Pancreatogenic Diabetes: Triggering Effects of Alcohol and HIV
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology of Pancreatitis and Diabetes
3. HIV-Induced Pancreatitis
3.1. Clinical Significance
3.2. HIV Entry into the Pancreas
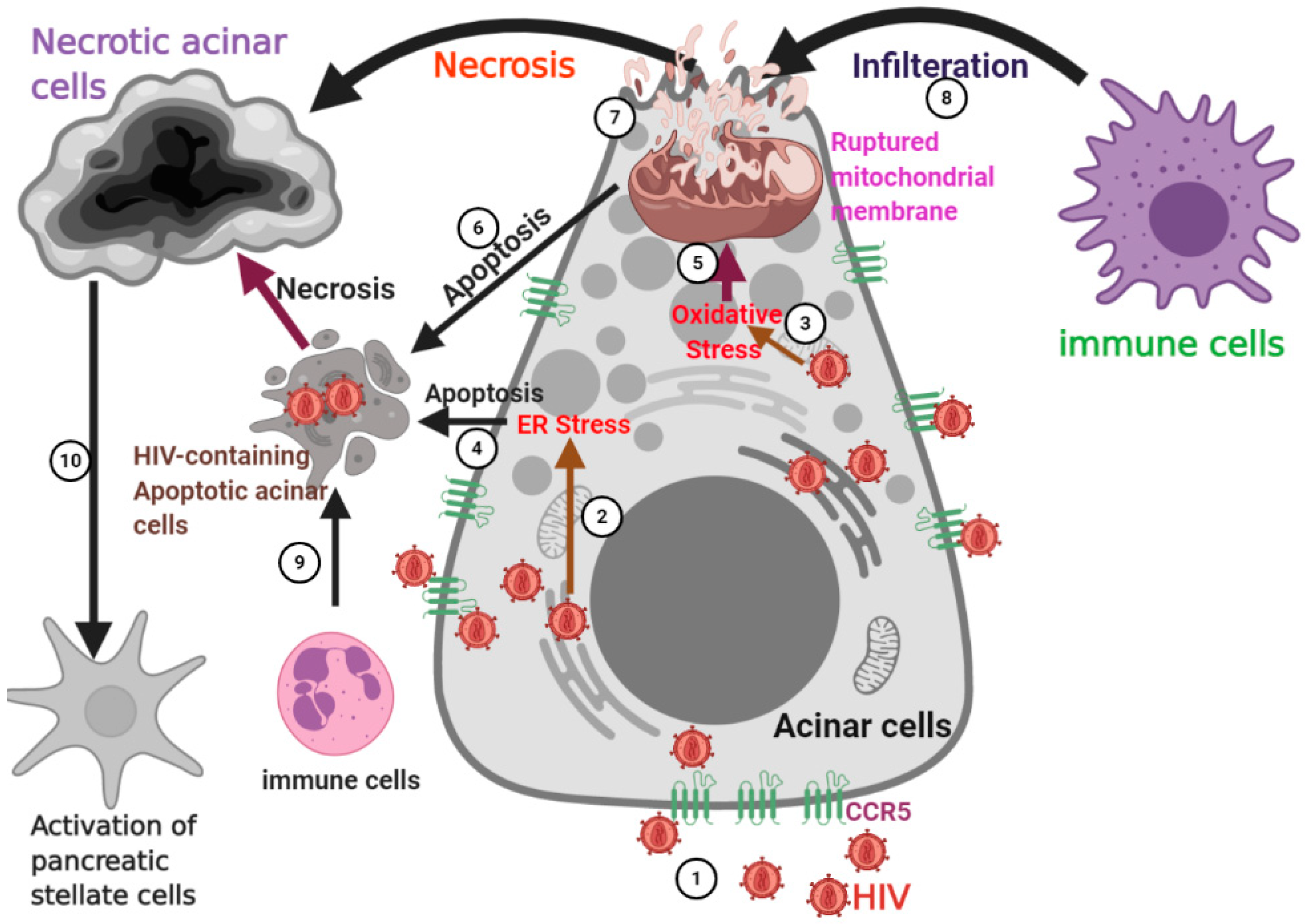
3.3. HIV-Induced Damage in Acinar Cells
4. Alcohol Potentiates HIV-Induced Pancreatitis
4.1. Significance
4.2. Pancreatic alcohol metabolism
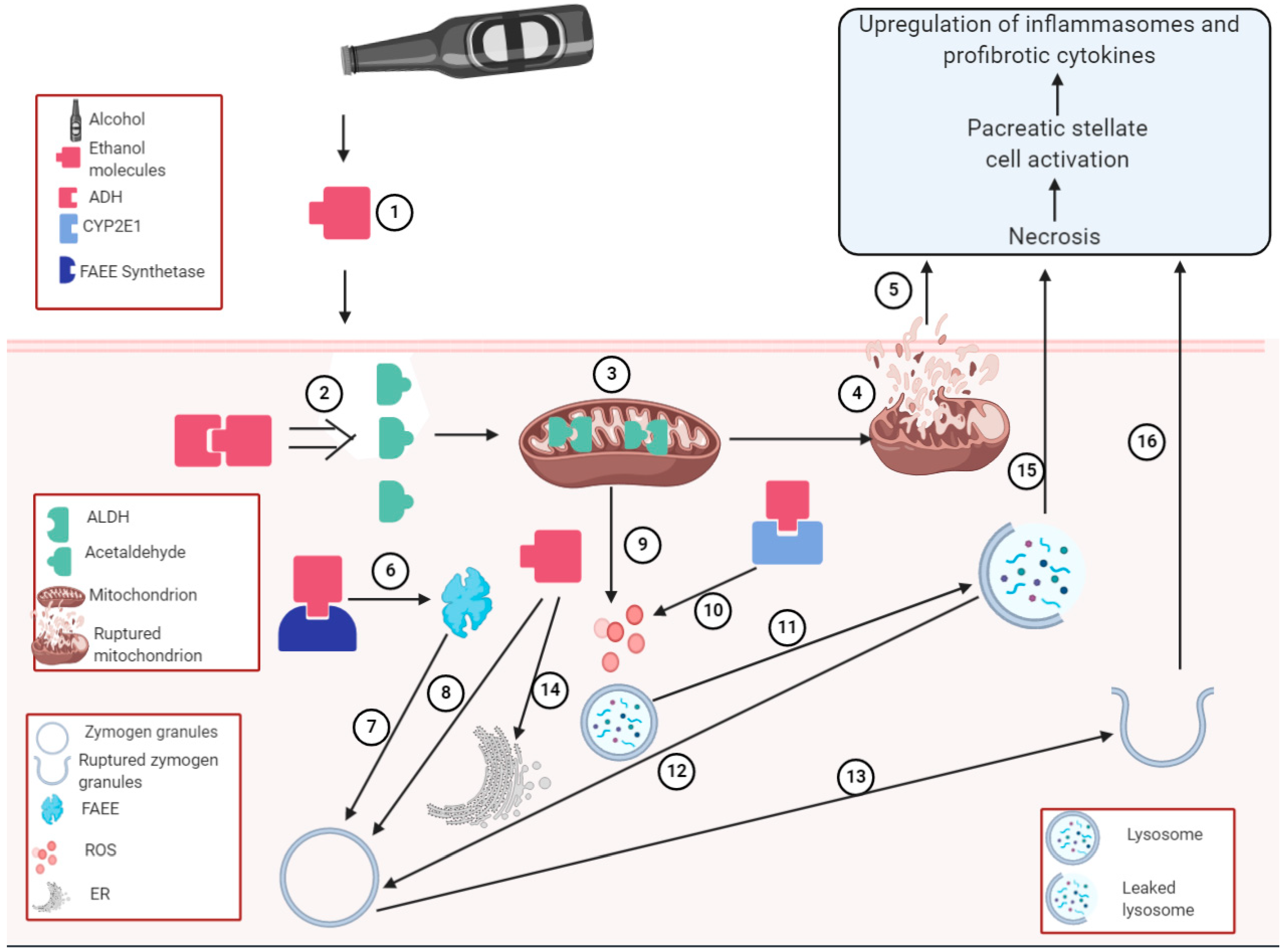
