A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Circadian Rhythm of the Body Temperature
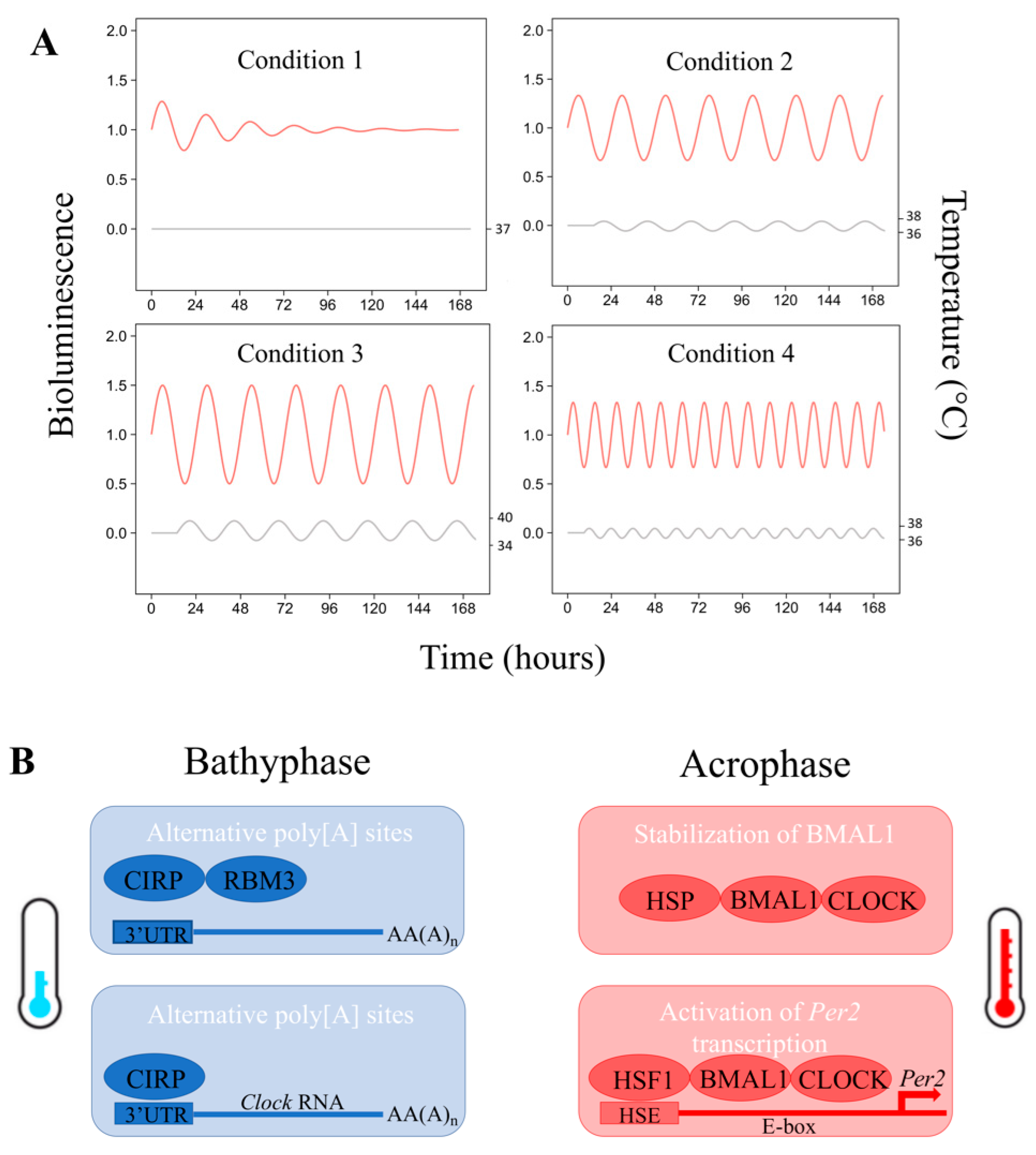
2.1. Clock Control of the CRBT
2.2. Measurements of the CRBT
2.3. Physiological Modulations of the CRBT
3. Molecular, Immune, and Biological Effects of Temperature Variations
3.1. Transcriptional Effect of Temprature Variations
3.2. Effect of Temperature Change on Immune Function
3.3. Effect of Temperature Variations on Microbes
3.4. Effect of Temperature Variations on Vectors
4. Circadian Disruption of the Temperature and Diseases
4.1. Fever and Hypothermia
4.2. Disruption of the CRBT and Trauma
4.3. Disruption of the CRBT and Infection
4.4. Disruption of the CRBT and Cancer
4.5. Disruption of the CRBT and Inflammatory Diseases
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Refinetti, R. The circadian rhythm of body temperature. Front. Biosci. 2010, 15, 564–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kräuchi, K.; Wirz-Justice, A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. 1994, 267, R819–R829. [Google Scholar] [CrossRef]
- Himms-Hagen, J. Cellular thermogenesis. Annu. Rev. Physiol. 1976, 38, 315–351. [Google Scholar] [CrossRef]
- Blondin, D.P.; Haman, F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb. Clin. Neurol. 2018, 156, 153–173. [Google Scholar]
- Frank, S.M.; Raja, S.N.; Bulcao, C.F.; Goldstein, D.S. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J. Appl. Physiol. 1999, 86, 1588–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cain, J.B.; Livingstone, S.D.; Nolan, R.W.; Keefe, A.A. Respiratory heat loss during work at various ambient temperatures. Respir. Physiol. 1990, 79, 145–150. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Logan, R.W.; Sarkar, D.K. Circadian nature of immune function. Mol. Cell Endocrinol. 2012, 349, 82–90. [Google Scholar] [CrossRef]
- Saini, C.; Morf, J.; Stratmann, M.; Gos, P.; Schibler, U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012, 26, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Ruby, N.F.; Dark, J.; Burns, D.E.; Heller, H.C.; Zucker, I. The suprachiasmatic nucleus is essential for circadian body temperature rhythms in hibernating ground squirrels. J. Neurosci. 2002, 22, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Nomoto, S.; Shibata, M.; Iriki, M.; Riedel, W. Role of afferent pathways of heat and cold in body temperature regulation. Int. J. Biometeorol. 2004, 49, 67–85. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Brauchi, S.; Orio, P.; Latorre, R. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc. Natl. Acad. Sci. USA 2004, 101, 15494–15499. [Google Scholar] [CrossRef] [Green Version]
- Nam, D.; Yechoor, V.K.; Ma, K. Molecular clock integration of brown adipose tissue formation and function. Adipocyte 2015, 12, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Kelly, G. Body temperature variability (Part 1): A review of the history of body temperature and its variability due to site selection, biological rhythms, fitness, and aging. Altern. Med. Rev. 2006, 11, 278–293. [Google Scholar]
- Edwards, B.; Waterhouse, J.; Reilly, T.; Atkinson, G. A comparison of the suitabilities of rectal, gut, and insulated axilla temperatures for measurement of the circadian rhythm of core temperature in field studies. Chronobiol. Int. 2002, 19, 579–597. [Google Scholar] [CrossRef]
- Bogh, M.; Minors, D.S.; Waterhouse, J.M. Can insulated skin temperature act as a substitute for rectal temperature when studying circadian rhythms? Chronobiol. Int. 1994, 11, 332–339. [Google Scholar] [CrossRef]
