholar] [CrossRef]
- Mengoni, A.; Cecchi, L.; Gonnelli, C. Nickel hyperaccumulating plants and Alyssum bertolonii: Model systems for studying biogeochemical interactions in serpentine soils. In Bio-Geo Interactions in Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2012; pp. 279–296. [Google Scholar]
- Echevarria, G. Genesis and behaviour of ultramafic soils and consequences for nickel biogeochemistry. In Agromining: Farming for Metals; Springer: Cham, Switzerland, 2018; pp. 135–156. [Google Scholar]
- Rahbek, C.; Borregaard, M.K.; Antonelli, A.; Colwell, R.K.; Holt, B.G.; Nogues-Bravo, D.; Rasmussen, C.M.; Richardson, K.; Rosling, M.T.; Whittaker, R.J.; et al. Building mountain biodiversity: Geological and evolutionary processes. Science 2019, 365, 1114–1119. [Google Scholar] [CrossRef]
- Pérez-García, F.J.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Merlo, M.E.; Sola, F.; Salmerón-Sánchez, E.; Garrido-Becerra, J.A.; Mota, J.F. Towards a global checklist of the of world gypsophytes: A qualitative approach. Plant Sociol. 2017, 54, 61–76. [Google Scholar] [CrossRef]
- Pérez-García, F.J.; Akhani, H.; Parsons, R.F.; Silcock, J.L.; Kurt, L.; Özdeniz, E.; Spampinato, G.; Musarella, C.; Salmerón-Sánchez, E.; Sola, F.; et al. A first inventory of gypsum flora in the Palearctic and Australia. Mediterr. Bot. 2018, 39, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Marchand, D.E. Edaphic control of plant distribution in the White Mountains, eastern California. Ecology 1973, 54, 233–250. [Google Scholar] [CrossRef]
- Carreira, J.A.; Lajtha, K.; Niell, F.X. Phosphorus transformations along a soil/vegetation series of fire–prone, dolomitic, semi–arid shrublands of southern Spain soil P and Mediterranean shrubland dynamic. Biogeochemistry 1997, 39, 87–120. [Google Scholar] [CrossRef]
- Carreira, J.A.; Niell, F.X.; Lajtha, K. Soil nitrogen availability and nitrification in Mediterranean shrublands of varying fire history and successional stage. Biogeochemistry 1994, 26, 189–209. [Google Scholar] [CrossRef]
- Campillo, N.; Martín, F.; Simón, M.; Iriarte, A. Cuantificación de la degradación de las propiedades de los suelos en explotaciones mineras a cielo abierto. Edafología 2000, 7, 31–42. [Google Scholar]
- Salmerón-Sánchez, E.; Martínez-Nieto, M.I.; Martínez-Hernández, F.; Garrido-Becerra, J.A.; Mendoza-Fernández, A.J.; Gil de Carrasco, C.; Ramos-Miras, J.J.; Lozano, R.; Merlo, M.E.; Mota, J.F. Ecology, genetic diversity and phylogeography of the Iberian endemic plant Jurinea pinnata (Lag.) DC. (Compositae) on two special edaphic substrates: Dolomite and gypsum. Plant Soil 2014, 374, 233–250. [Google Scholar] [CrossRef]
- Copp, C.J. The Development of Protocols to Restore the Globally At–Risk Limestone Barrens Ecosystem. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2014. [Google Scholar]
- Baier, R. Ernährungszustand und mögliche Anpassungsmechanismen der Fichte (Picea abies (L.) Karst.) auf Dolomitstandorten der Bayerischen Kalkalpen: Ergebnisse eines Düngeversuches an jungen Schutzwaldsanierungspflanzen| Nutrition status and possible adaptation mechanisms of Norway spruce (Picea abies (L.) Karst.) On dolomite sites in the Bavarian Limestone Alps: Results from a fertilisation experiment on young plantations in protection forests. Schweiz. Z. Fur Forstwes. 2004, 155, 378–391. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.E.; Garrido-Becerra, J.A.; Mota, J.F.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.; Pérez-García, F.J. Threshold ionic contents for defining the nutritional strategies of gypsophile flora. Ecol. Indic. 2019, 97, 247–259. [Google Scholar] [CrossRef]
