Taphonomic and Diagenetic Pathways to Protein Preservation, Part I: The Case of Tyrannosaurus rex Specimen MOR 1125
Abstract
:Simple Summary
Abstract
1. Introduction
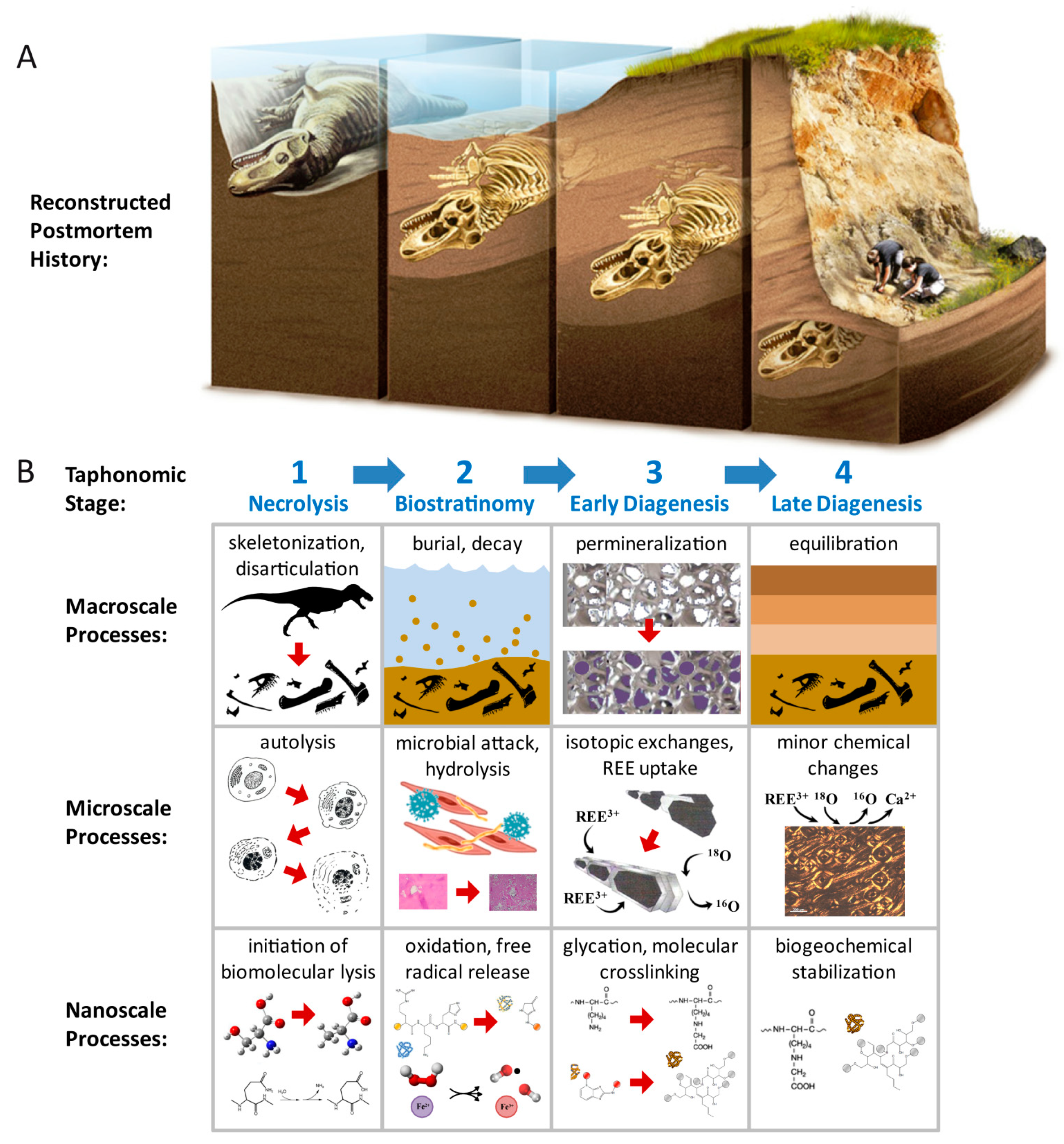
1.1. Biomolecular Preservation in Fossils
1.2. Trace Element Taphonomy of Fossil Bone
2. Taphonomic and Geologic Context
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Sample Preparation
3.2.2. LA-ICPMS Analyses
4. Results
4.1. Overall REE Composition
4.2. Intra-Bone REE Depth Profiles
4.3. NASC-Normalized REE Patterns
4.4. (La/Yb)N vs. (La/Sm)N Ratio Patterns
4.5. REE Anomalies
5. Discussion
5.1. Clarifying MOR 1125′s Paleoenvironmental and Taphonomic Context
5.2. Reconstructing the Geochemical History of MOR 1125
5.3. Emerging Taphonomic and Diagenetic Themes
6. Conclusions
- Our results strengthen the hypothesis that oxidizing depositional environments or, more specifically, oxidizing microenvironmental conditions during early diagenesis, can foster chemical reactions which stabilize bone cells, soft tissues, and their component biomolecules;
- In general, middle and internal cortical tissues usually experience less chemical alteration onaverage than the external cortex of any given fossil bone, implying that these interior regions should be the primary targets of future paleomolecular sampling;
- Diagenetic circumstances which restrict exposure to percolating pore fluids (i.e., burial in compact, fine-grained sediments, early/rapid cementation of sediments, encasement in early-diagenetic concretion) elevate molecular preservation potential by minimizing gross chemical alteration;
- Avoidance of protracted interactions with late-diagenetic pore fluids is almost certainly crucial to sustain long-term stability of biomolecules in a fossil bone.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curry, G.B. Molecular palaeontology: New life for old molecules. Trends Ecol. Evol. 1987, 2, 161–165. [Google Scholar] [CrossRef]
- Schweitzer, M.H. Soft tissue preservation in terrestrial Mesozoic vertebrates. Annu. Rev. Earth Planet. Sci. 2011, 39, 187–216. [Google Scholar] [CrossRef]
- Hofreiter, M.; Collins, M.; Stewart, J.R. Ancient biomolecules in Quaternary palaeoecology. Quat. Sci. Rev. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Goodwin, M.B.; Boatman, E.; Theil, E.; Marcus, M.A.; Fakra, S.C. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proc. R. Soc. B 2014, 281, 20132741. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, M.H.; Schroeter, E.R.; Cleland, T.P.; Zheng, W. Paleoproteomics of Mesozoic dinosaurs and other Mesozoic fossils. Proteomics 2019, 19, 1800251. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Taylor, S. Proteomes of the past: The pursuit of proteins in paleontology. Expert Rev. Proteom. 2019, 16, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, A.M.; Li, Z. DNA staining in fossil cells beyond the Quaternary: Reassessment of the evidence and prospects for an improved understanding of DNA preservation in deep time. Earth-Sci. Rev. 2021, 216, 103600. [Google Scholar] [CrossRef]
- Orlando, L.; Bonjean, D.; Bocherens, H.; Thenot, A.; Argant, A.; Otte, M.; Hanni, C. Ancient DNA and the population genetics of cave bears (Ursus spelaeus) through space and time. Mol. Biol. Evol. 2002, 19, 1920–1933. [Google Scholar] [CrossRef] [Green Version]
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef]
