Simple Summary
Toluene, as one of the volatile organic solvents, has important industrial applications and can be used in a wide range of consumer and commercial products. It can be inhaled and absorbed easily by the human body and can therefore affect the individual’s health through damaging different body tissues, including the ovary. This organic solvent has been one of the most well-studied neurotoxins in recent decades. Studies reporting its effects on ovarian function are still limited. To advance our knowledge on the effect of toluene on female reproduction, an in vivo study using female Wistar rats was investigated. We found that toluene exposure affected ovarian structure and hormone balance by increasing progesterone and testosterone levels. In addition, it has disrupted most of the ovarian markers involved in granulosa cell proliferation and differentiation. Interestingly, Toluene exposure induced both apoptosis and autophagy, confirming the crosstalk between both mechanisms. The promising results of this study may contribute to the prevention of reproductive problems in society by raising the awareness about the use of this hydrocarbon.
Abstract
Toluene has been shown to be highly toxic to humans and animals and can cause damage to various tissues. However, studies reporting its effects on ovarian function are still limited. In this study, we investigated the in vivo effect of toluene using female Wistar rats. We found that toluene exposure decreased ovarian weight and affected ovarian structure by increasing the number of abnormally growing follicles. Moreover, it significantly increased progesterone and testosterone levels. We also showed that toluene exposure decreased GDF-9 protein and its encoding gene. In addition, it inhibited the expression of most of the genes involved in granulosa cell proliferation and differentiation, such as Insl3, ccnd2 and actb. The TUNEL assay showed that apoptosis occurred at the middle and high doses only (4000 and 8000 ppm, respectively), whereas no effect was observed at the low dose (2000 ppm). Interestingly, we showed that toluene exposure induced autophagy as LC3 protein and its encoding gene significantly increased for all doses of treatment. These results may suggest that the activation of autophagy at a low dose of exposure was to protect ovarian cells against death by inhibiting apoptosis, whereas its activation at high doses of exposure triggered apoptosis leading to cell death.
1. Introduction
Volatile organic solvents have important industrial applications and can be used in a wide range of consumer and commercial products, such as paints, cleaners and adhesives. Toluene, as one of these volatile organic compounds, represents one of the major precursors of secondary organic aerosols [1,2]. It can enter the environment mostly through emissions from automobiles and airplanes and losses during the marketing of gasoline, spills and cigarette smoke [3]. It has been identified as potentially dangerous to human health and the environment because it can be inhaled and absorbed by the human body [4]. Exposure to toluene can vary among countries and cities but the safe exposure levels are normally between 10 and 100 ppm, while the immediately harmful to life and health limit has been set at 500 ppm [5]. Nevertheless, the exposure may be at a high concentration abused by humans at a time point reaching, in some cases, 10,000 ppm [6,7].
Toluene exposure can affect an individual’s health [8] and can cause a variety of physiological problems. It can damage different body tissues, including the kidneys [9], liver tissue [8,10,11] and central nervous system [12]. This organic solvent has been one of the most well-studied neurotoxins in recent decades [13,14,15,16,17,18,19]. Indeed, toluene vapor exposure appears to have significant action on the dopaminergic system since it has increased the release of the neurotransmitter dopamine in both the whole brain and the striatum, while no effect was found on the secretion of norepinephrine and serotonin levels in the brain. The Norepinephrine, however, was found to increase in the medulla and midbrain whereas serotonin was increased in the cerebellum, medulla, and striatum [20]. In addition, toluene was described to have an antidepressant-like effect through an interaction with NMDA, GABAA and dopamine receptors [21]. Despite the advances, research into the molecular and cellular targets of inhaled toluene is still limited, owing to the widespread usage of this solvent as an inhalant. It has been defined that the inhaled toluene affected both voltage-gated and ligand-gated ion channels [22]. It can also trigger oxidative stress [23], apoptotic effects [24] and epigenetic changes that might have a long-term impact on gene expression and behavior [25].
Interestingly, toluene has been reported to cause renal and liver damage and toxicity in humans [26,27,28]. Animal study findings revealed that as toluene concentrations increased, fecundity in Drosophila melanogaster females and offspring survivability decreased, suggesting that toluene’s effects are dose-dependent and detrimental to fly development [29]. Different species of mammals, including rats, rabbits and cows, have been used in a variety of experiments to investigate the reproductive and developmental toxicity of toluene [30]. These studies conclusively showed that toluene exposure may adversely affect female reproduction. It can freely pass through the placenta to the fetus, thereby inducing fetotoxicity revealed by fetal morphological anomalies as well as a decrease in fecundity [31,32]. Moreover, inhalation of toluene has been shown to increase the incidence of maternal and fetal morbidity and embryonal malformations in women [33,34], cows [35] and rats [36,37]. However, in vivo evidence in support of the main mechanisms of the toluene effect on ovary function is still limited, particularly the induction of autophagic and apoptosis. In our recent in vitro studies, we demonstrated, using cultured healthy mare ovarian granulosa cells, that toluene inhibited viability, proliferation, apoptosis, progesterone, prostaglandin F and IGF-I release [38,39,40]. To advance our knowledge on the effect of toluene on female reproduction, an in vivo study using female Wistar rats is investigated. To our knowledge, this is the first study to test the in vivo mechanisms of the toluene effect on ovarian function, including the involvement of apoptosis and autophagy processes in ovarian cell toxicity.
2. Materials and Methods
2.1. Animal Treatment and Sampling
This study was approved by the Scientific Research Ethics Committee at King Saud University (Reference No: KSU-SE-20-76). The Animal Welfare Center provided us with forty female Wistar rats weighing 200–250 g. They were held in single cages (22 to 24 °C) with a 12 h light/dark cycle and free access to food and water. The females were randomly divided into four groups of ten rats that were exposed to different concentrations of toluene for 28 days by inhalation for 30 min per day. The first group is composed of females that did not receive any toluene treatment. Rats in Group 2 were exposed to 2000 ppm toluene; rats in Group 3 were exposed to 4000 ppm toluene and rats in Group 4 were exposed to 8000 ppm toluene. After 28 days of treatment, animals were transferred individually to a transparent plastic box connected to a carbon dioxide tube at a flow rate of 10 L/hour for ten minutes. Blood samples were drawn from the heart and transferred directly to tubes containing an anticoagulant (EDTA) to obtain the serum after centrifugation. The ovaries were removed carefully, cleaned of adherent tissue and weighed. The sampled ovaries were labeled according to their origin (groups), and a portion of them were fixed in neutral buffered formalin (NBF) for classic histological study, immunostaining and TUNEL assay. A second portion was collected and stabilized in RNA later and stored at −80 °C for the molecular assays.
2.2. Histology
Ovarian samples were collected, weighed, and fixed in 10% neutral buffered formalin (NBF) for 24 h after 28 days of treatment. For classic histological analysis, samples were embedded in paraffin blocks that were serially cut into 5 µm-thick parts using a rotary microtome and stained with hematoxylin-eosin. For immunostaining and TUNEL assay, some blocks were cut into 3 µm-thick pieces. The follicular count for each ovary was calculated using the method mentioned in our previous research [41].
2.3. Hormones Analysis
To obtain plasma, blood samples were collected into lithium heparin tubes and centrifuged at 3000 rpm for 10 min. The levels of progesterone, estradiol, testosterone and IGF-1 were measured using competitive enzyme-linked immunosorbent assays (ELISAs) as directed by the manufacturer (Vector Laboratories, New Jersey, USA).
2.4. TUNEL Assay
The ovaries were fixed in 10% NBF for 24 h at room temperature for the TUNEL assay. Paraffin wax blocks were made, and 3 µm-thick paraffin parts were prepared and placed on coated slides. Dewaxed sections were rehydrated using normal methods and washed in phosphate-buffered saline (PBS). The tissue parts were then permeabilized in 0.1% Triton X-100 with 0.1% sodium citrate and incubated with a Proteinase K working solution at 37 °C for 15 min. TUNEL staining was performed according to the manufacturer’s instructions for the TMR red 12156792910 In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany). A positive control was created for one of the ovarian tissue sections, and a negative control was created by excluding the TdT from one of the other ovarian tissue sections. The nuclei were stained with Hoechst, and the labeled parts were examined with a Nikon TE 2000 fluorescence microscope using confocal microscopy (Nikon Co., Japan) to look for DNA fragmentation, chromatin condensation, and other apoptotic changes. The signal for TMR-red was analyzed by Zen 3.1 service (ZEN lite) and quantified using GraphPad Prism 9 program (GraphPad Software, San Diego, USA).
2.5. Immunostaining
Slides containing tissue sections were placed on hotplates (60 °C) and dewaxed with xylene (2× 10 min). Then, they were rehydrated with decreasing concentrations of ethanol and washed twice with distilled water and with 1× PBS. The slides were removed from the washing process and dried by placing them diagonally on a surface containing dry tissues to ensure that the washing solution was removed. After drying, the slices were placed in a suitable container on the floor covered with layers of tissues moistened with water to reduce the rate of evaporation of the solution that the tissue sections would be treated with in the following stages. The tissue sections were then placed in 0.1% Triton X-100 with 0.1% sodium citrate. Blocking buffer (1% BSA in PBS) was used to block nonspecific staining (45 min at RT). The slides were placed into a humid box, and were then incubated with anti-GDF-9 (1:100 dilutions) and anti-LC-3 (1:100 dilutions) primary antibody. The humid box was closed carefully with tin paper and kept overnight at 4 °C on a flat balanced surface in the dark. On the second day, the slides were washed with 1× PBS, and were incubated with antirabbit FITC-conjugated secondary antibody (1:2000 dilutions (ab6717, Abcam, UK) for 45 min at RT in the dark. During the next step, slides were washed with PBS and then TE buffer before adding Hoechst solution (dilute 1:15,000, Hoechst 33342, life technologies, Grand Island, NJ, USA). Finally, the slices were washed, dried, and covered with cover slides, ensuring that the edges were covered with nail polish. Photomicrographs of GDF-9 and LC-3 specific signals and nuclear stains were captured with spinning disk confocal microscope from Zeiss (Germany). The immunofluorescence signal for protein expression was analyzed by Zen 3.1 service (ZEN lite, blue Edition, Germany) and quantified using GraphPad Prism 9 program (GraphPad Software, San Diego, CA, USA).
2.6. Analysis of Gene Expression
RNA was extracted from ovarian tissues that had been previously stabilized with RNAlater (Qiagen, Westburg, The Netherlands). We used an RNeasy Mini Kit (Qiagen, Westburg, The Netherlands) with on-column DNase treatment with an RNase-Free DNase Package for RNA extraction (Qiagen). SYBR green and an Applied Biosynthesis 7500 Quick RT–PCR system (Carlsbad, CA, USA) were used to perform real-time PCR (RT–PCR) with the gene-specific primers described in Table 1. RT–PCR and several primer sets were used to obtain cDNA from these samples using an iScriptTM cDNA synthesis package (Applied Biosystem, Carlsbad, CA, USA).