4.3. Alcoholic Pancreatitis
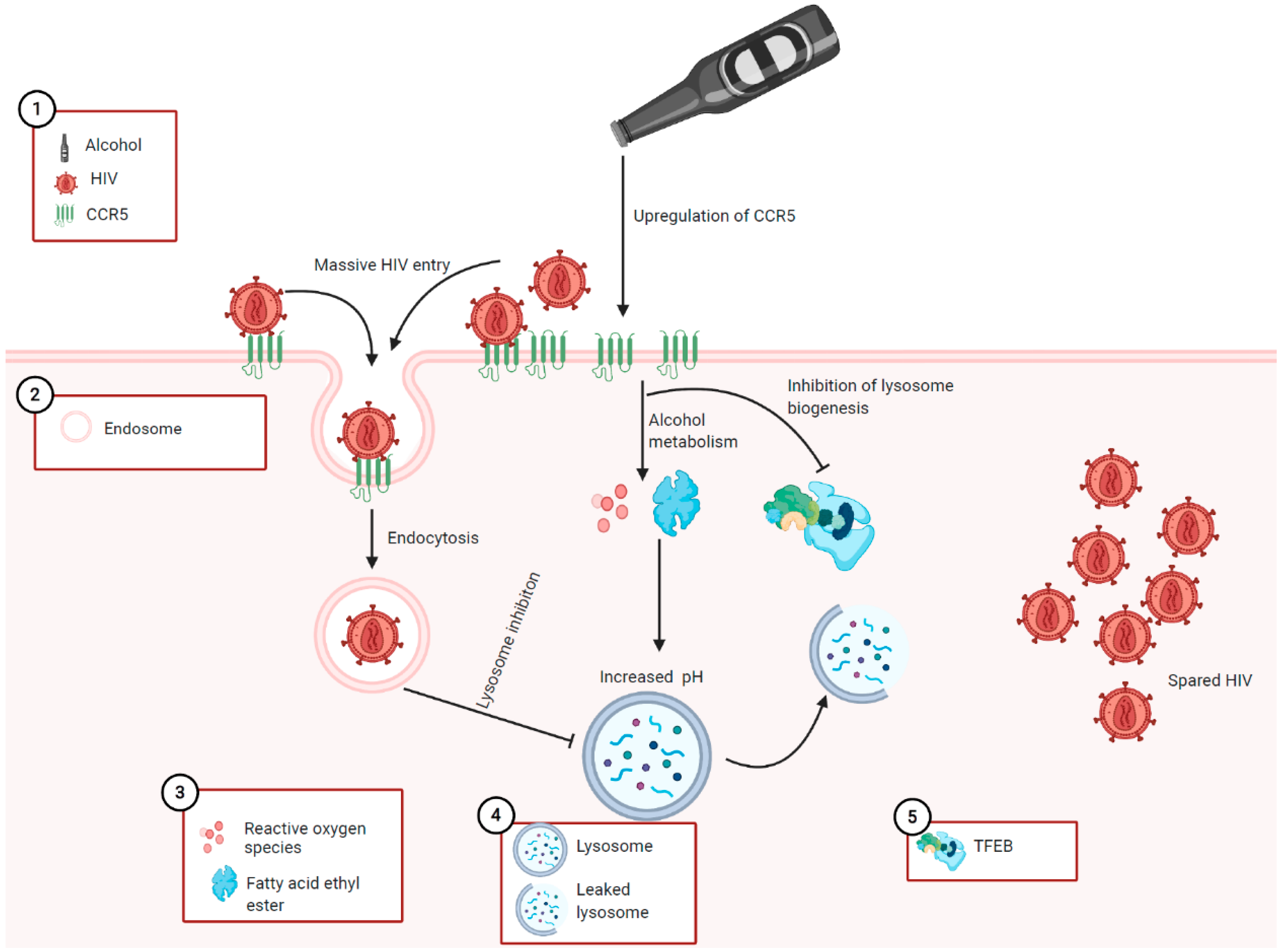
4.4. Proposed Mechanisms for the Role of Alcohol in HIV-Induced Pancreatitis
5. Potential Therapeutic Strategies for Alcohol and HIV-Induced Tissue Damage: A Reflection for HIV-Induced Pancreatitis Potentiated by Alcohol
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Global Health Observatory Data. Available online: https://www.who.int/gho/hiv/en/ (accessed on 13 July 2020).