- Noonan, J.P.; Coop, G.; Kudaravalli, S.; Smith, D.; Krause, J.; Alessi, J.; Chen, F.; Platt, D.; Pääbo, S.; Pritchard, J.K.; et al. Sequencing and analysis of Neanderthal genomic DNA. Science 2006, 314, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Oskam, C.L.; Haile, J.; McLay, E.; Rigby, P.; Allentoft, M.E.; Olsen, M.E.; Bengtsson, C.; Miller, G.H.; Schwenninger, J.-L.; Jacomb, C.; et al. Fossil avian eggshell preserves ancient DNA. Proc. R. Soc. B 2010, 277, 1991–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, R.; Westbury, M.V.; Sandoval-Velasco, M.; Vieira, F.G.; Jeon, S.; Zazula, G.; Martin, M.D.; Ho, S.Y.W.; Mather, N.; Gopalakrishnan, S.; et al. Genomic adaptations and evolutionary history of the extinct scimitar-toothed cat, Homotherium latidens. Curr. Biol. 2020, 30, 5018–5025. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, T.; Pečnerová, P.; Díez-del-Molino, D.; Bergström, A.; Oppenheimer, J.; Hartmann, S.; Xenikoudakis, G.; Thomas, J.A.; Dehasque, M.; Sağlican, E.; et al. Million-year-old DNA sheds light on the genomic history of mammoths. Nature 2021, 591, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zheng, W.; Moyer, A.E.; O’Connor, J.K.; Wang, M.; Zheng, X.; Wang, X.; Schroeter, E.R.; Zhou, Z.; Schweitzer, M.H. Molecular evidence of keratin and melanosomes in feathers of the Early Cretaceous bird Eoconfuciusornis. Proc. Natl. Acad. Sci. USA 2016, 113, 7900–7907. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zheng, W.; Sawyer, R.H.; Pennington, M.W.; Zheng, X.; Wang, X.; Wang, M.; Hu, L.; O’Connor, J.; Zhao, T.; et al. The molecular evolution of feathers with direct evidence from fossils. Proc. Natl. Acad. Sci. USA 2019, 116, 3018–3023. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, J.; Kuriyama, T.; Madsen, H.; Sjövall, P.; Zheng, W.; Uvdal, P.; Engdahl, A.; Moyer, A.E.; Gren, J.A.; Kamezaki, N.; et al. Biochemistry and adaptive colouration of an exceptionally preserved juvenile fossil sea turtle. Sci. Rep. 2017, 7, 13324. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Zheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, E.R.; DeHart, C.J.; Cleland, T.P.; Zheng, W.; Thomas, P.M.; Kelleher, N.L.; Bern, M.; Schweitzer, M.H. Expansion for the Brachylophosaurus canadensis collagen I sequence and additional evidence of the preservation of Cretaceous protein. J. Proteome Res. 2017, 16, 920–932. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, M.H.; Zheng, W.; Moyer, A.E.; Sjövall, P.; Lindgren, J. Preservation potential of keratin in deep time. PLoS ONE 2018, 13, e0206569. [Google Scholar]
- Bailleul, A.M.; Zheng, W.; Horner, J.R.; Hall, B.K.; Holliday, C.M.; Schweitzer, M.H. Evidence of proteins, chromosomes and chemical markers of DNA in exceptionally preserved dinosaur cartilage. Natl. Sci. Rev. 2020, 7, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, P.V.; Voegele, K.K.; Grandstaff, D.E.; Ash, R.D.; Zheng, W.; Schroeter, E.R.; Schweitzer, M.H.; Lacovara, K.J. Molecular tests support the viability of rare earth elements as proxies for fossil biomolecule preservation. Sci. Rep. 2020, 10, 15566. [Google Scholar] [CrossRef] [PubMed]
- Asara, J.M.; Schweitzer, M.H.; Freimark, L.M.; Phillips, M.; Cantley, L.C. Protein sequences from mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science 2007, 316, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Vander Heiden, M.G.; Neveu, J.M.; et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Organ, C.L.; Schweitzer, M.H.; Zheng, W.; Freimark, L.M.; Cantley, L.C.; Asara, J.M. Molecular phylogenetics of Mastodon and Tyrannosaurus rex. Science 2008, 320, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, P.G.; Briggs, D.E.G. Fossilization of feathers. Geology 1995, 23, 783–786. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Kear, A.J. Fossilization of soft tissue in the laboratory. Science 1993, 259, 1439–1442. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R.; Zamdborg, L.; Zheng, W.; Lee, J.E.; Tran, J.C.; Bern, M.; Duncan, M.B.; Lebleu, V.S.; Ahlf, D.R.; et al. Mass spectrometry and antibody-based characterization of blood vessels from Brachylophosaurus canadensis. J. Proteome Res. 2015, 14, 5252–5262. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R. Soft tissue and cellular preservation in vertebrate skeletal elements from the Cretaceous to the present. Proc. R. Soc. B 2007, 274, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, M.H.; Schroeter, E.R.; Goshe, M.B. Protein molecular data from ancient (>1 million years old) fossil material: Pitfalls, possibilities, and grand challenges. Anal. Chem. 2014, 86, 6731–6740. [Google Scholar] [CrossRef]
- Boatman, E.M.; Goodwin, M.B.; Holman, H.-Y.N.; Fakra, S.; Zheng, W.; Gronsky, R.; Schweitzer, M.H. Mechanisms of soft tissue and protein preservation in Tyrannosaurus rex. Sci. Rep. 2019, 9, 15678. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Bern, M. Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone 2013, 52, 414–423. [Google Scholar] [CrossRef]
- Sykes, G.A.; Collins, M.J.; Walton, D.I. The significance of a geochemically isolated intracrystalline organic fraction within biominerals. Org. Geochem. 1995, 23, 1059–1065. [Google Scholar] [CrossRef]
- Edwards, N.P.; Barden, H.E.; van Dongen, B.E.; Manning, P.L.; Larson, P.L.; Bergmann, U.; Sellers, W.I.; Wogelius, R.A. Infrared mapping resolves soft tissue preservation in 50 million year-old reptile skin. Proc. R. Soc. B 2011, 278, 3209–3218. [Google Scholar] [CrossRef] [Green Version]
- Allison, P.A. Konservat-Lagerstätten: Cause and classification. Paleobiology 1988, 14, 331–344. [Google Scholar] [CrossRef]
- Eglinton, G.; Logan, G.A. Molecular preservation. Philos. Trans. R. Soc. Lond. B 1991, 333, 315–328. [Google Scholar]
- Trueman, C.N. Rare earth element geochemistry and taphonomy of terrestrial vertebrate assemblages. PALAIOS 1999, 14, 555–568. [Google Scholar] [CrossRef]
- Trueman, C.N. Trace element geochemistry of bonebeds. In Bonebeds: Genesis, Analysis, and Paleobiological Significance; Rogers, R.R., Eberth, D.A., Fiorillo, A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2007; pp. 397–435. [Google Scholar]
- Trueman, C.N.; Tuross, N. Trace elements in Recent and fossil bone apatite. Rev. Miner. Geochem. 2002, 48, 489–521. [Google Scholar] [CrossRef]
- Trueman, C.N.; Palmer, M.R.; Field, J.; Privat, K.; Ludgate, N.; Chavagnac, V.; Eberth, D.A.; Cifelli, R.; Rogers, R.R. Comparing rates of recrystallisation and the potential for preservation of biomolecules from the distribution of trace elements in fossil bones. C. R. Palevol 2008, 7, 145–158. [Google Scholar] [CrossRef]
- Trueman, C.N.; Kocsis, L.; Palmer, M.R.; Dewdney, C. Fractionation of rare earth elements within bone mineral: A natural cation exchange system. Palaeogeogr. Palaeocl. 2011, 310, 124–132. [Google Scholar] [CrossRef]
- Hinz, E.A.; Kohn, M.J. The effect of tissue structure and soil chemistry on trace element uptake in fossils. Geochim. Cosmochim. Acta 2010, 74, 3213–3231. [Google Scholar] [CrossRef]
- Kocsis, L.; Trueman, C.N.; Palmer, M.R. Protracted diagenetic alteration of REE contents in fossil bioapatites: Direct evidence from Lu-Hf isotope systematics. Geochim. Cosmochim. Acta 2010, 74, 6077–6092. [Google Scholar] [CrossRef]
- Herwartz, D.; Tütken, T.; Jochum, K.P.; Sander, P.M. Rare earth element systematics of fossil bone revealed by LA-ICPMS analysis. Geochim. Cosmochim. Acta 2013, 103, 161–183. [Google Scholar] [CrossRef]
- Kohn, M.J. Models of diffusion-limited uptake of trace elements in fossils and rates of fossilization. Geochim. Cosmochim. Acta 2008, 72, 3758–3770. [Google Scholar] [CrossRef]