- Grassly, N.C.; Fraser, C. Seasonal infectious disease epidemiology. Proc. Biol. Sci. 2006, 273, 2541–2550. [Google Scholar] [CrossRef] [Green Version]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef]
- Harding, C.; Pompei, F.; Bordonaro, S.F.; McGillicuddy, D.C.; Burmistrov, D.; Sanchez, L.D. The daily, weekly, and seasonal cycles of body temperature analyzed at large scale. Chronobiol. Int. 2019, 36, 1646–1657. [Google Scholar] [CrossRef]
- Weinert, D. Circadian temperature variation and ageing. Ageing Res. Rev. 2010, 9, 51–60. [Google Scholar] [CrossRef]
- Weinert, D.; Waterhouse, J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol. Behav. 2007, 90, 246–256. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Dumont, M.; Duffy, J.F.; Steinberg, J.D.; Richardson, G.S.; Brown, E.N.; Sanchez, R.; Rios, C.D.; Ronda, J.M. Association of sleep–wake habits in older people with changes in output of circadian pacemaker. Lancet 1992, 340, 933–936. [Google Scholar] [CrossRef]
- Mortola, J.P. Gender and the circadian pattern of body temperature in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2017, 245, 4–12. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakagawa, T.; Hiyama, T.; Katsu, H.; Wakutsu, N.; Koga, M.; Usijima, S. Circadian changes in body temperature during the menstrual cycle of healthy adult females and patients suffering from premenstrual syndrome. Int. J. Clin. Pharmacol. Res. 1997, 17, 155–164. [Google Scholar]
- Duffy, J.F.; Cain, S.W.; Chang, A.M.; Phillips, A.J.K.; Münch, M.Y.; Gronfier, C.; Wyatt, J.K.; Dijk, D.J.; Wright, K.P., Jr.; Czeisler, C.A. Sex differences in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108, 15602–15608. [Google Scholar] [CrossRef] [Green Version]
- Te Lindert, B.H.W.; Van Someren, E.J.W. Skin temperature, sleep, and vigilance. Handb. Clin. Neurol. 2018, 156, 353–365. [Google Scholar]
- Monk, T.H.; Buysse, D.J.; Billy, B.D.; Fletcher, M.E.; Kennedy, K.S. Polysomnographic Sleep and Circadian Temperature Rhythms as a Function of Prior Shift Work Exposure in Retired Seniors. Healthy Aging Clin. Care Elder 2013, 2013, 9–19. [Google Scholar]
- Jang, T.W.; Kim, H.; Kang, S.H.; Choo, S.H.; Lee, I.S.; Choi, K.H. Circadian Rhythm of Wrist Temperature among Shift Workers in South Korea: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2017, 14, 1109. [Google Scholar] [CrossRef] [Green Version]
- Ki, Y.; Ri, H.; Lee, H.; Yoo, E.; Choe, J.; Lim, C. Warming Up Your Tick-Tock: Temperature-Dependent Regulation of Circadian Clocks. Neuroscientist 2015, 21, 503–518. [Google Scholar] [CrossRef]
- Glaser, F.T.; Stanewsky, R. Temperature synchronization of the Drosophila circadian clock. Curr. Biol. 2005, 15, 1352–1363. [Google Scholar] [CrossRef] [Green Version]
- Buhr, E.D.; Yoo, S.H.; Takahashi, J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Ostberg, J.R.; Taylor, S.L.; Baumann, H.; Repasky, E.A. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J. Leukoc. Biol. 2000, 68, 815–820. [Google Scholar]
- Morf, J.; Rey, G.; Schneider, K.; Stratmann, M.; Fujita, J.; Naef, F.; Schibler, U. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science 2012, 338, 379–383. [Google Scholar] [CrossRef]
- Tamaru, T.; Hattori, M.; Honda, K.; Benjamin, I.; Ozawa, T.; Takamatsu, K. Synchronization of circadian Per2 rhythms and HSF1-BMAL1:CLOCK interaction in mouse fibroblasts after short-term heat shock pulse. PLoS ONE 2011, 6, e24521. [Google Scholar] [CrossRef]
