Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Design and Setting
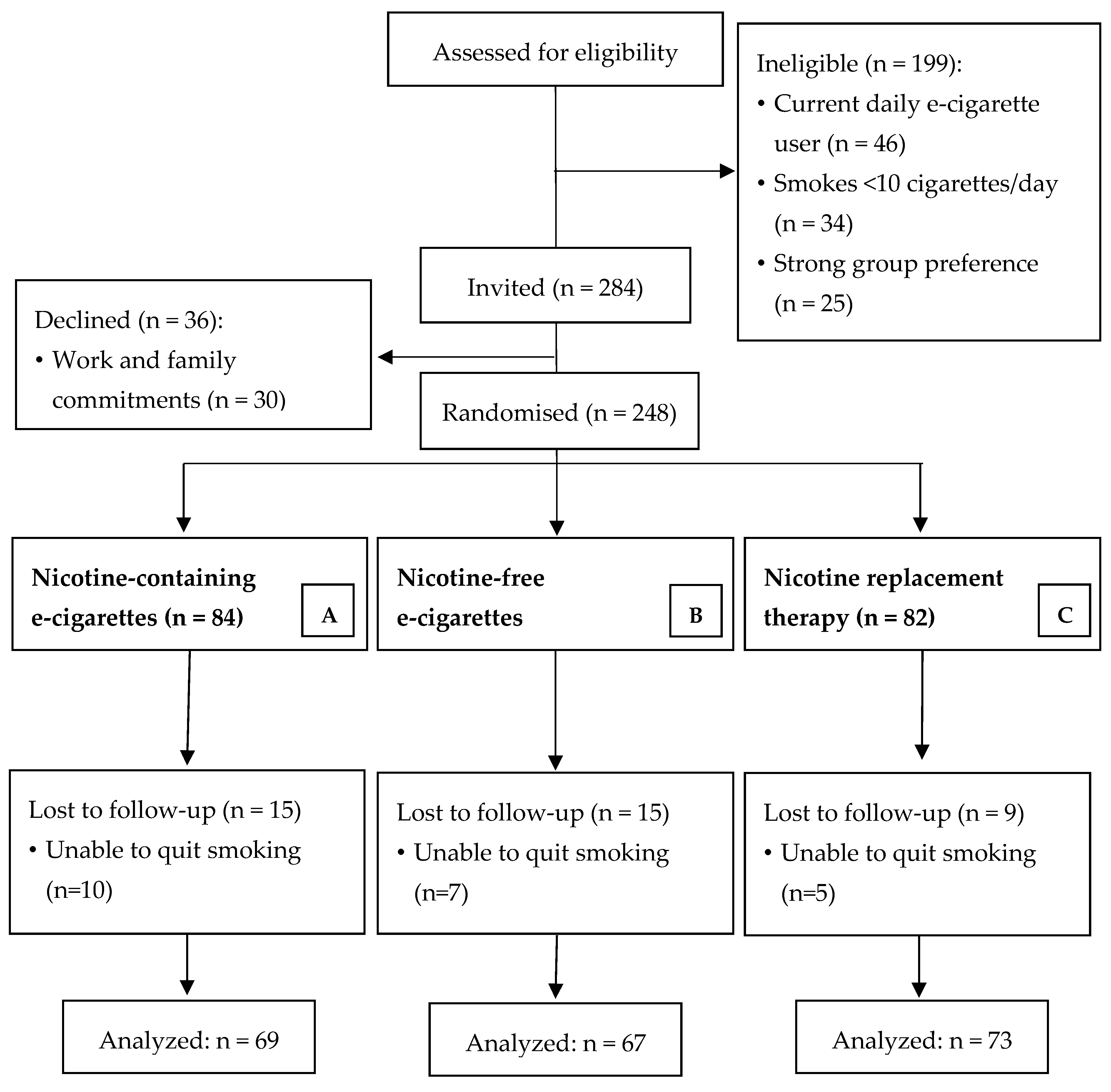
2.2. Participant Recruitment
2.3. Procedures
2.4. Intervention
2.5. Measures
2.5.1. Baseline
| Timeline | Duration |
| Baseline recording period | 4 min |
| 1st Dose (0.2 mA → 2 mC) | 10 s |
| 2nd Recording period | 4 min |
| 2nd Dose (0.2 mA → 3 mC) | 15 s |
| 3rd Recording period | 4 min |
| 3rd Dose (0.2 mA → 4 mC) | 20 s |
| 4th Recording period | 4 min |
| 4th Dose (0.3 mA → 6 mC) | 20 s |
| 5th Recording period | 4 min |
| mA: micro-amber, mC: micro-coulomb. | |
2.5.2. Outcomes
| Outcome Measure | Area of Focus | Reasons of Choice |
| Flow Mediated Dilation (%FMD) | Macrovascular function (NO bioavailability at arterial level). | It is a non-invasive, highly reproducible assessment. Reduced altered brachial artery FMD is considered an early marker for endothelial dysfunction, a CVD risk factor and can predict long-term adverse cardiovascular events. |
| Cutaneous vascular conductance (CVC) responses to acetylcholine (ACh) | Microvascular function (endothelial-dependent vasodilation; NO bioavailability at microvascular level). | It is the only non-invasive measure that can assess reliably endothelial-dependent vasodilatory function in general and NO bioavailability in particular, at a microcirculatory level. |
| Cutaneous vascular conductance (CVC) responses to sodium nitroprusside (SNP) | Microvascular function (endothelial-independent vasodilation; smooth muscle cell function at microvascular level). | It is the only non-invasive measure that can assess reliably endothelial-independent vasodilatory function. Smooth muscle cells are the most numerous components of the arterial and venous wall, playing a key role in vasodilation and in the progression of pathological conditions such as atherosclerosis. |
| Mean arterial pressure (MAP) | Macrovascular function (surrogate marker for arterial stiffness). | This is a readily available, non-invasive measure (i.e., measured through Systolic and Diastolic blood pressure measurements), that can indicate changes in arterial stiffness when direct measures (e.g., pulse wave velocity) are not available or there is a need to reduce participant burden. |
