Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Human Adipose-Derived Mesenchymal Stem Cells
2.2. Culture of NSCLC Lines
2.3. Production of MSC-TRAIL
2.4. Isolation and Characterisation of CD133+ CSCs
2.5. Analysis of Population Doubling Time
2.6. Analysis of IC50 Values of Different Chemotherapies
2.7. Chemo-Sensitization of CD133+ CSCs to MSC-TRAIL
2.8. Statistical Analysis
3. Results
3.1. Population Doublings of MSCs and NSCLC
3.2. Chemo-Sensitivity of MSCs versus NSCLC Cell Lines
3.3. IC50 Values of Chemotherapies in NSCLC and MSCs
3.4. Chemo-Sensitization of A549-Derived CD133+ CSCs
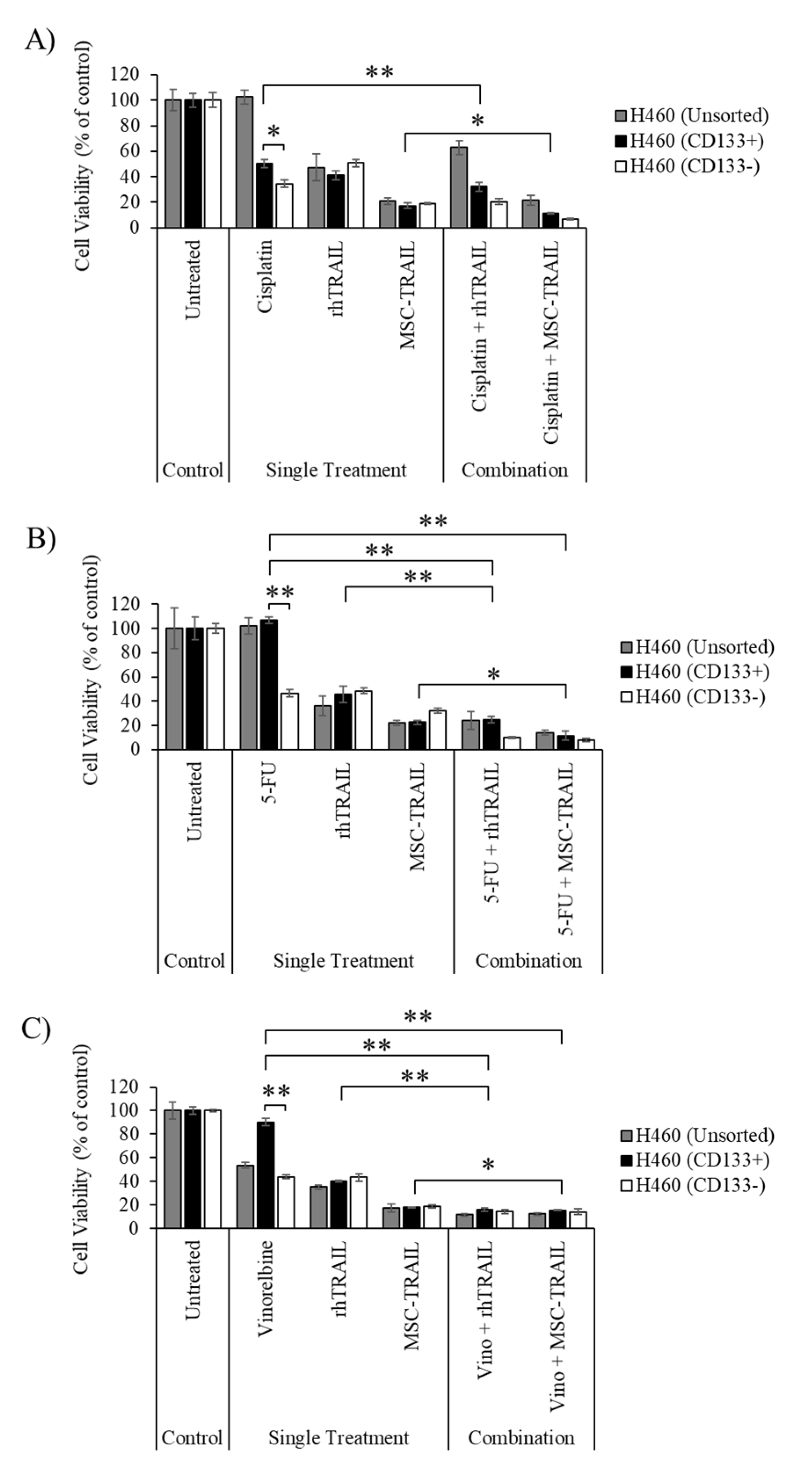
3.5. Chemo-Sensitization of H460-Derived CD133+ CSCs
3.6. Chemo-Sensitizing of H2170-Derived CD133+ CSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travis, W.D.; Travis, L.B.; Devesa, S.S. Lung Cancer. Cancer 1995, 75 (Suppl. 1), 191–202. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Latimer, K.M. Lung Cancer: Clinical Presentation and Diagnosis. FP Essent. 2018, 464, 23–26. [Google Scholar]
- Stewart, D.J. Mechanisms of Resistance to Cisplatin and Carboplatin. Crit. Rev. Oncol. Hematol. 2007, 63, 12–31. [Google Scholar] [CrossRef]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly Tumorigenic Lung Cancer CD133+ Cells Display Stem-like Features and Are Spared by Cisplatin Treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef] [Green Version]
- Hong, I.S.; Lee, H.Y.; Nam, J.S. Cancer Stem Cells: The “Achilles” Heel’ of Chemo-Resistant Tumors. Recent Pat. Anti-Cancer Drug Discov. 2014, 10, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL Death Receptor-Based Cancer Therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Marini, P.; Denzinger, S.; Schiller, D.; Kauder, S.; Welz, S.; Humphreys, R.; Daniel, P.T.; Jendrossek, V.; Budach, W.; Belka, C. Combined Treatment of Colorectal Tumours with Agonistic TRAIL Receptor Antibodies HGS-ETR1 and HGS-ETR2 and Radiotherapy: Enhanced Effects in Vitro and Dose-Dependent Growth Delay in Vivo. Oncogene 2006, 25, 5145–5154. [Google Scholar] [CrossRef] [Green Version]
- Nagane, M.; Pan, G.; Weddle, J.J.; Dixit, V.M.; Cavenee, W.K.; Huang, H.J. Increased Death Receptor 5 Expression by Chemotherapeutic Agents in Human Gliomas Causes Synergistic Cytotoxicity with Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Vitro and in Vivo. Cancer Res. 2000, 60, 847–853. [Google Scholar] [PubMed]
- Joshi, P.; Jeon, Y.-J.; Laganà, A.; Middleton, J.; Secchiero, P.; Garofalo, M.; Croce, C.M. MicroRNA-148a Reduces Tumorigenesis and Increases TRAIL-Induced Apoptosis in NSCLC. Proc. Natl. Acad. Sci. USA 2015, 112, 8650–8655. [Google Scholar] [CrossRef] [Green Version]