- Duursma, R.A.; Blackman, C.J.; Lopéz, R.; Martin-StPaul, N.K.; Cochard, H.; Medlyn, B.E. On the minimum leaf conductance: Its role in models of plant water use, and ecological and environmental controls. New Phytol. 2019, 221, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Petruzzellis, F.; Marusig, D.; Tomasella, M.; Natale, S.; Altobelli, A.; Calligaris, C.; Floridda, G.; Cucchi, F.; Forte, E.; et al. Water ‘on the rocks’: A summer drink for thirsty trees? New Phytol. 2021, 229, 199–212. [Google Scholar] [CrossRef]
- Wright, R.D.; Mooney, H.A. Substrate-oriented distribution of bristlecone pine in the White Mountains of California. Am. Midl. Nat. 1965, 73, 257–284. [Google Scholar] [CrossRef]
- Navarro-Fernández, C.M.; Aroca, R.; Barea, J.M. Influence of arbuscular mycorrhizal fungi and water regime on the development of endemic Thymus species in dolomitic soils. Appl. Soil Ecol. 2011, 48, 31–37. [Google Scholar] [CrossRef]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY–a global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Peñuelas, J.; Fernández-Martínez, M.; Ciais, P.; Jou, D.; Piao, S.; Obersteiner, M.; Vicca, S.; Janssens, I.A.; Sardans, J. The bioelements, the elementome, and the biogeochemical niche. Ecology 2019, 100, e02652. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Yamada, S.; Ishizawa, T.; Watanabe, T.; Shinano, T. Mineral characteristics of leaves of plants from different phylogeny grown in various soil types in the temperate region. Plant Food Hum. Nutr. 2003, 58, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Pope, N.; Harris, T.B.; Rajakaruna, N. Vascular plants of adjacent serpentine and granite outcrops on the deer isles, Maine, USA. Rhodora 2010, 112, 105–141. [Google Scholar] [CrossRef]
- Cera, A.; Montserrat-Martí, G.; Ferrio, J.P.; Drenovsky, R.E.; Palacio, S. Gypsum–exclusive plants accumulate more leaf S than non–exclusive species both in and off gypsum. Environ. Exp. Bot. 2021, 182, 104294. [Google Scholar] [CrossRef]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; De Ruiter, P.C. Species richness–productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Müllerová, V.; Hejcman, M.; Hejcmanová, P.; Pavlů, V. Effect of fertilizer application on Urtica dioica and its element concentrations in a cut grassland. Acta Oecol. 2014, 59, 1–6. [Google Scholar] [CrossRef]
- Wang, M.; Moore, T.R. Carbon, nitrogen, phosphorus, and potassium stoichiometry in an ombrotrophic peatland reflects plant functional type. Ecosystems 2014, 17, 673–684. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Bakshi, P.; Bhardwaj, R.; Thukral, A.K. Multivariate analysis on the distribution of elements in plants. Acta Physiol. Plant. 2018, 40, 187. [Google Scholar] [CrossRef]
- Allahham, A.; Kanno, S.; Zhang, L.; Maruyama-Nakashita, A. Sulfur deficiency increases phosphate accumulation, uptake, and transport in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2971. [Google Scholar] [CrossRef] [PubMed]