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007, 5, e34. [Google Scholar] [CrossRef]
- Doi, M.; Shimatani, H.; Atobe, Y.; Murai, I.; Hayashi, H.; Takahashi, Y.; Fustin, J.M.; Yamaguchi, Y.; Kiyonari, H.; Koike, N.; et al. Non-coding cis-element of Period2 is essential for maintaining organismal circadian behaviour and body temperature rhythmicity. Nat. Commun. 2019, 10, 2563. [Google Scholar] [CrossRef]
- Archer, S.N.; Laing, E.E.; Möller-Levet, C.S.; van der Veen, D.R.; Bucca, G.; Lazar, A.S.; Santhi, N.; Slak, A.; Kabiljo, R.; von Schantz, M.; et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl. Acad. Sci. USA 2014, 111, E682–E691. [Google Scholar] [CrossRef] [Green Version]
- Möller-Levet, C.S.; Archer, S.N.; Bucca, G.; Laing, E.E.; Slak, A.; Kabiljo, R.; Lo, J.C.; Santhi, N.; von Schantz, M.; Smith, C.P.; et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc. Natl. Acad. Sci. USA 2013, 110, E1132–E1141. [Google Scholar] [CrossRef] [Green Version]
- Bollinger, T.; Bollinger, A.; Oster, H.; Solbach, W. Sleep, immunity, and circadian clocks: A mechanistic model. Gerontology 2010, 56, 574–580. [Google Scholar] [CrossRef]
- Lange, T.; Dimitrov, S.; Born, J. Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sci. 2010, 1193, 48–59. [Google Scholar] [CrossRef]
- Hatzfeld-Charbonnier, A.S.; Lasek, A.; Castera, L.; Gosset, P.; Velu, T.; Formstecher, P.; Mortier, L.; Marchetti, P. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J. Leukoc. Biol. 2007, 81, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Knippertz, I.; Stein, M.F.; Dorrie, J.; Schaft, N.; Müller, I.; Deinzer, A.; Steinkasserer, A.; Nettelbeck, D.M. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int. J. Hyperth. 2011, 27, 591–603. [Google Scholar] [CrossRef]
- Zynda, E.R.; Grimm, M.J.; Yuan, M.; Zhong, L.; Mace, T.A.; Capitano, M.; Ostberg, J.R.; Lee, K.P.; Pralle, A.; Repasky, E.A. A role for the thermal environment in defining co-stimulation requirements for CD4+ T cell activation. Cell Cycle 2015, 14, 2340–2354. [Google Scholar] [CrossRef] [Green Version]
- Mace, T.A.; Zhong, L.; Kilpatrick, C.; Zynda, E.; Lee, C.-T.; Capitano, M.; Minderman, H.; Repasky, E.A. Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J. Leukoc. Biol. 2011, 90, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Sultan, M.F.; Tompkins, W.A.F.; Cain, C.A. Hyperthermic Enhancement of Antibody-Complement Cytotoxicity against Normal Mouse B Lymphocytes and Its Relation to Capping. Radiat. Res. 1983, 96, 251–260. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Ackermann, K.; Revell, V.L.; Lao, O.; Rombouts, E.J.; Skene, D.J.; Kayser, M. Diurnal rhythms in blood cell populations and the effect of acute sleep deprivation in healthy young men. Sleep 2012, 35, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutolo, M.; Straub, R.H. Circadian rhythms in arthritis: Hormonal effects on the immune/inflammatory reaction. Autoimmun. Rev. 2008, 7, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Melchart, D.; Martin, P.; Hallek, M.; Holzmann, M.; Jurcic, X.; Wagner, H. Circadian variation of the phagocytic activity of polymorphonuclear leukocytes and of various other parameters in 13 healthy male adults. Chronobiol. Int. 1992, 9, 35–45. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimba, S.; Tezuka, M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 2007, 30, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Gatti, G.; Del Ponte, D.; Cavallo, R.; Sartori, M.L.; Salvadori, A.; Carignola, R.; Carandente, F.; Angeli, A. Circadian changes in human natural killer-cell activity. Prog. Clin. Biol. Res. 1987, 227A, 399–409. [Google Scholar]