Table 1.
Primers for real-time RT-PCR.
2.7. Statistical Analysis
The mean and standard error of the mean were used to express the data (SEM). The statistical significance of the differences in mean values between the treatment and control groups was determined using GraphPad Prism version 5. For statistical comparisons, one-way analysis of variance (ANOVA) was used, followed by Tukey’s multiple comparison test. The difference was deemed statistically important when the p value was less than 0.05.
3. Results
3.1. Effect of Toluene on Body Weight
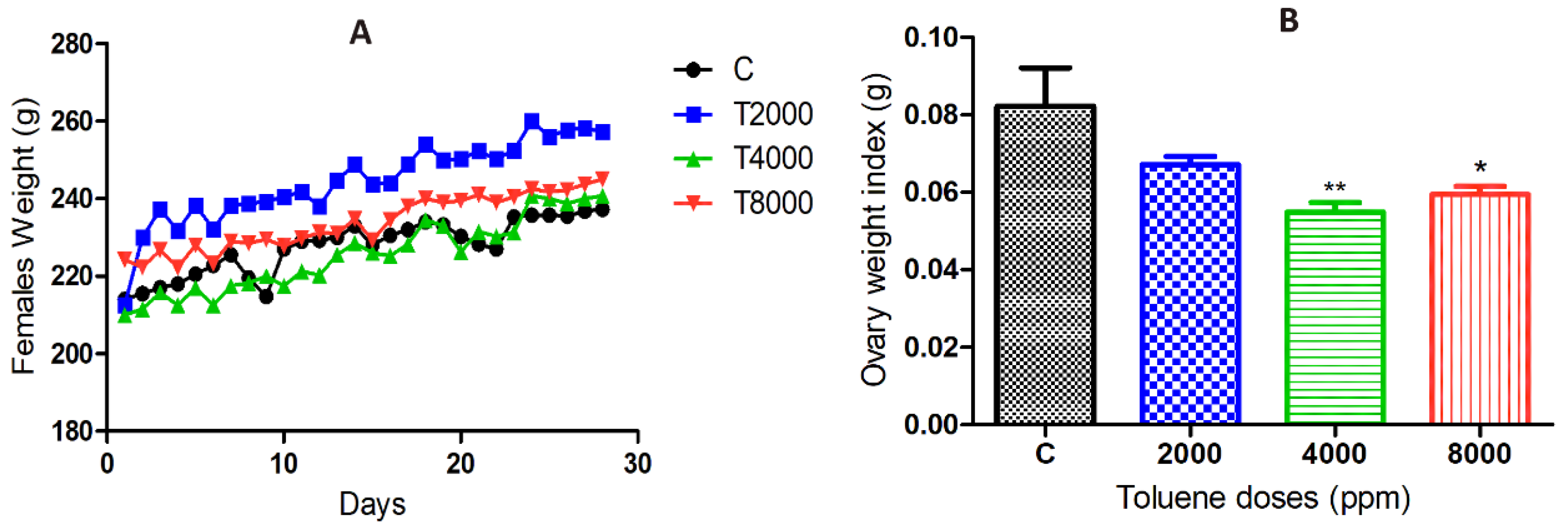
The results showed a significant increase in body weight of the 2000 ppm-treated group compared to the control group, whereas no significant difference was found between the 4000 and 8000 ppm-treated groups compared to the control group (Figure 1A).
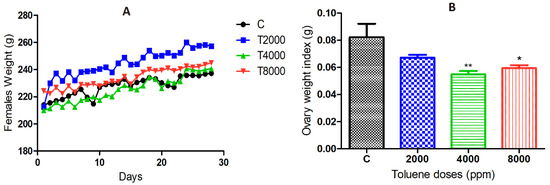
Figure 1.
(A) Body weight changes over 28 days in the different groups exposed to toluene. (B) Ovarian weight changes in the toluene-exposed females compared to the control group. Values are means ± S.E.M. *, p < 0.05; **, p < 0.01.
3.2. Effect of Toluene on Ovary Weight
Toluene exposure significantly reduced the ovarian weights of rats treated with doses of 4000 and 8000 ppm, whereas the dose of 2000 ppm did not affect ovarian weights (Figure 1B).
3.3. Effect of Toluene on the Number of Follicles
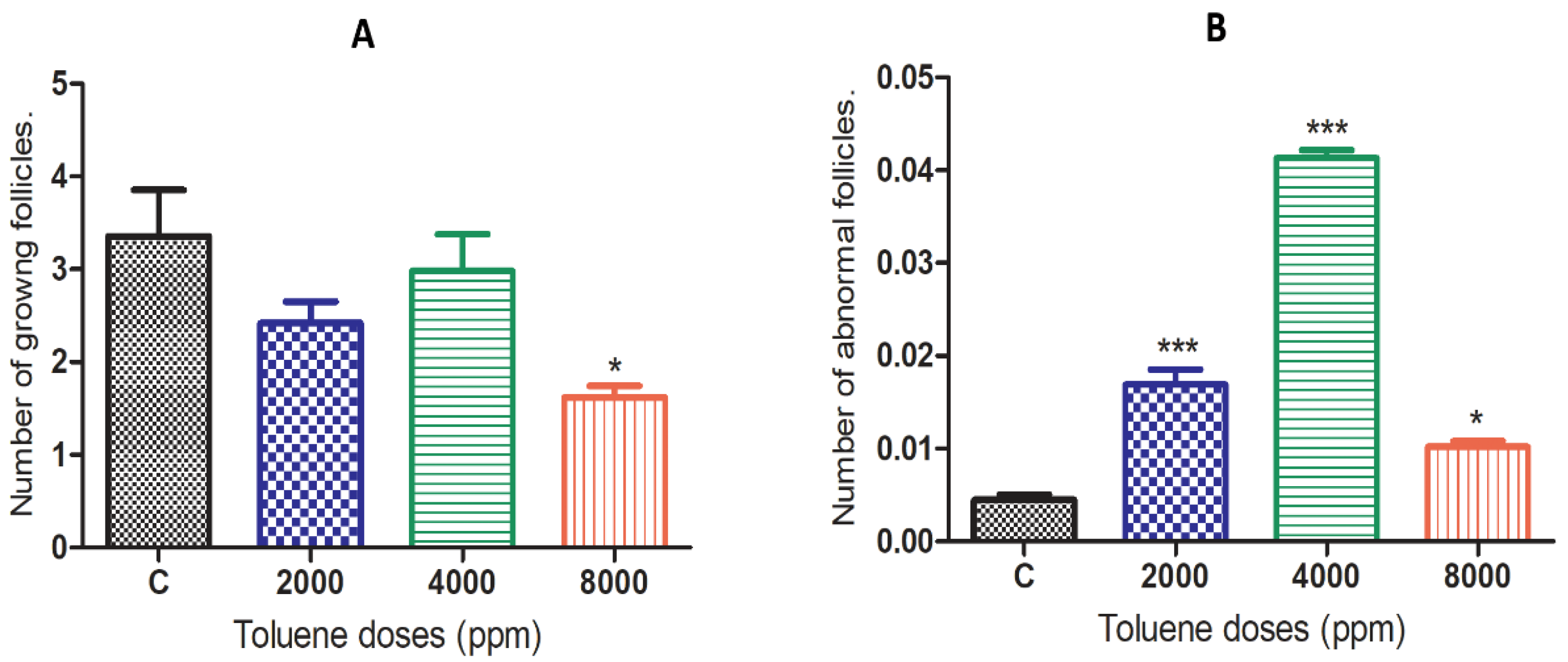
The effects of toluene on the number of growing ovarian follicles and abnormal ovarian follicles are presented in Figure 2. The results showed that toluene significantly decreased the number of growing follicles only in the 8000 ppm-treated group compared to the control (Figure 2A). However, it significantly increased the number of abnormal follicles in ovaries from all treated groups, and the highest number was obtained in the group treated with 4000 ppm (Figure 2B).
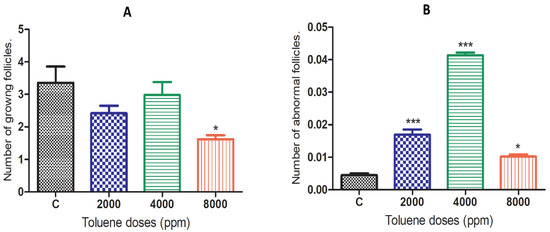
Figure 2.
Effects of different doses of toluene on the (A) number of growing follicles and (B) abnormal follicles. Values are means ± S.E.M. *, p < 0.05; ***, p < 0.001.
3.4. Effect of Toluene on Steroid Hormone Levels
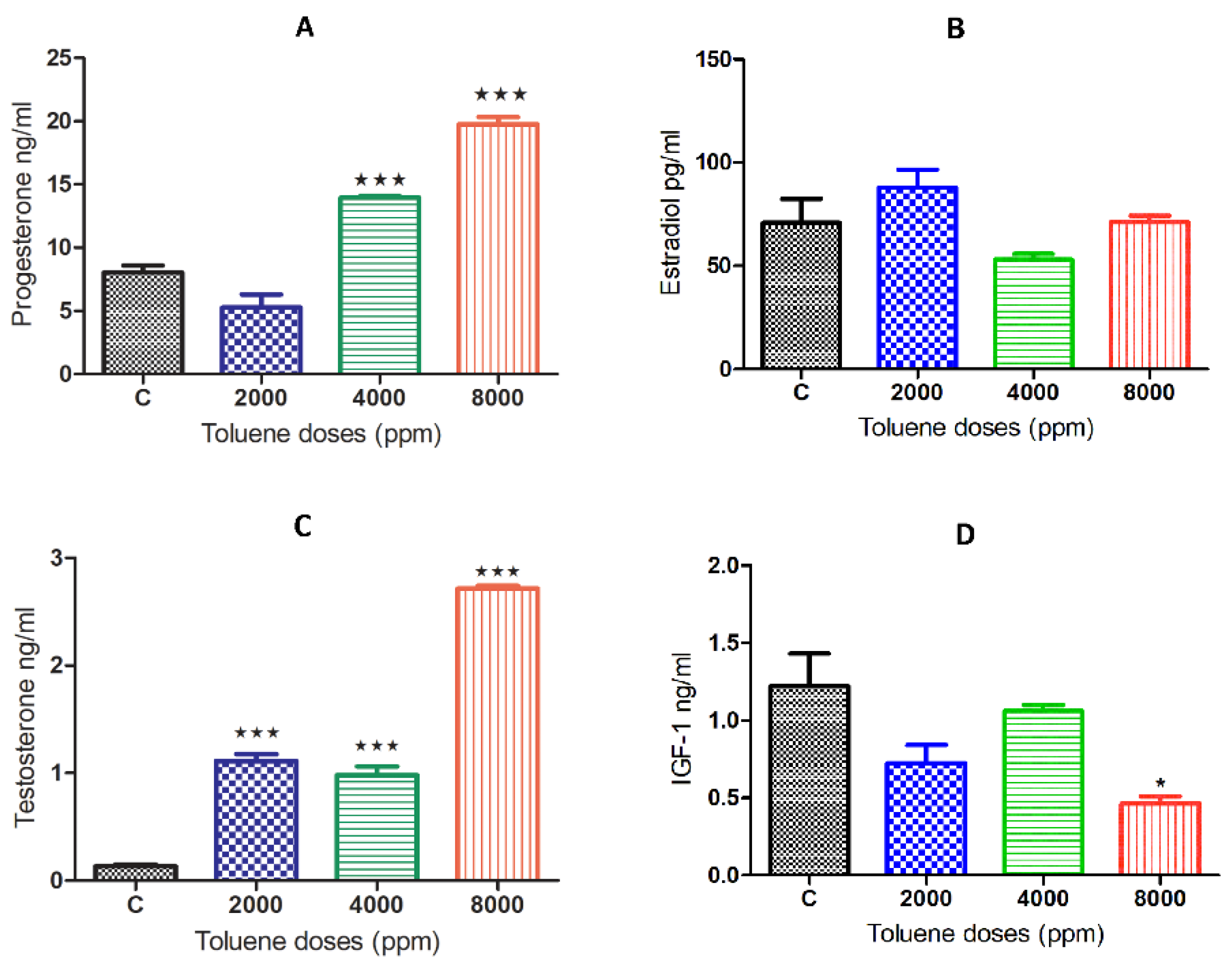
While there was no significant effect on estradiol (Figure 3B), exposure to toluene caused a significant increase in the secretion of the hormones progesterone and testosterone (Figure 3A,C) when compared to the control group. This increase was obtained for progesterone only at doses of 4000 and 8000 ppm, whereas it was observed among all treated groups for testosterone (Figure 3B,D). However, IGF-1 significantly decreased only with the high dose of treatment (Figure 3D).