- Haynes, B.F.; Burton, D.R.; Mascola, J.R. Multiple roles for HIV broadly neutralizing antibodies. Sci. Transl. Med. 2019, 11, eaaz2686. [Google Scholar] [CrossRef]
- Trejos-Castillo, E. Technology Platforms and Family Engagement for HIV/AIDS Prevention: Addressing the Needs of Minority Rural Youth. J. Adolesc. Health 2019, 65, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention CDC Fact Sheet. Available online: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/todaysepidemic-508.pdf (accessed on 15 July 2020).
- Center for Disease Control and Prevention AIDS and HIV. Available online: https://www.cdc.gov/nchs/fastats/aids-hiv.htm (accessed on 15 July 2020).
- Palella, F.J., Jr.; Chmiel, J.S.; Moorman, A.C.; Holmberg, S.D.; Investigators, H.O.S. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. Aids 2002, 16, 1617–1626. [Google Scholar] [CrossRef]
- Johnson, L.F.; May, M.T.; Dorrington, R.E.; Cornell, M.; Boulle, A.; Egger, M.; Davies, M.-A. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study. PLoS Med. 2017, 14, e1002468. [Google Scholar] [CrossRef]
- Mocroft, A.; Ledergerber, B.; Katlama, C.; Kirk, O.; Reiss, P.; Monforte, A.D.; Knysz, B.; Dietrich, M.; Phillips, A.N.; Lundgren, J.D. Decline in the AIDS and death rates in the EuroSIDA study: An observational study. Lancet 2003, 362, 22–29. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Beymer, M.R.; Suen, S.-C. Chronic Disease Onset Among People Living with HIV and AIDS in a Large Private Insurance Claims Dataset. Sci. Rep. 2019, 18514. [Google Scholar] [CrossRef]
- Harrison, K.M.; Song, R.; Zhang, X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. JAIDS J. Acquir. Immune Defic. Syndr. 2010, 53, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Teeraananchai, S.; Kerr, S.; Amin, J.; Ruxrungtham, K.; Law, M. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med. 2017, 18, 256–266. [Google Scholar] [CrossRef]
- Pettit, A.C.; Giganti, M.J.; Ingle, S.M.; May, M.T.; Shepherd, B.E.; Gill, M.J.; Fätkenheuer, G.; Abgrall, S.; Saag, M.S.; Del Amo, J. Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. J. Int. AIDS Soc. 2018, 21, e25031. [Google Scholar] [CrossRef] [PubMed]
- Prevention, HIV among People Aged 50 and over. Available online: https://www.cdc.gov/hiv/group/age/olderamericans/index.html (accessed on 1 January 2021).
- Escota, G.V.; O’Halloran, J.A.; Powderly, W.G.; Presti, R.M. Understanding mechanisms to promote successful aging in persons living with HIV. Int. J. Infect. Dis. 2018, 66, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J. Clin. Endocrinol. Metab. 2007, 92, 2506–2512. [Google Scholar] [CrossRef]
- Neuhaus, J.; Angus, B.; Kowalska, J.D.; La Rosa, A.; Sampson, J.; Wentworth, D.; Mocroft, A. Risk of all-cause mortality associated with non-fatal AIDS and serious non-AIDS events among adults infected with HIV. Aids 2010, 24, 697. [Google Scholar] [CrossRef] [PubMed]
- Galli, L.; Salpietro, S.; Pellicciotta, G.; Galliani, A.; Piatti, P.; Hasson, H.; Guffanti, M.; Gianotti, N.; Bigoloni, A.; Lazzarin, A. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur. J. Epidemiol. 2012, 27, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Herrin, M.; Tate, J.P.; Akgün, K.M.; Butt, A.A.; Crothers, K.; Freiberg, M.S.; Gibert, C.L.; Leaf, D.A.; Rimland, D.; Rodriguez-Barradas, M.C. Weight gain and incident diabetes among HIV infected-veterans initiating antiretroviral therapy compared to uninfected individuals. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 73, 228. [Google Scholar] [CrossRef] [PubMed]
- Willig, A.L.; Overton, E.T. Metabolic complications and glucose metabolism in HIV infection: A review of the evidence. Curr. HIV/AIDS Rep. 2016, 13, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Hermann, K.; Schalk, H.; Haider, B.; Hutterer, J.; Gmeinhart, B.; Pichler, K.; Brath, H.; Dorner, T.E. Impaired lipid profile and insulin resistance in a cohort of Austrian HIV patients. J. Infect. Chemother. 2016, 22, 248–253. [Google Scholar] [CrossRef]
- Noumegni, S.R.N.; Nansseu, J.R.; Ama, V.J.M.; Bigna, J.J.; Assah, F.K.; Guewo-Fokeng, M.; Leumi, S.; Katte, J.-C.; Dehayem, M.; Kengne, A.P. Insulin resistance and associated factors among HIV-infected patients in sub-Saharan Africa: A cross sectional study from Cameroon. Lipids Health Dis. 2017, 16, 148. [Google Scholar] [CrossRef]
- Natsag, J.; Erlandson, K.M.; Sellmeyer, D.E.; Haberlen, S.A.; Margolick, J.; Jacobson, L.P.; Palella, F.J., Jr.; Koletar, S.L.; Lake, J.E.; Post, W.S. HIV infection is associated with increased fatty infiltration of the thigh muscle with aging independent of fat distribution. PLoS ONE 2017, 12, e0169184. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.M.M.; Greco, D.B.; Moreira, A.N.; Guimarães, N.S.; Freire, C.M.V.; Rohlfs, B.G.; Machado, L.J.D.C. Lipid accumulation product index in HIV-infected patients: A marker of cardiovascular risk. Braz. J. Infect. Dis. 2018, 22, 171–176. [Google Scholar] [CrossRef]
