The Balance between Orthodontic Force and Radiation in the Jawbone: Microstructural, Histological, and Molecular Study in a Rat Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Irradiation
2.3. Application of Orthodontic Force
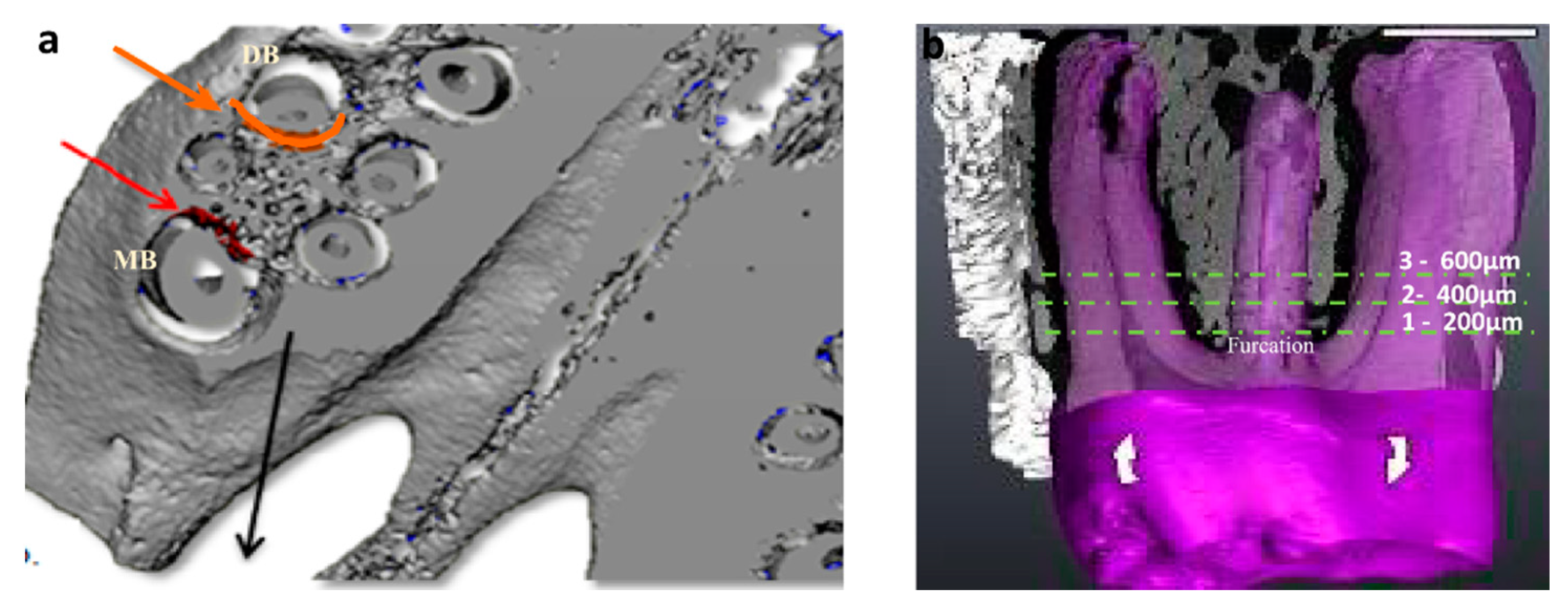
2.4. Micro-CT Analysis
2.5. Histological Preparation
2.6. Histomorphometric Analysis
2.7. Immunohistochemical Stains
2.8. Immunomorphometry
2.9. Tartrate-Resistant Acid Phosphatase (TRAP) Staining and Assessment
2.10. Statistical Analysis
3. Results
3.1. General
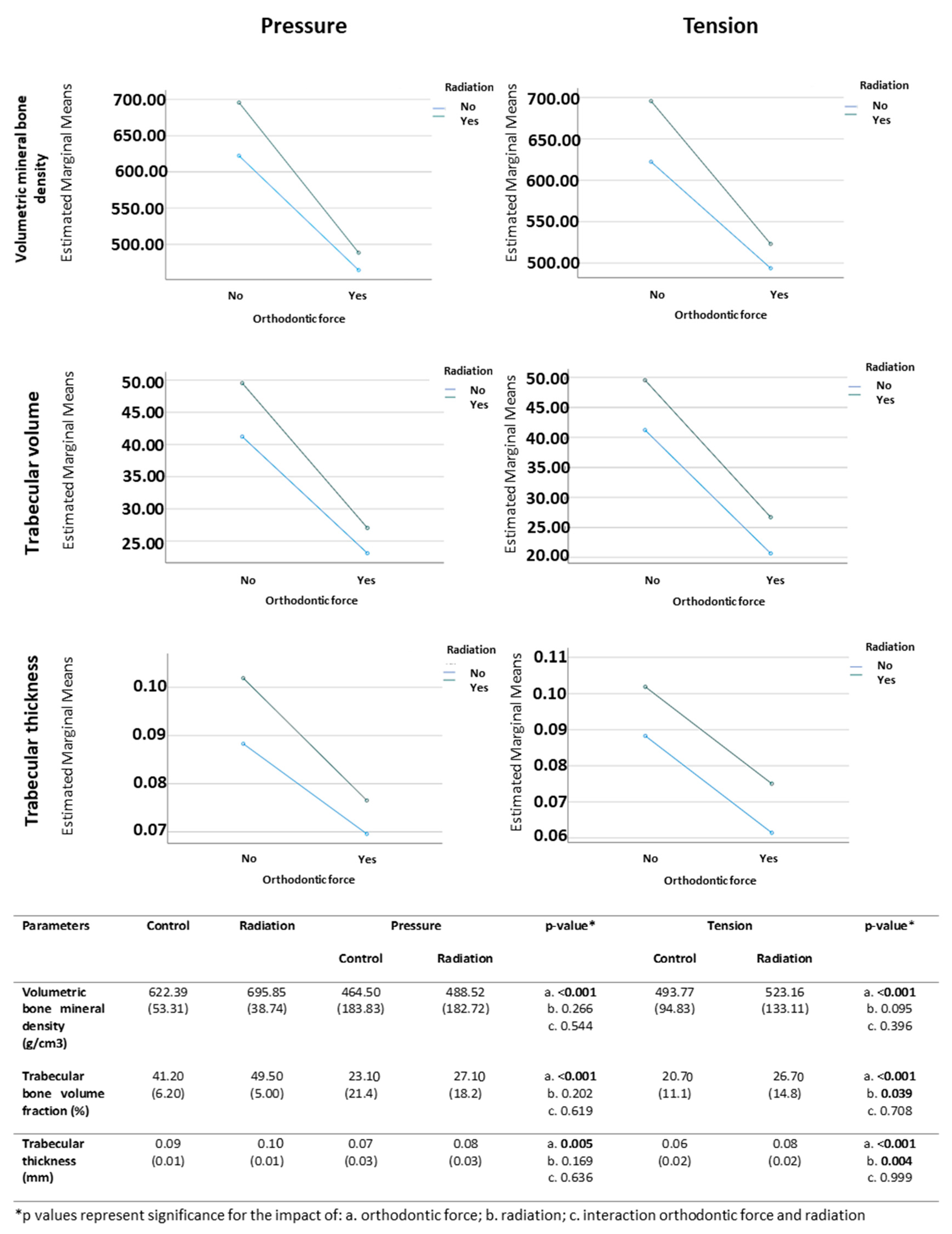
3.2. Micro-CT Analysis
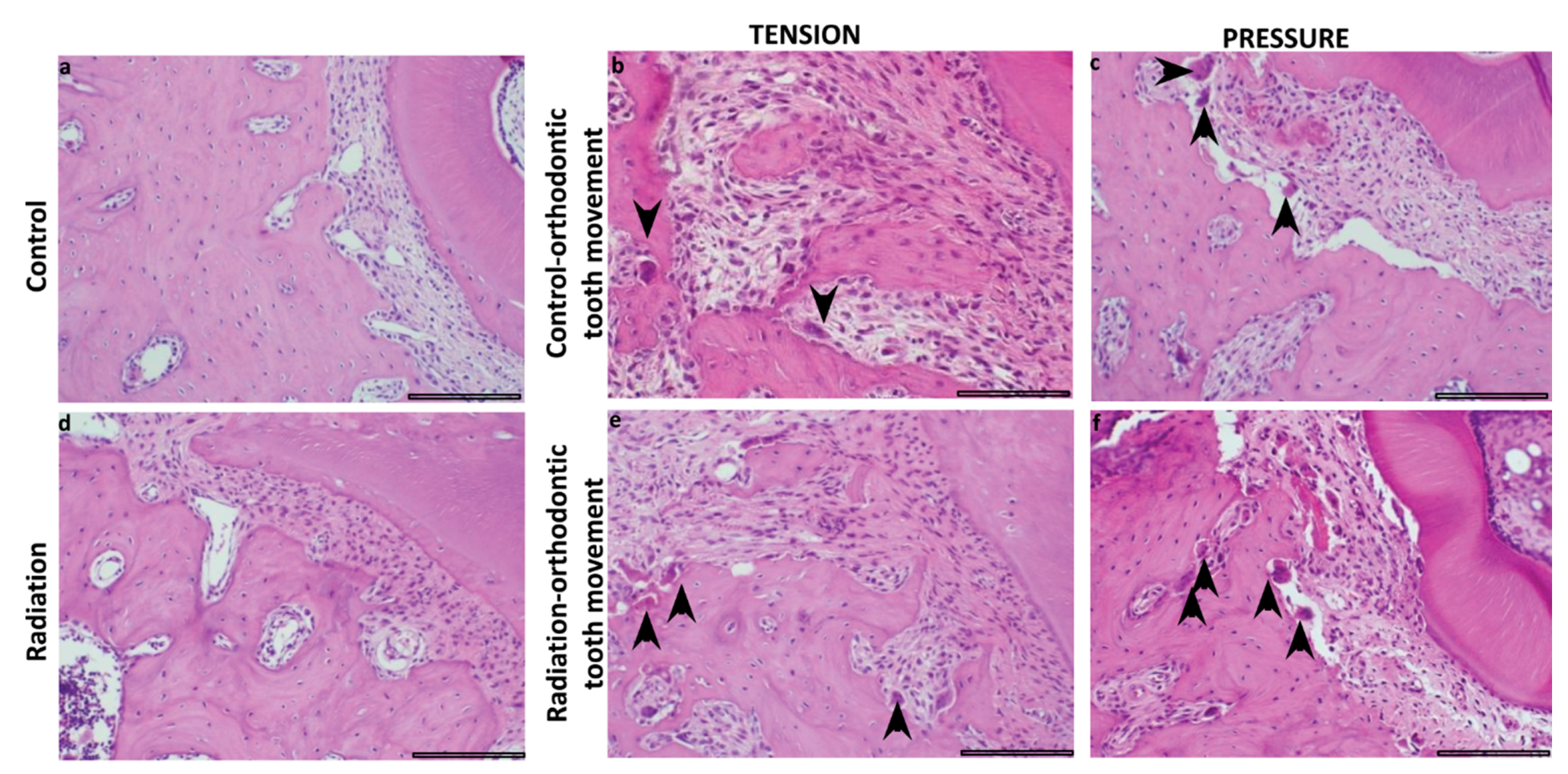
3.3. Histomorphometric Analysis
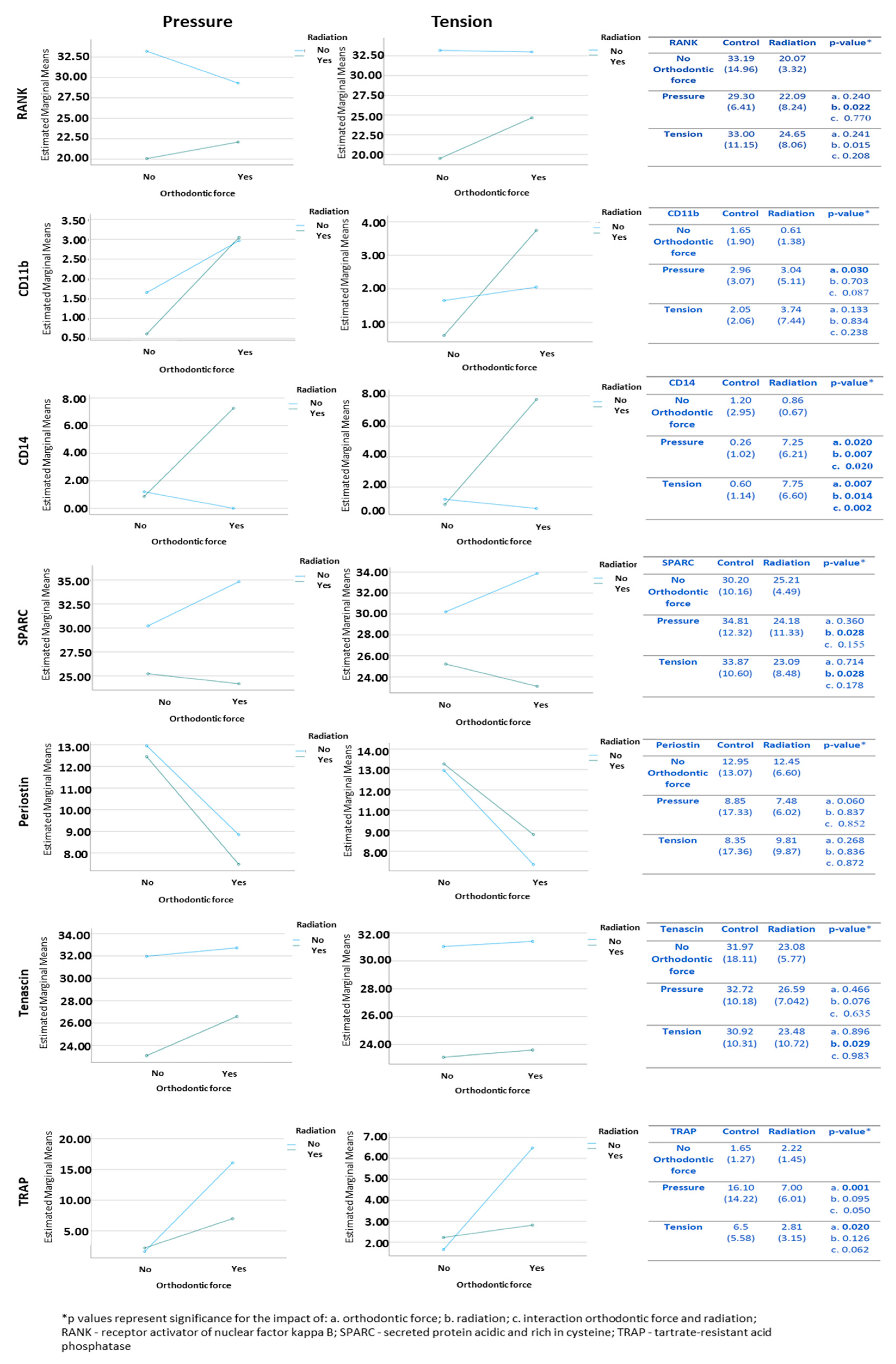
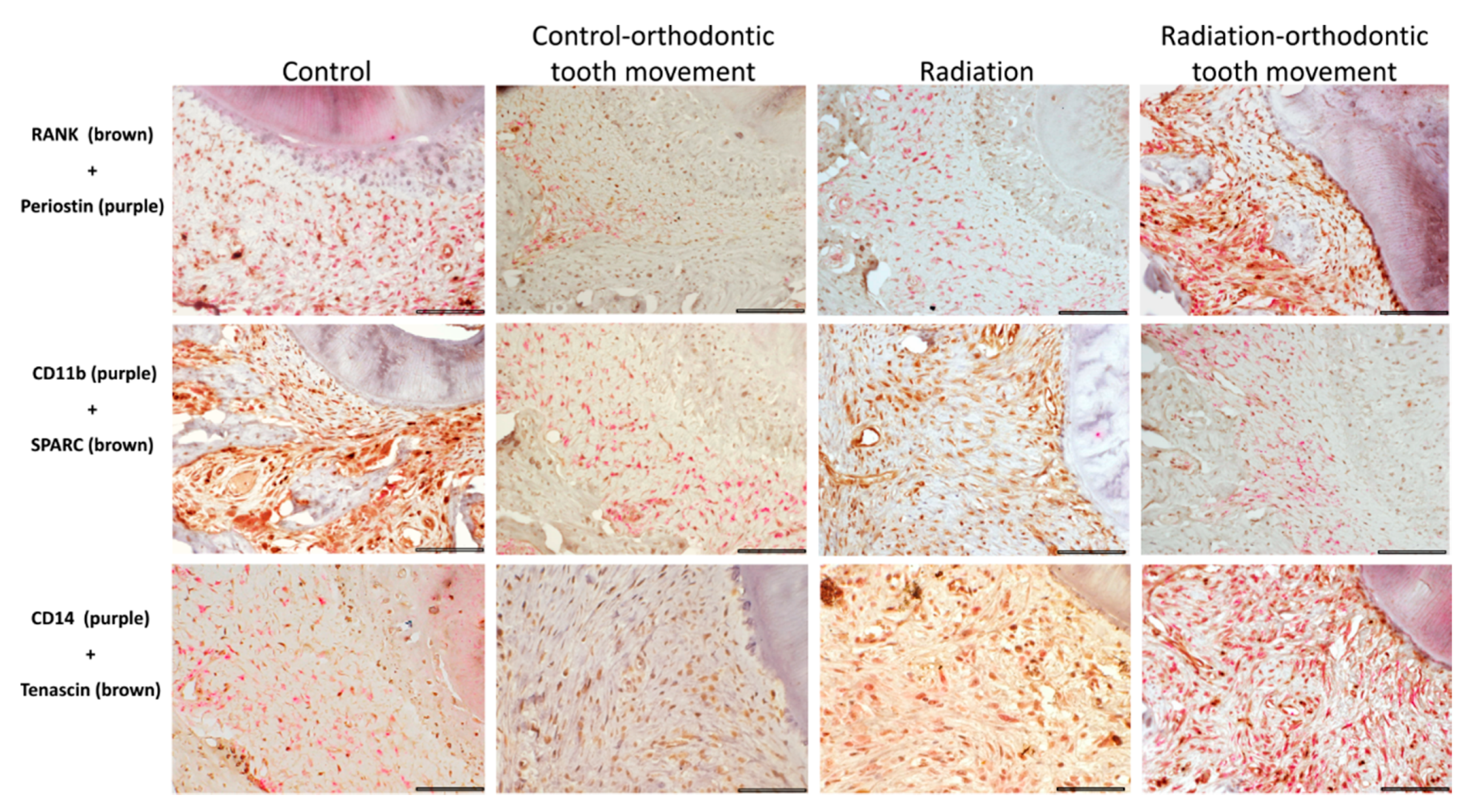
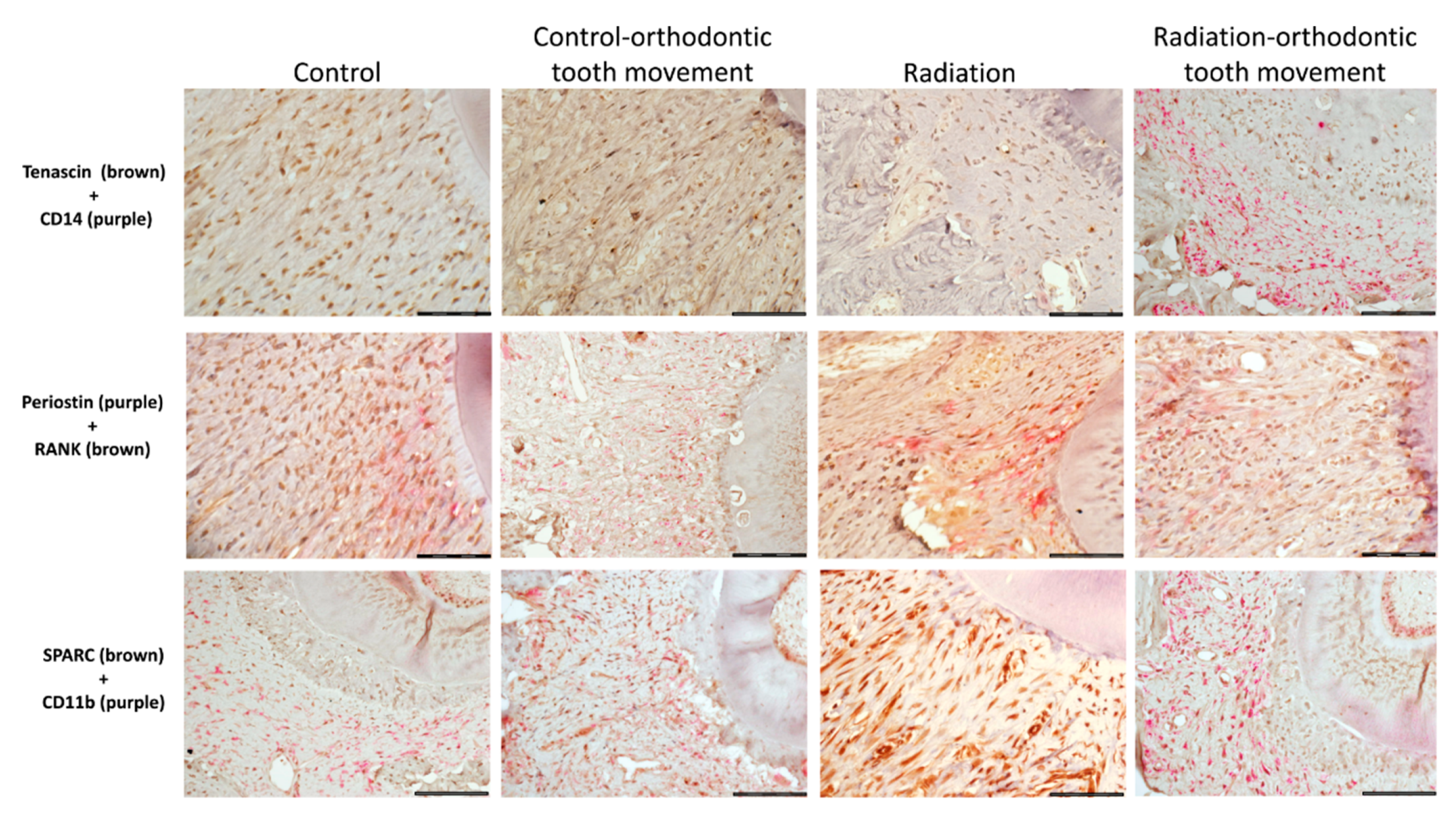
3.4. Immunohistochemical Stains
3.4.1. Cells Expressing Markers of Pre-Osteoclasts: RANK, CD11b, and CD14
3.4.2. Cells Expressing Markers of Pre-Osteoblasts: SPARC, Periostin, and Tenascin
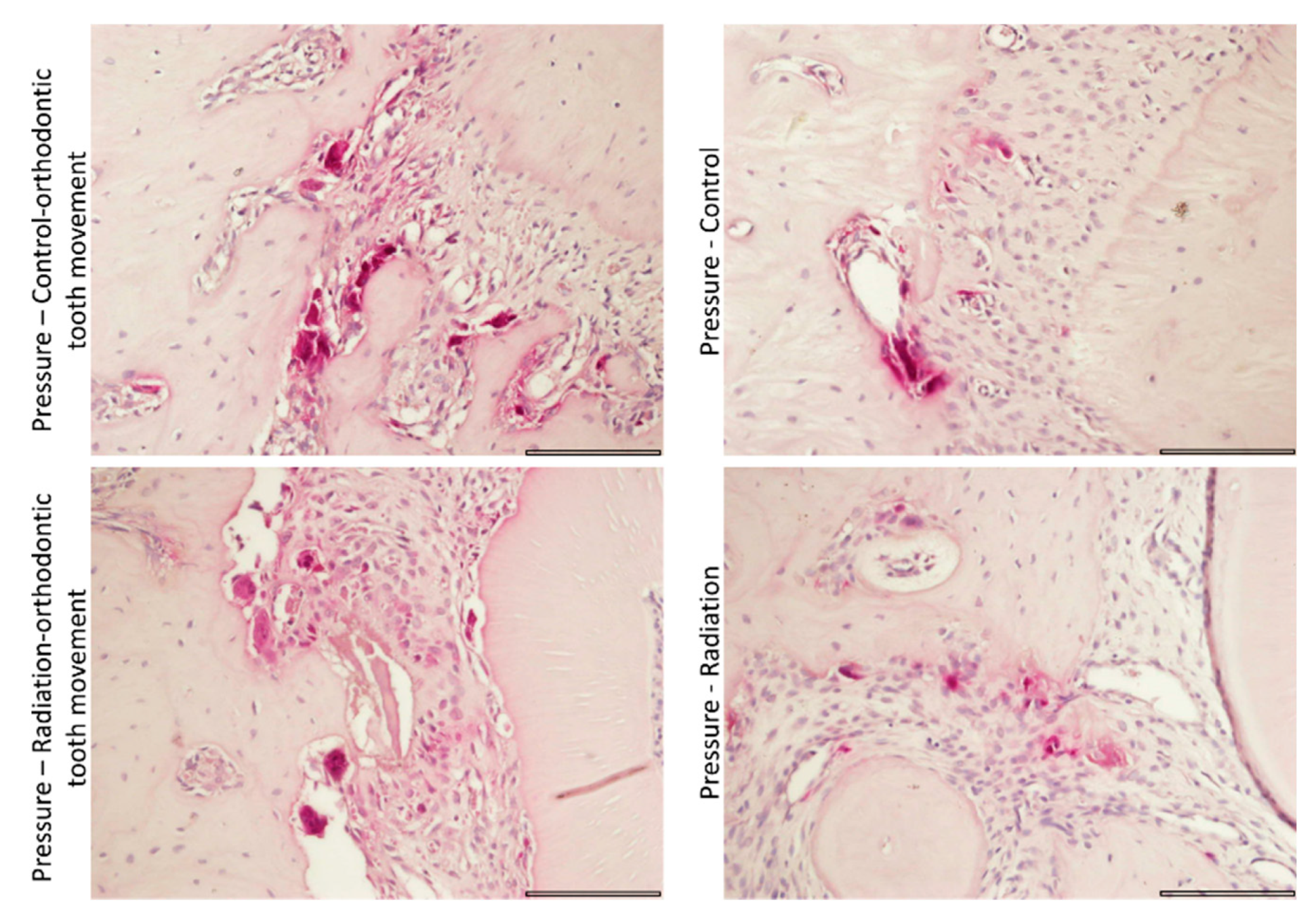
3.4.3. Tartrate-Resistant Acid Phosphatase (TRAP) Staining for Activity of Osteoclasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, S.S.; Massa, S.T.; Varvares, M.A. Improved overall survival and mortality in head and neck cancer with adjuvant concurrent chemoradiotherapy in national databases. Head Neck 2016, 38, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Eurocare Working Group. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Dahllöf, G.; Huggare, J. Orthodontic considerations in the pediatric cancer patient: A review. Semin. Orthod. 2004, 10, 266–276. [Google Scholar] [CrossRef]
