1. Introduction
The severity of burn injuries is related to the rate at which heat is transferred from the heating agent to the skin. Thermal burns account for the majority of human burn injuries and include contact and flame burns bearing different temperature transmission profiles. Contact burns often result from direct contact with a hot object and are confined to the part of skin that touched the hot object, whereas flames usually affect larger areas by indirect transmission of heat [
1]. Our current knowledge of local tissue damage by burns has been obtained using animal burn models. However, heat transmission properties of human cutaneous tissue have not yet been studied experimentally.
Thermal burns mostly involve the cutaneous and subcutaneous adipose tissues that direct a local inflammatory response, which not only protects against pathogens but also promotes tissue formation and remodeling [
2,
3]. In particular, subcutaneous adipose tissue contains numerous macrophages, fibroblasts and stem cells that play significant roles in the initiation, elongation and termination of inflammation [
4].
Tissue-resident macrophages emerge during embryonic development and persist in many tissues via self-renewal. They are capable of this by local proliferation and produce a variety of factors that stimulate the activation, proliferation, and differentiation of many cells, including fibroblasts and stem cells [
5,
6]. Earlier studies with cultured human bone marrow-derived macrophages indicated that two main phenotypes of macrophages can be induced in vitro: type M1, which expresses proinflammatory cytokines and type M2, which expresses anti-inflammatory cytokines [
7]. This classification has provided a useful system to distinguish macrophages using a few non-overlapping markers. Animal studies revealed that macrophage classification may be more complex during acute injury, when tissue-resident and monocyte-derived macrophages coexist at the site of injury [
8]. Thus, the classification of macrophages in M1 and M2 is particularly useful in well-defined in vitro conditions.
Following injury, subcutaneous resident macrophages function as sentinels and become rapidly activated and contribute to the local proinflammatory environment within the wound [
9,
10]. They secrete and respond to a number of different cytokines and chemokines to attract different sets of immune cells to the injury site, thereby affecting the proliferation and differentiation of stem cells. In addition, the interaction between resident macrophages and fibroblasts was hypothesized to mediate important signals towards wound healing [
11]. As M1/M2 polarization governs tissue damage, the reversibility from both states is considered to have a critical therapeutic value but has not been established in reproducible human tissue models in vitro [
12].
To study subcutaneous resident macrophage polarization in response to flame burns, we established a 3-dimensional human composite tissue model consisting of hypodermis and subcutaneous tissue [
13]. A series of flame burn experiments previously confirmed the utility of our model for the study of local cellular responses to burn damage [
13]. In the present study, we used this model to compare the spatiotemporal activation of subcutaneous macrophages and fibroblasts in response to controlled contact burns at 100 °C and flame burns at 300 °C bearing different temperature transmission profiles.
2. Materials and Methods
2.1. Tissue Specimens
Human composite subcutaneous/cutaneous tissue samples were obtained from 6 healthy donors undergoing elective surgery at the RWTH Aachen University Hospital as follows: donor 1, 31 years old, male, breast; donor 2, 44 years old, female, breast; donor 3, 41 years old, female, breast; donor 4, 27 years old, male, abdomen; donor 5, 49 years old, male, abdomen; and donor 6, 36 years old, female, abdomen. Ethical approval for the use of discarded surgical tissues was obtained from the Independent Ethics Committee of RWTH Aachen University Hospital and registered under identification number 206/13 on 11 December 2013. Donor consent was obtained before surgery according to the regulations of the Independent Ethics Committee of RWTH Aachen University Hospital.