- Pillon, Y.; Petit, D.; Gady, C.; Soubrand, M.; Joussein, E.; Saladin, G. Ionomics suggests niche differences between sympatric heathers (Ericaceae). Plant Soil 2019, 434, 481–489. [Google Scholar] [CrossRef]
- Medina-Cazorla, J.M. Conservación y Biogeografia de la Flora Dolomitófila Bética. Ph.D. Thesis, Universidad de Almería, Almería, Spain, 2015. [Google Scholar]
- Mota-Poveda, J.F.; Salmerón-Sánchez, E.; Pérez-García, F.J.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Medina-Cazorla, J.M.; Merlo, M.E. Catálago Delphi de la flora edafoendémica de los blanquizales dolomíticos béticos: Bases para su conocimiento y conservación. In Biología de la Conservación de Plantas en Sierra Nevada: Principios y Retos Para su Preservación; Peñas de Giles, J., Lorite, J., Eds.; Editorial Universidad de Granada: Granada, Spain, 2019; pp. 193–210. ISBN 9788433865120. [Google Scholar]
- Moore, M.J.; Mota, J.F.; Douglas, N.A.; Flores-Olvera, H.; Ochoterena, H. The ecology, assembly, and evolution of gypsophile floras. In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R.S., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 97–128. [Google Scholar]
- Muller, C.T.; Moore, M.J.; Feder, Z.; Tiley, H.; Drenovsky, R.E. Phylogenetic patterns of foliar mineral nutrient accumulation among gypsophiles and their relatives in the Chihuahuan Desert. Am. J. Bot. 2017, 104, 1442–1450. [Google Scholar] [CrossRef] [Green Version]
- Kruckeberg, A.R. California Serpentines: Flora, Vegetation, Geology, Soils, and Management Problems; University of California Press: Berkeley, CA, USA, 1984. [Google Scholar]
- Kazakou, E.; Adamidis, G.C.; Baker, A.J.; Reeves, R.D.; Godino, M.; Dimitrakopoulos, P.G. Species adaptation in serpentine soils in Lesbos Island (Greece): Metal hyperaccumulation and tolerance. Plant Soil 2010, 332, 369–385. [Google Scholar] [CrossRef]
- Galey, M.L.; van der Ent, A.; Iqbal, M.C.M.; Rajakaruna, N. Ultramafic geoecology of south and Southeast Asia. Bot. Stud. 2017, 58, 18. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Shivay, Y.S. Sulphur in soil, plant and human nutrition. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 429–434. [Google Scholar] [CrossRef]
- Guo, W. Magnesium homeostasis mechanisms and magnesium use efficiency in plants. In Plant Macronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Hossain, M.A., Kamiya, T., Burritt, D., Tran, L.S.P., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 197–213. [Google Scholar] [CrossRef]
- Conn, S.J.; Conn, V.; Tyerman, S.D.; Kaiser, B.N.; Leigh, R.A.; Gilliham, M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011, 190, 583–594. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef]
- Tyndall, R.W.; Hull, J.C. Vegetation, flora, and plant physiological ecology of serpentine barrens of eastern North America. In Savannas, Barrens, and Rock Outcrop Plant Communities of North America; Anderson, R.C., Fralish, J.S., Baskin, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 67–82. [Google Scholar] [CrossRef]