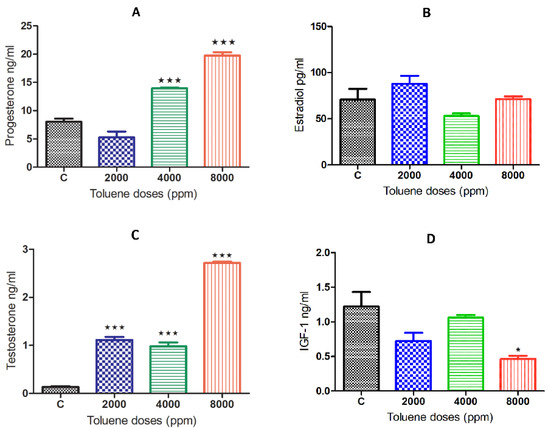
Figure 3.
Serum hormone levels of female rats exposed to toluene compared to the control group. The levels of progesterone (A) significantly increased in females from the 4000 and 8000 groups, whereas testosterone levels (C) significantly increased among all treatments. However, no significant effect of toluene exposure was found on estradiol (B,D) IGF1. Values are means ± S.E.M. *, p < 0.05; ***, p < 0.001.
3.5. Effect of Toluene on Gene Expression Levels
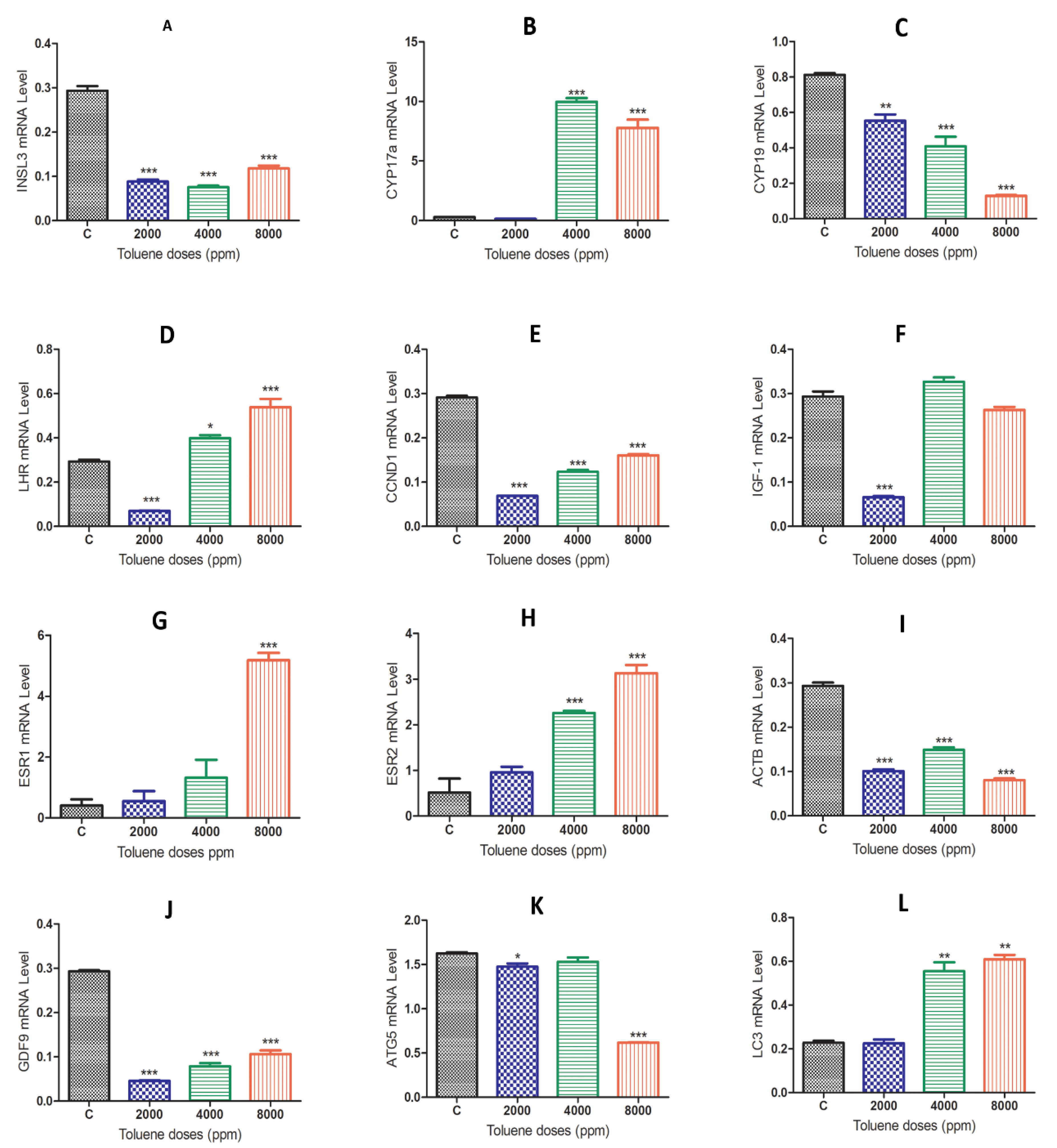
When comparing the gene expression results between the control group and the toluene-treated groups, we found that rats exposed to toluene at different doses significantly decreased the mRNA levels of the Insl3, Cyp19, ccnd1, Igf-1, Actb, GDF-9 and Atg5 genes (Figure 4A,C,E,F,I–K). Exposure to toluene significantly increased the expression of the Cyp17a, Lhr, Esr2 and Lc3 genes at 4000 and 8000 ppm (Figure 4B,D,H,L) and significantly increased the Esr1 gene in the 2000 ppm-treated group (Figure 4G).
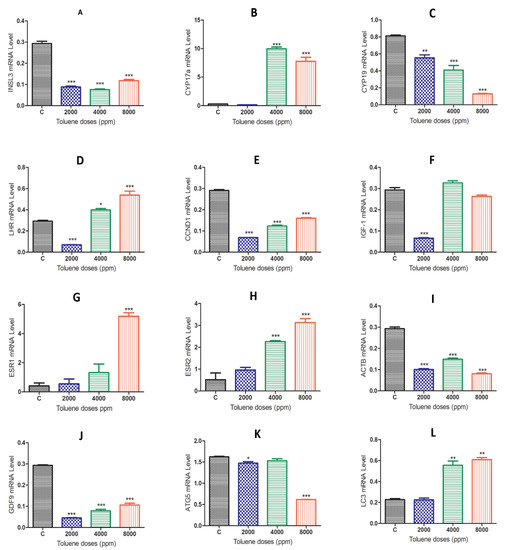
Figure 4.