2.2. In Vitro Burn Experiments
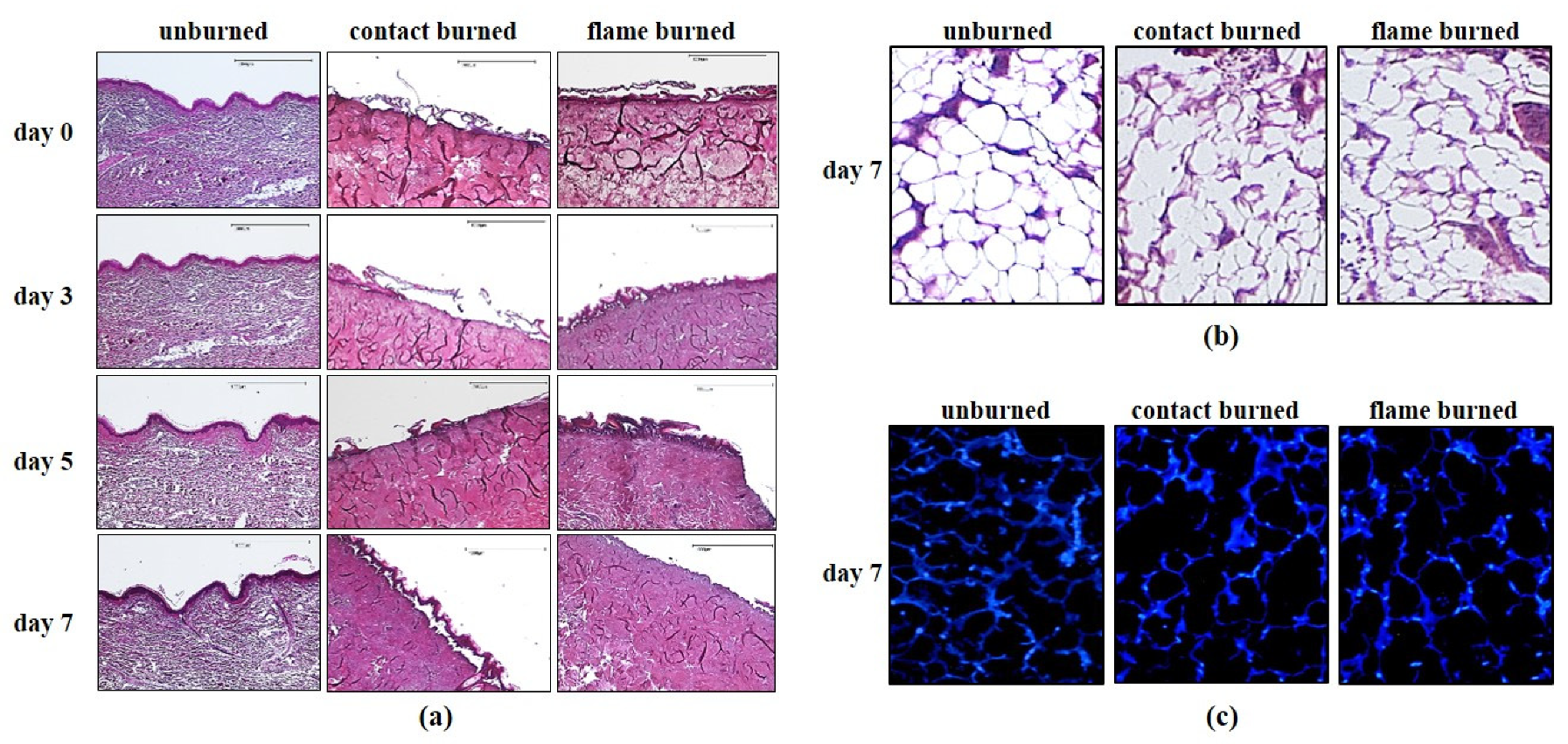
Tissue samples from every patient were cut into 2 equal pieces of cutaneous surface (2 × 2 cm) that included a 1-cm-thick layer of underlying subcutaneous fat tissue that did not reach the superficial fascia. The processed tissue pieces from every patient were subjected to contact or flame burns (
Supplementary Figure S1). For contact burns, the composite tissue pieces were placed on a culture dish and burned on day 0 using a stainless steel cylindrical bar of 10-mm-diameter and 10-mm-length (1.9 g) for 30 s without extra pressure; the bar was preheated to 120 °C using an alcohol burner (RSG Solingen, Germany), and its temperature was continuously monitored using a thermocouple. Then, the steel bar was moved away from the gun flame and placed on the tissue as soon as it was cooled down to 100 °C. This was applied three times on the same position of the tissue surface, which was labeled using a surgical marking pen before burn injuries were inflicted. All contact burns were performed using the same stainless steel bar. For flame burns, the composite tissue pieces were burned on day 0 using a pre-adjusted stable flame surface at 300 °C for 15 s produced by a McKenna burner with adjustable air and fuel flow and integrated thermocouple on flame surface. The setup of the burner was designed to allow a homogeneous gas mixture past the cooled sintered metal matrix with a diameter of 60 mm, thereby providing a one-dimensional flow field at the burner exit and a well-defined flat flame downstream of the matrix. A cool water stream was introduced through an annular ring around the burner matrix to avoid any environmental air entrainment into the flame. Under these conditions, the McKenna burner was able to repeatedly produce highly stable and well-defined temperatures. Furthermore, a unique steel holder with a 10-mm-diameter orifice was fabricated to place the human tissue at an adjustable distance from the flame. During the experiments, the holder orifice was concentrically aligned with the burner. Therefore, all of the inflicted burn wounds in this study were of equal shape and size. The container also included a mount for a thermocouple at a distance of 1 mm from the tissue sample to detect the ambient gas temperature. Before each experiment, the holder position was adjusted to achieve 300 °C at a distance 1 mm from the epidermis. The cutaneous surface of the tissue samples was completely protected, except for a circle with a diameter of 1 cm that was exposed to the flame for a precise time frame. During contact and flame burn experiments, the temperature at the bottom of the tissue was measured between 20 to 25 °C. After inflicting burn injuries, human composite pieces bearing one burned zone (~0.785 cm