- Ramos, G.V.; Pinheiro, C.M.; Messa, S.P.; Delfino, G.B.; de Cássia Marqueti, R.; de Fátima Salvini, T.; Durigan, J.L.Q. Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci. Rep. 2016, 6, 18525. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Wei, Z.Z.; Cao, W.; Won, S.; Gu, X.; Winter, M.; Dix, T.A.; Wei, L.; Yu, S.P. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol. Dis. 2016, 96, 248–260. [Google Scholar] [CrossRef] [Green Version]
- Kokolus, K.M.; Capitano, M.L.; Lee, C.-T.; Eng, J.W.-L.; Waight, J.D.; Hylander, B.L.; Sexton, S.; Hong, C.-C.; Gordon, C.J.; Abrams, S.I.; et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 20176–20181. [Google Scholar] [CrossRef] [Green Version]
- Chichelnitskiy, E.; Himmelseher, B.; Bachmann, M.; Pfeilschifter, J.; Mühl, H. Hypothermia promotes interleukin-22 expression and fine-tunes its biological activity. Front. Immunol. 2017, 8, 742. [Google Scholar] [CrossRef]
- Leigh, N.D.; Kokolus, K.M.; O’Neill, R.E.; Du, W.; Eng, J.W.-L.; Qiu, J.; Chen, J.L.; McCarthy, P.L.; Farrar, J.D.; Cao, X.; et al. Housing temperature-induced stress is suppressing murine graft versus-host disease through beta2-adrenergic receptor signaling. J. Immunol. 2015, 195, 5045–5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostberg, J.R.; Gellin, C.; Patel, R.; Repasky, E.A. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J. Immunol. 2001, 167, 2666–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.P.; Barani Lonbani, Z.; Woodruff, M.A.; Parker, T.J.; Steck, R.; Peake, J.M. Effects of Topical Icing on Inflammation, Angiogenesis, Revascularization, and Myofiber Regeneration in Skeletal Muscle Following Contusion Injury. Front. Physiol. 2017, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appenheimer, M.M.; Evans, S.S. Temperature and adaptive immunity. Handb. Clin. Neurol. 2018, 156, 397–415. [Google Scholar]
- Peres Bota, D.; Lopes Ferreira, F.; Mélot, C.; Vincent, J.L. Body temperature alterations in the critically ill. Intensive Care Med. 2004, 30, 811–816. [Google Scholar] [CrossRef]
- Gibbs, J.; Ince, L.; Matthews, L.; Mei, J.; Bell, T.; Yang, N.; Saer, B.; Begley, N.; Poolman, T.; Pariollaud, M.; et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 2014, 20, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Araki, T.; Endo, M. Circadian clock during plant development. J. Plant Res. 2018, 131, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Hua, J. Modulation of plant immunity by light, circadian rhythm, and temperature. Curr. Opin. Plant Biol. 2013, 16, 406–413. [Google Scholar] [CrossRef]
- Terrell, K.A.; Quintero, R.P.; Murray, S.; Kleopfer, J.D.; Murphy, J.B.; Evans, M.J.; Nissen, B.D.; Gratwicke, B. Cryptic impacts of temperature variability on amphibian immune function. J. Exp. Biol. 2013, 216, 4204–4211. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Bai, S.; Du, L. Temperature regulates circadian rhythms of immune responses in red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2015, 45, 641–647. [Google Scholar] [CrossRef]