mRNA expression levels of different genes in the ovaries of rats in the treatment groups compared to the control group. (A), INSL3; (B), CYP17a; (C) CYP19; (D) LHR; (E) CCND1; (F) IGF-1; (G) ESR1; (H) ESR2; (I) ACTB; (J) GDF-9; (K) ATG5; (L) LC-3; Values are means ± S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
3.6. Effect of Toluene on LC3 and GDF9 Protein Expression
3.6.1. GDF9 Protein
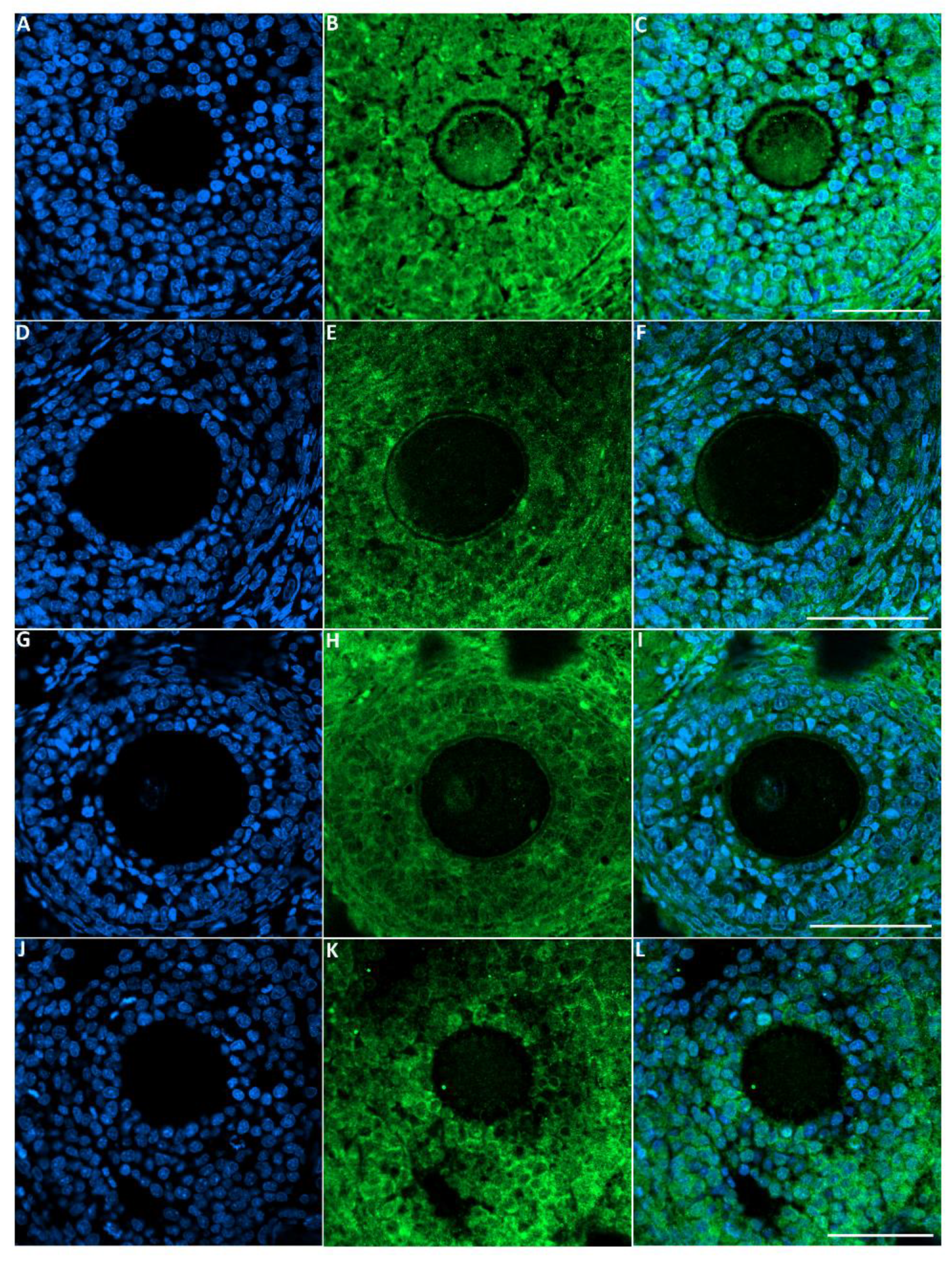
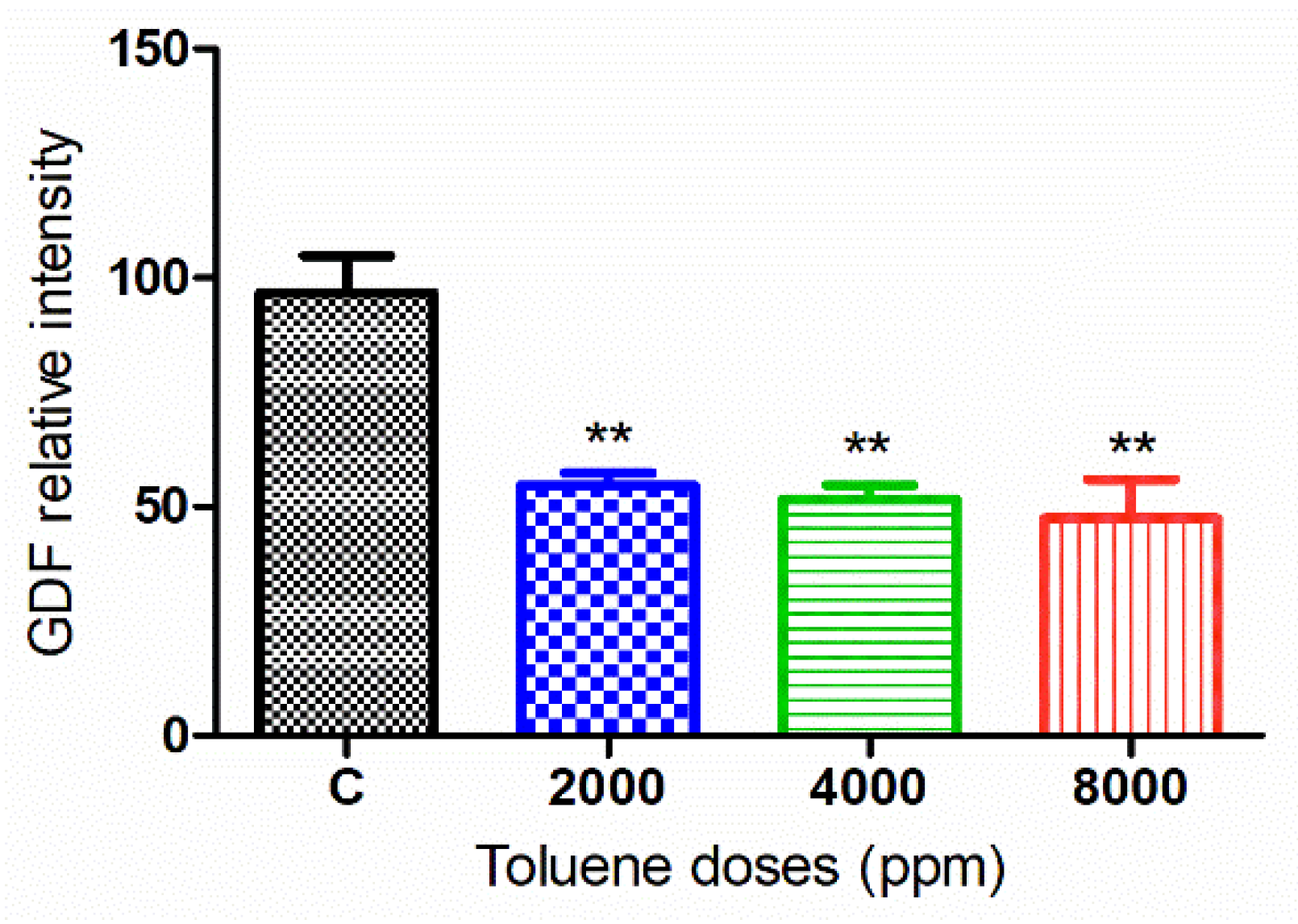
The green fluorescence intensity of GDF9 was significantly reduced in the ovaries of rats exposed to toluene at the different concentrations of treatment (2000, 4000 and 8000 ppm) compared to the control group (Figure 5 and Figure 6).
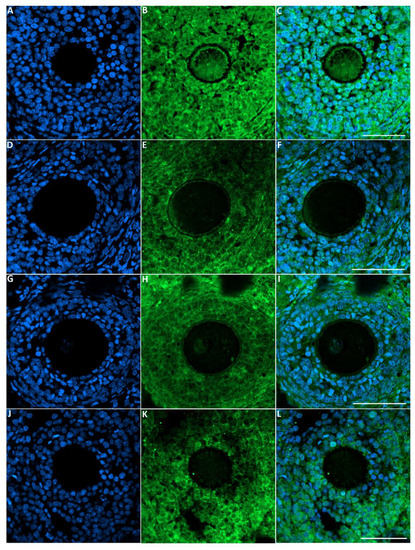
Figure 5.
Representative images of GDF-9 immunofluorescence staining in rats of the different groups of the experiment. Control group (A–C), 2000 ppm-treated group (D–F), 4000 ppm-treated group (G–I) and 8000 ppm-treated group (J–L). Scale bars = 100 µm.
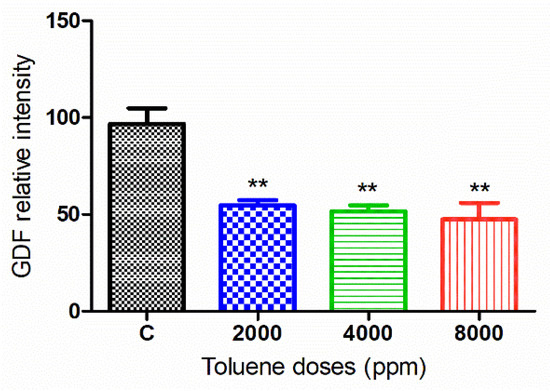
Figure 6.
Relative fluorescence intensity of GDF-9 levels in the control and exposed groups. Values are means ± S.E.M. **, p < 0.01.