2) and the distal posterior unburned zone (~3.215 cm
2) surrounding the burn were obtained.
2.3. Human Composite Tissue and 3D Culture In Vitro
After burn injuries were inflicted, the tissue samples were embedded in a polysaccharide polymer (agarose) for extended maintenance in vitro. The embedding procedure consisted of 2 consecutive steps: sealing using superficial dehydration and embedding in a precise gradient of ultrapure polysaccharide polymer LowMelt-Agarose (Bio-Budget, Krefeld, Germany) in DMEM/F-12 cell culture medium (Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, PAA, Cölbe, Germany) and penicillin and streptomycin (100 U/mL each; Sigma-Aldrich, Munich, Germany). The sealing step ensures direct contact with the embedding matrix. For embedding, a thin layer of agarose-medium mixture (1–1.5 mL) was added to the well of a 6-well culture plate. Following polymerization for 2 min, the tissue was mounted on the top of the first layer and surrounded by agarose-medium mixture to reach, but not cover the tissue surface. After complete polymerization of 5 min, 0.5 mL of fresh medium was added to form a thin layer and cover the tissue surface. The embedded sections were then incubated at 37 °C with 5% CO2, and the medium on the top of the polymer was replaced at days 3 and 6. At days 3, 5, and 7 after burning, the human tissue pieces were carefully separated from the embedding material and preserved in a freshly prepared mixture of methanol (VWR, Radnor, PA, USA), chloroform (Carl Roth, Karlsruhe, Germany), and acetic acid (Carl Roth, Karlsruhe, Germany) at a ratio of 6:3:1 for 24 h.
2.4. Hematoxylin and Eosin Staining
The preserved tissue pieces were then dehydrated, embedded in paraffin and sectioned into 3-μm sections using a SLIDE4003E microtome (pfm medical, Cologne, Germany) before staining. The sections were fixed on glass slides, deparaffinized and stained using a standard hematoxylin and eosin (HE) staining protocol (Merck Millipore, Darmstadt, Germany). Briefly, the procedure included nuclear staining with hematoxylin for 5–10 min, rinsing in warm water for 10 min, staining in 0.3% eosin for 5 min, rinsing with tap water, dehydrating in an ethanol gradient (80%, 96%, and 100%), treating with xylene, and sealing.