- Anding, A.L.; Jones, J.D.; Newton, M.A.; Curley, R.W.J.; Clagett-Dame, M. 4-HPR Is an Endoplasmic Reticulum Stress Aggravator and Sensitizes Breast Cancer Cells Resistant to TRAIL/Apo2L. Anticancer Res. 2018, 38, 4403–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.-C.; Jayaram, S.; Ganesh, L.; Qian, L.; Rotmensch, J.; Maker, A.V.; Prabhakar, B.S. Knockdown of MADD and C-FLIP Overcomes Resistance to TRAIL-Induced Apoptosis in Ovarian Cancer Cells. Am. J. Obstet. Gynecol. 2011, 205, 362.e12–362.e25. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Z.; Xu, F.; Yuan, K.; Sun, Y.; Zhou, T.; Zhao, X.; McDonald, J.M.; Chen, Y. Regulation of Pancreatic Cancer TRAIL Resistance by Protein O-GlcNAcylation. Lab. Investig. 2020, 100, 777–785. [Google Scholar] [CrossRef]
- Safa, A.R. Resistance to Cell Death and Its Modulation in Cancer Stem Cells. Crit. Rev. Oncog. 2016, 21, 203–219. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, W.; Liu, S.; Chi, J.; Zhang, J.; Sui, A.; Wang, L.; Liang, Z.; Li, D.; Chen, Y.; et al. N-Acetyl-Glucosamine Sensitizes Non-Small Cell Lung Cancer Cells to TRAIL-Induced Apoptosis by Activating Death Receptor 5. Cell. Physiol. Biochem. 2018, 45, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Obaidi, I.; Cassidy, H.; Gaspar, V.I.; McCaul, J.; Higgins, M.; Halász, M.; Reynolds, A.L.; Kennedy, B.N.; McMorrow, T. Curcumin Sensitizes Kidney Cancer Cells to TRAIL-Induced Apoptosis via ROS Mediated Activation of JNK-CHOP Pathway and Upregulation of DR4. Biology 2020, 9, 92. [Google Scholar] [CrossRef]
- Kelley, S.K.; Harris, L.A.; Xie, D.; Deforge, L.; Totpal, K.; Bussiere, J.; Fox, J.A. Preclinical Studies to Predict the Disposition of Apo2L/Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Humans: Characterization of in Vivo Efficacy, Pharmacokinetics, and Safety. J. Pharmacol. Exp. Ther. 2001, 299, 31–38. [Google Scholar] [PubMed]
- Wong, S.H.M.; Kong, W.Y.; Fang, C.-M.; Loh, H.-S.; Chuah, L.-H.; Abdullah, S.; Ngai, S.C. The TRAIL to Cancer Therapy: Hindrances and Potential Solutions. Crit. Rev. Oncol. Hematol. 2019, 143, 81–94. [Google Scholar] [CrossRef]
- Duiker, E.W.; Dijkers, E.C.F.; Lambers Heerspink, H.; de Jong, S.; van der Zee, A.G.J.; Jager, P.L.; Kosterink, J.G.W.; de Vries, E.G.E.; Lub-de Hooge, M.N. Development of a Radioiodinated Apoptosis-Inducing Ligand, RhTRAIL, and a Radiolabelled Agonist TRAIL Receptor Antibody for Clinical Imaging Studies. Br. J. Pharmacol. 2012, 165, 2203–2212. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Tung, C.-H.; Yang, K.; Weissleder, R.; Breakefield, X.O. Inducible Release of TRAIL Fusion Proteins from a Proapoptotic Form for Tumor Therapy. Cancer Res. 2004, 64, 3236–3242. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Wang, Y.; Wang, X.; Wang, Y.; Ma, Y. Tagged and Untagged TRAIL Show Different Activity against Tumor Cells. Oncol. Lett. 2012, 4, 1301–1304. [Google Scholar] [CrossRef]
- Müller, N.; Schneider, B.; Pfizenmaier, K.; Wajant, H. Superior Serum Half Life of Albumin Tagged TNF Ligands. Biochem. Biophys. Res. Commun. 2010, 396, 793–799. [Google Scholar] [CrossRef]
- Singh, M.; Sori, H.; Ahuja, R.; Meena, J.; Sehgal, D.; Panda, A.K. Effect of N-Terminal Poly Histidine-Tag on Immunogenicity of Streptococcus Pneumoniae Surface Protein SP0845. Int. J. Biol. Macromol. 2020, 163, 1240–1248. [Google Scholar] [CrossRef]