- Kilbourne, A.; Justice, A.; Rabeneck, L.; Rodriguez-Barradas, M.; Weissman, S. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J. Clin. Epidemiol. 2001, 54, S22–S28. [Google Scholar] [CrossRef]
- Sarkar, S.; Brown, T.T. Diabetes in People Living with HIV. In Endotext [Internet]; MDText.com Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Longenecker, C.T.; Jiang, Y.; Yun, C.-H.; Debanne, S.; Funderburg, N.T.; Lederman, M.M.; Storer, N.; Labbato, D.E.; Bezerra, H.G.; McComsey, G.A. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int. J. Cardiol. 2013, 168, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Beires, M.T.; Silva-Pinto, A.; Santos, A.C.; Madureira, A.J.; Pereira, J.; Carvalho, D.; Sarmento, A.; Freitas, P. Visceral adipose tissue and carotid intima-media thickness in HIV-infected patients undergoing cART: A prospective cohort study. BMC Infect. Dis. 2018, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; McGinnis, K.; Rodriguez-Barradas, M.C.; Crystal, S.; Simberkoff, M.; Goetz, M.B.; Leaf, D.; Justice, A.C. HIV infection and the risk of diabetes mellitus. Aids 2009, 23, 1227. [Google Scholar] [CrossRef]
- Gebrie, A.; Tesfaye, B.; Gebru, T.; Adane, F.; Abie, W.; Sisay, M. Diabetes mellitus and its associated risk factors in patients with human immunodeficiency virus on anti-retroviral therapy at referral hospitals of Northwest Ethiopia. Diabetol. Metab. Syndr. 2020, 12, 20. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Huang, M.-Y.; Hsu, C.-Y.; Su, Y.-C. Bidirectional relationship between diabetes and acute pancreatitis: A population-based cohort study in Taiwan. Medicine 2016, 95, e2448. [Google Scholar] [CrossRef]
- Shen, H.-N.; Yang, C.-C.; Chang, Y.-H.; Lu, C.-L.; Li, C.-Y. Risk of diabetes mellitus after first-attack acute pancreatitis: A national population-based study. Am. J. Gastroenterol. 2015, 110, 1698–1706. [Google Scholar] [CrossRef]
- Grinspoon, S.K.; Bilezikian, J.P. HIV disease and the endocrine system. N. Engl. J. Med. 1992, 327, 1360–1365. [Google Scholar] [PubMed]
- Schwartz, M.S.; Brandt, L.J. The spectrum of pancreatic disorders in patients with the acquired immune deficiency syndrome. Am. J. Gastroenterol. 1989, 84, 459–462. [Google Scholar]
- Cappell, M.S.; Marks, M. Acute pancreatitis in HIV-seropositive patients: A case control study of 44 patients. Am. J. Med. 1995, 98, 243–248. [Google Scholar] [CrossRef]
- Dutta, S.; Ting, C.; Lai, L. Study of prevalence, severity, and etiological factors associated with acute pancreatitis in patients infected with human immunodeficiency virus. Am. J. Gastroenterol. 1997, 92, 2044–2048. [Google Scholar]
- Dassopoulos, T.; Ehrenpreis, E.D. Acute pancreatitis in human immunodeficiency virus–infected patients: A review. Am. J. Med. 1999, 107, 78–84. [Google Scholar] [CrossRef]
- Ito, T.; Otsuki, M.; Itoi, T.; Shimosegawa, T.; Funakoshi, A.; Shiratori, K.; Naruse, S.; Kuroda, Y. The Research Committee of Intractable Diseases of the Pancreas. Pancreatic diabetes in a follow-up survey of chronic pancreatitis in Japan. J. Gastroenterol. 2007, 42, 291–297. [Google Scholar] [CrossRef]
- Monroe, A.K.; Glesby, M.J.; Brown, T.T. Diagnosing and managing diabetes in HIV-infected patients: Current concepts. Clin. Infect. Dis. 2015, 60, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Cooper, D.A. Adverse effects of antiretroviral therapy. Lancet 2000, 356, 1423–1430. [Google Scholar] [CrossRef]
- Salehian, B.; Bilas, J.; Bazargan, M.; Abbasian, M. Prevalence and incidence of diabetes in HIV-infected minority patients on protease inhibitors. J. Natl. Med. Assoc. 2005, 97, 1088. [Google Scholar] [PubMed]
- Kalra, S.; Agrawal, N. Diabetes and HIV: Current understanding and future perspectives. Curr. Diabetes Rep. 2013, 13, 419–427. [Google Scholar] [CrossRef]
- Dragovic, G. Acute pancreatitis in HIV/AIDS patients: An issue of concern. Asian Pac. J. Trop. Biomed. 2013, 3, 422–425. [Google Scholar] [CrossRef]
- Apostolova, N.; Blas-Garcia, A.; Esplugues, J.V. Mitochondrial toxicity in HAART: An overview of in vitro evidence. Curr. Pharm. Des. 2011, 17, 2130–2144. [Google Scholar] [CrossRef]
- Moore, R.D.; Keruly, J.C.; Chaisson, R.E. Incidence of pancreatitis in HIV-infected patients receiving nucleoside reverse transcriptase inhibitor drugs. Aids 2001, 15, 617–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dragovic, G.; Milic, N.; Jevtovic, D. Incidence of acute pancreatitis and nucleoside reverse transcriptase inhibitors usage. Int. J. STD AIDS 2005, 16, 427. [Google Scholar] [CrossRef]
- Smith, C.J.; Olsen, C.H.; Mocroft, A.; Viard, J.P.; Staszewski, S.; Panos, G.; Staub, T.; Blaxhult, A.; Vetter, N.; Lundgren, J.D. The role of antiretroviral therapy in the incidence of pancreatitis in HIV-positive individuals in the EuroSIDA study. Aids 2008, 22, 47–56. [Google Scholar] [CrossRef]
- Nachega, J.B.; Trotta, M.P.; Nelson, M.; Ammassari, A. Impact of metabolic complications on antiretroviral treatment adherence: Clinical and public health implications. Curr. HIV/AIDS Rep. 2009, 6, 121. [Google Scholar] [CrossRef]
