Activity of TREK-2-like Channels in the Pyramidal Neurons of Rat Medial Prefrontal Cortex Depends on Cytoplasmic Calcium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunofluorescence Staining
2.2. Electrophysiological Recordings
3. Results
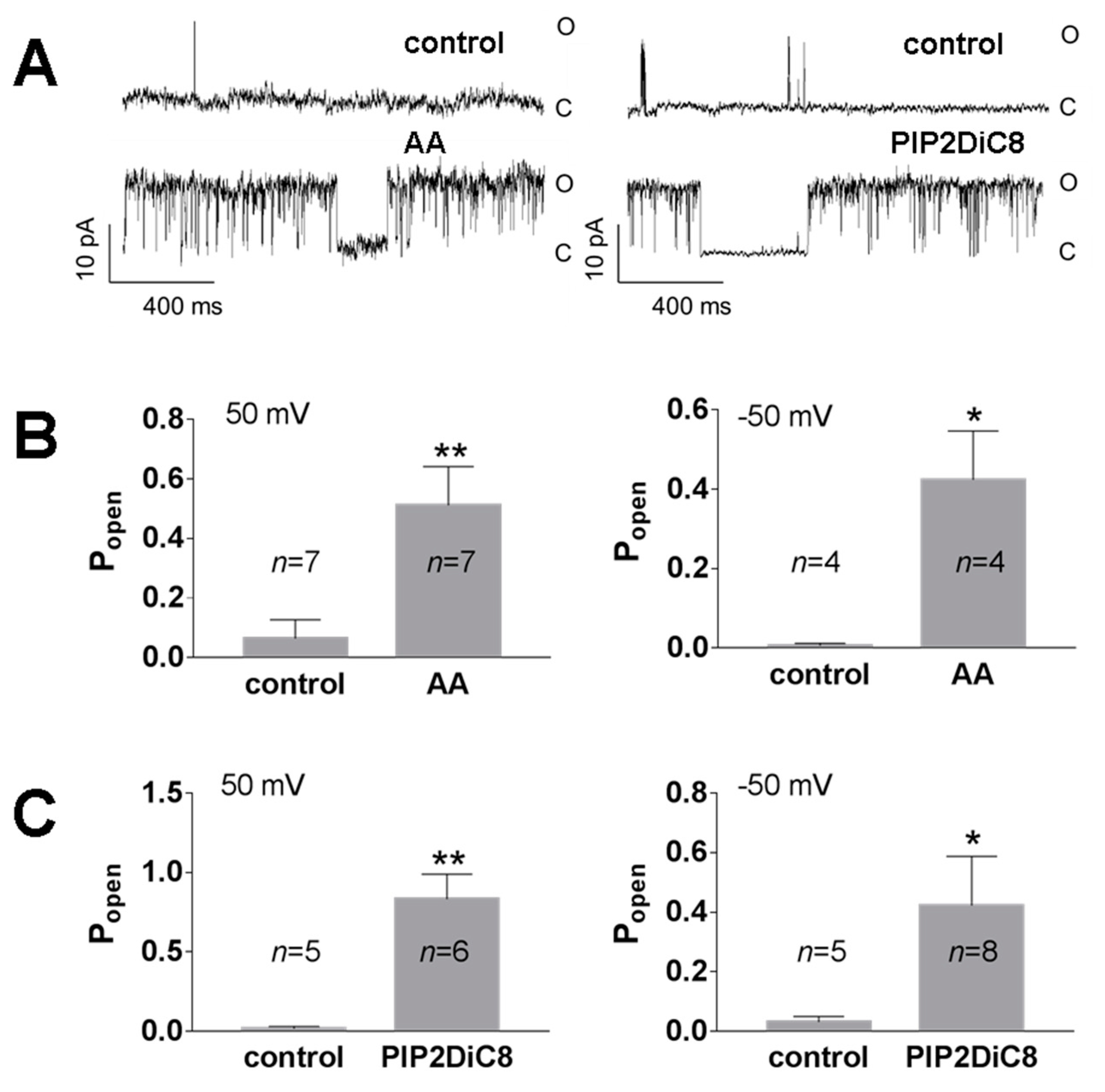
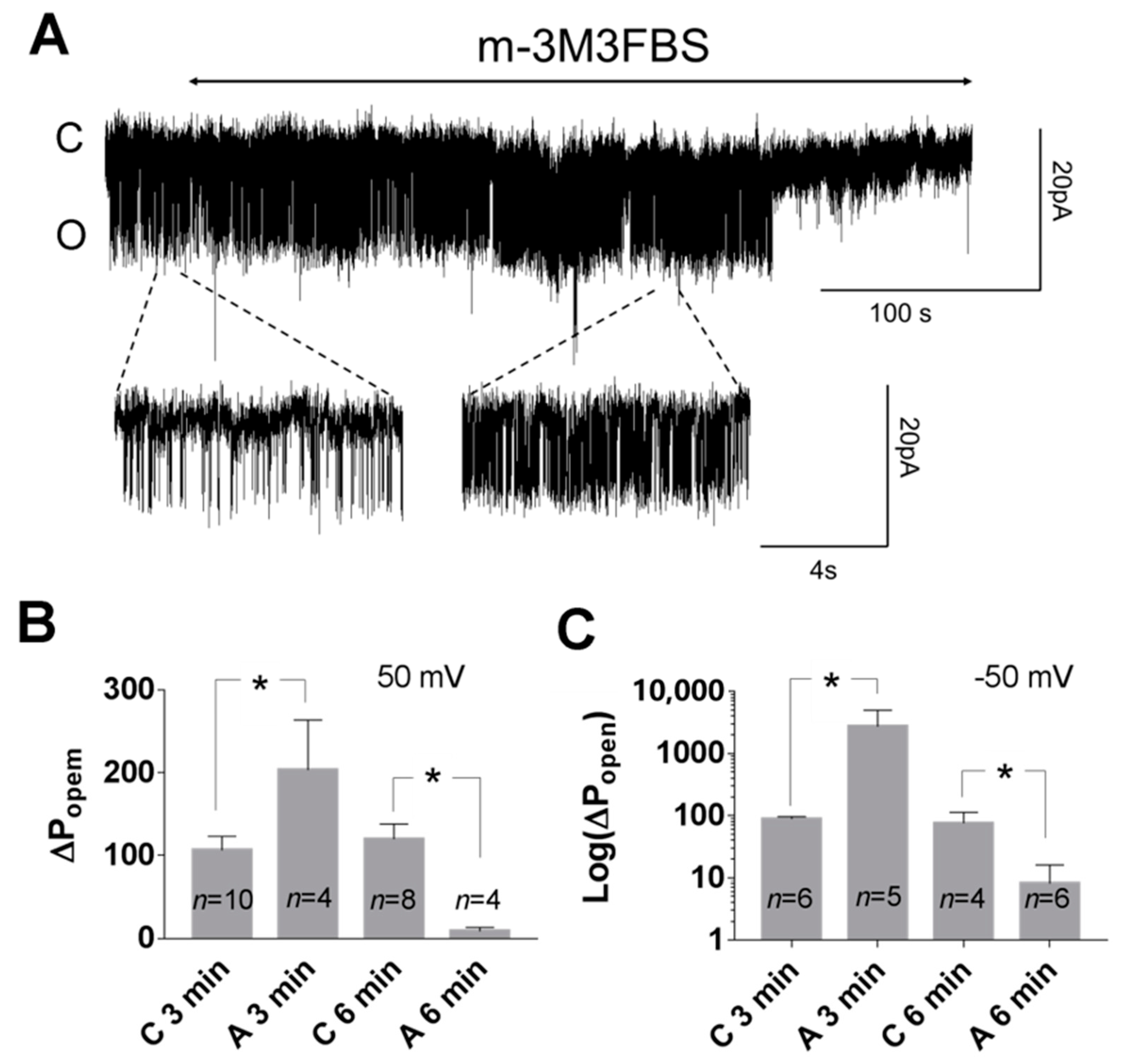
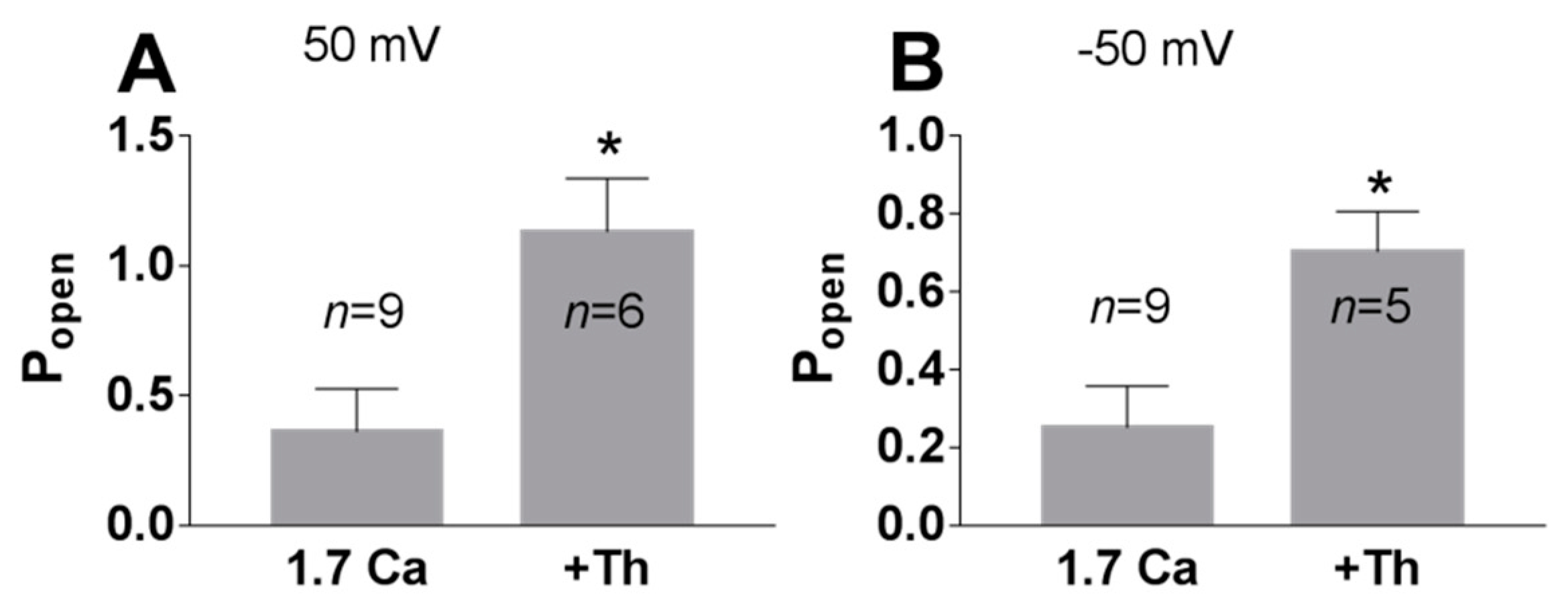
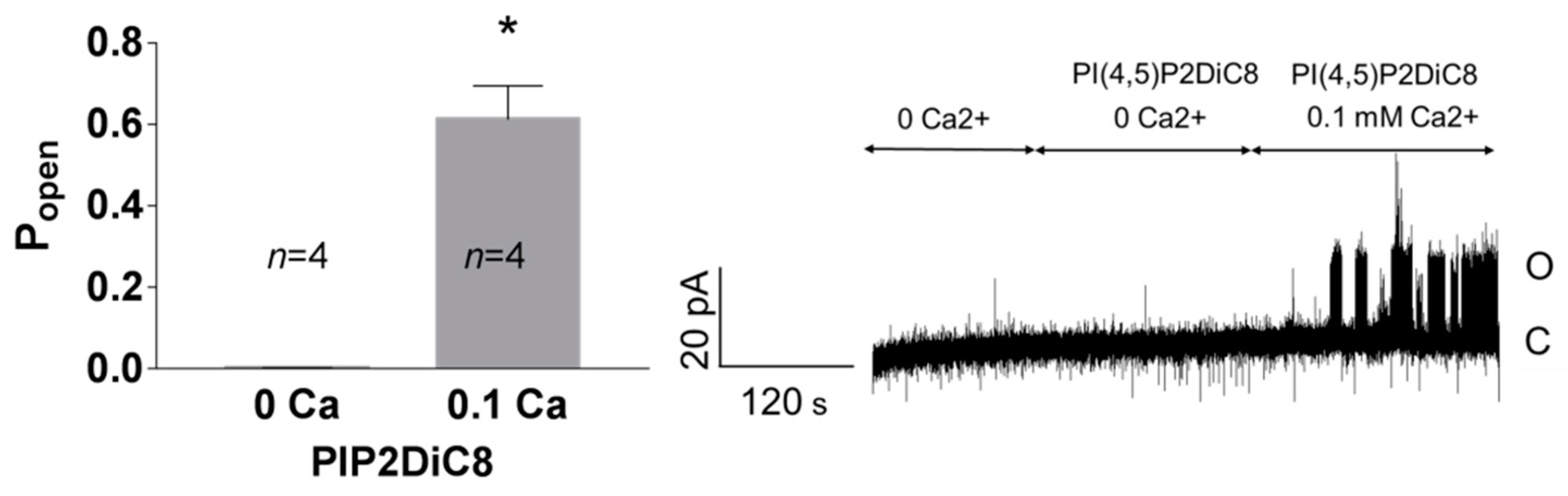
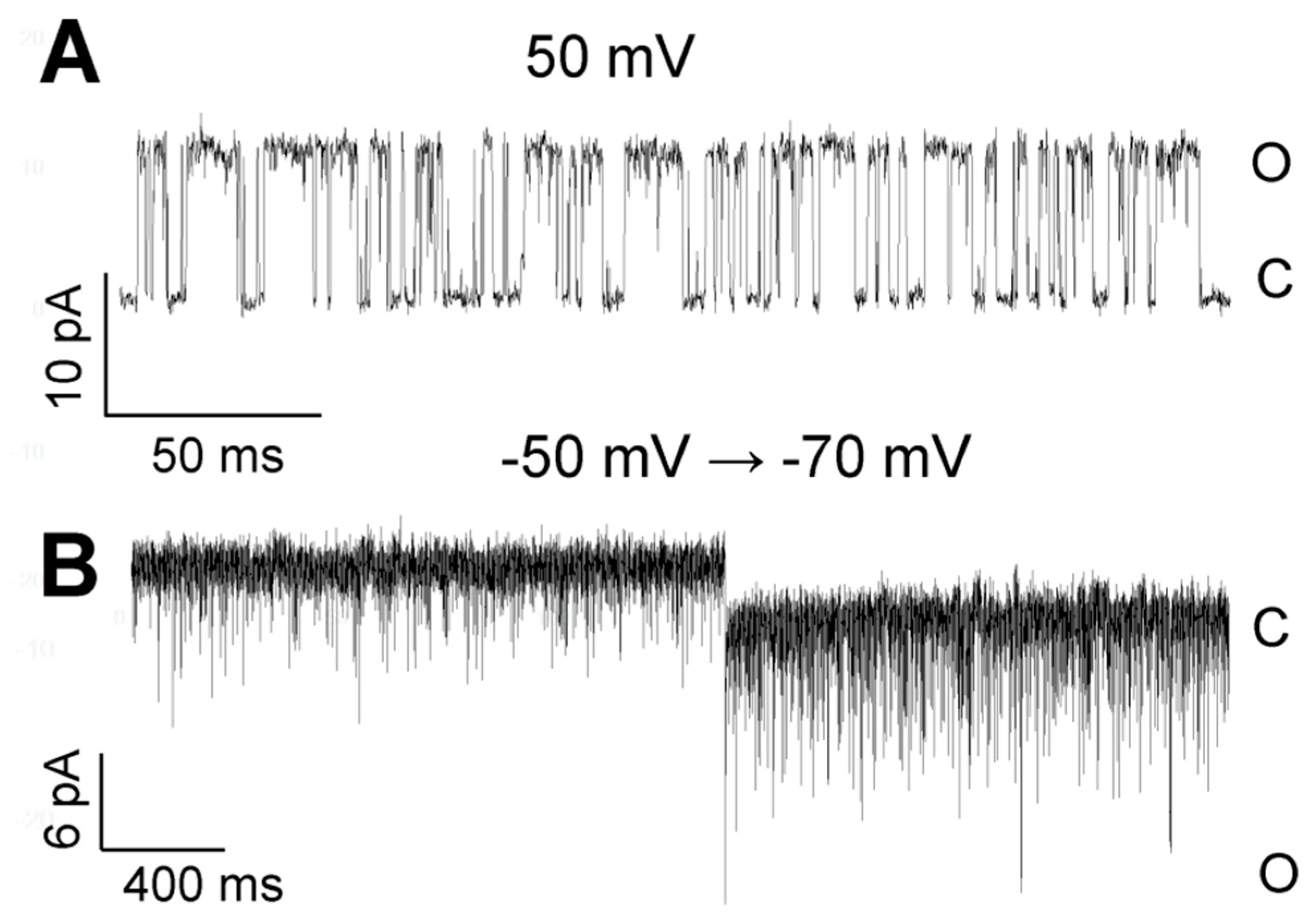
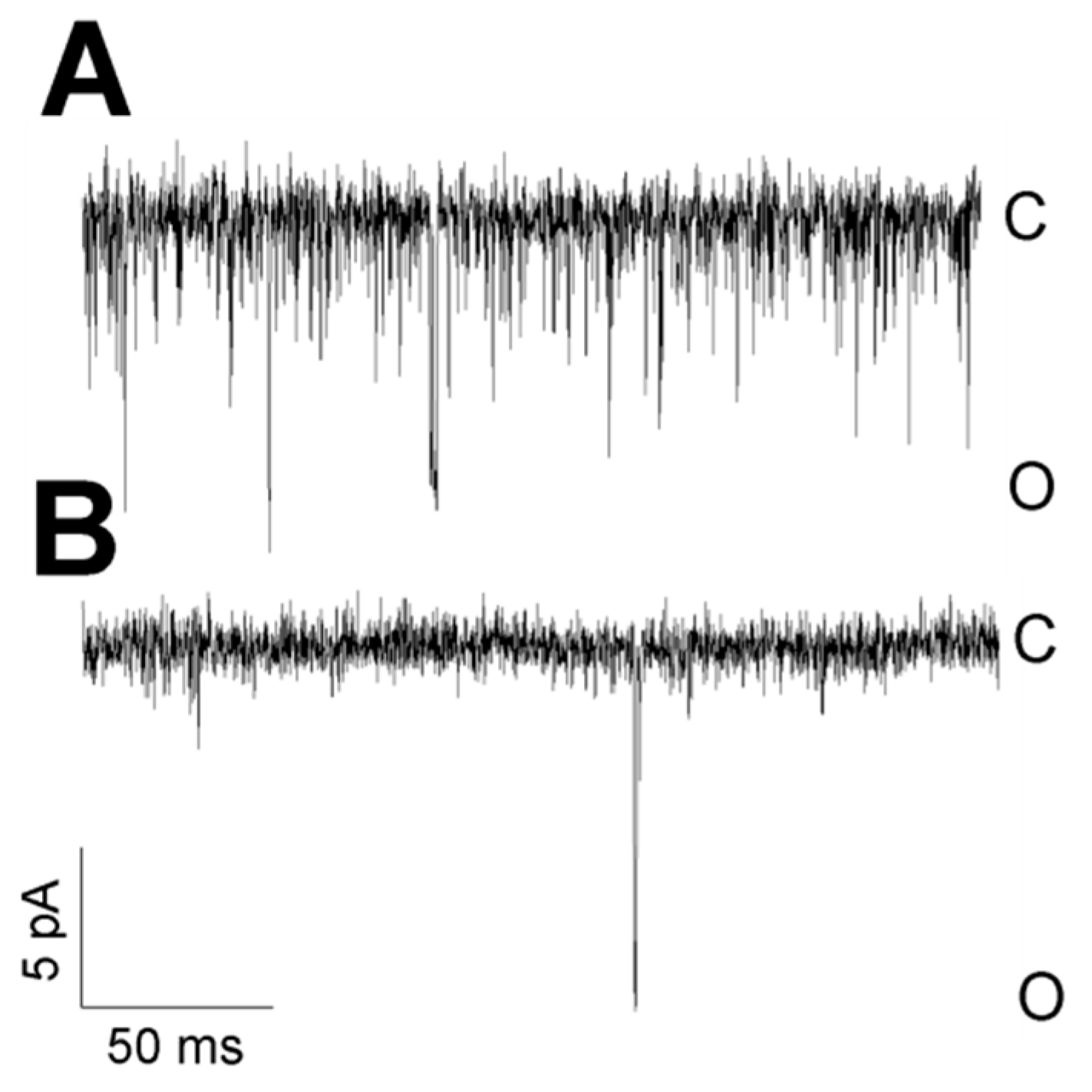
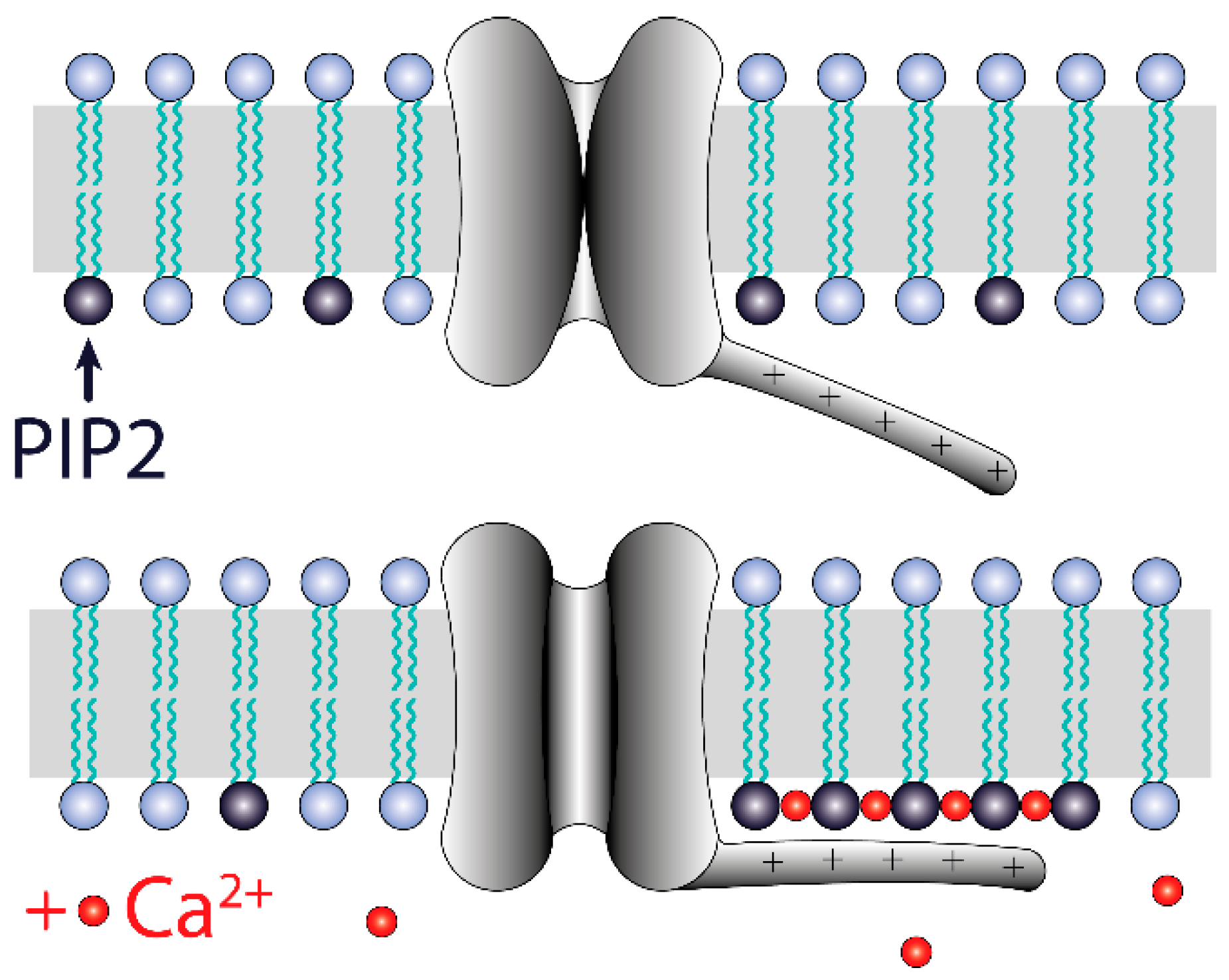
3.1. Role of Calcium in TREK-2-Like Channel Activation
3.2. Pattern of TREK-2-Like Channel Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, J.N.; Grafman, J.H. Human prefrontal cortex: Processing and representational perspectives. Nat. Rev. Neurosci. 2003, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021, 3, fcab125. [Google Scholar] [CrossRef]
- Xu, P.; Chen, A.; Li, Y.; Xing, X.; Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019, 51, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Agarwal, P.; Ravichandiran, V. Two-Pore Domain Potassium Channel in Neurological Disorders. J. Membr. Biol. 2021, 254, 367–380. [Google Scholar] [CrossRef]
- Lesage, F.; Terrenoire, C.; Romey, G.; Lazdunski, M. Human TREK2, a 2P Domain Mechano-sensitive K+Channel with Multiple Regulations by Polyunsaturated Fatty Acids, Lysophospholipids, and Gs, Gi, and Gq Protein-coupled Receptors. J. Biol. Chem. 2000, 275, 28398–28405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heurteaux, C.; Lucas, G.; Guy, N.; El Yacoubi, M.; Thümmler, S.; Peng, X.-D.; Noble, F.; Blondeau, N.; Widmann, C.; Borsotto, M.; et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat. Neurosci. 2006, 9, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sun, H.; Gong, W.; Li, X.; Pan, Z.; Shan, H.; Zhang, Z. Genetic and pharmacological inhibition of two-pore domain potassium channel TREK-1 alters depression-related behaviors and neuronal plasticity in the hippocampus in mice. CNS Neurosci. Ther. 2021, 27, 220–232. [Google Scholar] [CrossRef]
- Thümmler, S.; Duprat, F.; Lazdunski, M. Antipsychotics inhibit TREK but not TRAAK channels. Biochem. Biophys. Res. Commun. 2007, 354, 284–289. [Google Scholar] [CrossRef]