- Lawrence, D.; Shahrokh, Z.; Marsters, S.; Achilles, K.; Shih, D.; Mounho, B.; Hillan, K.; Totpal, K.; DeForge, L.; Schow, P.; et al. Differential Hepatocyte Toxicity of Recombinant Apo2L/TRAIL Versions. Nat. Med. 2001, 7, 383–385. [Google Scholar] [CrossRef]
- Zhong, H.-H.; Wang, H.-Y.; Li, J.; Huang, Y.-Z. TRAIL-Based Gene Delivery and Therapeutic Strategies. Acta Pharmacol. Sin. 2019, 40, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Jia, Z.; Yin, X.; Zhang, X.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Cartilage, and Adipose Tissue. Stem Cells Dev. 2008, 17, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Zhu, L.; Oh, J.M.; Gangadaran, P.; Lee, H.W.; Baek, S.H.; Rajendran, R.L.; Gopal, A.; Jeong, S.Y.; Lee, S.-W.; et al. Migration of Mesenchymal Stem Cells to Tumor Xenograft Models and in Vitro Drug Delivery by Doxorubicin. Int. J. Med. Sci. 2018, 15, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Chulpanova, D.S.; Gilazieva, Z.E.; Kletukhina, S.K.; Aimaletdinov, A.M.; Garanina, E.E.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Cytochalasin B-Induced Membrane Vesicles from Human Mesenchymal Stem Cells Overexpressing IL2 Are Able to Stimulate CD8(+) T-Killers to Kill Human Triple Negative Breast Cancer Cells. Biology 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Chen, Y.; Lu, L.; Hu, X.; Shao, C.; Zhang, Y.; Zhou, X.; Zhou, Y.; Wu, L.; Liu, R.; et al. Human Umbilical Cord Blood- Derived Mesenchymal Stem Cells Producing IL-15 Eradicate Established Pancreatic Tumor in Syngeneic Mice. Mol. Cancer Ther. 2014, 13, 2127–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone Marrow-Derived Mesenchymal Stem Cells as Vehicles for Interferon-β Delivery into Tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar] [PubMed]
- Un Choi, Y.; Yoon, Y.; Jung, P.Y.; Hwang, S.; Hong, J.E.; Kim, W.-S.; Sohn, J.H.; Rhee, K.-J.; Bae, K.S.; Eom, Y.W. TRAIL-Overexpressing Adipose Tissue-Derived Mesenchymal Stem Cells Efficiently Inhibit Tumor Growth in an H460 Xenograft Model. Cancer Genom. Proteom. 2021, 18, 569–578. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Ma, T.; Zeng, J.; Zhou, X.; Li, H.; Tang, M.; Liu, X.; Li, F.; Jiang, B.; et al. Secreted TRAIL Gene-Modified Adipose-Derived Stem Cells Exhibited Potent Tumor-Suppressive Effect in Hepatocellular Carcinoma Cells. Immun. Inflamm. Dis. 2021, 9, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Chu, T.; Brooke, G.N.; Zwacka, R.M. MSC.STRAIL Has Better Efficacy than MSC.FL-TRAIL and in Combination with AKTi Blocks Pro-Metastatic Cytokine Production in Prostate Cancer Cells. Cancers 2019, 11, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.R.; Park, S.A.; Ahn, S.; Jeun, S.-S.; Ryu, C.H. Evaluation of Combination Treatment Effect With TRAIL-Secreting Mesenchymal Stem Cells and Compound C Against Glioblastoma. Anticancer Res. 2019, 39, 6635–6643. [Google Scholar] [CrossRef]