2.5.3. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary Outcome: Macrovascular Assessment
3.3. Secondary Outcomes: Microvascular Assessment
3.3.1. Acetylcholine (ACh)
3.3.2. Sodium Nitroprusside (SNP)
3.3.3. Mean Arterial Pressure (MAP)
3.4. Heavy Smokers Subgroup
4. Discussion
4.1. Limitations
4.2. Strengths
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US) & Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010.
- Office on Smoking and Health (US). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2006.
- U.S. Department of Health & Human Services. Smoking Cessation: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Stead, L.F.; Koilpillai, P.; Fanshawe, T.R.; Lancaster, T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst. Rev. 2016, 3, CD008286. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Hong, B.; Livingstone-Banks, J.; Wheat, H.; Fanshawe, T.R. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst. Rev. 2019, 6, CD009670. [Google Scholar] [CrossRef] [PubMed]
- Brose, L.S.; West, R.; Mcdermott, M.S.; Fidler, J.A.; Croghan, E.; Mcewen, A. What makes for an effective stop-smoking service? Thorax 2011, 66, 924–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.E.; Mcgowan, J.A.; Ubhi, H.K.; Proudfoot, H.; Shahab, L.; Brown, J.; West, R. Modelling continuous abstinence rates over time from clinical trials of pharmacological interventions for smoking cessation. Addiction 2019, 114, 787–797. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Brown, J. Smoking in England—Latest Statistics Smoking Toolkit Study. 2020. Available online: http://www.smokinginengland.info/latest-statistics/ (accessed on 25 March 2020).
- Jackson, S.E.; Kotz, D.; West, R.; Brown, J. Moderators of real-world effectiveness of smoking cessation aids: A population study. Addiction 2019, 114, 1627–1638. [Google Scholar] [CrossRef]
- Beard, E.; West, R.; Michie, S.; Brown, J. Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: A time-series analysis between 2006 and 2017. Addiction 2019, 115, 961–974. [Google Scholar] [CrossRef] [Green Version]
- McNeill, A.; Brose, L.S.; Calder, R.; Simonavicius, E.; Robson, D. Vaping in England: An Evidence Update Including Vaping for Smoking Cessation, February 2021; A Report Commissioned by Public Health England; Public Health England: London, UK, 2021.