3.6.2. LC3 Protein
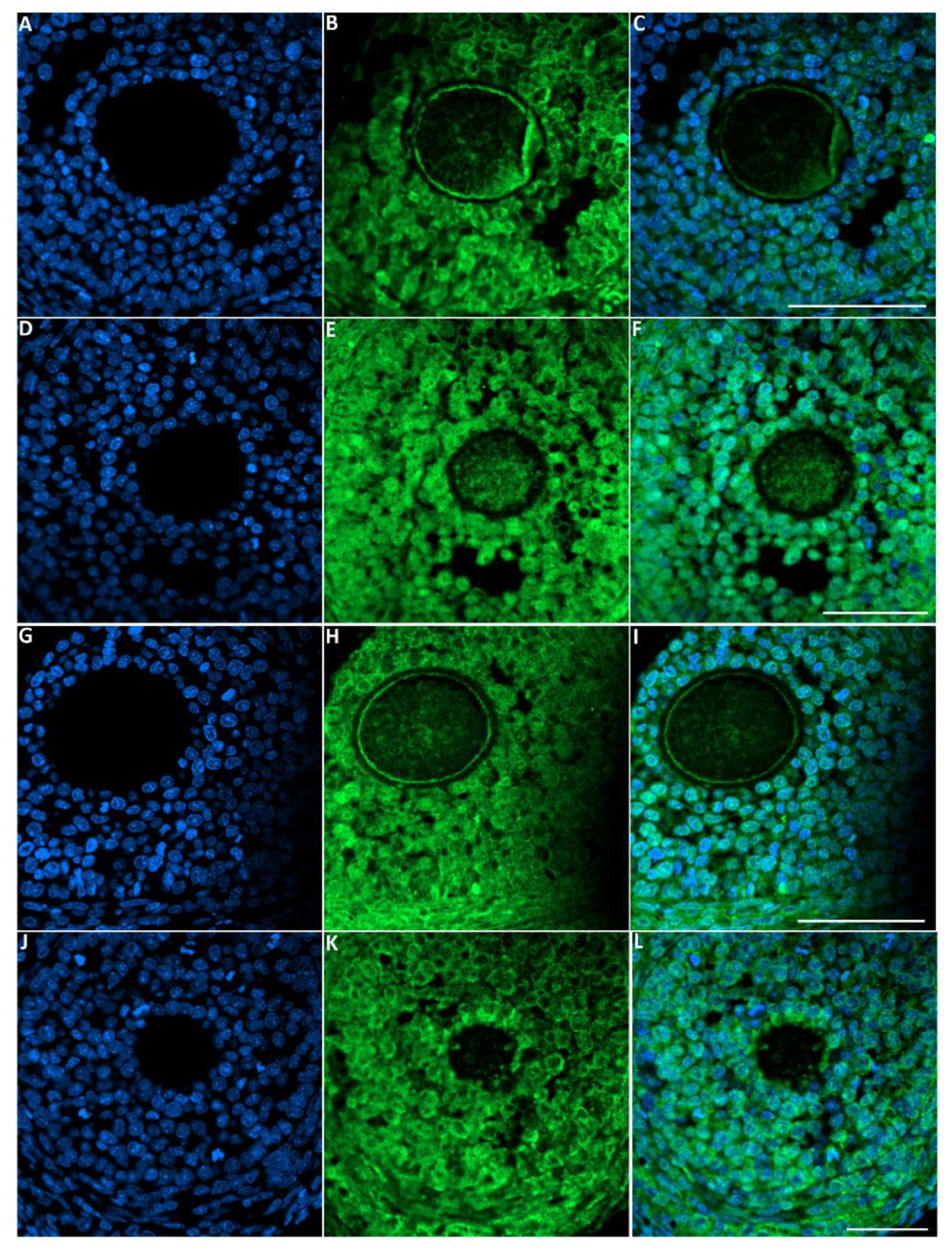
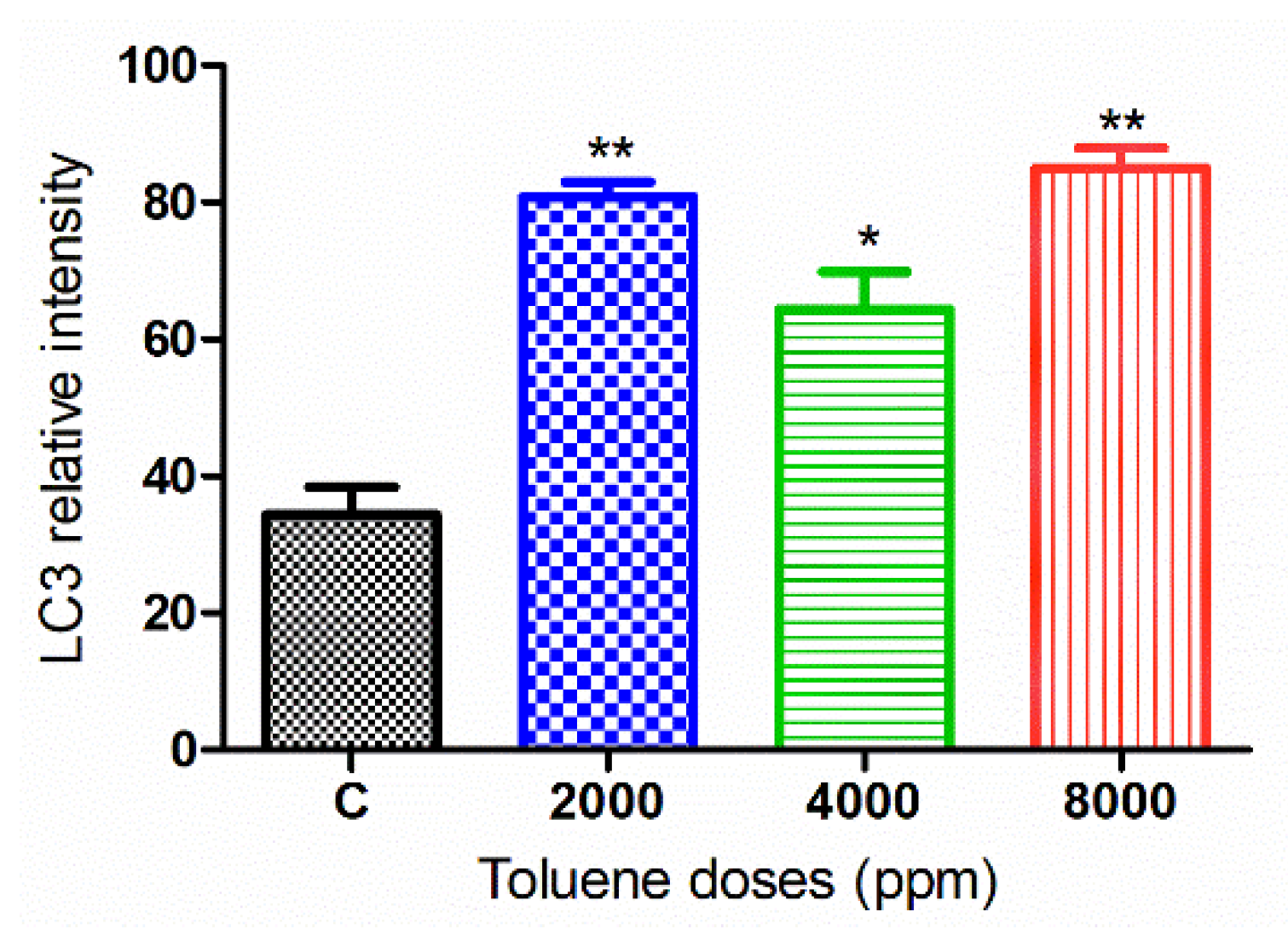
The green fluorescence intensity of LC3 was significantly increased in the ovaries of rats exposed to toluene at different concentrations (2000, 4000 and 8000 ppm) compared to the control group (Figure 7 and Figure 8).
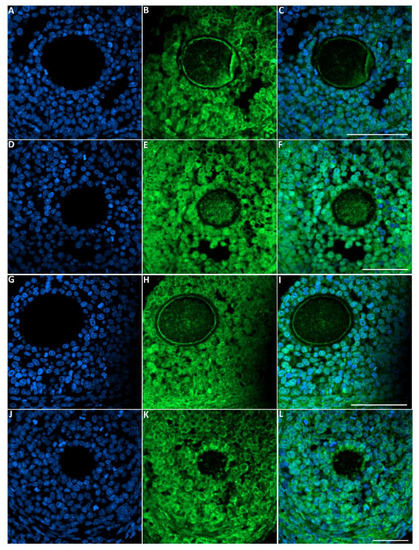
Figure 7.
Representative images of LC-3 immunofluorescence staining in female rats that were exposed to toluene compared to the control. Control group (A–C), 2000 ppm-treated group (D–F), 4000 ppm-treated group (G–I) and 8000 ppm-treated group (J–L). Scale bars = 100 µm.
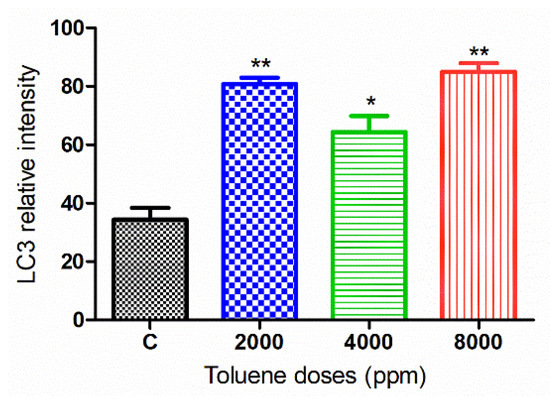
Figure 8.
Relative fluorescence intensity of LC-3 levels in the control and exposed groups. Values are means ± S.E.M. *, p < 0.05; **, p < 0.01.
3.7. DNA Damage in Cells Treated with Toluene
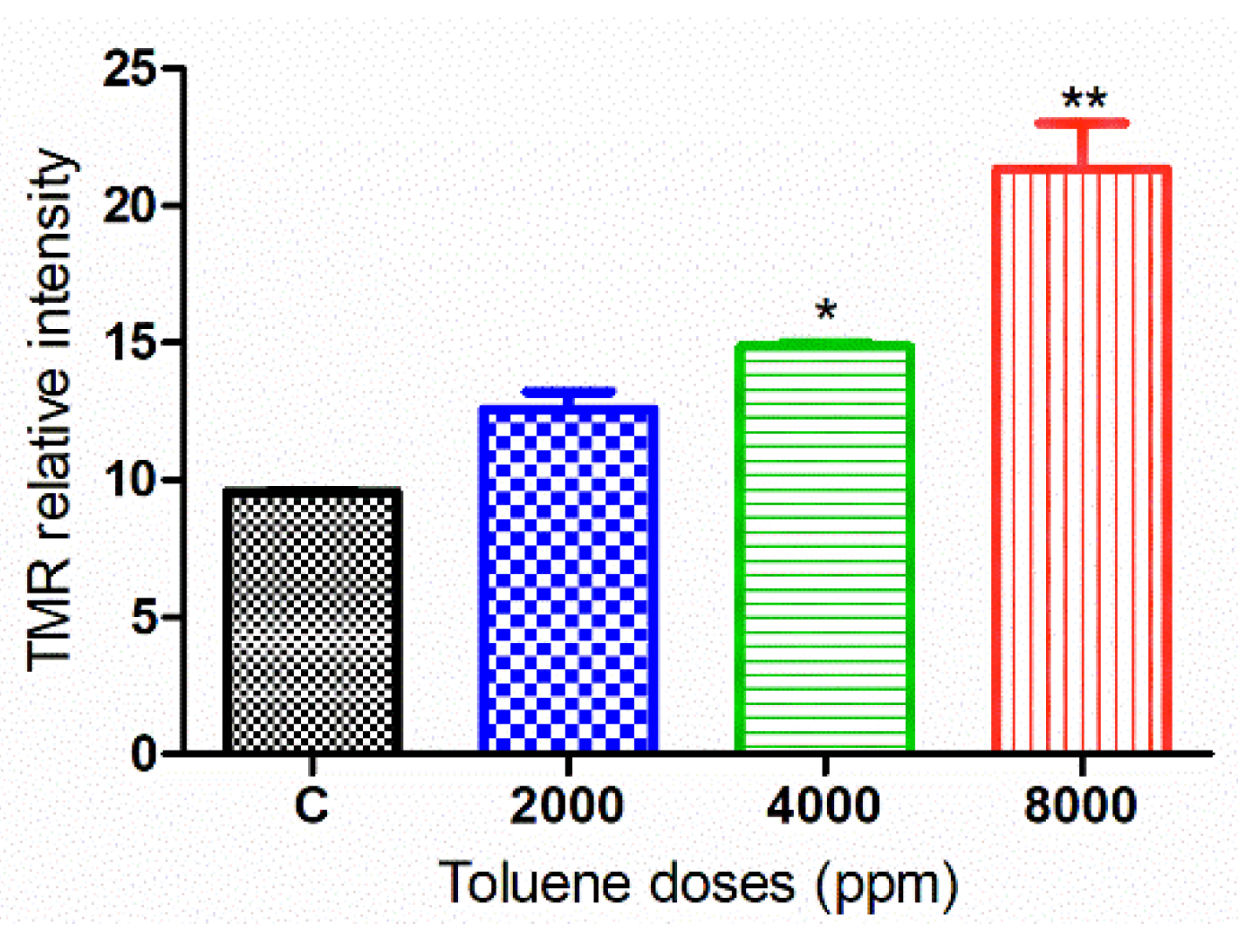
Apoptosis was measured in ovarian tissues from various treatment groups (Figure 9). When comparing the treated groups to the control, the relative intensity of TMR-Red revealed a dose-dependent increase in apoptosis. However, this increase was not significant in ovaries from females treated with the dose 2000 ppm (Figure 10).