2.5. DAPI (4′,6-Diamidino-2-phenylindole) Staining
Embedded tissue sections were carefully separated from the embedding material and were fixed and sectioned as described for HE staining. The sections were deparaffinized, washed with PBS, treated with protein blocking solution (Dako, Hamburg, Germany) for 30 min at room temperature, and stained for DAPI (final concentration 1:100,000) for 10–15 min according to the manufacturer’s protocol (Dako, Hamburg, Germany). After they were rinsed 3 times with PBS, the sections were sealed with Mowiol (Sigma-Aldrich, Munich, Germany) and maintained at 4 °C overnight. Finally, sections were photographed under a fluorescence microscope (DM microscope 600).
2.6. Immunohistochemistry/Light Microscopy
Tissue sections subjected to HE staining or immunohistochemistry were analyzed under a light microscope. For immunohistochemistry, CD68, CD80, CD163, and HSP47 were chosen as the protein markers of subcutaneous monocytes, M1 macrophages, M2 macrophages and fibroblasts, respectively (Ito and Nagata 2017, Jaguin et al., 2013, Vogel et al., 2014). Embedded tissue pieces were sectioned into 3-μm sections, which were then deparaffinized according to the manufacturer’s protocol (Acris, Los Angeles, CA, USA) before they were incubated with a 1:250 dilution of the following primary antibodies: mouse anti-human CD68 (Agilent Technologies, Waldbronn, Germany), rabbit anti-human HSP47 (Abcam, Berlin, Germany), mouse anti-human CD80, and rabbit anti-human CD163 (Acris, Los Angeles, CA, USA). After the sections were treated with the 1:250 dilution of horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Medac, Wedel, Germany), the stain was developed with diaminobenzidine (DAB, Agilent Technologies, Waldbronn, Germany).
Macroscopic changes were viewed under a DM microscope 600 (Leica, Wetzlar, Germany), and images were processed using DISKUS software (Leica, Wetzlar, Germany). The damage depth was defined as the distance from the most superficial core of the burn site to the most distal visible tissue damage. Semi-quantitative analysis was performed with at least 5 randomly selected fields per slice at high magnification (400×) to calculate the number of positively stained cells within a photographic area (approximately 0.073 mm2), which was determined in at least 5 equivalent microscopic fields in different locations within the burned and unburned subcutaneous zones at the indicated days postburn.
2.7. Statistical Analysis
All results are reported as the mean ± standard deviation (SD). Statistical analysis was performed with SPSS 18.0. Results were compared using two-way repeated ANOVA with Bonferroni’s multiple comparisons. Differences with a p value ≤ 0.05 were considered statistically significant.
4. Discussion
Burn wound progression refers to an unsolved phenomenon in the clinic that can induce the conversion of a burn wound from a superficial burn to a deep subcutaneous burn with severe consequences for burn victims. In current clinical practice, burn wounds are initially evaluated only for extent, depth, and circumferential components [
2]. All decisions regarding the wound care, hospitalization, and transfer are made based on this information. The main findings of our study suggest that thermal forces decisively influence burn wound progression in human subcutaneous tissue within days after injury. Thus, the type of burns and their thermal characteristics, like heath source and duration, may be relevant to initial decision-making in primary care. In our experimental model, adipose tissue damage and macrophage and fibroblast activation progress together with analog changes in spatiotemporal dynamics. Furthermore, macrophage and fibroblast activation is a hallmark of inflammation and tissue regeneration, suggesting that both processes are initiated within the damaged network of subcutaneous adipose tissue immediately after burn injury. This highlights the crucial role of subcutaneous adipose tissue in human burn wound progression.