- George, J.; Hussain, M.; Vadiveloo, T.; Ireland, S.; Hopkinson, P.; Struthers, A.D.; Donnan, P.T.; Khan, F.; Lang, C.C. Cardiovascular Effects of Switching from Tobacco Cigarettes to Electronic Cigarettes. J. Am. Coll. Cardiol. 2019, 74, 3112–3120. [Google Scholar] [CrossRef]
- Kennedy, C.D.; Van Schalkwyk, M.C.I.; Mckee, M.; Pisinger, C. The cardiovascular effects of electronic cigarettes: A systematic review of experimental studies. Prev. Med. 2019, 127, 105770. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Fraiman, J.B. Cardiovascular effects of electronic cigarettes. Nat. Rev. Cardiol. 2017, 14, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Crank, H.; Gumber, A.; Brose, L.S. Smokers making a quit attempt using e-cigarettes with or without nicotine or prescription nicotine replacement therapy: Impact on cardiovascular function (ISME-NRT)—A study protocol. BMC Public Health 2017, 17, 293. [Google Scholar] [CrossRef] [Green Version]
- The National Centre for Smoking Cessation and Training (NCSCT). Standard Treatment Programme—A Guide to Providing Behavioural Support for Smoking Cessation. 2014. Available online: http://www.ncsct.co.uk/usr/pub/NCSCT%20STP.pdf (accessed on 25 September 2021).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.F.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exer. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Klonizakis, M.; Winter, E. Effects of arm-cranking exercise in cutaneous microcirculation in older, sedentary people. Microvasc. Res. 2011, 81, 331–336. [Google Scholar] [CrossRef]
- Tew, G.A.; Gumber, A.; McIntosh, E.; Kesterton, S.; King, B.; Michaels, J.A.; Klonizakis, M. Effects of supervised exercise training on lower-limb cutaneous microvascular reactivity in adults with venous ulcers. Eur. J. Appl. Physiol. 2018, 118, 321–329. [Google Scholar] [CrossRef] [Green Version]
- West, R.; Hajek, P.; Stead, L.; Stapleton, J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction 2005, 100, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. The prevention and handling of the missing data. Korean J. Anesthesiol. 2013, 64, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Sciarretta, S.; Violi, F.; Nocella, C.; Loffredo, L.; Perri, L.; Peruzzi, M.; Marullo, A.G.; De Falco, E.; Chimenti, I.; et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 2016, 150, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Haptonstall, K.P.; Choroomi, Y.; Moheimani, R.; Nguyen, K.; Tran, E.; Lakhani, K.; Ruedisueli, I.; Gornbein, J.; Middlekauff, H.R. Differential effects of tobacco cigarettes and electronic cigarettes on endothelial function in healthy young people. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H547–H556. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.; Green, D.J.; Weisbrod, C.; Best, M.; Dembo, L.; O’Driscoll, G.; Tschakovsky, M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H119–H126. [Google Scholar] [CrossRef] [Green Version]