- Kocsis, L.; Gheerbrant, E.; Mouflih, M.; Cappetta, H.; Ulianov, A.; Chiaradia, M.; Bardet, N. Gradual changes in upwelled seawater conditions (redox, pH) from the late Cretaceous through early Paleogene at the northwest coast of Africa: Negative Ce anomaly trend recorded in fossil bio-apatite. Chem. Geol. 2016, 421, 44–54. [Google Scholar] [CrossRef]
- Ullmann, P.V.; Grandstaff, D.E.; Ash, R.D.; Lacovara, K.J. Geochemical taphonomy of the Standing Rock Hadrosaur Site: Exploring links between rare earth elements and cellular and soft tissue preservation. Geochim. Cosmochim. Acta 2020, 269, 223–237. [Google Scholar] [CrossRef]
- Grandstaff, D.E.; Terry, D.O. Rare earth element composition of Paleogene vertebrate fossils from Toadstool Geologic Park, Nebraska, USA. Appl. Geochem. 2009, 24, 733–745. [Google Scholar] [CrossRef]
- Bosio, G.; Gioncada, A.; Gariboldi, K.; Bonaccorsi, E.; Collareta, A.; Pasero, M.; Celma, C.D.; Malinverno, E.; Urbina, M.; Bianucci, G. Mineralogical and geochemical characterization of fossil bones from a Miocene marine Konservat-Lagerstätte. J. S. Am. Earth Sci. 2021, 105, 102924. [Google Scholar] [CrossRef]
- Ullmann, P.V.; Shaw, A.; Nellermoe, R.; Lacovara, K.J. Taphonomy of the Standing Rock Hadrosaur Site, Corson County, South Dakota. PALAIOS 2017, 32, 779–796. [Google Scholar] [CrossRef]
- Ullmann, P.V.; Pandya, S.H.; Nellermoe, R. Patterns of soft tissue and cellular preservation in relation to fossil bone microstructure and overburden depth at the Standing Rock Hadrosaur Site, Maastrichtian Hell Creek Formation, South Dakota, USA. Cretaceous Res. 2019, 99, 1–13. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R.; Toporski, J.K. Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science 2005, 307, 1952–1955. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gates, T.A. The Late Jurassic Cleveland-Lloyd Dinosaur Quarry as a drought-induced assemblage. PALAIOS 2005, 20, 363–375. [Google Scholar] [CrossRef]
- Fowler, D. The Hell Creek Formation, Montana: A stratigraphic review and revision based on a sequence stratigraphic approach. Geosciences 2020, 10, 435. [Google Scholar] [CrossRef]
- San Antonio, J.D.; Schweitzer, M.H.; Jensen, S.T.; Kalluri, R.; Buckley, M.; Orgel, J.P.R.O. Dinosaur peptides suggest mechanisms of protein survival. PLoS ONE 2011, 6, e20381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, J.H.; Butler, R.D.; Weiler, M.W.; Schumaker, K.K. Context, naming and formal designation of the Cretaceous Hell Creek Formation lectostratotype, Garfield County, Montana. In Through the End of the Cretaceous in the Type Locality of the Hell Creek Formation in Montana and Adjacent Areas; Wilson, G.P., Clemens, W.A., Horner, J.R., Hartman, J.H., Eds.; Geological Society of America Special Paper 503; Geological Society of America: Boulder, CO, USA, 2014; pp. 89–121. [Google Scholar]
- Horner, J.R.; Goodwin, M.B.; Myhrvold, N. Dinosaur census reveals abundant Tyrannosaurus and rare ontogenetic stages in the Upper Cretaceous Hell Creek Formation (Maastrichtian), Montana, USA. PLoS ONE 2011, 6, e16574. [Google Scholar] [CrossRef] [PubMed]
- Waage, K.M. Cretaceous transitional environments and faunas in central South Dakota. In A Symposium on Paleoenvironments of the Cretaceous Seaway in the Western Interior, Geological Society of America Rocky Mountain Section 20th Annual Meeting; Colorado School of Mines: Golden, CO, USA, 1968; pp. 237–266. [Google Scholar]
- Frye, C.I. Stratigraphy of the Hell Creek Formation in North Dakota. N. Dak. Geol. Surv. Bull. 1969, 54, 1–65. [Google Scholar]