- Ratajczak, H.V.; Lange, R.W.; Sothern, R.B.; Hagen, K.L.; Vescei, P.; Wu, J.; Halberg, F.; Thomas, P.T. Surgical influence on murine immunity and tumor growth: Relationship of body temperature and hormones with splenocytes. Proc. Soc. Exp. Biol. Med. 1992, 199, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Takahashi, J.S.; Figueiredo, L.M. Circadian rhythms in parasites. PLoS Pathog. 2017, 13, e1006590. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, U.; Thakkar, N.; Das, P.; Pal Bhadra, M. Evolution of circadian rhythms: From bacteria to human. Sleep Med. 2017, 35, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Swan, J.A.; Golden, S.S.; LiWang, A.; Partch, C.L. Structure, function, and mechanism of the core circadian clock in cyanobacteria. J. Biol. Chem. 2018, 293, 5026–5034. [Google Scholar] [CrossRef] [Green Version]
- Schmelling, N.M.; Lehmann, R.; Chaudhury, P.; Beck, C.; Albers, S.V.; Axmann, I.M.; Wiegard, A. Minimal tool set for a prokaryotic circadian clock. BMC Evol. Biol. 2017, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- Rust, M.J.; Golden, S.S.; OShea, E.K. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science 2011, 331, 220–223. [Google Scholar] [CrossRef] [Green Version]
- Rijo-Ferreira, F.; Pinto-Neves, D.; Barbosa-Morais, N.L.; Takahashi, J.S.; Figueiredo, L.M. Trypanosoma brucei metabolism is under circadian control. Nat. Microbiol. 2017, 2, 17032. [Google Scholar] [CrossRef]
- Paulose, J.K.; Cassone, C.V.; Graniczkowska, K.B.; Cassone, V.M. Entrainment of the Circadian Clock of the Enteric Bacterium Klebsiella aerogenes by Temperature Cycles. iScience 2019, 19, 1202–1213. [Google Scholar] [CrossRef]
- Liang, X.; FitzGerald, G.A. Timing the Microbes: The Circadian Rhythm of the Gut Microbiome. J. Biol. Rhythms 2017, 32, 505–515. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef] [Green Version]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdock, C.C.; Paaijmans, K.P.; Cox-Foster, D.; Read, A.F.; Thomas, M.B. Rethinking vector immunology: The role of environmental temperature in shaping resistance. Nat. Rev. Microbiol. 2012, 10, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Koelzow, N.; Hoeltje, H.; Karl, I. Assay conditions in laboratory experiments: Is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 2011, 166, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Sundén-Cullberg, J.; Rylance, R.; Svefors, J.; Norrby-Teglund, A.; Björk, J.; Inghammar, M. Fever in the Emergency Department Predicts Survival of Patients with Severe Sepsis and Septic Shock Admitted to the ICU. Crit. Care Med. 2017, 45, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kiekkas, P.; Velissaris, D.; Karanikolas, M.; Aretha, D.; Samios, A.; Skartsani, C.; Baltopoulos, G.I.; Filos, K.S. Peak body temperature predicts mortality in critically ill patients without cerebral damage. Heart Lung 2010, 39, 208–216. [Google Scholar] [CrossRef]
- Garami, A.; Steiner, A.A.; Romanovsky, A.A. Fever and hypothermia in systemic inflammation. Handb. Clin. Neurol. 2018, 157, 565–597. [Google Scholar]
- Schwarz, S.; Häfner, K.; Aschoff, A.; Schwab, S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000, 54, 354–361. [Google Scholar] [CrossRef]
- Bao, L.; Chen, D.; Ding, L.; Ling, W.; Xu, F. Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS ONE 2014, 9, e90956. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.; Shaikh, D.H.; Mantri, N.; Alemam, A.; Zhang, A.; Adrish, M. Fever is associated with higher morbidity and clot burden in patients with acute pulmonary embolism. BMJ Open Respir. Res. 2018, 5, e000327. [Google Scholar] [CrossRef]