- Benetos, A.; Adamopoulos, C.; Bureau, J.M.; Temmar, M.; Labat, C.; Bean, K.; Thomas, F.; Pannier, B.; Asmar, R.; Zureik, M.; et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002, 105, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Quinn, U.; Tomlinsonm, L.A.; Cockcroft, J.R. Arterial stiffness. JRSM Cardiovasc. Dis. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Moheimani, R.S.; Bhetraratana, M.; Peters, K.M.; Yang, B.K.; Yin, F.; Gornbein, J.; Araujo, J.A.; Middlekauff, H.R. Sympathomimetic Effects of Acute E-Cigarette Use: Role of Nicotine and Non-Nicotine Constituents. J. Am. Heart Assoc. 2017, 6, e006579. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neunteufl, T.; Heher, S.; Kostner, K.; Mitulovic, G.; Lehr, S.; Khoschsorur, G.; Schmid, R.W.; Maurer, G.; Stefenelli, T. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J. Am. Coll. Cardiol. 2002, 39, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Black, M.A.; Cable, N.T.; Thijssen, D.H.; Green, D.J. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1109–H1116. [Google Scholar] [CrossRef]
- Kisch, T.; Helmke, A.; Schleusser, S.; Song, J.; Liodaki, E.; Stang, F.H.; Mailaender, P.; Kraemer, R. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): Results of a controlled, prospective cohort study. Microvasc. Res. 2016, 104, 55–62. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Nicotine-Containing E-Cigarettes, n = 84 | Nicotine-Free E-Cigarettes, n = 82 | Nicotine Replacement Therapy (NRT), n = 82 |
|---|---|---|---|
| Sex, Female/Male, n | 46/38 | 41/41 | 36/46 |
| Age, Mean years (SD) | 44 (14) | 44 (13) | 44 (13) |
| BMI a, Mean (SD) | 27.6 (5.9) | 27.3 (5.4) | 26.3 (5.1) |
| Smoking Years, Mean (SD) | 24 (13) | 25 (13) | 25 (13) |
| Cigarettes per day, Mean (SD) | 18 (7) | 16 (7) | 18 (7) |
| Percentage of Heavy Smokers (≥20 cigarettes per day), Mean (SD) | 48% | 36% | 49% |
| Smoking packet years, Mean (SD) | 23 (17) | 22 (17) | 24 (19) |
| Exhaled air carbon monoxide, Mean parts per million (SD) | 15.2 (7.9) | 14.3 (8.1) | 16.9 (6.9) |
| Physical Activity, Mean weekly MET b minutes (SD) | 2772 (2669) | 3082 (3191) | 2756 (2627) |
| Artery Diameter (pre-inflation), Mean (SD) | 4.22 (0.66) | 4.13 (0.65) | 4.10 (0.56) |
| Artery Diameter (post-inflation), Mean (SD) | 4.47 (0.64) | 4.34 (0.61) | 4.36 (0.55) |
| %FMD c, Mean (SD) | 6.2 (4.4) | 5.6 (3.6) | 6.7 (4.0) |
| Baseline flow (mL/min) | 115.8 (74.8) | 116.1 (73.5) | 116.3 (73.5) |
| Reactive hyperaemia blood flow (mL/min) | 676.8 (269.3) | 678.5 (270.7) | 674.5 (263.1) |
| Peak CVC d for Ach e, Mean PU/mmHg (SD) | 1.48 (1.02) | 1.49 (0.98) | 1.34 (1.00) |
| Peak CVC for SNP f, Mean PU/mmHg (SD) | 1.45 (1.02) | 1.15 (0.72) | 1.35 (0.98) |
| MAP g, Mean mm Hg (SD) | 98.3 (11.3) | 97.4 (12.2) | 98.2 (11.5) |
| 3 Days Post Quit | Nicotine-Containing E-Cigarettes, n = 69 | Nicotine-Free E-Cigarettes, n = 67 | Nicotine Replacement Therapy (NRT), n = 73 | F Value, p Value and Degrees of Freedom | η2 |
|---|---|---|---|---|---|
| %FMD a,b | |||||
| Unadjusted, Mean (SD) | 9.0 (4.1) | 10.2 (5.2) | 9.4 (4.5) | F = 1.03, p = 0.360, df = 2,207 | 0.01 |
| Adjusted for baseline, Mean (95% C.I.) | 9.0 (7.9–10.0) | 10.3 (9.2–11.4) | 9.4 (8.4–10.5) | F = 8.99, p < 0.001, df = 3,207 | 0.117 |
| Fully adjusted, Mean (95% C.I.) | 9.0 (7.9–10) | 10.3 (9.2–11.3) | 9.5 (8.4–10.5) | F = 5.75, p < 0.001, df = 7,207 | 0.167 |
| Peak CVC c for ACh d,e | |||||
| Unadjusted Mean PU/mmHg (SD) | 1.64 (1.06) | 1.76 (1.42) | 1.64 (1.18) | F = 0.172, p = 0.84, df = 2,207 | 0.001 |
| Adjusted for baseline, Mean PU/mmHg (95% C.I.) | 1.64 (1.38–1.91) | 1.73 (1.46–2.01) | 1.68 (1.42–1.95) | F = 8.43, p < 0.001, df = 3,207 | 0.110 |
| Fully adjusted, Mean PU/mmHg (95% C.I.) | 1.63 (1.37–1.90) | 1.77 (1.49–2.05) | 1.67 (1.38–1.91) | F = 4.73, p < 0.001, df = 7,207 | 0.145 |
| Peak CVC for SNP e,f | |||||