- Moore, W.L. Stratigraphy and Environments of Deposition of the Cretaceous Hell Creek Formation (Reconnaissance) and the Paleocene Ludlow Formation (Detailed), Southwestern North Dakota. Report of Investigations No. 56. United States. 1976. Available online: https://www.osti.gov/biblio/7346007 (accessed on 3 November 2021).
- Murphy, E.C.; Hoganson, J.W.; Johnson, K.R. Lithostratigraphy of the Hell Creek Formation in North Dakota. Geol. Soc. Am. Spec. Pap. 2002, 361, 9–34. [Google Scholar]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R. Gender-specific reproductive tissue in ratites and Tyrannosaurus rex. Science 2005, 308, 1456–1460. [Google Scholar] [CrossRef] [Green Version]
- Gromet, L.P.; Dymek, R.F.; Haskin, L.A.; Korotev, R.L. The “North American shale composite”: Its compilation, major and trace element characteristics. Geochim. Cosmochim. Acta 1984, 48, 2469–2482. [Google Scholar] [CrossRef]
- Haskin, L.A.; Haskin, M.A.; Frey, F.A.; Wildeman, T.R. Relative and absolute terrestrial abundances of the rare earths. In Origin and Distribution of the Elements; Ahrens, L.H., Ed.; Pergamon: Oxford, UK, 1968; pp. 889–912. [Google Scholar]
- Herwartz, D.; Tütken, T.; Munker, C.; Jochum, K.P.; Stoll, B.; Sander, P.M. Timescales and mechanisms of REE and Hf uptake in fossil bones. Geochim. Cosmochim. Acta 2011, 75, 82–105. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sc. Lett. 1996, 143, 245–255. [Google Scholar] [CrossRef]
- Pack, A.; Russell, S.S.; Shelley, J.M.G.; van Zuilen, M. Geo- and cosmochemistry of the twin elements yttrium and holmium. Geochim. Cosmochim. Acta 2007, 71, 4592–4608. [Google Scholar] [CrossRef]
- Colson, M.C.; Colson, R.O.; Nellermoe, R. Stratigraphy and depositional environments of the upper Fox Hills and lower Hell Creek Formations at the Concordia Hadrosaur Site in northwestern South Dakota. Rocky Mt. Geol. 2004, 39, 93–111. [Google Scholar] [CrossRef]
- Staub, J.R.; Cohen, A.D. The Snuggedy Swamp of South Carolina: A back-barrier estuarine coal-forming environment. J. Sediment. Petrol. 1979, 49, 133–144. [Google Scholar]
- McCabe, P.J. Depositional environments of coal and coal-bearing strata. Spec. Publ. Int. 1984, 7, 13–42. [Google Scholar]
- Reineck, H.-E.; Singh, I.B. Depositional Sedimentary Environments; Springer: Berlin, Germany, 1980; pp. 1–439. [Google Scholar]
- Kroeger, T.J. Palynology of the Hell Creek Formation (Upper Cretaceous, Maastrichtian) in northwestern South Dakota: Effects of paleoenvironment on the composition of palynomorph assemblages. Geol. Soc. Am. Spec. Pap. 2002, 361, 457–472. [Google Scholar]
- Flight, J.N. Sequence Stratigraphic Analysis of the Fox Hills and Hell Creek Fms (Maastrichtian), Eastern Montana and Its Relationship to Dinosaur Paleontology. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2004. [Google Scholar]
- Schlesinger, P.M. The formation of caliche in soils of the Mojave Desert, California. Geochim. Cosmochim. Acta 1992, 49, 57–66. [Google Scholar] [CrossRef]
- Rogers, R.R. Taphonomy of three dinosaur bone beds in the Upper Cretaceous Two Medicine Formation of northwestern Montana: Evidence for drought-related mortality. PALAIOS 1990, 5, 394–413. [Google Scholar] [CrossRef]
- Rogers, R.R.; Kidwell, S.M. A conceptual framework for the genesis and analysis of vertebrate skeletal concentrations. In Bonebeds: Genesis, Analysis, and Paleobiological Significance; Rogers, R.R., Eberth, D.A., Fiorillo, A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2007; pp. 1–64. [Google Scholar]
- Behrensmeyer, A.K. Taphonomic and ecologic information from bone weathering. Palaeogeogr. Palaeocl. 1978, 4, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Retallack, G.J. Dinosaurs and dirt. In Dinofest International Proceedings; Wolberg, D.L., Stump, E., Rosenberg, G.D., Eds.; Academy of Natural Sciences: Philadelphia, PA, USA, 1997; pp. 345–359. [Google Scholar]