- Djillani, A.; Mazella, J.; Heurteaux, C.; Borsotto, M. Role of TREK-1 in Health and Disease, Focus on the Central Nervous System. Front. Pharmacol. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Yu, B. Recent advance and possible future in TREK-2: A two-pore potassium channel may involved in the process of NPP, brain ischemia and memory impairment. Med. Hypotheses 2008, 70, 618–624. [Google Scholar] [CrossRef]
- Lamas, J.A.; Fernández-Fernández, D. Tandem pore TWIK-related potassium channels and neuroprotection. Neural Regen. Res. 2019, 14, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Méndez-Reséndiz, K.A.; Oviedo, N.; Murbartián, J. PKC- and PKA-dependent phosphorylation modulates TREK-1 function in naïve and neuropathic rats. J. Neurochem. 2021, 157, 2039–2054. [Google Scholar] [CrossRef]
- Kim, E.-J.; Lee, D.K.; Hong, S.-G.; Han, J.; Kang, D. Activation of TREK-1, but Not TREK-2, Channel by Mood Stabilizers. Int. J. Mol. Sci. 2017, 18, 2460. [Google Scholar] [CrossRef] [Green Version]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G.; et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsotto, M.; Veyssiere, J.; Maati, H.M.O.; DeVader, C.; Mazella, J.; Heurteaux, C. Targeting two-pore domain K+channels TREK-1 and TASK-3 for the treatment of depression: A new therapeutic concept. Br. J. Pharmacol. 2014, 172, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.; Choe, C.; Kim, D. Thermosensitivity of the two-pore domain K+channels TREK-2 and TRAAK. J. Physiol. 2005, 564, 103–116. [Google Scholar] [CrossRef]
- Brohawn, S. How ion channels sense mechanical force: Insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann. N. Y. Acad. Sci. 2015, 1352, 20–32. [Google Scholar] [CrossRef]
- Noël, J.; Sandoz, G.; Lesage, F. Molecular regulations governing TREK and TRAAK channel functions. Channels 2011, 5, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.R.; Anderson, E.O.; Gracheva, E.O.; Bagriantsev, S.N. Temperature Sensitivity of Two-Pore (K2P) Potassium Channels. In Current Topics in Membranes; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 74, pp. 113–133. [Google Scholar]