| Unadjusted Mean PU/mmHg (SD) | 1.65 (1.17) | 1.71 (1.61) | 1.50 (1.21) | F = 0.382, p = 0.68, df = 2,207 | 0.004 |
| Adjusted for baseline, Mean PU/mmHg (95% C.I.) | 1.62 (1.31–1.93) | 1.71 (1.39–2.03) | 1.49 (1.19–1.80) | F = 0.73, p = 0.54, df = 3,207 | 0.011 |
| Fully adjusted, Mean PU/mmHg (95% C.I.) | 1.61 (1.30–1.92) | 1.72 (1.40–2.05) | 1.52 (1.21–1.83) | F = 1.00, p = 0.44, df = 7,207 | 0.034 |
| MAP g | |||||
| Unadjusted, Mean mmHg (SD) | 95.7 (11.1) | 96.6 (12.3) | 95.9 (11.4) | F = 0.176, p = 0.84, df = 2,207 | 0.002 |
| Adjusted for baseline, Mean mmHg (95% C.I.) | 94.3 (93.3–97.4) | 96.3 (95.4–98.6) | 95.4 (95.1–98.3) | F = 72.42, p < 0.001, df = 3,207 | 0.516 |
| Fully adjusted, Mean mmHg (95% C.I.) | 94.3 (93.3–97.4) | 96.3 (95.4–98.6) | 95.4 (95.1–98.3) | F = 34.23, p < 0.001, df = 7,207 | 0.545 |
| 3 Days Post Quit | Nicotine-Containing E-Cigarettes, n = 35 | Nicotine-Free E-Cigarettes, n = 30 | Nicotine Replacement Therapy (NRT), n = 24 | F Value, p Value and Degrees of Freedom | η2 |
|---|---|---|---|---|---|
| %FMD a,b | |||||
| Unadjusted, Mean (SD) | 8.4 (3.9) | 10.7 (6.0) | 10.0 (4.8) | F = 1.672, p = 0.19, df = 2,88 | 0.038 |
| Adjusted for baseline, Mean (95% C.I.) | 8.1 (6.4–9.8) | 11.0 (9.1–13.0) | 10.2 (8.6–11.8) | F = 5.53, p < 0.01, df = 3,88 | 0.163 |
| Fully adjusted, Mean (95% C.I.) | 8.1 (6.4–9.8) | 11.2 (9.4–13.1) | 10.1 (8.6–11.7) | F = 14.53, p < 0.001, df = 7,88 | 0.268 |
| Peak CVC c for ACh d,e | |||||
| Unadjusted Mean PU/mmHg (SD) | 1.73 (1.17) | 1.31 (0.77) | 1.80 (1.25) | F = 1.371, p = 0.26, df = 2,88 | 0.031 |
| Adjusted for baseline, Mean PU/mmHg (95% C.I.) | 1.75 (1.37–2.12) | 1.36 (0.94–1.78) | 1.76 (1.41–2.11) | F = 3.98, p < 0.01, df = 3,88 | 0.123 |
| Fully adjusted, Mean PU/mmHg (95% C.I.) | 1.70 (1.34–2.06) | 1.38 (0.94–1.82) | 1.70 (1.34–2.06) | F = 2.24, p < 0.04, df = 7,88 | 0.168 |
| Peak CVC for SNP f,e | |||||
| Unadjusted Mean PU/mmHg (SD) | 1.41 (0.84) | 1.42 (1.34) | 1.56 (1.27) | F = 0.17, p = 0.84, df = 2,88 | 0.004 |
| Adjusted for baseline, Mean PU/mmHg (95% C.I.) | 1.39 (0.99–1.79) | 1.48 (1.02–1.93) | 1.53 (1.15–1.90) | F = 1.14, p = 0.38, df = 3,88 | 0.039 |
| Fully adjusted, Mean PU/mmHg (95% C.I.) | 1.36 (0.95–1.78) | 1.50 (1.02–1.98) | 1.56 (1.20–1.98) | F = 1.04, p = 0.41, df = 7,88 | 0.085 |
| MAP g,b | |||||
| Unadjusted, Mean mmHg (SD) | 99 (13) | 99 (12) | 97 (11) | F = 0.77, p = 0.47, df = 2,88 | 0.018 |
| Adjusted for baseline, Mean mmHg (95% C.I.) | 98 (95–101) | 99 (96–102) | 98 (95–101) | F = 29.25, p < 0.001, df = 3,88 | 0.508 |
| Fully adjusted, Mean mmHg (95% C.I.) | 97 (94–100) | 99 (97–102) | 98 (95–101) | F = 4.24, p < 0.001, df = 7,88 | 0.557 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klonizakis, M.; Gumber, A.; McIntosh, E.; Brose, L.S. Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial. Biology 2021, 10, 1208. https://doi.org/10.3390/biology10111208
Klonizakis M, Gumber A, McIntosh E, Brose LS. Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial. Biology. 2021; 10(11):1208. https://doi.org/10.3390/biology10111208
Chicago/Turabian StyleKlonizakis, Markos, Anil Gumber, Emma McIntosh, and Leonie S. Brose. 2021. "Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial" Biology 10, no. 11: 1208. https://doi.org/10.3390/biology10111208
APA StyleKlonizakis, M., Gumber, A., McIntosh, E., & Brose, L. S. (2021). Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial. Biology, 10(11), 1208. https://doi.org/10.3390/biology10111208