- Dahllöf, G.; Jönsson, A.; Ulmner, M.; Huggare, J. Orthodontic treatment in long-term survivors after pediatric bone marrow transplantation. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 459–465. [Google Scholar] [CrossRef]
- Runge, M.E.; Edwards, M.S. Orthodontic treatment for an adolescent with a history of acute lymphoblastic leukemia. Pediatr. Dent. 2000, 22, 494–498. [Google Scholar] [PubMed]
- Ott, S.M. Histomorphometric measurements of bone turnover, mineralization, and volume. Clin. J. Am. Soc. Nephrol. 2008, 3, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.J.; Keeling, S.D.; Wronski, T.J. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone 1991, 12, 401–409. [Google Scholar] [CrossRef]
- Perinetti, G.; Varvara, G.; Salini, L.; Tetè, S. Alkaline phosphatase activity in dental pulp of orthodontically treated teeth. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Reher, P.; Sousa, A.A.; Harris, M. Osteoradionecrosis of the jaws—A current overview—Part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac. Surg. 2010, 14, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Chrcanovic, B.R.; Reher, P.; Sousa, A.A.; Harris, M. Osteoradionecrosis of the jaws—A current overview—Part 2: Dental management and therapeutic options for treatment. Oral Maxillofac. Surg. 2010, 14, 81–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zheng, Z.; Fang, D.; Gao, R.; Liu, Y.; Fan, Z.P.; Zhang, C.M.; Wang, S.L. Early-stage pathogenic sequence of jaw osteoradionecrosis in vivo. J. Dent. Res. 2012, 91, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Nadella, K.R.; Kodali, R.M.; Guttikonda, L.K.; Jonnalagadda, A. Osteoradionecrosis of the jaws: Clinico-therapeutic management: A literature review and update. J. Maxillofac. Oral Surg. 2015, 14, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchanque-Fossuo, C.N.; Donneys, A.; Deshpande, S.S.; Nelson, N.S.; Boguslawski, M.J.; Gallagher, K.K.; Sarhaddi, D.; Poushanchi, B.; Goldstein, S.A.; Buchman, S.R. Amifostine remediates the degenerative effects of radiation on the mineralization capacity of the murine mandible. Plast. Reconstr. Surg. 2012, 129, 646e–655e. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Maltha, J.C.; Kuijpers-Jagtman, A.M. Optimum force magnitude for orthodontic tooth movement: A systematic literature review. Angle Orthod. 2003, 73, 86–92. [Google Scholar] [PubMed]
- Zaki, A.E.; Van Huysen, G. Histology of the periodontium following tooth movement. J. Dent. Res. 1963, 42, 1373–1379. [Google Scholar] [CrossRef]
- Shimpo, S.; Horiguchi, Y.; Nakamura, Y.; Lee, M.; Oikawa, T.; Noda, K.; Kuwahara, Y.; Kawasaki, K. Compensatory bone formation in young and old rats during tooth movement. Eur. J. Orthod. 2003, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Katona, T.R.; Paydar, N.H.; Akay, H.U.; Roberts, W.E. Stress analysis of bone modeling response to rat molar orthodontics. J. Biomech. 1995, 28, 27–38. [Google Scholar] [CrossRef]