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Jun, Y.K.; Zhang, Y.-H.; Nam, J.H.; Shin, D.H.; Kim, S.J. Identification of critical amino acids in the proximal C-terminal of TREK-2 K+ channel for activation by acidic pHi and ATP-dependent inhibition. Pflug. Arch. Eur. J. Physiol. 2018, 470, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.T.; de Groot, B.L. Mechanism of Mechanosensitive Gating of the TREK-2 Potassium Channel. Biophys. J. 2018, 114, 1336–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClenaghan, C.; Schewe, M.; Aryal, P.; Carpenter, L.; Baukrowitz, T.; Tucker, S.J. Polymodal activation of the TREK-2 K2P channel produces structurally distinct open states. J. Gen. Physiol. 2016, 147, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Ramírez, P.; Reboreda, A.; Rueda-Ruzafa, L.; Herrera-Pérez, S.; Lamas, J.A. Contribution of KCNQ and TREK Channels to the Resting Membrane Potential in Sympathetic Neurons at Physiological Temperature. Int. J. Mol. Sci. 2020, 21, 5796. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Pérez, S.; Campos-Ríos, A.; Rueda-Ruzafa, L.; Lamas, J.A. Contribution of K2P potassium channels to cardiac phys-iology and pathophysiology. Int. J. Mol. Sci. 2021, 22, 6635. [Google Scholar] [CrossRef]
- Chemin, J.; Patel, A.J.; Duprat, F.; Lauritzen, I.; Lazdunski, M.; Honoré, E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005, 24, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Gnatenco, C.; Bang, H.; Kim, D. Localization of TREK-2 K + channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflug. Arch. 2001, 442, 952–960. [Google Scholar] [CrossRef]
- Maingret, F.; Patel, A.J.; Lesage, F.; Lazdunski, M.; Honore, E. Mechano- or Acid Stimulation, Two Interactive Modes of Activation of the TREK-1 Potassium Channel. J. Biol. Chem. 1999, 274, 26691–26696. [Google Scholar] [CrossRef] [Green Version]
- Ben Soussia, I.; Choveau, F.S.; Blin, S.; Kim, E.-J.; Feliciangeli, S.; Chatelain, F.C.; Kang, D.; Bichet, D.; Lesage, F. Antagonistic Effect of a Cytoplasmic Domain on the Basal Activity of Polymodal Potassium Channels. Front. Mol. Neurosci. 2018, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, G.; Bell, S.C.; Isacoff, E. Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc. Natl. Acad. Sci. USA 2011, 108, 2605–2610. [Google Scholar] [CrossRef] [Green Version]