- Chapman, S.J.; Woolley, I.J.; Visvanathan, K.; Korman, T.M. Acute pancreatitis caused by tipranavir/ritonavir-induced hypertriglyceridaemia. Aids 2007, 21, 532–533. [Google Scholar] [CrossRef]
- Petrov, M.S.; Yadav, D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, J.; Justice, A.C.; Gordon, A.J.; Bryant, K.; Alcohol, V.; Group, B.C.R. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med. Care 2006, 44, S1–S6. [Google Scholar] [CrossRef]
- Galvan, F.H.; Bing, E.G.; Fleishman, J.A.; London, A.S.; Caetano, R.; Burnam, M.A.; Longshore, D.; Morton, S.C.; Orlando, M.; Shapiro, M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study. J. Stud. Alcohol 2002, 63, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zagaria, M. Acute pancreatitis: Risks, causes, and mortality in older adults. US Pharm. 2011, 36, 20–24. [Google Scholar]
- Testoni, P.A. Acute recurrent pancreatitis: Etiopathogenesis, diagnosis and treatment. World J. Gastroenterol. 2014, 20, 16891–16901. [Google Scholar] [CrossRef]
- Xiao, A.Y.; Tan, M.L.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Kurti, F.; Shpata, V.; Kuqo, A.; Duni, A.; Roshi, E.; Basho, J. Incidence of acute pancreatitis in Albanian population. Mater. Socio-Med. 2015, 27, 376. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef]
- Karimi, M.; Bruns, A.; Maisonneuve, P.; Lowenfels, A.B. Temporal trends in incidence and severity of acute pancreatitis in Lüneburg County, Germany: A population-based study. Pancreatology 2009, 9, 420–426. [Google Scholar]
- Spanier, B.W.M.; Dijkgraaf, M.G.; Bruno, M.J. Trends and forecasts of hospital admissions for acute and chronic pancreatitis in the Netherlands. Eur. J. Gastroenterol. Hepatol. 2008, 20, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Stimac, D.; Mikolasevic, I.; Krznaric-Zrnic, I.; Radic, M.; Milic, S. Epidemiology of acute pancreatitis in the North Adriatic Region of Croatia during the last ten years. Gastroenterol. Res. Pract. 2013, 2013. [Google Scholar] [CrossRef]
- Worning, H. Acute pancreatitis in Denmark. Ugeskr. Laeger 1994, 156, 2086–2089. [Google Scholar]
- McKay, C.; Evans, S.; Sinclair, M.; Carter, C.; Imrie, C. High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br. J. Surg. 1999, 86, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Bailón, M.; de Miguel Yanes, J.M.; Jiménez-García, R.; Hernández-Barrera, V.; Pérez-Farinós, N.; López-de-Andrés, A. National trends in incidence and outcomes of acute pancreatitis among type 2 diabetics and non-diabetics in Spain (2001–2011). Pancreatology 2015, 15, 64–70. [Google Scholar] [CrossRef]
- Jaakkola, M.; Nordback, I. Pancreatitis in Finland between 1970 and 1989. Gut 1993, 34, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Głuszek, S.; Kozieł, D. Prevalence and progression of acute pancreatitis in the Świętokrzyskie Voivodeship population. Pol. Prz. Chir. 2012, 84, 618–625. [Google Scholar]
- Ouyang, G.; Pan, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Zhou, Z.; Wen, Y. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020, 18, 388. [Google Scholar] [CrossRef]
- Lew, D.; Afghani, E.; Pandol, S. Chronic Pancreatitis: Current Status and Challenges for Prevention and Treatment. Dig Dis Sci 2017, 62, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.L.; Vadhavkar, S.; Singh, G.; Omary, M.B. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch. Intern. Med. 2008, 168, 649–656. [Google Scholar] [CrossRef]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187.e3. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Y.; Liao, Z.; Zou, D.-W.; Jin, Z.-D.; Zou, D.-J.; Jin, G.; Hu, X.-G.; Li, Z.-S. Occurrence of and risk factors for diabetes mellitus in Chinese patients with chronic pancreatitis. Pancreas 2011, 40, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ewald, N.; Kaufmann, C.; Raspe, A.; Kloer, H.; Bretzel, R.; Hardt, P. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes/Metab. Res. Rev. 2012, 28, 338–342. [Google Scholar] [CrossRef]
- Abu-El-Haija, M.; Hornung, L.; Denson, L.A.; Husami, A.; Lin, T.K.; Matlock, K.; Nathan, J.D.; Palermo, J.J.; Thompson, T.; Valencia, C.A. Prevalence of abnormal glucose metabolism in pediatric acute, acute recurrent and chronic pancreatitis. PLoS ONE 2018, 13, e0204979. [Google Scholar] [CrossRef]
- Tripathi, A.; Liese, A.; Jerrell, J.; Zhang, J.; Rizvi, A.; Albrecht, H.; Duffus, W. Incidence of diabetes mellitus in a population-based cohort of HIV-infected and non-HIV-infected persons: The impact of clinical and therapeutic factors over time. Diabet. Med. 2014, 31, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Romieu, A.C.; Garg, S.; Rosenberg, E.S.; Thompson-Paul, A.M.; Skarbinski, J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res. Care 2017, 5, e000304. [Google Scholar] [CrossRef]
- San Francisco Department of Public Health HIV Epidemiology. Annual Report 2017. Available online: https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2017-Green-20180904-Web.pdf (accessed on 23 November 2020).