- Bellhouse, D.R. Area estimation by point-counting techniques. Biometrics 1981, 37, 303–312. [Google Scholar] [CrossRef]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: Histomorphometric evaluations at 9 months. J. Periodontol. 2000, 71, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Garnero, P.; Ferrari, S. Periostin action in bone. Mol Cell Endocrinol. 2016, 432, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Spring, J.; Beck, K.; Chiquet-Ehrismann, R. Two contrary functions of tenascin: Dissection of the active sites by recombinant tenascin fragments. Cell 1989, 59, 325–334. [Google Scholar] [CrossRef]
- Nies, D.E.; Hemesath, T.J.; Kim, J.H.; Gulcher, J.R.; Stefansson, K. The complete cDNA sequence of human hexabrachion (Tenascin): A multidomain protein containing unique epidermal growth factor repeats. J. Biol. Chem. 1991, 266, 2818–2823. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Diverse biological functions of the SPARC family of proteins. Int. J. Biochem. Cell Biol. 2012, 44, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Murphy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Klein, Y.; Fleissig, O.; Stabholz, A.; Chaushu, S.; Polak, D. Bone regeneration with bovine bone impairs orthodontic tooth movement despite proper osseous wound healing in a novel mouse model. J. Periodontol. 2019, 90, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Tsay, T.P.; Chen, M.H.; Oyen, O.J.; Hahn, S.S.; Marty, J.J. The effect of cobalt-60 irradiation on bone marrow cellularity and alveolar osteoclasts. Proc. Natl. Sci. Counc. Repub. China B 1995, 19, 185–195. [Google Scholar] [PubMed]