- Petersen, E.N.; Pavel, M.A.; Wang, H.; Hansen, S.B. Disruption of palmitate-mediated localization; a shared pathway of force and anesthetic activation of TREK-1 channels. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183091. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Ramírez, P.; Reboreda, A.; Rueda-Ruzafa, L.; Herrera-Pérez, S.; Lamas, J.A. PIP2 Mediated Inhibition of TREK Potassium Currents by Bradykinin in Mouse Sympathetic Neurons. Int. J. Mol. Sci. 2020, 21, 389. [Google Scholar] [CrossRef] [Green Version]
- Bista, P.; Pawlowski, M.; Cerina, M.; Ehling, P.; Leist, M.; Meuth, P.; Aissaoui, A.; Borsotto, M.; Heurteaux, C.; Decher, N.; et al. Differential phospholipase C-dependent modulation of TASK and TREK two-pore domain K+channels in rat thalamocortical relay neurons. J. Physiol. 2015, 593, 127–144. [Google Scholar] [CrossRef] [Green Version]
- Murbartián, J.; Lei, Q.; Sando, J.J.; Bayliss, D.A. Sequential Phosphorylation Mediates Receptor- and Kinase-induced Inhibition of TREK-1 Background Potassium Channels. J. Biol. Chem. 2005, 280, 30175–30184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.; Han, J.; Kim, D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am. J. Physiol. Physiol. 2006, 291, C649–C656. [Google Scholar] [CrossRef]
- Ładno, W.; Gawlak, M.; Szulczyk, P.; Nurowska, E. Kinetic properties and adrenergic control of TREK-2-like channels in rat medial prefrontal cortex (mPFC) pyramidal neurons. Brain Res. 2017, 1665, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.; Remon, J.I.; Matavel, A.; Sui, J.L.; Keselman, I.; Medei, E.; Shen, Y.; Rosenhouse-Dantsker, A.; Logothetis, D.E. Protein Kinase A Modulates PLC-Dependent Regulation and PIP2-Sensitivity of K+Channels. Channels 2007, 1, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Chemin, J.; Patel, A.J.; Duprat, F.; Sachs, F.; Lazdunski, M.; Honore, E. Up- and down-regulation of the mechano-gated K2P channel TREK-1 by PIP2 and other membrane phospholipids. Pflug. Arch. Eur. J. Physiol. 