A study of tissue samples from burn victims two to six days postburn suggested that a higher proinflammatory tissue status can explain the high conversion rate from partial to full thickness burns [
17]. Notably, it is not feasible to capture details on thermal burn conditions upon clinical admission, and tissue assessments generally occur at the wound site days after injury. Previous studies revealed that circulating macrophages infiltrate into the wound between 24 h and 46 days after burns and thereby obscure the true inflammatory state of the damaged tissue [
18]. In our model, we were able to assess the tissue before and immediately after the onset of damage in samples from six donors. Among them, two donors exhibited between 2- and 11-fold higher numbers of subcutaneous macrophages, indicating a higher proinflammatory tissue status before burn exposure (data not shown). However, the progression of tissue damage in both donors was not significantly different than that in other donors. Although our donor collection did not encompass a multitude of different inflammatory preconditions, our experimental model implies that the dynamics of wound progression in contact burns are independent of the inflammatory tissue environment. Moreover, contact and flame burns inflicted distinct dynamics of wound progression in the same donor. Therefore, we assume that the inflammatory environment at the injury site has a minor impact on burn wound progression.
Previous studies have suggested that oxidative stress and apoptosis play important roles in prolonged inflammation and tissue damage [
19]. However, as many studies have used animal burn models, there is a strong limitation to the clinical applicability of the results on human burns [
1]. Among many reported animal burn models, a porcine model has reported burn wound progression up to 7 days postburn [
20]. Scald burns conferred by temperatures from 50 °C to 90 °C inflicted visible damage on the dermis, which progressed to a depth of 3 mm 7 days postburn. However, no harm to the underlying adipose tissue was detected in the porcine model. We observed that contact burns at 100 °C inflicted significant progressive damage on human subcutaneous adipose tissue. This discrepancy is either due to the difference between 90 °C and 100 °C or to interspecies differences in the epidermal thickness and the vascular network between porcine and human models, despite their well-recognized translational relevance. Thus, the human composite tissue model is a useful approach used to investigate the local impact of thermal forces on the human dermis.
A large number of macrophage markers have been identified in murine models, but the majority of them have not been translated to humans [
8,
21]. The current classification of human macrophages is based on few protein markers from blood-derived monocytes, which differentiate into M1- and M2-type macrophages in vitro [
8,
21]; however, further studies revealed that the M1/M2 paradigm may be misleading during acute tissue injury, when circulating macrophages infiltrate the tissue and exceed the number of resident macrophages [
22,
23]. Thus, our human composite tissue model is undoubtedly subjected to the same limitations as the M1/M2 paradigm [
24,
25], but the migration of circulating blood-derived macrophages could be excluded, thus allowing the number of macrophages to remain constant in the unharmed zone of all samples (
Figure 3). Therefore, the use of M1/M2 polarization may be limited to the well-defined in vitro conditions as in the human tissue burn model. Independent of the possibly disparate inflammatory functions of M1 (CD80+) and M2 (CD163+) macrophages, both subtypes emerge simultaneously at the site of adipose tissue damage. Furthermore, the total number of all macrophages (CD68+) increases at the site of injury. We assume that preconditioned M1 and M2 subtypes reside within the adipose tissue and become independently activated in response to damage to mature adipocytes. Macrophages maintain adipose tissue by eliminating dead adipocytes, but they can also regulate the inflammatory microenvironment during weight loss or obesity and excessive accumulation of fat [
4,
26,
27,
28].
Human fibroblasts represent a complex group of cells with distinct functions in the regulation of tissue matrix deposition and likely serve as pluripotent mesenchymal stem cells [
29]. Previous reports suggested that tissue regeneration follows the sequential activation of pro- and anti-inflammatory signals that ultimately activate fibroblasts and their proliferation [
9,
30]. Our results show that the activation of fibroblasts and polarization of M1 and M2 macrophages occur simultaneously in the same spatiotemporal dynamics. Thus, activation of subcutaneous fibroblasts may participate or be a part of the inflammatory response at the early stages of wound healing.