- Smid, J.; Scherner, M.; Wolfram, O.; Groscheck, T.; Wippermann, J.; Braun-Dullaeus, R.C. Cardiogenic Causes of Fever. Dtsch. Arztebl. Int. 2018, 115, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Feng, F.; Tian, Y.; Yang, X.; Sun, L.; Hong, L.; Yang, J.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Postoperative fever predicts poor prognosis of gastric cancer. Oncotarget 2017, 8, 62622–62629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.G.; Ahn, C.; Shin, H.; Kim, W.; Lim, T.H.; Jang, B.H.; Cho, Y.; Choi, K.S.; Lee, J.; Na, M.K. Efficacy of the cooling method for targeted temperature management in post-cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2020, 148, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.J.; Nichol, A.D.; Bailey, M.; Bernard, S.; Cameron, P.A.; Pili-Floury, S.; Forbes, A.; Gantner, D.; Higgins, A.M.; Huet, O.; et al. POLAR Trial Investigators and the ANZICS Clinical Trials Group. Effect of Early Sustained Prophylactic Hypothermia on Neurologic Outcomes Among Patients with Severe Traumatic Brain Injury: The POLAR Randomized Clinical Trial. JAMA 2018, 320, 2211–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.R.; Munn, D.D.; Meredith, J.W.; Chang, M.C. Systemic inflammatory response syndrome in the trauma intensive care unit: Who is infected? J. Trauma 1999, 47, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.; Moore, F.A.; Moore, E.E.; Balogh, Z. Postinjury multiple organ failure. Injury 2009, 40, 912–918. [Google Scholar] [CrossRef]
- Grima, N.A.; Ponsford, J.; Hilaire, M.A.S.; Mansfield, D.; Rajaratnam, S.M.W. Circadian Melatonin Rhythm Following Traumatic Brain Injury. Neurorehabilit. Neural Repair 2016, 30, 972–977. [Google Scholar] [CrossRef]
- Coiffard, B.; Diallo, A.B.; Culver, A.; Mezouar, S.; Hammad, E.; Vigne, C.; Nicolino-Brunet, C.; Dignat-George, F.; Baumstarck, K.; Boucekine, M.; et al. Circadian Rhythm Disruption and Sepsis in Severe Trauma Patients. Shock 2019, 52, 29–36. [Google Scholar] [CrossRef]
- Blume, C.; Lechinger, J.; Santhi, N.; del Giudice, R.; Gnjezda, M.T.; Pichler, G.; Scarpatetti, M.; Donis, J.; Michitsch, G.; Schabus, M. Significance of circadian rhythms in severely brain-injured patients: A clue to consciousness? Neurology 2017, 88, 1933–1941. [Google Scholar] [CrossRef] [Green Version]
- Culver, A.; Coiffard, B.; Antonini, F.; Duclos, G.; Hammad, E.; Vigne, C.; Mege, J.L.; Baumstarck, K.; Boucekine, M.; Zieleskiewicz, L.; et al. Circadian disruption of core body temperature in trauma patients: A single-center retrospective observational study. J. Intensive Care 2020, 8, 4. [Google Scholar] [CrossRef]
- Boone, D.R.; Sell, S.L.; Micci, M.A.; Crookshanks, J.M.; Parsley, M.; Uchida, T.; Prough, D.S.; DeWitt, D.S.; Hellmich, H.L. Traumatic brain injury-induced dysregulation of the circadian clock. PLoS ONE 2012, 7, e46204. [Google Scholar] [CrossRef]
- Diallo, A.B.; Coiffard, B.; Leone, M.; Mezouar, S.; Mege, J.L. For Whom the Clock Ticks: Clinical Chronobiology for Infectious Diseases. Front. Immunol. 2020, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.; Mebazaa, A.; Plaud, B.; Legrand, M. ‘Chronomics’ in ICU: Circadian aspects of immune response and therapeutic perspectives in the critically ill. Intensive Care Med. Exp. 2014, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tweedie, I.E.; Bell, C.F.; Clegg, A.; Campbell, I.T.; Minors, D.S.; Waterhouse, J.M. Retrospective study of temperature rhythms of intensive care patients. Crit. Care Med. 1989, 17, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, J.A.C.; Van Dongen, H.P.A.; Grant, D.A.; Freedman, N.S.; Zwaveling, J.H.; Schwab, R.J. Altered