2007, 455, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Shin, D.H.; Kim, H.J.; Yoo, H.Y.; Zhang, Y.-H.; Nam, J.H.; Kim, W.K.; Kim, S.J. Inhibition of TREK-2 K+ channels by PI(4,5)P2: An intrinsic mode of regulation by intracellular ATP via phosphatidylinositol kinase. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Jeon, Y.K.; Zhang, Y.H.; Nam, J.H.; Shin, N.H.; Kim, S.J. Triple arginine residues in the proximal C-terminus of TREK K+ channels are critical for biphasic regulation by phosphatidylinositol 4,5-bisphosphate. Am. J. Physiol. Cell Physiol. 2019, 316, C312–C324. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.-H.; Woo, J.; Kim, A.S.J. Dual regulatory effects of PI(4,5)P2 on TREK-2 K+ channel through antagonizing interaction between the alkaline residues (K330 and R355-357) in the cytosolic C-terminal helix. Korean J. Physiol. Pharmacol. 2020, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Cabanos, C.; Wang, M.; Han, X.; Hansen, S.B. A Soluble Fluorescent Binding Assay Reveals PIP 2 Antagonism of TREK-1 Channels. Cell Rep. 2017, 20, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Gawlak, M.; Szulczyk, B.; Berłowski, A.; Grzelka, K.; Stachurska, A.; Pełka, J.; Czarzasta, K.; Małecki, M.; Kurowski, P.; Nurowska, E.; et al. Age-dependent expression of Nav1.9 channels in medial prefrontal cortex pyramidal neurons in rats. Dev. Neurobiol. 2017, 77, 1371–1384. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Dworakowska, B.; Nurowska, E.; Dołowy, K. Hydrocortisone inhibition of wild-type and αD200Q nicotinic acetylcholine receptors. Chem. Biol. Drug Des. 2018, 92, 1610–1617. [Google Scholar] [CrossRef]
- Nurowska, E.; Adamiec, M.; Dworakowska, B. Extracellular divalent ions modulate TREK-2-like channel conductance in prefrontal pyramidal neurons in rats. Med. Res. J. 2019, 4, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Gu, W.; Schlichthörl, G.; Hirsch, J.R.; Engels, H.; Karschin, C.; Karschin, A.; Derst, C.; Steinlein, O.K.; Daut, J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J. Physiol. 2002, 539, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Truell, J.; Gnatenco, C.; Kim, D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J. Physiol. 2002, 542, 431–444. [Google Scholar] [CrossRef]