- Li, M.; Sun, S.; Dangelmajer, S.; Zhang, Q.; Wang, J.; Hu, F.; Dong, F.; Kahlert, U.D.; Zhu, M.; Lei, T. Exploiting Tumor-Intrinsic Signals to Induce Mesenchymal Stem Cell-Mediated Suicide Gene Therapy to Fight Malignant Glioma. Stem Cell Res. Ther. 2019, 10, 88. [Google Scholar] [CrossRef]
- Kretz, A.L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. Trailblazing Strategies for Cancer Treatment. Cancers 2019, 11, 456. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Deedigan, L.; Albarenque, S.M.; Mohr, A.; Zwacka, R.M. Delivery of STRAIL Variants by MSCs in Combination with Cytotoxic Drug Treatment Leads to P53-Independent Enhanced Antitumor Effects. Cell Death Dis. 2013, 4, e503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.A.; Lee, C.; Kwak, P.A.; Park, C.-K.; Wang, K.-C.; Phi, J.H.; Lee, J.Y.; Chong, S.; Kim, S.-K. Histone Deacetylase Inhibitor Panobinostat Potentiates the Anti-Cancer Effects of Mesenchymal Stem Cell-Based STRAIL Gene Therapy against Malignant Glioma. Cancer Lett. 2018, 442, 161–169. [Google Scholar] [CrossRef]
- Fakiruddin, K.S.; Ghazalli, N.; Lim, M.N.; Zakaria, Z.; Abdullah, S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. Int. J. Mol. Sci. 2018, 19, 2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redjal, N.; Zhu, Y.; Shah, K. Combination of Systemic Chemotherapy with Local Stem Cell Delivered S-TRAIL in Resected Brain Tumors. Stem Cells 2015, 33, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Shan, H.; Li, D.; Li, Z.R.; Zhu, K.S.; Jiang, Z.B. The Inhibitory Effect of MSCs Expressing TRAIL as a Cellular Delivery Vehicle in Combination with Cisplatin on Hepatocellular Carcinoma. Cancer Biol. Ther. 2012, 13, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Yoon, N.; Park, M.S.; Peltier, G.C.; Lee, R.H. Pre-Activated Human Mesenchymal Stromal Cells in Combination with Doxorubicin Synergistically Enhance Tumor-Suppressive Activity in Mice. Cytotherapy 2015, 17, 1332–1341. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Zhu, W.; Gou, H.; Cao, D.; Yang, Y.; Huang, Y.; Yi, C. Synergistic Effect of Subtoxic-Dose Cisplatin and TRAIL to Mediate Apoptosis by down-Regulating Decoy Receptor 2 and up-Regulating Caspase-8, Caspase-9 and Bax Expression on NCI-H460 and A549 Cells. Iran. J. Basic Med. Sci. 2013, 16, 710–718. [Google Scholar] [PubMed]
- Reinhardt, A.; Liu, H.; Ma, Y.; Zhou, Y.; Zang, C.; Habbel, J.-P.; Possinger, K.; Eucker, J. Tumor Cell-Selective Synergism of TRAIL- and ATRA-Induced Cytotoxicity in Breast Cancer Cells. Anticancer Res. 2018, 38, 2669–2682. [Google Scholar] [CrossRef] [Green Version]
- Kamalabadi-Farahani, M.; Vasei, M.; Ahmadbeigi, N.; Ebrahimi-Barough, S.; Soleimani, M.; Roozafzoon, R. Anti-Tumour Effects of TRAIL-Expressing Human Placental Derived Mesenchymal Stem Cells with Curcumin-Loaded Chitosan Nanoparticles in a Mice Model of Triple Negative Breast Cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S1011–S102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Hyun, S.-K.; Kim, H.-B.; Kang, C.-D.; Kim, S.