- Tsay, T.P.; Chen, M.H.; Oyen, O.J. Osteoclast activation and recruitment after application of orthodontic force. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 323–330. [Google Scholar] [CrossRef]
- Rody, W.J.; King, G.J.; Gu, G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Kuijpers-Jagtman, A.M.; Maltha, J.C. Osteoclast differentiation during experimental tooth movement by a short-term force application: An immunohistochemical study in rats. Acta Odontol. Scand. 2008, 66, 314–320. [Google Scholar] [CrossRef]
- Vandevska-Radunovic, V.; Kvinnsland, I.H.; Kvinnsland, S.; Jonsson, R. Immunocompetent cells in rat periodontal ligament and their recruitment incident to experimental orthodontic tooth movement. Eur. J. Oral Sci. 1997, 105, 36–44. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Q.; Xu, W.; She, C.; Xie, Z.G.; Mao, Y.T.; Dong, Q.R.; Ling, M. Low-dose X-ray irradiation promotes osteoblast proliferation, differentiation and fracture healing. PLoS ONE 2014, 9, e104016. [Google Scholar]
- Dare, A.; Hachisu, R.; Yamaguchi, A.; Yokose, S.; Yoshiki, S.; Okano, T. Effects of ionizing radiation on proliferation and differentiation of osteoblast-like cells. J. Dent. Res. 1997, 76, 658–664. [Google Scholar] [CrossRef]
- Despars, G.; Carbonneau, C.L.; Bardeau, P.; Coutu, D.L.; Beauséjour, C.M. Loss of the osteogenic differentiation potential during senescence is limited to bone progenitor cells and is dependent on p53. PLoS ONE 2013, 8, e73206. [Google Scholar]