- Goldstein, S.A.N.; Bockenhauer, D.; O’Kelly, I.; Zilberberg, N. Potassium leak channels and the KCNK family of two-p-domain subunits. Nat. Rev. Neurosci. 2001, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Han, T.W.; Nassar, L.; Zubia, M.; Jan, Y.N.; Jan, L.Y. Phosphatidylinositol-(4, 5)-bisphosphate regulates calcium gating of small-conductance cation channel TMEM16F. Proc. Natl. Acad. Sci. USA 2018, 115, E1667–E1674. [Google Scholar] [CrossRef] [Green Version]
- Castro, H.; Bermeo, K.; Arenas, I.; Garcia, D.E. Maintenance of CaV2.2 channel-current by PIP2 unveiled by neomycin in sympathetic neurons of the rat. Arch. Biochem. Biophys. 2020, 682, 108261. [Google Scholar] [CrossRef] [PubMed]
- Myeong, J.; Park, C.-G.; Suh, B.-C.; Hille, B. Compartmentalization of phosphatidylinositol 4,5-bisphosphate metabolism into plasma membrane liquid-ordered/raft domains. Proc. Natl. Acad. Sci. USA 2021, 118, e2025343118. [Google Scholar] [CrossRef]
- Horowitz, L.F.; Hirdes, W.; Suh, B.-C.; Hilgemann, D.W.; Mackie, K.; Hille, B. Phospholipase C in Living Cells. J. Gen. Physiol. 2005, 126, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Krjukova, J.; Holmqvist, T.; Danis, A.S.; Åkerman, K.E.; Kukkonen, J.P. Phospholipase C activatorm-3M3FBS affects Ca2+ homeostasis independently of phospholipase C activation. Br. J. Pharmacol. 2004, 143, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Pagán, A.F.; Rivera-Aponte, D.E.; Melnik-Martínez, K.V.; Zayas-Santiago, A.; Kucheryavykh, L.Y.; Martins, A.H.; Cubano, L.A.; Skatchkov, S.N.; Eaton, M.J. Up-Regulation of TREK-2 Potassium Channels in Cultured Astrocytes Requires De Novo Protein Synthesis: Relevance to Localization of TREK-2 Channels in Astrocytes after Transient Cerebral Ischemia. PLoS ONE 2015, 10, e0125195. [Google Scholar] [CrossRef] [Green Version]
- Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Inyushin, M.; Shuba, Y.M.; Sanabria, P.; Cubano, L.A.; Skatchkov, S.N.; Eaton, M.J. Ischemia Increases TREK-2 Channel Expression in Astrocytes: Relevance to Glutamate Clearance. Open Neurosci. J. 2009, 3, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honoré, E. The neuronal background K2P channels: Focus on TREK1. Nat. Rev. Neurosci. 2007, 8, 251–261. [Google Scholar] [CrossRef]