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Ferreira, F.A.Y.; Yonamine, R.Y.; Chehter, E.Z. Antiretroviral drugs and acute pancreatitis in HIV/AIDS patients: Is there any association? A literature review. Einstein (Sao Paulo) 2014, 12, 112–119. [Google Scholar] [CrossRef]
- Dowell, S.; Holt, E.; Murphy, F. Pancreatitis Associated with HIV infection. In Proceedings of the Fifth International Conference on AIDS, Montreal, QC, Canada, 4–9 June 1989. [Google Scholar]
- Pezzilli, R.; Gullo, L.; Ricchi, E.; Costigliola, P.; Sprovieri, G.; Pilati, G.; Fontana, G. Serum pancreatic enzymes in HIV-seropositive patients. Dig. Dis. Sci. 1992, 37, 286–288. [Google Scholar] [CrossRef]
- Carroccio, A.; Fontana, M.; Spagnuolo, M.; Zuin, G.; Montalto, G.; Canani, R.B.; Verghi, F.; Di Martino, D.; Bastoni, K.; Buffardi, F. Pancreatic dysfunction and its association with fat malabsorption in HIV infected children. Gut 1998, 43, 558–563. [Google Scholar] [CrossRef]
- Carroccio, A.; Di Prima, L.; Di Grigoli, C.; Soresi, M.; Farinella, E.; Di Martino, D.; Guarino, A.; Notarbartolo, A.; Montalto, G. Exocrine pancreatic function and fat malabsorption in human immunodeficiency virus-infected patients. Scand. J. Gastroenterol. 1999, 34, 729–734. [Google Scholar]
- Bitar, A.; Altaf, M.; Sferra, T.J. Acute pancreatitis: Manifestation of acute HIV infection in an adolescent. Am. J. Case Rep. 2012, 13, 17. [Google Scholar] [CrossRef]
- Parenti, D.M.; Steinberg, W.; Kang, P. Infectious causes of acute pancreatitis. Pancreas 1996, 13, 356–371. [Google Scholar] [CrossRef]
- Rizzardi, G.P.; Tambussi, G.; Lazzarin, A. Acute pancreatitis during primary HIV-1 infection. N. Engl. J. Med. 1997, 336, 1836–1837. [Google Scholar] [CrossRef]
- Mortier, E.; Gaba, S.; Mari, I.; Vinceneux, P.; Pouchot, J. Acute pancreatitis during primary HIV-1 infection. Am. J. Gastroenterol. 2002, 97, 504. [Google Scholar] [CrossRef]
- Tyner, R.; Turett, G. Primary human immunodeficiency virus infection presenting as acute pancreatitis. South. Med. J. 2004, 97, 393–395. [Google Scholar] [CrossRef]
- Paño-Pardo, J.R.; Alcaide, M.L.; Abbo, L.; Dickinson, G. Primary HIV infection with multisystemic presentation. Int. J. Infect. Dis. 2009, 13, e177–e180. [Google Scholar] [CrossRef] [PubMed]
- Bhurwal, A.; Sapru, S.; Ramasamy, D. Diffuse Pancreatic Inflammation in an HIV Infected Individual with Elevated IgG4 Levels. J. Med. Cases 2018, 9, 109–111. [Google Scholar] [CrossRef][Green Version]
- Bhurwal, A.; Sapru, S.; Ramasamy, D. Diffuse Pancreatic Inflammation in an HIV Patient Masquerading as Autoimmune Pancreatitis: A Case Report: 1306. Am. J. Gastroenterol. 2016, 111, S584. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Simon, D.; Wittner, M. Gastrointestinal manifestations. Med. Clin. N. Am. 1992, 76, 45–62. [Google Scholar] [CrossRef]
- Schwartz, M.S.; Cappell, M.S. Pentamidine-associated pancreatitis. Dig. Dis. Sci. 1989, 34, 1617–1620. [Google Scholar] [CrossRef]
- Murphey, S.A.; Josephs, A.S. Acute pancreatitis associated with pentamidine therapy. Arch. Intern. Med. 1981, 141, 56–58. [Google Scholar] [CrossRef]
- Blanchard, J.N.; Wohlfeiler, M.; Canas, A.; King, K.; Lonergan, J.T. Pancreatitis treated with didanosine and tenofovir disoproxil fumarate. Clin. Infect. Dis. 2003, 37, e57–e62. [Google Scholar] [CrossRef][Green Version]
- Callens, S.; De Schacht, C.; Huyst, V.; Colebunders, R. Pancreatitis in an HIV-infected person on a tenofovir, didanosine and stavudine containing highly active antiretroviral treatment. J. Infect. 2003, 47, 188–189. [Google Scholar] [CrossRef]
- Longhurst, H.; Pinching, A. Pancreatitis associated with hydroxyurea in combination with didanosine. BMJ 2001, 322, 81. [Google Scholar] [CrossRef] [PubMed]
- Barrios, A.; Negredo, F.; Vilaro-Rodriguez, J.; Domingo, P.; Estrada, V.; Labarga, P.; Asensi, V.; Morales, D.; Santos, J.; Terron, J. Safety and efficacy of a QD simplification regimen. In Proceedings of the 11th Conference on Retroviruses and Opportunistic Infections, Francisco, CA, USA, 8–11 February 2004. [Google Scholar]
- Barbosa, A.G.; Chehter, E.Z.; Bacci, M.R.; Mader, A.A.; Fonseca, F.L. AIDS and the pancreas in the HAART era: A cross sectional study. Int. Arch. Med. 2013, 6, 28. [Google Scholar] [CrossRef]
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed]
- Permanyer, M.; Ballana, E.; Ruiz, A.; Badia, R.; Riveira-Munoz, E.; Gonzalo, E.; Clotet, B.; Esté, J.A. Antiretroviral agents effectively block HIV replication after cell-to-cell transfer. J. Virol. 2012, 86, 8773–8780. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Siliciano, R.F. Targeting the latent reservoir for HIV-1. Immunity 2018, 48, 872–895. [Google Scholar] [CrossRef]
- Shan, L.; Deng, K.; Gao, H.; Xing, S.; Capoferri, A.A.; Durand, C.M.; Rabi, S.A.; Laird, G.M.; Kim, M.; Hosmane, N.N. Transcriptional reprogramming during effector-to-memory transition renders CD4+ T cells permissive for latent HIV-1 infection. Immunity 2017, 47, 766–775.e3. [Google Scholar] [CrossRef]
- Dahabieh, M.S.; Battivelli, E.; Verdin, E. Understanding HIV latency: The road to an HIV cure. Annu. Rev. Med. 2015, 66, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.-W.; Engel, D.; Berrey, M.M.; Shea, T.; Corey, L.; Fauci, A.S. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 1998, 95, 8869–8873. [Google Scholar] [CrossRef]
- Chauhan, A. Enigma of HIV-1 latent infection in astrocytes: An in-vitro study using protein kinase C agonist as a latency reversing agent. Microbes Infect. 2015, 17, 651–659. [Google Scholar] [CrossRef]
- Chauhan, A.; Tikoo, A.; Patel, J.; Abdullah, A.M. HIV-1 endocytosis in astrocytes: A kiss of death or survival of the fittest? Neurosci. Res. 2014, 88, 16–22. [Google Scholar] [CrossRef]
- Gray, L.R.; Roche, M.; Flynn, J.K.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. Is the central nervous system a reservoir of HIV-1? Curr. Opin. HIV AIDS 2014, 9, 552. [Google Scholar] [CrossRef]