- Szymczyk, K.H.; Shapiro, I.M.; Adams, C.S. Ionizing radiation sensitizes bone cells to apoptosis. Bone 2004, 34, 148–156. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qiu, W.; Zhang, Z.; Wang, Z.; Zhang, X.; He, Y. Effects of irradiation on growth and differentiation-related gene expression in osteoblasts. J. Craniofac. Surg. 2011, 22, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Wilde, J.; Yokozeki, M.; Terai, K.; Kudo, A.; Moriyama, K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res. 2003, 312, 345–351. [Google Scholar] [CrossRef]
- Watanabe, T.; Yasue, A.; Fujihara, S.; Tanaka, E. PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK pathway in human periodontal ligament cells. Arch. Oral Biol. 2012, 57, 52–59. [Google Scholar] [CrossRef]
- Rangiani, A.; Jing, Y.; Ren, Y.; Yadav, S.; Taylor, R.; Feng, J.Q. Critical roles of periostin in the process of orthodontic tooth movement. Eur. J. Orthod. 2016, 38, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Liu, H.; Cui, J.; Hasegawa, T.; Hongo, H.; Feng, W.; Li, J.; Sun, B.; Kudo, A.; Amizuka, N.; et al. Histochemical examination of cathepsin K, MMP1 and MMP2 in compressed periodontal ligament during orthodontic tooth movement in periostin deficient mice. J. Mol. Histol. 2014, 45, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Nie, E.M.; Deng, G.; Lai, L.Z.; Sun, F.Y.; Tian, H.; Fang, F.C.; Zou, Y.G.; Wu, B.L.; Ou-Yang, J. Periostin is essential for periodontal ligament remodeling during orthodontic treatment. Mol. Med. Rep. 2017, 15, 1800–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; He, Y.; Feng, J.Q.; Shu, R.; Liu, Z.; Li, J.; Wang, Y.; Xu, Y.; Zeng, H.; Xu, X.; et al. Wnt3α and transforming growth factor-β induce myofibroblast differentiation