- Wen, Y.; Vogt, V.M.; Feigenson, G.W. Multivalent Cation-Bridged PI(4,5)P2 Clusters Form at Very Low Concentrations. Biophys. J. 2018, 114, 2630–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmento, M.J.; Coutinho, A.; Fedorov, A.; Prieto, M.; Fernandes, F. Ca2+ induces PI(4,5)P2 clusters on lipid bilayers at physiological PI(4,5)P2 and Ca2+ concentrations. Biochim. Biophys. Acta Biomembr. 2014, 1838, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Collins, A.; Guo, L.; Smith-Dupont, K.B.; Gai, F.; Svitkina, T.; Janmey, P.A. Divalent Cation-Induced Cluster Formation by Polyphosphoinositides in Model Membranes. J. Am. Chem. Soc. 2012, 134, 3387–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.; Rohacs, T.; Hansen, S.B. Tools for Understanding Nanoscale Lipid Regulation of Ion Channels. Trends Biochem. Sci. 2019, 44, 795–806. [Google Scholar] [CrossRef]
- 9Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Lipp, P.; Reither, G. Protein Kinase C: The “Masters” of Calcium and Lipid. Cold Spring Harb. Perspect. Biol. 2011, 3, a004556. [Google Scholar] [CrossRef]
- Carver, C.M.; Shapiro, M.S. Gq-coupled muscarinic receptor enhancement of KCNQ2/3 channels and activation of TRPC channels in multimodal control of excitability in dentate gyrus granule cells. J. Neurosci. 2019, 39, 1566–1587. [Google Scholar] [CrossRef] [PubMed]
- Falkenburger, B.H.; Jensen, J.B.; Hille, B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 2010, 135, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lee, S.-H.; Ho, W.-K. Hydrogen peroxide selectively increases TREK-2 currents via myosin light chain kinases. Front. Biosci. 2007, 12, 1642. [Google Scholar] [CrossRef] [Green Version]