-H. Potential Role of CD133 Expression in the Susceptibility of Human Liver Cancer Stem-Like Cells to TRAIL. Oncol. Res. 2016, 24, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Fakiruddin, K.S.; Lim, M.; Nordin, N.; Rosli, R.; Zakaria, Z.; Abdullah, S. Targeting of CD133+ Cancer Stem Cells by Mesenchymal Stem Cell Expressing TRAIL Reveals a Prospective Role of Apoptotic Gene Regulation in Non-Small Cell Lung Cancer. Cancers 2019, 11, 1261. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.Q.; Kolluri, K.K.; Sage, E.K.; Gowers, K.H.C.; Janes, S.M. Mesenchymal Stromal Cell Delivery of Full-Length Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Is Superior to Soluble Type for Cancer Therapy. Cytotherapy 2015, 17, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Wang, W.; Bai, Y. Claudin-7 Suppresses the Cytotoxicity of TRAIL-Expressing Mesenchymal Stem Cells in H460 Human Non-Small Cell Lung Cancer Cells. Apoptosis 2014, 19, 491–505. [Google Scholar] [CrossRef]
- Mohr, A.; Lyons, M.; Deedigan, L.; Harte, T.; Shaw, G.; Howard, L.; Barry, F.; O’Brien, T.; Zwacka, R. Mesenchymal Stem Cells Expressing TRAIL Lead to Tumour Growth Inhibition in an Experimental Lung Cancer Model. J. Cell. Mol. Med. 2008, 12, 2628–2643. [Google Scholar] [CrossRef] [Green Version]
- Artykov, A.A.; Belov, D.A.; Shipunova, V.O.; Trushina, D.B.; Deyev, S.M.; Dolgikh, D.A.; Kirpichnikov, M.P.; Gasparian, M.E. Chemotherapeutic Agents Sensitize Resistant Cancer Cells to the DR5-Specific Variant DR5-B More Efficiently than to TRAIL by Modulating the Surface Expression of Death and Decoy Receptors. Cancers 2020, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Li, J.Z.; Ma, Y.Y.; Qian, D.; Zhong, J.Y.; Jin, M.M.; Huang, P.; Che, L.Y.; Pan, B.; Wang, Y.; et al. Shikonin Sensitizes A549 Cells to TRAIL-Induced Apoptosis through the JNK, STAT3 and AKT Pathways. BMC Cell Biol. 2018, 19, 29. [Google Scholar] [CrossRef]
- Wang, P.; Aguirre, A. New Strategies and In Vivo Monitoring Methods for Stem Cell-Based Anticancer Therapies. Stem Cells Int. 2018, 2018, 7315218. [Google Scholar] [CrossRef]
- Bazzett, L.B.; Watkins, C.S.; Gercel-Taylor, C.; Taylor, D.D. Modulation of Proliferation and Chemosensitivity by Procathepsin D and Its Peptides in Ovarian Cancer. Gynecol. Oncol. 1999, 74, 181–187. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and Their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Dong, J.; Haiech, J.; Kilhoffer, M.-C.; Zeniou, M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016, 2016, 1740936. [Google Scholar] [CrossRef] [Green Version]
- Sterlacci, W.; Savic, S.; Fiegl, M.; Obermann, E.; Tzankov, A. Putative Stem Cell Markers in Non-Small-Cell Lung Cancer: A Clinicopathologic Characterization. J. Thorac. Oncol. 2014, 9, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Tachezy, M.; Zander, H.; Wolters-Eisfeld, G.; Müller, J.; Wicklein, D.; Gebauer, F.; Izbicki, J.R.; Bockhorn, M. Activated Leukocyte Cell Adhesion Molecule (CD166): An “Inert” Cancer Stem Cell Marker for Non-Small Cell Lung Cancer? Stem Cells 2014, 32, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, K.; Wang, Q.; Chen, S.; Gou, Y.; Cui, Y.; Li, Q. Cisplatin-Mediated c-Myc Overexpression and Cytochrome c (Cyt c) Release Result in the up-Regulation of the Death Receptors DR4 and DR5 and the Activation of Caspase 3 and Caspase 9, Likely Responsible for the TRAIL-Sensitizing Effect of Cisplatin. Med. Oncol. 2015, 32, 133. [Google Scholar] [CrossRef]