circadian rhythmicity in patients in the ICU. Chest 2013, 144, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaioannou, V.E.; Sertaridou, E.N.; Chouvarda, I.G.; Kolios, G.C.; Pneumatikos, I.N. Determining rhythmicity and determinism of temperature curves in septic and non-septic critically ill patients through chronobiological and recurrence quantification analysis: A pilot study. Intensive Care Med. Exp. 2019, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Sothern, R.B.; Rhame, F.; Suarez, C.; Fletcher, C.; Sackett-Lundeen, L.; Haus, E.; Hrushesky, W.J. Oral temperature rhythmometry and substantial within-day variation in zidovudine levels following steady-state dosing in human immunodeficiency virus (HIV) infection. Prog. Clin. Biol. Res. 1990, 341A, 67–76. [Google Scholar]
- Magona, J.W.; Walubengo, J.; Olaho-Mukani, W.; Jonsson, N.N.; Eisler, M.C. Diagnostic value of rectal temperature of African cattle of variable coat colour infected with trypanosomes and tick-borne infections. Vet. Parasitol. 2009, 160, 301–305. [Google Scholar] [CrossRef]
- Huitron-Resendiz, S.; Marcondes, M.C.; Flynn, C.T.; Lanigan, C.M.; Fox, H.S. Effects of simian immunodeficiency virus on the circadian rhythms of body temperature and gross locomotor activity. Proc. Natl. Acad. Sci. USA 2007, 104, 15138–15143. [Google Scholar] [CrossRef] [Green Version]
- Gautherie, M.; Gros, C. Circadian rhythm alteration of skin temperature in breast cancer. Chronobiologia 1977, 4, 1–17. [Google Scholar]
- Bailleul, F.; Lévi, F.; Reinberg, A.; Mathé, G. Interindividual differences in the circadian hematologic time structure of cancer patients. Chronobiol. Int. 1986, 3, 47–54. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Gilchrist, J.M.; Chen, K.; Gautam, S.; Freedman, R.R. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause 2004, 11, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Roche, V.P.; Mohamad-Djafari, A.; Innominato, P.F.; Karaboué, A.; Gorbach, A.; Lévi, F.A. Thoracic surface temperature rhythms as circadian biomarkers for cancer chronotherapy. Chronobiol. Int. 2014, 31, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, C.G.; Karaboué, A.; Liu, W.M.; Meyer, J.; Innominato, P.F.; Chon, K.H.; Gorbach, A.M.; Lévi, F. Skin surface temperature rhythms as potential circadian biomarkers for personalized chronotherapeutics in cancer patients. Interface Focus 2011, 1, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Okyar, A.; Dulong, S.; Innominato, P.F.; Clairambault, J. Circadian timing in cancer treatments. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 377–421. [Google Scholar] [CrossRef] [Green Version]
- Smolensky, M.H.; Hermida, R.C.; Reinberg, A.; Sackett-Lundeen, L.; Portaluppi, F. Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol. Int. 2016, 33, 1101–1119. [Google Scholar] [CrossRef]
- Barnes, P.; FitzGerald, G.; Brown, M.; Dollery, C. Nocturnal asthma and changes in circulating epinephrine, histamine, and cortisol. N. Engl. J. Med. 1980, 303, 263–267. [Google Scholar] [CrossRef]
- Hand, L.E.; Hopwood, T.W.; Dickson, S.H.; Walker, A.L.; Loudon, A.S.; Ray, D.W.; Bechtold, D.A.; Gibbs, J.E. The circadian clock regulates inflammatory arthritis. FASEB J. 2016, 30, 3759–3770. [Google Scholar] [CrossRef] [Green Version]
- Gombert, M.; Carrasco-Luna, J.; Pin-Arboledas, G.; Codoñer-Franch, P. The connection of circadian rhythm to inflammatory bowel disease. Transl. Res. 2019, 206, 107–118. [Google Scholar] [CrossRef]