- Boi, M.E.; Medas, D.; Aquilanti, G.; Bacchetta, G.; Birarda, G.; Cappai, G.; Carlomagno, I.; Casu, M.A.; Gianoncelli, A.; Meneghini, C.; et al. Mineralogy and Zn Chemical Speciation in a Soil-Plant System from a Metal-Extreme Environment: A Study on Helichrysum microphyllum subsp. tyrrhenicum (Campo Pisano Mine, SW Sardinia, Italy). Minerals 2020, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Palacio, S.; Aitkenhead, M.; Escudero, A.; Montserrat-Martí, G.; Maestro, M.; Robertson, A.J. Gypsophile chemistry unveiled: Fourier transform infrared (FTIR) spectroscopy provides new insight into plant adaptations to gypsum soils. PLoS ONE 2014, 9, e107285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and ecological significance of biomineralization in plants. Trends Plant Sci. 2014, 19, 166–174. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Visscher, A.M.; Paul, A.L.; Kirst, M.; Guy, C.L.; Schuerger, A.C.; Ferl, R.J. Growth performance and root transcriptome remodeling of Arabidopsis in response to Mars–like levels of magnesium sulfate. PLoS ONE 2010, 5, e12348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oze, C.; Skinner, C.; Schroth, A.W.; Coleman, R.G. Growing up green on serpentine soils: Biogeochemistry of serpentine vegetation in the Central Coast Range of California. Appl. Geochem. 2008, 23, 3391–3403. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Mooney, H.A. Influence of soil type on the distribution of two closely related species of Erigeron. Ecology 1966, 47, 950–958. [Google Scholar] [CrossRef]
- Burghardt, M.; Burghardt, A.; Gall, J.; Rosenberger, C.; Riederer, M. Ecophysiological adaptations of water relations of Teucrium chamaedrys L. to the hot and dry climate of xeric limestone sites in Franconia (Southern Germany). Flora 2008, 203, 3–13. [Google Scholar] [CrossRef]
- Mota, J.F.; Sánchez-Gómez, P.; Guirado Romero, J.S. Diversidad Vegetal de las Yeseras Ibéricas: El Reto de los Archipiélagos Edáficos Para la Biología de la Conservación; ADIF–Mediterráneo Asesores Consultores: Almería, Spain, 2011. [Google Scholar]
- Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Martínez-Nieto, M.I.; Garrido-Becerra, J.A.; Salmerón-Sánchez, E.; Merlo, M.E.; Gil, C.; Mota, J.F. Areas of endemism as a conservation criterion for Iberian gypsophilous flora: A multi–scale test using the NDM/VNDM program. Plant Biosyst. 2015, 149, 483–493. [Google Scholar] [CrossRef]
- Van Gils, H.A.M.J.; Conti, F.; Ciaschetti, G.; Westinga, E. Fine resolution distribution modelling of endemics in Majella National Park, Central Italy. Plant Biosyst. 2012, 146, 276–287. [Google Scholar] [CrossRef]
- Tomaselli, M.; Foggi, B.; Carbognani, M.; Gennai, M.; Petraglia, A. The rock-face vegetation in the northern Apennines and neighbouring mountain areas, from the coast line to the highest summits. Phytocoenologia 2019, 49, 7–70. [Google Scholar] [CrossRef]
- DeChaine, E.G.; Martin, A.P. Marked genetic divergence among sky island populations of Sedum lanceolatum (Crassulaceae) in the Rocky Mountains. Am. J. Bot. 2005, 92, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Boucher, F.C.; Zimmermann, N.E.; Conti, E. Allopatric speciation with little niche divergence is common among alpine Primulaceae. J. Biogeogr. 2016, 43, 591–602. [Google Scholar] [CrossRef]
- Cole, C.T. Genetic variation in rare and common plants. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 213–237. [Google Scholar] [CrossRef]
- Wang, I.J.; Bradburd, G.S. Isolation by environment. Mol. Ecol. 2014, 23, 5649–5662. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.M.; O’Dwyer, C. Genetic variability and population structure of the endangered golden sun moth, Synemon plana. Biol. Conserv. 2000, 92, 371–381. [Google Scholar] [CrossRef]