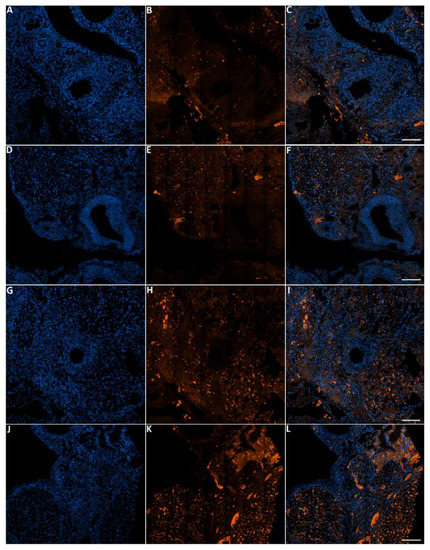
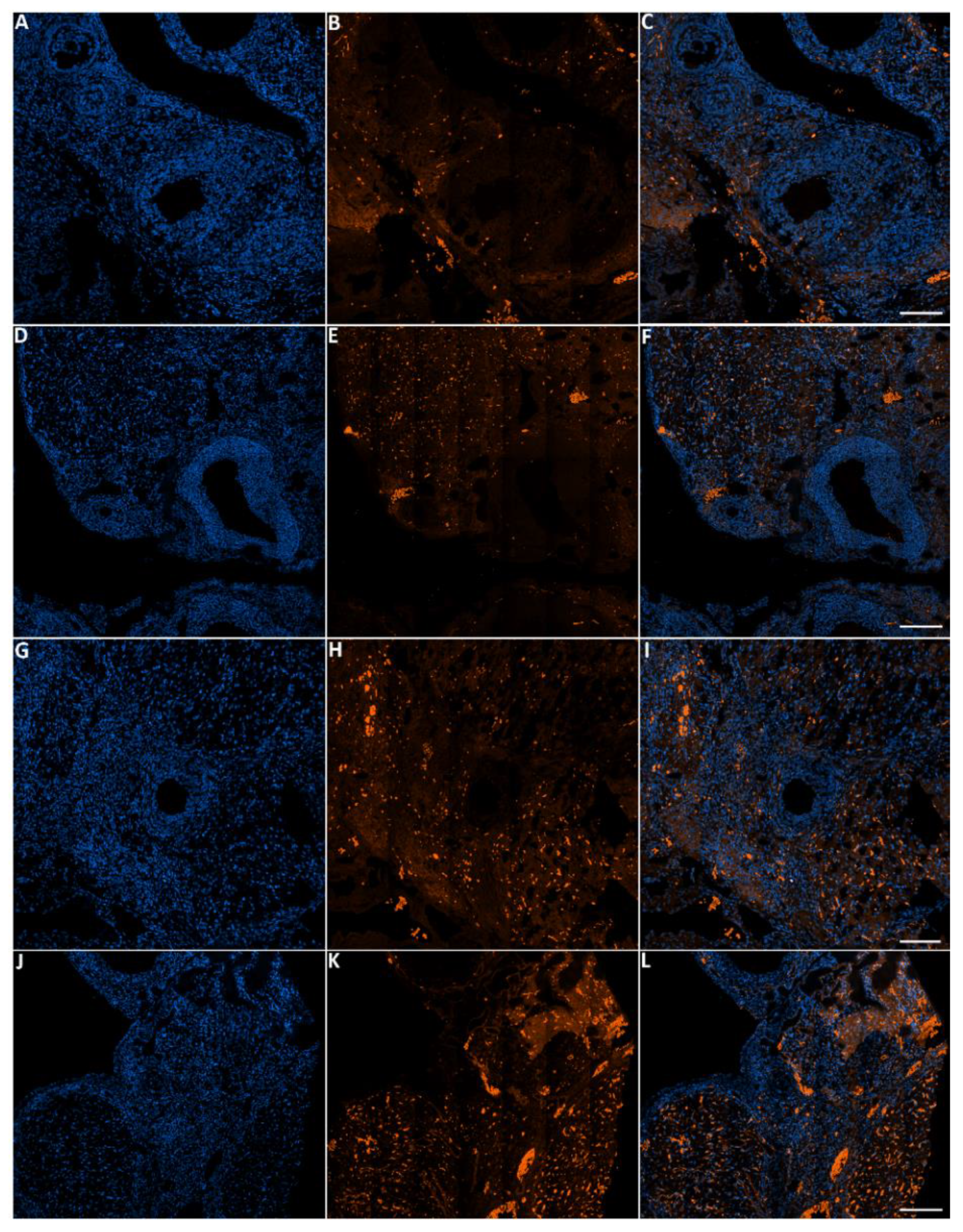
Figure 9.
Representative images of apoptosis in ovaries from the control and treated groups stained with Hoechst and TMR red. Ovaries from control (A–C), ovaries from females that were exposed to 2000 ppm (D–F), 4000 ppm (G–I) and 8000 ppm (J–L). Scale bars = 100 µm.
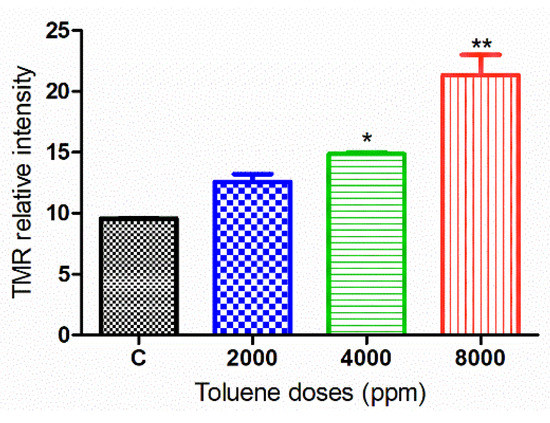
Figure 10.
TMR-Red relative fluorescence intensity in the control and exposure groups. Values are means ± S.E.M. *, p < 0.05; **, p < 0.01.
4. Discussion
Exposure to hazardous chemicals is currently the main occupational disease and the main clinical concern, especially when we know that the direct effect may affect the next generation [42,43]. Among these toxicants, toluene has been shown to cause multiple injuries and damage to different tissues of the body. These include cancer and many other chronic diseases, such as shortness of breath and leukemia [3]. However, studies reporting its effects on ovarian function are still limited. In our previous in vitro studies, we demonstrated that toluene inhibited ovarian cell viability, proliferation and estrogen release [38,44]. In this study, we investigated the in vivo effect of toluene using female Wistar rats. We found that exposure to a low dose (2000 ppm) significantly increased body weight, whereas no effect was observed with the middle and high doses. The effect of toluene exposure on animal body weight varies in the literature. While some studies reported a decrease in body weight, others reported an increase in body weight following exposure to toluene [45]. This variation may be due to many factors, including the method of administration, whether it was administered through gavage, injection or inhalation [46].
In the present study, we identified the reproductive toxic effect of toluene exposure on ovarian function and follicle development potential and initially confirmed that this toxicity was derived from ovarian structure disruption, inhibition of folliculogenesis and steroidogenesis-related markers, induction of apoptosis and autophagy. We first observed a reduced ovarian weight in rats treated with doses of 4000 and 8000 ppm. This reduction was associated with an increase in the number of abnormal follicles, whereas the number of growing follicles was reduced at 8000 ppm. Our results are consistent with previous studies that reported that toluene exposure caused multiple injuries in adult female mouse ovaries, leading to the disruption of the follicular development process and altering their histological structure [47,48]. Based on the fact that hydrocarbons are considered endocrine disruptors, we analyzed some reproductive hormones that have a direct effect on ovarian function. We found that toluene exposure caused a significant increase in the secretion of the hormones progesterone and testosterone when compared to the control group. Based on the fact that the steroidogenic factor Cyp17a is responsible for androgen production, including testosterone [49,50], this increase in testosterone levels is in accordance with the significant increase in Cyp17a mRNA levels shown by RT–PCR. The increase in progesterone release discords the results of our previous study in which we used cultured ovarian granulosa cells, in which we found that the addition of toluene resulted in suppression of progesterone secretion [39]. It has been shown that progesterone may have antioxidative and protective effects since it inhibits the production of oxygen species (ROS) in the ovaries of benzene-treated rats [51] and protects against bropirimine-mediated embryolethality [52]. Thus, the upward trend of progesterone with increasing doses of toluene may be interpreted as a strategy of protection created by the ovary against toluene exposure and not due to the increase in the number of the corpus luteum which has the secretion of progesterone as its primary function. This significant increase in progesterone could also be due to the effect of other factors, since the corpora lutea are also under the control of other organs rather than the ovary, including the hypothalamus and pituitary and even the uterus [53]. Indeed, a previous study showed that toluene caused a decrease in the level of follicle stimulating hormone (FSH) responsible for the growth of ovarian follicles [54], which may have, as a consequence, an increase in progesterone as a result of the feedback interactions between ovarian and gonadotropic hormones. Intestinally, it has been previously reported that progesterone activates autophagy as a strategic neuroprotective mechanism [55] which may suggest that the significant progesterone release after toluene exposure has a protective effect of ovarian cells through triggering autophagy.
During follicular growth and development, Insl3, Ccnd2, Actb and Gdf are essential for granulosa cell proliferation and differentiation [56]. Our data show that toluene exposure caused a significant downward trend in the mRNA levels of these genes with increasing doses, which may have negatively affected granulosa cell proliferation and follicle development. In particular, INSL3 in females of Mammalia is produced from ovarian follicular theca cells [57] and plays a role in the control of the number of healthy growing follicles [58,59]. The downregulation of this gene under the effect of toluene exposure may have increased the number of abnormal follicles. Among the positive regulators of granulosa cell proliferation are D-type cyclins (CCND1, CCND2 and CCND3) that activate the cyclin-dependent kinases CDK 4 and 6 [60]. The knockout of cyclin D2 (Ccnd2) led to the impairment of granulosa cell proliferation and the failure of ovulation [61], which is in accordance with our results. Interestingly, toluene exposure caused a significant decrease in the expression of the GDF protein, which negatively affected the number of developing follicles, mainly at 8000 ppm. This is evident since GDF-9 is needed for early stages of follicle development in several mammalian species [62] by suppressing granulosa cell apoptosis and follicular atresia [63]. In other words, the downregulation of GDF-9 increases the occurrence of apoptosis in ovarian cells. This is in accordance with the TUNEL assay results, which demonstrated that apoptotic cells increased in a dose-dependent manner in response to toluene exposure, even though the increase was not significant at 2000 ppm. Consistent with previous studies, environmental toxins, including benzene, toluene and xylene, have been shown to cause cellular processes to be disrupted by inducing apoptosis and genotoxicity [10,64,65,66,67,68,69].