- Radaelli, A.; Carandente, F.; Tadini, G.; Ronchi, M.; Zoccoli, A.; Caccialanza, P. Circadian temporal structure in psoriasis. Chronobiologia 1982, 9, 203–209. [Google Scholar]
- Xu, Z.; Huang, C.; Su, H.; Turner, L.R.; Qiao, Z.; Tong, S. Diurnal temperature range and childhood asthma: A time-series study. Environ. Health 2013, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Y.; Chai, H. Airway cooling and nocturnal asthma. Chest 1982, 81, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.J. Seasonal immune function and sickness responses. Trends Immunol. 2004, 25, 187–192. [Google Scholar] [CrossRef] [PubMed]


| Trend | Immune System Variation | Species | Model | Ref. | |
|---|---|---|---|---|---|
| Fever | ⬈ | DC migration | Human (healthy) | In vitro | [45] |
| ⬈ | APC and T-cell interactions | Human (healthy) | In vitro | [45,46] | |
| ⬈ | TNF and IL-12 production | Mice (WT) | In vivo and in vitro | [36,46] | |
| ⬈ | IL-2 CD4+ T-cell production | Human (healthy) | In vitro | [47] | |
| ⬈ | IFN-γ CD8+ T-cell production | Mice (WT) | In vitro | [48] | |
| ⬈ | Ab-dependent complement-mediated lysis | Mice (healthy) | In vitro | [49] | |
| 37.5 °C | ⬈ | Lymphocyte adhesion and trafficking | Human/Mice (healthy) | In vitro | [50] |
| ~ | Blood and tissue leukocyte number | Human (healthy)/Mice (WT/KO) | In vitro and in vivo | [51,52] | |
| ~ | Neutrophils ICAM-1 expression | Mice (WT/KO) | In vitro and in vivo | [52] | |
| ~ | Cytokine production | Human (arthritis) | In vivo and in vitro | [53] | |
| ~ | Phagocytic activity | Human (healthy)/Mice (WT/siRNA) | In vivo and in vitro | [54,55] | |
| ~ | Natural killer-cell activity | Human (healthy) | In vitro | [56] | |
| ~ | Whole-blood transcriptome | Human (healthy) | In vivo and in vitro | [41] | |
| 36.5 °C | ⬊ | DC tissue infiltration | Rat (WT) | In vivo and in vitro | [57] |
| Hypothermia | ⬊ | TNF and IL-12 production | Mice (WT with ischemic stroke) | In vivo and in vitro | [58] |
| ⬈ | IL-10 production | Mice (WT with ischemic stroke) | In vivo and in vitro | [58] | |
| ⬊ | DC maturation | Mice (WT with tumor) | In vivo and in vitro | [59] | |
| ⬊ | T-cell-activating function | Mice (WT with tumor) | In vivo and in vitro | [59] | |
| ⬊ | IFN-γ T-cell production | Mice (endotoxemic) | In vivo and in vitro | [60] | |
| ⬊ | Lymphocyte tissue infiltration | Mice (WT with acute GVHD) | In vivo and in vitro | [61] | |
| ⬊ | Homing of lymphocytes | Mice (WT experiencing CHS) | In vivo and in vitro | [62] | |
| ⬈ | Neutrophil and macrophage infiltration | Rat (Muscle contusion injury) | In vivo and in vitro | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coiffard, B.; Diallo, A.B.; Mezouar, S.; Leone, M.; Mege, J.-L. A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System. Biology 2021, 10, 65. https://doi.org/10.3390/biology10010065
Coiffard B, Diallo AB, Mezouar S, Leone M, Mege J-L. A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System. Biology. 2021; 10(1):65. https://doi.org/10.3390/biology10010065
Chicago/Turabian StyleCoiffard, Benjamin, Aïssatou Bailo Diallo, Soraya Mezouar, Marc Leone, and Jean-Louis Mege. 2021. "A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System" Biology 10, no. 1: 65. https://doi.org/10.3390/biology10010065
APA StyleCoiffard, B., Diallo, A. B., Mezouar, S., Leone, M., & Mege, J. -L. (2021). A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System. Biology, 10(1), 65. https://doi.org/10.3390/biology10010065