- Książek, A.; Ładno, W.; Szulczyk, B.P.; Grzelka, K.; Szulczyk, P.J. Properties of BK-type Ca++-dependent K+ channel currents in medial prefrontal cortex pyramidal neurons in rats of different ages. Front. Cell. Neurosci. 2013, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzkiewicz-Jałowiecka, A.; Trybek, P.; Machura, Ł.; Dworakowska, B.; Grzywna, Z.J. Mechanosensitivity of the BK Channels in Human Glioblastoma Cells: Kinetics and Dynamical Complexity. J. Membr. Biol. 2018, 251, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Maingret, F.; Honore, E.; Lazdunski, M.; Patel, A.J. Molecular Basis of the Voltage-Dependent Gating of TREK-1, a Mechano-Sensitive K+ Channel. Biochem. Biophys. Res. Commun. 2002, 292, 339–346. [Google Scholar] [CrossRef]
- Schewe, M.; Nematian-Ardestani, E.; Sun, H.; Musinszki, M.; Cordeiro, S.; Bucci, G.; de Groot, B.L.; Tucker, S.J.; Rapedius, M.; Baukrowitz, T. A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K+ Channels. Cell 2016, 164, 937–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulczyk, B.; Pasierski, M.; Nurowska, E. Valproic acid potently inhibits interictal-like epileptiform activity in prefrontal cortex pyramidal neurons. Neurosci. Lett. 2019, 708, 134350. [Google Scholar] [CrossRef]
- Pasierski, M.; Szulczyk, B. Capsaicin inhibits sodium currents and epileptiform activity in prefrontal cortex pyramidal neurons. Neurochem. Int. 2020, 135, 104709. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dworakowska, B.; Gawlak, M.; Nurowska, E. Activity of TREK-2-like Channels in the Pyramidal Neurons of Rat Medial Prefrontal Cortex Depends on Cytoplasmic Calcium. Biology 2021, 10, 1119. https://doi.org/10.3390/biology10111119
Dworakowska B, Gawlak M, Nurowska E. Activity of TREK-2-like Channels in the Pyramidal Neurons of Rat Medial Prefrontal Cortex Depends on Cytoplasmic Calcium. Biology. 2021; 10(11):1119. https://doi.org/10.3390/biology10111119
Chicago/Turabian StyleDworakowska, Beata, Maciej Gawlak, and Ewa Nurowska. 2021. "Activity of TREK-2-like Channels in the Pyramidal Neurons of Rat Medial Prefrontal Cortex Depends on Cytoplasmic Calcium" Biology 10, no. 11: 1119. https://doi.org/10.3390/biology10111119
APA StyleDworakowska, B., Gawlak, M., & Nurowska, E. (2021). Activity of TREK-2-like Channels in the Pyramidal Neurons of Rat Medial Prefrontal Cortex Depends on Cytoplasmic Calcium. Biology, 10(11), 1119. https://doi.org/10.3390/biology10111119