from periodontal ligament cells via different pathways. Exp. Cell Res. 2017, 353, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bai, D.; Ruest, L.B.; Feng, J.Q.; Guo, Y.W.; Tian, Y.; Jing, Y.; He, Y.; Han, X.L. Expression analysis of α-smooth muscle actin and tenascin-C in the periodontal ligament under orthodontic loading or in vitro culture. Int. J. Oral Sci. 2015, 7, 232–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Zhao, D.; Wu, Y.; Xu, C.; Zhang, F. Cyclic stretch induced gene expression of extracellular matrix and adhesion molecules in human periodontal ligament cells. Arch. Oral Biol. 2015, 60, 447–455. [Google Scholar] [CrossRef] [PubMed]

| Coupled Antibodies | Coupled Antibodies | Coupled Antibodies | ||||
|---|---|---|---|---|---|---|
| RANK 1 | Periostin | CD14 | Tenascin C | CD11b | SPARC 2 | |
| Source of antigen; manufacturer; concentration | Mouse; Novus Biologicals, Centennial, CO, USA; Catalog number NB100-56508; 1:200 | Rabbit; Novus Biologicals, Centennial, CO, USA; Catalog number NBP1-30042; 1:200 | Rabbit; Bioss, Woburn, MA, USA; Catalog number bs-1192R; 1:250 | Mouse; Novus Biologicals, Centennial, CO, USA; Catalog number NB110-68136H; 1:40 | Rabbit; OriGene, Rockville, MD, USA; Catalog number TA323950; 1:50 | Rabbit, Proteintech Group, Rosemont, IL, USA; Catalog number 15274-1-AP; 1:100 |
| Identified phenotype: pre-osteoblasts/osteoblasts | + | + | + | |||
| Identified phenotype: pre-osteoclasts/osteoclasts | + | + | + | |||