- Behrensymer, A.K. Vertebrate preservation in fluvial channels. Palaeogeogr. Palaeocl. 1988, 63, 183–199. [Google Scholar] [CrossRef]
- Suarez, C.A.; Suarez, M.B.; Terry, D.O.; Grandstaff, D.E. Rare earth element geochemistry and taphonomy of the Early Cretaceous Crystal Geyser Dinosaur Quarry, east-central Utah. PALAIOS 2007, 22, 500–512. [Google Scholar] [CrossRef]
- Suarez, C.A.; Morschhauser, E.M.; Suarez, M.B.; You, H.; Li, D.; Dodson, P. Rare earth element geochemistry of bone beds from the Lower Cretaceous Zhonggou Formation of Gansu Province, China. J. Vertebr. Paleontol. 2019, 38, 22–35. [Google Scholar] [CrossRef]
- Rogers, R.R.; Fricke, H.C.; Addona, V.; Canavan, R.R.; Dwyer, C.N.; Harwood, C.L.; Koenig, A.E.; Murray, R.; Thole, J.T.; Williams, J. Using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) to explore geochemical taphonomy of vertebrate fossils in the Upper Cretaceous Two Medicine and Judith River Formations of Montana. PALAIOS 2010, 25, 183–195. [Google Scholar] [CrossRef]
- Suarez, C.A.; Kohn, M.J. Caught in the act: A case study on microscopic scale physicochemical effects of fossilization on stable isotopic composition of bone. Geochim. Cosmochim. Acta 2020, 268, 277–295. [Google Scholar] [CrossRef]
- Cantrell, K.J.; Byrne, R.H. Rare earth element complexation by carbonate and oxalate ions. Geochim. Cosmochim. Acta 1987, 51, 597–605. [Google Scholar] [CrossRef]
- Luo, Y.-R.; Byrne, R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochim. Cosmochim. Acta 2004, 68, 691–699. [Google Scholar] [CrossRef]
- Livingstone, D.A. Chemical composition of rivers and lakes. In Data of Geochemistry; Fleischer, M., Ed.; USGS: Washington, DC, USA, 1963; pp. G1–G64. [Google Scholar]
- Zhang, J.; Létolle, R.; Jusserand, C. Major element chemistry of the Huanghe (Yellow River), China—Weathering processes and chemical fluxes. J. Hydrol. 1995, 168, 173–203. [Google Scholar] [CrossRef]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon 3. Weathering chemistry and limits to dissolved inputs. J. Geophys. Res. 1987, 92, 8293–8302. [Google Scholar] [CrossRef]
- Berner, R.A. Calcium carbonate concretions formed by the decomposition of organic matter. Science 1968, 159, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Pourret, O.; Davranche, M.; Gruau, G.; Dia, A. Rare earth elements complexation with humic acid. Chem. Geol. 2007, 243, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Föllmi, K.B. The phosphorous cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Hu, C.; Liang, R.; Qiu, J.; Wang, Y. Phosphorous release during decomposition of the submerged macrophyte Potamogeton crispus. Limnology 2018, 19, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Byrne, R.H.; Liu, X.; Schijf, J. The influence of phosphate coprecipitation on rare earth distributions in natural waters. Geochim. Cosmochim. Acta 1996, 60, 3341–3346. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H.; Schijf, J. Comparative coprecipitation of phosphate and arsenate with yttrium and the rare earths: The influence of solution complexation. J. Solut. Chem. 1997, 26, 1187–1198. [Google Scholar] [CrossRef]
- Brunet, R.-C.; Astin, K.B. Variation in phosphorus flux during a hydrological season: The River Ardour. Water Res. 1998, 32, 547–558. [Google Scholar] [CrossRef]
- Elderfield, H.; Upstill-Goddard, R.; Sholkovitz, E.R. The rare earth elements in rivers, estuaries, and coastal seas and their significance to the composition of ocean waters. Geochim. Cosmochim. Acta 1990, 54, 971–991. [Google Scholar] [CrossRef]