- Canaud, G.; Dejucq-Rainsford, N.; Avettand-Fenoël, V.; Viard, J.-P.; Anglicheau, D.; Bienaimé, F.; Muorah, M.; Galmiche, L.; Gribouval, O.; Noël, L.-H. The kidney as a reservoir for HIV-1 after renal transplantation. J. Am. Soc. Nephrol. 2014, 25, 407–419. [Google Scholar] [CrossRef]
- Devadoss, D.; Singh, S.P.; Acharya, A.; Do, K.C.; Periyasamy, P.; Manevski, M.; Mishra, N.; Tellez, C.; Ramakrishnan, S.; Belinsky, S. Lung Bronchial Epithelial Cells are HIV Targets for Proviral Genomic Integration. bioRxiv 2020. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Jenabian, M.A. The lungs as anatomical reservoirs of HIV infection. Rev. Med. Virol. 2014, 24, 35–54. [Google Scholar] [CrossRef]
- Cribbs, S.K.; Lennox, J.; Caliendo, A.M.; Brown, L.A.; Guidot, D.M. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. Aids Res. Hum. Retrovir. 2015, 31, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chikwari, C.D.; Dringus, S.; Ferrand, R.A. Barriers to, and emerging strategies for, HIV testing among adolescents in sub-Saharan Africa. Curr. Opin. HIV AIDS 2018, 13, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Blackard, J.T.; Ma, G.; Martin, C.M.; Rouster, S.D.; Shata, M.T.; Sherman, K.E. HIV variability in the liver and evidence of possible compartmentalization. Aids Res. Hum. Retrovir. 2011, 27, 1117–1126. [Google Scholar] [CrossRef]
- Igarashi, T.; Brown, C.R.; Endo, Y.; Buckler-White, A.; Plishka, R.; Bischofberger, N.; Hirsch, V.; Martin, M.A. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 2001, 98, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.J.; Sugawara, S.; Goyal, A.; Durand, C.M.; Quinn, J.; Sachithanandham, J.; Cameron, A.M.; Bailey, J.R.; Perelson, A.S.; Balagopal, A. No recovery of replication-competent HIV-1 from human liver macrophages. J. Clin. Investig. 2018, 128, 4501–4509. [Google Scholar] [CrossRef]
- Kandathil, A.; Durand, C.; Quinn, J.; Cameron, A.; Thomas, D.; Balagopal, A. Liver macrophages and HIV-1 persistence. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, USA, 23–26 February 2015. [Google Scholar]
- Ganesan, M.; New-Aaron, M.; Dagur, R.S.; Makarov, E.; Wang, W.; Kharbanda, K.K.; Kidambi, S.; Poluektova, L.Y.; Osna, N.A. Alcohol Metabolism Potentiates HIV-Induced Hepatotoxicity: Contribution to End-Stage Liver Disease. Biomolecules 2019, 9, 851. [Google Scholar] [CrossRef]
- Kong, L.; Maya, W.C.; Moreno-Fernandez, M.E.; Ma, G.; Shata, M.T.; Sherman, K.E.; Chougnet, C.; Blackard, J.T. Low-level HIV infection of hepatocytes. Virol. J. 2012, 9, 157. [Google Scholar] [CrossRef]
- Chehter, E.Z.; Longo, M.A.; Laudanna, A.A.; Duarte, M.I.S. Involvement of the pancreas in AIDS: A prospective study of 109 post-mortems. Aids 2000, 14, 1879–1886. [Google Scholar] [CrossRef]
- Bricaire, F.; Marche, C.; Zoubi, D.; Saimot, A.; Regnier, B. HIV and the pancreas. Lancet 1988, 331, 65–66. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Kim, B.O.; Gattone, V.H.; Li, J.; Nath, A.; Blum, J.; He, J.J. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: Requirement for the human mannose receptor. J. Virol. 2004, 78, 4120–4133. [Google Scholar] [CrossRef] [PubMed]
- Eitner, F.; Cui, Y.; Hudkins, K.L.; Stokes, M.B.; Segerer, S.; Mack, M.; Lewis, P.L.; Abraham, A.A.; Schlöndorff, D.; Gallo, G. Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J. Am. Soc. Nephrol. 2000, 11, 856–867. [Google Scholar]
- Silva, M.; Chen, F.; Shannon, K.; Geng, Y.; Klitzner, T.; Krogstad, P. Expression of CXCR4 in Human Fetal Cardiac Myocytes: A Role in HIV Related Cardiomyopathy? Pediatric Res. 1999, 45, 174. [Google Scholar] [CrossRef][Green Version]
- de Campos, W.R.L.; Chirwa, N.; London, G.; Rotherham, L.S.; Morris, L.; Mayosi, B.M.; Khati, M. HIV-1 subtype C unproductively infects human cardiomyocytes in vitro and induces apoptosis mitigated by an anti-Gp120 aptamer. PLoS ONE 2014, 9, e110930. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zepp, M.; Georges, R.B.; Jarahian, M.; Kazemi, M.; Eyol, E.; Berger, M.R. The CCR5 antagonist maraviroc causes remission of pancreatic cancer liver metastasis in nude rats based on cell cycle inhibition and apoptosis induction. Cancer Lett. 2020, 474, 82–93. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Lillard, J.W.; Singh, R. Expression of CCR5 and its ligand CCL5 in pancreatic cancer. Am. Assoc. Immnol. 2016, 196, 51.3. [Google Scholar]
- Goecke, H.; Forssmann, U.; Uguccioni, M.; Friess, H.; Conejo-Garcia, J.R.; Zimmermann, A.; Baggiolini, M.; Büchler, M.W. Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery 2000, 128, 806–814. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.; Wu, K.; Zhao, Y.; Hu, G. Pancreatic stellate cells increase the invasion of human pancreatic cancer cells through the stromal cell-derived factor-1/CXCR4 axis. Pancreatology 2010, 10, 186–193. [Google Scholar] [CrossRef]
- Sarmiento, L.; Frisk, G.; Anagandula, M.; Hodik, M.; Barchetta, I.; Netanyah, E.; Cabrera-Rode, E.; Cilio, C.M. Echovirus 6 infects human exocrine and endocrine pancreatic cells and induces pro-inflammatory innate immune response. Viruses 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Carroll-Anzinger, D.; Kumar, A.; Adarichev, V.; Kashanchi, F.; Al-Harthi, L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J. Virol. 2007, 81, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Brack-Werner, R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. Aids 1999, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kanmogne, G.D.; Kennedy, R.; Grammas, P. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: Possible pathway for HIV entry into the brain and HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2002, 61, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, F.S.; Pandol, S.; Jamieson, J.D. Structure-function relationships in the pancreatic acinar cell. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 869–894. [Google Scholar]