- O’Dell, R.E.; Rajakaruna, N. Intraspecific variation, adaptation, and evolution. In Serpentine: Evolution and Ecology in a Model System; Harrison, S.P., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 97–137. [Google Scholar]
- Melendo, M.; Giménez, E.; Cano, E.; Gómez-Mercado, F.; Valle, F. The endemic flora in the south of the Iberian Peninsula: Taxonomic composition, Biological spectrum, pollination, reproductive mode and dispersal. Flora 2003, 198, 260–276. [Google Scholar] [CrossRef] [Green Version]
- Turchetto, C.; Segatto, A.L.A.; Mäder, G.; Rodrigues, D.M.; Bonatto, S.L.; Freitas, L.B. High levels of genetic diversity and population structure in an endemic and rare species: Implications for conservation. AoB Plants 2016, 8, plw002. [Google Scholar] [CrossRef] [Green Version]
- Lutz, E.; Schneller, J.J.; Holderegger, R. Understanding population history for conservation purposes: Population genetics of Saxifraga aizoides (Saxifragaceae) in the lowlands and lower mountains north of the Alps. Am. J. Bot. 2000, 87, 583–590. [Google Scholar] [CrossRef]
- Cánovas, J.L.; Jiménez, J.F.; Mota, J.F.; Sánchez-Gómez, P. Genetic diversity of Viola cazorlensis Gand. an endemic species of Mediterranean dolomitic habitats: Implications for conservation. System. Biodivers. 2015, 13, 571–580. [Google Scholar] [CrossRef]
- Martín-Hernanz, S.; Martínez-Sánchez, S.; Albaladejo, R.G.; Lorite, J.; Arroyo, J.; Aparicio, A. Genetic diversity and differentiation in narrow versus widespread taxa of Helianthemum (Cistaceae) in a hotspot: The role of geographic range, habitat, and reproductive traits. Ecol. Evol. 2019, 9, 3016–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.C.; Hung, K.H.; Schaal, B.A.; Ge, X.J.; Hsu, T.W.; Chaing, T.Y. Contrasting phylogeographical patterns between mainland and island taxa of the Pinus luchuensis complex. Mol. Ecol. 2006, 15, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Salmerón-Sánchez, E.; Merlo, M.E.; Medina-Cazorla, J.M.; Pérez-García, F.J.; Martínez-Hernández, F.; Garrido-Becerra, J.A.; Mendoza-Fernández, A.J.; Valle, F.; Mota, J.F. Variability, genetic structure and phylogeography of the dolomitophilous species Convolvulus boissieri (Convolvulaceae) in the Baetic ranges, inferred from AFLPs, plastid DNA and ITS sequences. Bot. J. Linn. Soc. 2014, 176, 505–523. [Google Scholar] [CrossRef] [Green Version]
- Schönswetter, P.; Stehlik, I.; Holderegger, R.; Tribsch, A. Molecular evidence for glacial refugia of mountain plants in the European Alps. Mol. Ecol. 2005, 14, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Neel, M.C.; Ellstrand, N.C. Patterns of allozyme diversity in the threatened plant Erigeron parishii (Asteraceae). Am. J. Bot. 2001, 88, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Neel, M.C.; Ellstrand, N.C. Conservation of genetic diversity in the endangered plant Eriogonum ovalifolium var. vineum (Polygonaceae). Conserv. Genet. 2003, 4, 337–352. [Google Scholar] [CrossRef]
- Neel, M.C. Patch connectivity and genetic diversity conservation in the federally endangered and narrowly endemic plant species Astragalus albens (Fabaceae). Biol. Conserv. 2008, 141, 938–955. [Google Scholar] [CrossRef]