- Trueman, C.N.; Benton, M.J.; Palmer, M.R. Geochemical taphonomy of shallow marine vertebrate assemblages. Palaeogeog. Palaeocl. 2003, 197, 151–169. [Google Scholar] [CrossRef]
- Metzger, C.A.; Terry, D.O.; Grandstaff, D.E. Effect of paleosol formation on rare earth element signatures in fossil bone. Geology 2004, 32, 497–500. [Google Scholar] [CrossRef]
- Williams, C.T.; Potts, P.J. Element distribution maps in fossil bones. Archaeometry 1988, 30, 237–247. [Google Scholar] [CrossRef]
- Williams, C.T.; Henderson, P.; Marlow, C.A.; Molleson, T.I. The environment of deposition indicated by the distribution of rare earth elements in fossil bones from Olduvai Gorge, Tanzania. Appl. Geochem. 1997, 12, 537–547. [Google Scholar] [CrossRef]
- Kohn, M.J.; Moses, R.J. Trace element diffusivities in bone rule out simple diffusive uptake during fossilization but explain in vivo uptake and release. Proc. Natl. Acad. Sci. USA 2013, 110, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wings, O. Authigenic minerals in fossil bones from the Mesozoic of England: Poor correlation with depositional environments. Palaeogeog. Palaeocl. 2004, 204, 15–32. [Google Scholar] [CrossRef]
- Janssens, K.; Vincze, L.; Vekemans, B.; Williams, C.T.; Radtke, M.; Haller, M.; Knochel, A. The non-destructive determination of REE in fossilized bone using synchrotron radiation induced K-line X-ray microfluorescence analysis. J. Anal. Chem. 1999, 363, 413–420. [Google Scholar] [CrossRef]
- Herwartz, D.; Munker, C.; Tütken, T.; Hoffmann, J.E.; Wittke, A.; Barbier, B. Lu-Hf isotope systematics of fossil biogenic apatite and their effects on geochronology. Geochim. Cosmochim. Acta 2013, 101, 328–343. [Google Scholar] [CrossRef]
- Reynard, B.; Lecuyer, C.; Grandjean, P. Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chem. Geol. 1999, 155, 233–241. [Google Scholar] [CrossRef]
- Trueman, C.N.; Behrensmeyer, A.K.; Potts, R.; Tuross, N. High-resolution records of location and stratigraphic provenance from the rare earth element composition of fossil bones. Geochim. Cosmochim. Acta 2006, 70, 4343–4355. [Google Scholar] [CrossRef]
- Martin, J.E.; Patrick, D.; Kihm, A.J.; Foit, F.F.; Grandstaff, D.E. Lithostratigraphy, tephrochronology, and rare earth element geochemistry of fossils at the classical Pleistocene Fossil Lake area, south central Oregon. J. Geol. 2005, 113, 139–155. [Google Scholar] [CrossRef]
- Costa, I.A.; Barros, J.A.; Malta, L.; Viana, M.A.; Santos, R.P. Viva a Terra! 7; Porto Editora: Port, Portugal, 2016; pp. 1–48. [Google Scholar]
- Wiemann, J.; Fabbri, M.; Yang, T.-R.; Stein, K.; Sander, P.M.; Norell, M.A.; Briggs, D.E.G. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 2018, 9, 4741. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, M.H. Molecular paleontology: Some current advances and problems. Ann. Paleontol. 2004, 90, 81–102. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C. Apoptosis and programmed cell death in health and disease. Adv. Clin. Chem. 1994, 31, 177–246. [Google Scholar]
- Bayoumy, A.M.; Youssif, G.; Elgohary, E.A.; Husien, S.; El Deen, H.S.; Albeltagy, N.M.; Abdelnaby, D.R.; Elhaes, H.; Ibrahim, M.A. Impact of salvation on the geometrical parameters of some amino acids. Lett. Appl. NanoBioSci. 2019, 8, 567–570. [Google Scholar]
- Schroeter, E.R.; Cleland, T.P. Glutamine deamidation: An indicator of antiquity, or preservational quality? Rapid Commun. Mass Spectrom. 2016, 30, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, M.; Baynes, J.W. Glycation. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: London, UK, 2013; pp. 405–411. [Google Scholar]