Autophagy is an evolutionarily conserved cellular mechanism that contributes to the protection of cells against a variety of intracellular and extracellular stressors [70]. It is an important process that may support cell survival or trigger cell death pathways [71,72]. Our immunofluorescence results showed that exposure to toluene significantly increased the protein expression of LC3 and its encoding gene for all doses of treatment. On the other hand, toluene exposure triggered apoptosis for middle and high doses of treatment (4000 and 8000 ppm) whereas TMR-Red increase was not significant in ovaries from females treated with the low dose (2000 ppm). These results suggest that a low dose of toluene exposure may trigger the autophagy machinery which worked to protect ovarian cells from the damage by inhibiting apoptosis. However, the activated autophagy after treatment with both middle and high doses was associated with facilitation of apoptosis rather than its repression. Our results are consistent with what was mentioned about the role of environmental pollutants, especially industrial compounds, that are able to trigger autophagy, either by increasing it as a protective response against cellular damage or by a pathway to shift its protective role toward a pro-cell death mechanism [70]. In fact, it has been previously shown that autophagy and apoptosis are two closely interconnected processes that can occur simultaneously and share many factors in response to diverse stimuli [72,73]. In some cases of low-dose toxins, the activation of autophagy appears to be an initial event in the cell self-repair mechanism that protects cells against damage, leading to the inhibition of apoptosis [74]. Once unable to protect cells against stressful conditions, autophagy upregulates the apoptotic process, leading to cell death [75], which is in accordance with our TUNEL assay data.
5. Conclusions
We conclude from our in vivo study that toluene disrupted ovarian function injury by significantly decreasing the number of growing follicles and increasing the number of abnormal follicles, progesterone and testosterone levels. In addition, it inhibited GDF-9 protein expression and mRNA levels of genes responsible of follicular growth and development. At lower concentrations of toluene exposure, autophagy was activated to prevent ovarian injury by inhibiting apoptosis. Middle and higher concentrations of toluene induced autophagy and apoptosis that eventually caused ovarian cell death. Our findings provide novel information on the mechanism of disruption of hydrocarbons, and the potential mutual interactions between autophagy and apoptosis in reproductive toxicity that will aid in future research. For future studies, it will be critical to determine the in utero impacts of toluene exposure in order to determine the alterations and potential consequences that could have a long-term impact on the health of future generations.
Author Contributions
A.H.H. and S.A. designed the experiments. A.A. and N.A. carried out the experiments. A.H.H., L.M. and M.A. analyzed the data. A.A., A.H.H. and A.V.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (13-ENV1321-02).
Institutional Review Board Statement
This study was approved by the Scientific Research Ethics Committee at King Saud University (Reference No: KSU-SE-20-76).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (Abdel Halim Harrath), upon reasonable request.
Acknowledgments
The authors are grateful to the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, for funding this study (Award Number 13-ENV1321-02).
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
| Actb | Actin Beta |
| Atg5 | Autophagy related 5 |
| BSA | Bovine serum albumin |
| cDNA | Complementary DNA |
| Ccnd1 | Cyclin D1 |
| Ccnd2 | Cyclin D2 |
| Cdk | Cyclin-dependent kinases |
| Cyp17a | Cytochrome P450 family 17 subfamily A |
| Cyp19 | Cytochrome P450 family 19 |
| Esr1 | Estrogen receptor 1 |
| Esr2 | Estrogen receptor 2 |
| FITC | fluorescein isothiocyanate |
| Gdf-9 | Growth differentiation factor 9 |
| Igf-1 | Insulin-like growth factor-1 |
| Insl3 | Insulin-Like Peptide 3 |
| Lhr | Luteinizing hormone receptor |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| NBF | Neutral buffered formalin |
| PBS | Phosphate-buffered saline |
| RT-PCR | Real-time PCR |
| TUNEL | Terminal deoxynucleotidyl transferase mediated nick end-labeling |
| TdT | Terminal deoxynucleotidyl transferase |
References
- Zhang, J.; An, J.; Qu, Y.; Liu, X.; Chen, Y. Impacts of potential HONO sources on the concentrations of oxidants and secondary organic aerosols in the Beijing-Tianjin-Hebei region of China. Sci. Total. Environ. 2019, 647, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Zeng, L.; Guo, H.; Simpson, I.; Ling, Z.; Wang, Y.; Murray, F.; Louie, P.; Saunders, S.; Lam, S.; et al. Evaluation of the effectiveness of air pollution control measures in Hong Kong. Environ. Pollut. 2017, 220, 87–94. [Google Scholar] [CrossRef]
- Veerakumar, U.; Al Shaali, S.J.; Abbas, A. Subduing Harmful Exposure of BTEX by Improving Facility and Practices. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 8 November 2016. [Google Scholar]
- Chauhan, S.K.; Saini, N.; Yadav, V.B. Recent trends of volatile organic compounds in ambient air and its health impacts: A review. Int. J. Technol. Res. Eng. 2014, 1, 667. [Google Scholar]
- Administration OSaH, Toluene. Available online: https://www.osha.gov/SLTC/toluene/exposure_limits.html (accessed on 14 November 2013).
- Lubman, D.I.; Yücel, M.; Lawrence, A.J. Inhalant abuse among adolescents: Neurobiological considerations. Br. J. Pharmacol. 2008, 154, 316–326. [Google Scholar] [CrossRef]
- Duncan, J.R.; Dick, A.L.W.; Egan, G.; Kolbe, S.; Gavrilescu, M.; Wright, D.; Lubman, D.I.; Lawrence, A.J. Adolescent toluene inhalation in rats affects white matter maturation with the potential for recovery following abstinence. PLoS ONE 2012, e44790. [Google Scholar] [CrossRef] [PubMed]
- Bingham, E.; Cohrssen, B. Patty’s Toxicology, 6 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ballantyne, B. Perspectives in Basic and Applied Toxicology, 1987 ed.; Butterworth & Co.: London, UK, 1988. [Google Scholar]
- Ayan, M.; Tas, U.; Sogut, E.; Kuloglu, T.; Cayli, S.; Kocaman, N.; Karaca, Z.I.; Sahin, M. The apoptotic effect of a high dose of toluene on liver tissue during the acute phase: An experimental study. Toxicol. Ind. Health 2013, 29, 728–736. [Google Scholar] [CrossRef]
- Tas, U.; Ogeturk, M.; Kuloglu, T.; Sapmaz, H.I.; Kocaman, N.; Zararsiz, I.; Sarsilmaz, M. HSP70 immune reactivity and TUNEL positivity in the liver of toluene-inhaled and melatonin-treated rats. Toxicol. Ind. Health 2013, 29, 514–522. [Google Scholar] [CrossRef]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The Effects of Toluene on the Central Nervous System. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Soares, M.V.; Charão, M.F.; Jacques, M.T.; dos Santos, A.L.A.; Luchese, C.; Pinton, S.; Ávila, D.S. Airborne toluene exposure causes germline apoptosis and neuronal damage that promotes neurobehavioural changes in Caenorhabditis elegans. Environ. Pollut. 2020, 256, 113406. [Google Scholar] [CrossRef]
- Balster, R.L. Neural basis of inhalant abuse. Drug Alcohol Depend. 1998, 51, 207–214. [Google Scholar] [CrossRef]
- Berenguer, P.; Soulage, C.; Perrin, D.; Pequignot, J.-M.; Abraini, J.H. Behavioral and neurochemical effects induced by subchronic exposure to 40 ppm toluene in rats. Pharmacol. Biochem. Behav. 2003, 74, 997–1003. [Google Scholar] [CrossRef]
- Kondo, H.; Huang, J.; Ichihara, G.; Kamijima, M.; Saito, I.; Shibata, E.; Ono, Y.; Hisanaga, N.; Takeuchi, Y.; Nakahara, D. Toluene induces behavioral activation without affecting striatal dopamine metabolism in the rat: Behavioral and microdialysis studies. Pharmacol. Biochem. Behav. 1995, 51, 97–101. [Google Scholar] [CrossRef]
- Riegel, A.C.; French, E.D. An Electrophysiological Analysis of Rat Ventral Tegmental Dopamine Neuronal Activity During Acute Toluene Exposure. Pharmacol. Toxicol. 1999, 85, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Yang, M.; Song, M.-S.; Kim, J.-S.; Kim, S.-H.; Kim, J.-C.; Kim, H.; Shin, T.; Wang, H.; Moon, C. Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacol. Biochem. Behav. 2010, 94, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Von Euler, G.; Fuxe, K.; Hansson, T.; Ögren, S.O.; Agnati, L.F.; Eneroth, P.; Härfstrand, A.; Gustafsson, J.Å. Effects of chronic toluene exposure of central monoamine and peptide receptors and their interactions in the adult male rat. Toxicology 1988, 52, 103–126. [Google Scholar] [CrossRef]
- Eisenberg, D.P. Neurotoxicity and mechanism of toluene abuse. Einstein QJ Biol. Med. 2003, 19, 150–159. [Google Scholar]
- Cruz, S.L.; Soberanes-Chávez, P.; Páez-Martinez, N.; López-Rubalcava, C. Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs. Psychopharmacology 2009, 204, 279–286. [Google Scholar] [CrossRef]
- Cruz, S.L.; Rivera-García, M.T.; Woodward, J.J. Review of Toluene Actions: Clinical Evidence, Animal Studies, and Molecular Targets. J. Drug Alcohol Res. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Royland, J.E.; Richards, J.E.; Besas, J.; MacPhail, R.C. Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicol. Appl. Pharmacol. 2011, 256, 386–398. [Google Scholar] [CrossRef]
- Zhvania, M.G.; Chilachava, L.R.; Japaridze, N.J.; Gelazonia, L.K.; Lordkipanidze, T. Immediate and persisting effect of toluene chronic exposure on hippocampal cell loss in adolescent and adult rats. Brain Res. Bull. 2012, 87, 187–192. [Google Scholar] [CrossRef]
- Huerta-Rivas, A.; López-Rubalcava, C.; Sánchez-Serrano, S.L.; Valdez-Tapia, M.; Lamas, M.; Cruz, S.L. Toluene impairs learning and memory, has antinociceptive effects, and modifies histone acetylation in the dentate gyrus of adolescent and adult rats. Pharmacol. Biochem. Behav. 2012, 102, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Revilla, A.S.; Pestana, C.; Pardo-Andreu, G.L.; Santos, A.C.; Uyemura, S.A.; Gonzales, M.E.; Curti, C. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol. Vitr. 2007, 21, 782–788. [Google Scholar] [CrossRef]
- Wang, D.; Horike, T.; Mizuuchi, H.; Ishii, K.; Zhen, L.; Taketa, K. Liver Function Tests of Workers Exposed to Toluene and Toluene/Dimethylformamide at Low Concentrations. J. Occup. Health 1996, 38, 113–117. [Google Scholar] [CrossRef]