- Yoon, Y.-K.; Kim, H.-P.; Han, S.-W.; Oh, D.Y.; Im, S.-A.; Bang, Y.-J.; Kim, T.-Y. KRAS Mutant Lung Cancer Cells Are Differentially Responsive to MEK Inhibitor Due to AKT or STAT3 Activation: Implication for Combinatorial Approach. Mol. Carcinog. 2010, 49, 353–362. [Google Scholar] [CrossRef]
- Wang, H.; Yang, T.; Wu, X. 5-Fluorouracil Preferentially Sensitizes Mutant KRAS Non-Small Cell Lung Carcinoma Cells to TRAIL-Induced Apoptosis. Mol. Oncol. 2015, 9, 1815–1824. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and Antitumor Activity of Recombinant Soluble Apo2 Ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huynh, J.M.; Liu, G.-S.; Ballweg, R.; Aryeh, K.S.; Paek, A.L.; Zhang, T. Designing Combination Therapies with Modeling Chaperoned Machine Learning. PLoS Comput. Biol. 2019, 15, e1007158. [Google Scholar] [CrossRef]
- Ding, L.; Yuan, C.; Wei, F.; Wang, G.; Zhang, J.; Bellail, A.C.; Zhang, Z.; Olson, J.J.; Hao, C. Cisplatin Restores TRAIL Apoptotic Pathway in Glioblastoma-Derived Stem Cells through up-Regulation of DR5 and down-Regulation of c-FLIP. Cancer Investig. 2011, 29, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Huang, M.; Ren, D.; He, J.; Zhao, F.; Yi, C.; Huang, Y. The Synergistic Effects of Low Dose Fluorouracil and TRAIL on TRAIL-Resistant Human Gastric Adenocarcinoma AGS Cells. BioMed Res. Int. 2013, 2013, 293874. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Rasheduzzaman, M.; Lee, Y.-J.; Seol, D.-W.; Park, S.-Y. Enhancement of TRAIL-Induced Apoptosis by 5-Fluorouracil Requires Activating Bax and P53 Pathways in TRAIL-Resistant Lung Cancers. Oncotarget 2017, 8, 18095–18105. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cell Types | Cisplatin (µM) | 5-FU (mM) | Vinorelbine (µM) |
|---|---|---|---|
| MSC-WT | 73.3 ± 5.8 | 13.1 ± 1.8 | 36.3 ± 5.5 |
| MSC-EV | 40.3 ± 8.4 | 26.3 ± 6.7 | 36.0 ± 1.0 |
| MSC-TRAIL | 29.0 ± 2.6 | 10.0 ± 1.0 | 32.3 ± 3.21 |
| A549 | 26.0 ± 1.0 | 1.6 ± 0.2 | <1.5 |
| H2170 | 10.6 ± 1.5 | 1.5 ± 0.1 | 10.67 ± 1.5 |
| H460 | 1.5 ± 0.1 | 1.1 ± 0.2 | 8.57 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakiruddin, K.S.; Lim, M.N.; Nordin, N.; Rosli, R.; Abdullah, S. Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines. Biology 2021, 10, 1103. https://doi.org/10.3390/biology10111103
Fakiruddin KS, Lim MN, Nordin N, Rosli R, Abdullah S. Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines. Biology. 2021; 10(11):1103. https://doi.org/10.3390/biology10111103
Chicago/Turabian StyleFakiruddin, Kamal Shaik, Moon Nian Lim, Norshariza Nordin, Rozita Rosli, and Syahril Abdullah. 2021. "Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines" Biology 10, no. 11: 1103. https://doi.org/10.3390/biology10111103