- Mello, R.B.; Silva, M.R.R.; Alves, M.T.S.; Evison, M.P.; Guiamarães, M.A.; Francisco, R.A.; Astolphi, R.D.; Iwamura, E.S.M. Tissue microarray analysis applied to bone diagenesis. Sci. Rep. 2017, 7, 39987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, C.; Cavin, L.; Vennemann, T.; Martini, R. Histology and geochemistry of Allosaurus (Dinosauria: Theropoda) from the Cleveland-Lloyd Dinosaur Quarry (Late Jurassic, Utah): Paleobiological implications. Front. Earth Sci. 2021, 9, 641060. [Google Scholar] [CrossRef]
- Kowal-Linka, M.; Jochum, K.P.; Surmik, D. LA-ICP-MS analysis of rare earth elements in marine reptile bones from the Middle Triassic bonebed (Upper Silesia, S Poland): Impact of long-lasting diagenesis, and factors controlling the uptake. Chem. Geol. 2014, 363, 213–228. [Google Scholar] [CrossRef]
- McCormack, J.M.; Bahr, A.; Gerdes, A.; Tütken, T.; Prinz-Grimm, P. Preservation of successive diagenetic stages in Middle Triassic bonebeds: Evidence from in situ trace element and strontium isotope analysis of vertebrate fossils. Chem. Geol. 2015, 410, 108–123. [Google Scholar] [CrossRef]
- Decrée, S.; Herwartz, D.; Mercadier, J.; Miján, I.; de Buffrénil, V.; Leduc, T.; Lambert, O. The post-mortem history of a bone revealed by its trace element signature: The case of a fossil whale rostrum. Chem. Geol. 2018, 477, 137–150. [Google Scholar] [CrossRef]
- McLain, M.; Ullmann, P.V.; Ash, R.D.; Bohnstedt, K.; Nelsen, D.; Clark, R.; Brand, L.R.; Chadwick, A.V. Independent confirmation of fluvial reworking at a Lance Formation (Maastrichtian) bonebed by traditional and chemical taphonomic analyses. PALAIOS 2021, 36, 193–215. [Google Scholar] [CrossRef]
- Goodwin, M.B.; Grant, P.G.; Bench, G.; Holroyd, P.A. Elemental composition and diagenetic alteration of dinosaur bone: Distinguishing micron-scale spatial and compositional heterogeneity using PIXE. Palaeogeogr. Palaeocl. 2007, 253, 458–476. [Google Scholar] [CrossRef]








| Element | Concentration |
|---|---|
| Sc | 83.43 |
| Mn | 2439 |
| Fe | 0.73 |
| Sr | 2386 |
| Y | 1102 |
| Ba | 888 |
| La | 66.48 |
| Ce | 107.47 |
| Pr | 14.24 |
| Nd | 63.72 |
| Sm | 17.65 |
| Eu | 5.45 |
| Gd | 34.38 |
| Tb | 6.88 |
| Dy | 63.45 |
| Ho | 18.82 |
| Er | 74.13 |
| Tm | 12.51 |
| Yb | 93.69 |
| Lu | 16.68 |
| Th | 0.13 |
| U | 37.77 |
| ∑REE | 596 |
| (Ce/Ce*)N | 0.82 |
| (Pr/Pr*)N | 0.92 |
| (Ce/Ce**)N | 1.26 |
| (La/La*)N | 2.83 |
| Y/Ho | 58.55 |
| Clear DMD Kink for LREE? | Relative Noise in Outer Cortex for La | REE Suggest Flow in Marrow Cavity? | Relative ∑REE Content (Whole Bone) | Relative U Content (Whole Bone) | Relative Porosity of the Cortex |
|---|---|---|---|---|---|
| No | Low | No | Low | Low | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullmann, P.V.; Macauley, K.; Ash, R.D.; Shoup, B.; Scannella, J.B. Taphonomic and Diagenetic Pathways to Protein Preservation, Part I: The Case of Tyrannosaurus rex Specimen MOR 1125. Biology 2021, 10, 1193. https://doi.org/10.3390/biology10111193
Ullmann PV, Macauley K, Ash RD, Shoup B, Scannella JB. Taphonomic and Diagenetic Pathways to Protein Preservation, Part I: The Case of Tyrannosaurus rex Specimen MOR 1125. Biology. 2021; 10(11):1193. https://doi.org/10.3390/biology10111193
Chicago/Turabian StyleUllmann, Paul V., Kyle Macauley, Richard D. Ash, Ben Shoup, and John B. Scannella. 2021. "Taphonomic and Diagenetic Pathways to Protein Preservation, Part I: The Case of Tyrannosaurus rex Specimen MOR 1125" Biology 10, no. 11: 1193. https://doi.org/10.3390/biology10111193
APA StyleUllmann, P. V., Macauley, K., Ash, R. D., Shoup, B., & Scannella, J. B. (2021). Taphonomic and Diagenetic Pathways to Protein Preservation, Part I: The Case of Tyrannosaurus rex Specimen MOR 1125. Biology, 10(11), 1193. https://doi.org/10.3390/biology10111193