| n | Gravel | Sand | Silt | Clay | pH | Carbonates | OC % | N (%) | Ca (%) | Mg (%) | Ca:Mg | CEC | EC | Ca2+ | Mg2+ | Na+ | K+ | WR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dolomite | |||||||||||||||||||
| [25] | 7.5 | 6.89 | 0.999 | ||||||||||||||||
| [11] | 5 | 7.68 | 67.87 | 16.2 | 7.3 | 2.22 | |||||||||||||
| [67] | 4 | 42.61 | 71.9 | 21.00 | 7.53 | 8.6 | 85.43 | 1.22 | 0.07 | 12.63 | 5.75 | 0.05 | 0.11 | 9.65 | |||||
| [65,66] | 4 | 49.5–86.5 | 85.8–92.6 | 1.5–3.8 | 7.5–8.5 | 69.4 | 18.6 | 10.5 | 1.77 | 1.53–10.6 | |||||||||
| [64] | 4 | 7.5–8.2 | 25.4 | 2.23 | 32.5 | 6.22 | 0.29 | 0.59 | 8.2 | ||||||||||
| [75] | 1 | 63.7 | 34.1 | 2.2 | 8.0–8.1 | 14.3 | 11.5 | 3.4 | 0.1 | 0.4 | |||||||||
| [7] | 5 | 7.6 | 1.18 | 0.5296 | 2.2 | 20.2 | |||||||||||||
| [76] | 2 | 8.2 | 0.1 | 21.9 | 11.9 | 1.84 | 2.7 | ||||||||||||
| [68] | 14 | 44.43 | 51.21 | 48.79 * | 8.2 | 77.89 | 3.13 | 0.26 | 16.17 | 10.05 | 1.61 | 13.21 | 1.16 | 0.04 | 0.2 | 6.95 | |||
| [17] | 15 | 7.94 | 10.92 | 5.98 | 2.56 | ||||||||||||||
| Ultramafic | |||||||||||||||||||
| [25] | 6 | 6.5 | 0.918 | 0.999 | 0.92 | ||||||||||||||
| [17] | 10 | 6.81 | 8.21 | 12.32 | 0.86 | ||||||||||||||
| Gypsum | |||||||||||||||||||
| [68] | 10 | 14.65 | 17.77 | 82.23 * | 8.2 | 31.64 | 0.54 | 0.07 | 17.52 | 1.37 | 37.69 | 9.02 | 2.59 | 0.13 | 0.23 | 15.47 | |||
| Limestone | |||||||||||||||||||
| [25] | 3 | 7.3 | 0.77 | 0.02 | |||||||||||||||
| [11] | 15 | 7.53 | 54.24 | 25.74 | 0.31 | 95.61 | |||||||||||||
| [17] | 14 | 7.73 | 24.28 | 2.89 | 11.3 |
| n | N | P | K | N:P | ||
| Dolomite_Baetic | Non-Dolomitophytes | 142 | 1.71 (0.82) | 0.12 (0.07) | 0.82 (0.53) | 15.76 (7.14) |
| Dolomitophytes | 90 | 1.69 (0.45) | 0.07 (0.02) | 0.82 (0.19) | 25.43 (10.83) | |
| All | 232 | 1.70 (0.72) | 0.11 (0.06) | 0.82 (0.45) | 18.91 (9.65) | |
| Serpentine | 67 | 1.59 (0.9) | 0.14 (0.09) | 0.82 (0.07) | 12.08 (4.84) | |
| Gypsum | 73 | 2.34 (0.91) | 0.09 (0.05) | 1.23 (0.69) | 30.30 (13.96) | |
| n | Ca | Mg | S | Ca:Mg | ||
| Dolomite_Baetic | Non-Dolomitophytes | 142 | 2.24 (1.94) | 0.46 (0.26) | 0.24 (0.32) | 4.76 (3.13) |
| Dolomitophytes | 90 | 1.98 (0.72) | 0.71 (0.37) | 0.19 (0.20) | 2.95 (2.95) | |
| All | 232 | 2.15 (1.65) | 0.54 (0.32) | 0.22 (0.29) | 4.17 (2.73) | |
| Dolomite_Hungary | 28 | 1.06 (0.44) | 0.31 (0.08) | 0.32 (0.16) | 3.35 (1.19) | |
| Limestone_Hungary | 27 | 1.39 (0.61) | 0.26 (0.12) | 0.31 (0.17) | 6.00 (3.12) | |
| Serpentine | 109 | 0.43 (0.11) | 0.24 (0.12) | 0.22 (0.07) | 2.16 (1.03) | |
| Gypsum | 123 | 4.87 (3.13) | 0.80 (0.77) | 2.69 (2.43) | 12.36 (20.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Merlo, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Salmerón-Sánchez, E. Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon. Biology 2021, 10, 38. https://doi.org/10.3390/biology10010038
Mota J, Merlo E, Martínez-Hernández F, Mendoza-Fernández AJ, Pérez-García FJ, Salmerón-Sánchez E. Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon. Biology. 2021; 10(1):38. https://doi.org/10.3390/biology10010038
Chicago/Turabian StyleMota, Juan, Encarna Merlo, Fabián Martínez-Hernández, Antonio J. Mendoza-Fernández, Francisco Javier Pérez-García, and Esteban Salmerón-Sánchez. 2021. "Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon" Biology 10, no. 1: 38. https://doi.org/10.3390/biology10010038