- Pyykkö, K. Time-course of effects of toluene on microsomal enzymes in rat liver, kidney and lung during and after inhalation exposure. Chem. Interact. 1983, 44, 299–310. [Google Scholar] [CrossRef]
- Ullah, E.; Arredondo, D.; Moreno, A.; Campanas, M.; Lopez, F.; Kay, G.; Nguyen, A.; Rosell, R. Increased Toluene Exposure and Toxicity Effects Results in Low Fly Fecundity and Offspring Development in Female Drosophila melanogaster. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ungváry, G.; Tátrai, E. On the Embryotoxic Effects of Benzene and Its Alkyl Derivatives in Mice, Rats and Rabbits. Arch. Toxicol. 1985, 8, 425–430. [Google Scholar] [CrossRef]
- Webb, E.; Bushkin-Bedient, S.; Cheng, A.; Kassotis, C.; Balise, V.; Nagel, S.C. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev. Environ. Health 2014, 29, 307–318. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol. 2017, 71, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Kuczkowski, K.M. The effects of drug abuse on pregnancy. Curr. Opin. Obstet. Gynecol. 2007, 19, 578–585. [Google Scholar] [CrossRef]
- Hannigan, J.H.; Bowen, S.E. Reproductive Toxicology and Teratology of Abused Toluene. Syst. Biol. Reprod. Med. 2010, 56, 184–200. [Google Scholar] [CrossRef]
- Waldner, C.L. The Association Between Exposure to the Oil and Gas Industry and Beef Calf Mortality in Western Canada. Arch. Environ. Occup. Health 2008, 63, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.E.; Batis, J.C.; Paez-Martinez, N.; Cruz, S.L. The last decade of solvent research in animal models of abuse: Mechanistic and behavioral studies. Neurotoxicol. Teratol. 2006, 28, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.A.; Fata, E.; Bowen, S.E.; Jen, K.-L.C.; Coscina, D.V. Effects of abuse pattern of gestational toluene exposure on metabolism, feeding and body composition. Physiol. Behav. 2008, 93, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Tarko, A.; Fabová, Z.; Kotwica, J.; Valocký, I.; Alrezaki, A.; Alwasel, S.; Harrath, A.; Sirotkin, A. The inhibitory influence of toluene on mare ovarian granulosa cells can be prevented by fennel. Gen. Comp. Endocrinol. 2020, 295, 113491. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kadasi, A.; Baláži, A.; Kotwica, J.; Alrezaki, A.; Harrath, A.H. Mechanisms of the direct effects of oil-related contaminants on ovarian cells. Environ. Sci. Pollut. Res. 2020, 27, 5314–5322. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Tarko, A.; Kotwica, J.; Alrezaki, A.; Harrath, A.H. Interrelationships between metabolic hormones, leptin and ghrelin, and oil-related contaminants in control of oxytocin and prostaglandin F release by feline ovaries. Reprod. Biol. 2020, 20, 254–258. [Google Scholar] [CrossRef]
- Harrath, A.H.; Alrezaki, A.; Mansour, L.; Alwasel, S.H.; Palomba, S. Food restriction during pregnancy and female offspring fertility: Adverse effects of reprogrammed reproductive lifespan. J. Ovarian Res. 2017, 10, 77. [Google Scholar] [CrossRef]
- Painter, R.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.; Roseboom, T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1243–1249. [Google Scholar] [CrossRef]
- Susiarjo, M.; Hassold, T.J.; Freeman, E.; Hunt, P.A. Bisphenol a Exposure In Utero Disrupts Early Oogenesis in the Mouse. PLoS Genet. 2007, 3, e5. [Google Scholar] [CrossRef]
- Sirotkin, A.; Záhoranska, Z.; Tarko, A.; Fabova, Z.; Alwasel, S.; Harrath, A.H. Plant polyphenols can directly affect ovarian cell functions and modify toluene effects. J. Anim. Physiol. Anim. Nutr. 2021, 105, 80–89. [Google Scholar] [CrossRef]
- Roberts, L.; Nicolich, M.; Schreiner, C. Developmental and reproductive toxicity evaluation of toluene vapor in the rat: II. Developmental toxicity. Reprod. Toxicol. 2007, 23, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Callan, S.; Kott, J.; Cleary, J.; McCarthy, M.K.; Baltes, B.B.; Bowen, S.E. Changes in developmental body weight as a function of toluene exposure: A meta-analysis of animal studies. Hum. Exp. Toxicol. 2016, 35, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kareem, L.A.; Banna, H.B.; Naoom, K.M. Effects of toluene and formaldehyde on oogenesis in adult female mice. Diyala J. Med. 2014, 6, 33–40. [Google Scholar]
- Chen, H.; Song, L.; Wang, X.; Wang, S. Effect of exposure to low concentration of benzene and its analogues on luteal function of female workers. J. Hyg. Res. 2000, 29, 351–353. [Google Scholar]
- Murray, A.A.; Gosden, R.G.; Allison, V.; Spears, N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod. Fertil. 1998, 113, 27–33. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhang, C.-P.; Li, L.; Huang, L.; Ji, S.-Y.; Lu, C.-L.; Fan, C.-H.; Cai, H.; Ren, Y.; Hu, Z.-Y.; et al. Testosterone Induces Redistribution of Forkhead Box-3a and Down-Regulation of Growth and Differentiation Factor 9 Messenger Ribonucleic Acid Expression at Early Stage of Mouse Folliculogenesis. Endocrinology 2010, 151, 774–782. [Google Scholar] [CrossRef]
- Verma, Y.; Rana, S. Effects of Progesterone on Benzene Toxicity in Rats. Arch. Ind. Hyg. Toxicol. 2008, 59, 1–9. [Google Scholar] [CrossRef][Green Version]
- Black, D.L.; Marks, T.A.; Branstetter, D.G.; Kirton, K.T. Reversal of bropirimine developmental toxicity with progesterone. Toxicol. Appl. Pharmacol. 1991, 108, 121–128. [Google Scholar] [CrossRef]
- Stocco, C.; Telleria, C.; Gibori, G. The Molecular Control of Corpus Luteum Formation, Function, and Regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef]
- Salem, R.R.; Kelada, M.N. A Biochemichal and Ultrastructural Study on the Effect of Toluene on the Pars Distalis of Anterior Pituitary Glands of Adult Male Albino Rats. Egypt J. Histol. 2020, 43, 948–959. [Google Scholar] [CrossRef]
- Na Kim, H.; Lee, S.-J.; Koh, J.-Y. The neurosteroids, allopregnanolone and progesterone, induce autophagy in cultured astrocytes. Neurochem. Int. 2012, 60, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Yenuganti, V.R.; Viergutz, T.; Vanselow, J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen. Comp. Endocrinol. 2016, 232, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ivell, R.; Anand-Ivell, R. Insulin-like peptide 3 (INSL3) is a major regulator of female reproductive physiology. Hum. Reprod. Update 2018, 24, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Anand-Ivell, R.; Tremellen, K.; Dai, Y.; Heng, K.; Yoshida, M.; Knight, P.G.; Hale, G.E. Circulating insulin-like factor 3 (INSL3) in healthy and infertile women. Hum. Reprod. 2013, 28, 3093–3102. [Google Scholar] [CrossRef]
- Seyam, E.; Hefzy, E. Evaluation of the correlation between insulin like factor 3, polycystic ovary syndrome, and ovarian maldescent. Gynecol. Endocrinol. 2018, 34, 481–488. [Google Scholar] [CrossRef]
- Shimizu, T.; Hirai, Y.; Miyamoto, A. Expression of Cyclins and Cyclin-Dependent Kinase Inhibitors in Granulosa Cells from Bovine Ovary. Reprod. Domest. Anim. 2013, 48, e65–e69. [Google Scholar] [CrossRef]
- Sicinski, P.; Donaher, J.L.; Geng, Y.; Parker, S.B.; Gardner, H.P.; Park, M.Y.; Robker, R.; Richards, J.S.; McGinnis, L.; Biggers, J.D.; et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nat. Cell Biol. 1996, 384, 470–474. [Google Scholar] [CrossRef]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nat. Cell Biol. 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Orisaka, M.; Orisaka, S.; Jiang, J.-Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K.; Elliott, J. Growth Differentiation Factor 9 Is Antiapoptotic during Follicular Development from Preantral to Early Antral Stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef]
- Ross, D. The Role of Metabolism and Specific Metabolites in Benzene-Induced Toxicity: Evidence and Issues. J. Toxicol. Environ. Health Part A 2000, 61, 357–372. [Google Scholar] [CrossRef]
- Nakai, N.; Murata, M.; Nagahama, M.; Hirase, T.; Tanaka, M.; Fujikawa, T.; Nakao, N.; Nakashima, K.; Kawanishi, S. Oxidative DNA Damage Induced by Toluene is Involved in its Male Reproductive Toxicity. Free Radic. Res. 2003, 37, 69–76. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Q.; Wang, Y.; Zhu, S. Toxic effects of BTEX in water on Daphnia magna and Limnodrilus hoffmeisteri and safety assessment of the aquatic environment. Acta Sci. Circumstantiae 2009, 29, 1485–1490. [Google Scholar]
- Zhang, M.; Wang, Y.; Wang, Q.; Yang, J.; Yang, D.; Liu, J.; Li, J. Involvement of Mitochondria-Mediated Apoptosis in Ethylbenzene-Induced Renal Toxicity in Rat. Toxicol. Sci. 2010, 115, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Q.; Luo, Y.; Yang, G.; Zhou, T. Joint action and lethal levels of toluene, ethylbenzene, and xylene on midge (Chironomus plumosus) larvae. Environ. Sci. Pollut. Res. 2012, 20, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Neuparth, T.; Capela, R.; Pereira, S.P.P.; Moreira, S.M.; Santos, M.M.; Reis-Henriques, M.A. Toxicity effects of hazardous and noxious substances (HNS) to marine organisms: Acute and chronic toxicity of p-xylene to the amphipod Gammarus locusta. J. Toxicol. Environ. Health Part A 2014, 77, 1210–1221. [Google Scholar] [CrossRef]
- García, G.M.; Mariño, G. Autophagy role in environmental pollutants exposure. Prog. Mol. Biol. Transl. Sci. 2020, 172, 257–291. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshino, O.; Nakashima, A.; Ito, M.; Nishio, K.; Ono, Y.; Kusabiraki, T.; Kunitomi, C.; Takahashi, N.; Harada, M.; et al. Inhibition of autophagy in theca cells induces CYP17A1 and PAI-1 expression via ROS/p38 and JNK signalling during the development of polycystic ovary syndrome. Mol. Cell. Endocrinol. 2020, 508, 110792. [Google Scholar] [CrossRef]
- Zecchini, S.; Serafini, F.P.; Catalani, E.; Giovarelli, M.; Coazzoli, M.; Di Renzo, I.; De Palma, C.; Perrotta, C.; Clementi, E.; Buonanno, F.; et al. Dysfunctional autophagy induced by the pro-apoptotic natural compound climacostol in tumour cells. Cell Death Dis. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Yuan, Z.; Yi, J.; Chen, J.; Wang, N.; Tian, Y. Autophagy and Apoptosis Interact to Modulate T-2 Toxin-Induced Toxicity in Liver Cells. Toxins 2019, 11, 45. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Park, K.-I.; Kim, S.-H.; Yu, S.-N.; Park, S.-G.; Kim, Y.W.; Seo, Y.-K.; Ma, J.-Y.; Ahn, S.-C. Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).