| Cellular location of target antigen | Cytoplasm | Mainly extracellular matrix (as secreted protein) tissue); also cytoplasmic [20,21] | Cell surface | Mainly extracellular matrix (as secreted protein); also cytoplasmic [21,22,23] | Cell surface | Cytoplasmic and extracellular matrix (as secreted protein) tissue) [21,24,25,26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorchin-Ashkenazi, H.; Ginat-Koton, R.; Gabet, Y.; Klein, Y.; Chaushu, S.; Dorchin, H.; Brosh, T.; Vered, M. The Balance between Orthodontic Force and Radiation in the Jawbone: Microstructural, Histological, and Molecular Study in a Rat Model. Biology 2021, 10, 1203. https://doi.org/10.3390/biology10111203
Dorchin-Ashkenazi H, Ginat-Koton R, Gabet Y, Klein Y, Chaushu S, Dorchin H, Brosh T, Vered M. The Balance between Orthodontic Force and Radiation in the Jawbone: Microstructural, Histological, and Molecular Study in a Rat Model. Biology. 2021; 10(11):1203. https://doi.org/10.3390/biology10111203
Chicago/Turabian StyleDorchin-Ashkenazi, Hadas, Ravit Ginat-Koton, Yankel Gabet, Yehuda Klein, Stella Chaushu, Hezi Dorchin, Tamar Brosh, and Marilena Vered. 2021. "The Balance between Orthodontic Force and Radiation in the Jawbone: Microstructural, Histological, and Molecular Study in a Rat Model" Biology 10, no. 11: 1203. https://doi.org/10.3390/biology10111203
APA StyleDorchin-Ashkenazi, H., Ginat-Koton, R., Gabet, Y., Klein, Y., Chaushu, S., Dorchin, H., Brosh, T., & Vered, M. (2021). The Balance between Orthodontic Force and Radiation in the Jawbone: Microstructural, Histological, and Molecular Study in a Rat Model. Biology, 10(11), 1203. https://doi.org/10.3390/biology10111203