- Lodish, H.; Berk, A.; Zipursky, S.; Matsudaira, P.; Baltimore, D.; Darnell, J. Folding, Modification, and Degradation of Proteins. In Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kong, L.; Yu, X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 150–164. [Google Scholar] [CrossRef]
- Nooka, S.; Ghorpade, A. Organellar stress intersects the astrocyte endoplasmic reticulum, mitochondria and nucleolus in HIV associated neurodegeneration. Cell Death Dis. 2018, 9, 317. [Google Scholar] [CrossRef]
- Nooka, S.; Ghorpade, A. HIV-1-associated inflammation and antiretroviral therapy regulate astrocyte endoplasmic reticulum stress responses. Cell Death Discov 2017, 3, 17061. [Google Scholar] [CrossRef]
- Shah, A.; Vaidya, N.K.; Bhat, H.K.; Kumar, A. HIV-1 gp120 induces type-1 programmed cell death through ER stress employing IRE1α, JNK and AP-1 pathway. Sci. Rep. 2016, 6, 18929. [Google Scholar] [CrossRef]
- Fan, Y.; He, J.J. HIV-1 Tat induces unfolded protein response and endoplasmic reticulum stress in astrocytes and causes neurotoxicity through glial fibrillary acidic protein (GFAP) activation and aggregation. J. Biol. Chem. 2016, 291, 22819–22829. [Google Scholar] [CrossRef]
- Colli, M.L.; Paula, F.M.; Marselli, L.; Marchetti, P.; Roivainen, M.; Eizirik, D.L. Coxsackievirus B tailors the unfolded protein response to favour viral amplification in pancreatic β cells. J. Innate Immun. 2019, 11, 375–390. [Google Scholar] [CrossRef]
- Hirota, M.; Kitagaki, M.; Itagaki, H.; Aiba, S. Quantitative measurement of spliced XBP1 mRNA as an indicator of endoplasmic reticulum stress. J. Toxicol. Sci. 2006, 31, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Saveljeva, S.; Mc Laughlin, S.L.; Vandenabeele, P.; Samali, A.; Bertrand, M.J.M. Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death Dis. 2015, 6, e1587. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Kara, M.; Oztas, E. Endoplasmic reticulum stress-mediated cell death. In Programmed Cell Death; BoD—Books on Demand: Nordersted, Germany, 2019. [Google Scholar]
- Bhat, T.A.; Chaudhary, A.K.; Kumar, S.; O’Malley, J.; Inigo, J.R.; Kumar, R.; Yadav, N.; Chandra, D. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fribley, A.; Zhang, K.; Kaufman, R.J. Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 2009, 559, 191–204. [Google Scholar]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Morishima, N.; Nakanishi, K.; Nakano, A. Activating Transcription Factor-6 (ATF6) Mediates Apoptosis with Reduction of Myeloid Cell Leukemia Sequence 1 (Mcl-1) Protein via Induction of WW Domain Binding Protein 1*. J. Biol. Chem. 2011, 286, 35227–35235. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Proics, E.; De Bieville, C.; Rousseau, D.; Bonnafous, S.; Patouraux, S.; Adam, G.; Lavallard, V.; Rovere, C.; Le Thuc, O. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015, 6, e1879. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Li, Z.; Guo, B. ER stress-induced inflammasome activation contributes to hepatic inflammation and steatosis. J. Clin. Cell. Immunol. 2016, 7, 457. [Google Scholar] [CrossRef]
- Jones, B.A.; Gores, G.J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G1174–G1188. [Google Scholar] [CrossRef]
- Herzenberg, L.A.; De Rosa, S.C.; Dubs, J.G.; Roederer, M.; Anderson, M.T.; Ela, S.W.; Deresinski, S.C.; Herzenberg, L.A. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 1997, 94, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Suthanthiran, M.; Anderson, M.E.; Sharma, V.K.; Meister, A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc. Natl. Acad. Sci. USA 1990, 87, 3343–3347. [Google Scholar] [CrossRef] [PubMed]
- Sönnerborg, A.; Carlin, G.; Åkerlund, B.; Jarstrand, C. Increased production of malondialdehyde in patients with HIV infection. Scand. J. Infect. Dis. 1988, 20, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Reshi, M.L.; Su, Y.-C.; Hong, J.-R. RNA viruses: ROS-mediated cell death. Int. J. Cell Biol. 2014, 2014, 467452. [Google Scholar] [CrossRef]
- Watanabe, L.M.; Júnior, F.B.; Jordão, A.A.; Navarro, A.M. Influence of HIV infection and the use of antiretroviral therapy on selenium and selenomethionine concentrations and antioxidant protection. Nutrition 2016, 32, 1238–1242. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, H.; Zhang, H.; Shi, Y.; Wei, F.; Liu, D.; Liu, K.; Chen, D. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012, 1458, 1–11. [Google Scholar] [CrossRef]
- Haughey, N.J.; Cutler, R.G.; Tamara, A.; McArthur, J.C.; Vargas, D.L.; Pardo, C.A.; Turchan, J.; Nath, A.; Mattson, M.P. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 2004, 55, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Brundu, S.; Palma, L.; Picceri, G.G.; Ligi, D.; Orlandi, C.; Galluzzi, L.; Chiarantini, L.; Casabianca, A.; Schiavano, G.F.; Santi, M. Glutathione depletion is linked with Th2 polarization in mice with a retrovirus-induced immunodeficiency syndrome, murine AIDS: Role of proglutathione molecules as immunotherapeutics. J. Virol. 2016, 90, 7118–7130. [Google Scholar] [CrossRef]
- Kawauchi, Y.; Suzuki, K.; Watanabe, S.; Yamagiwa, S.; Yoneyama, H.; Han, G.D.; Palaniyandi, S.S.; Veeraveedu, P.T.; Watanabe, K.; Kawachi, H. Role of IP-10/CXCL10 in the progression of pancreatitis-like injury in mice after murine retroviral infection. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G345–G354. [Google Scholar] [CrossRef][Green Version]
- Salmen, S.; Colmenares, M.; Peterson, D.L.; Reyes, E.; Rosales, J.D.; Berrueta, L. HIV-1 Nef associates with p22-phox, a component of the NADPH oxidase protein complex. Cell. Immunol. 2010, 263, 166–171. [Google Scholar] [CrossRef]
- Pandhare, J.; Dash, S.; Jones, B.; Villalta, F.; Dash, C. A novel role of proline oxidase in HIV-1 envelope glycoprotein-induced neuronal autophagy. J. Biol. Chem. 2015, 290, 25439–25451. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, R.F.; Xu, Y.C.; Flores, S.C.; Terada, L.S. HIV Tat activates c-Jun amino-terminal kinase through an oxidant-dependent mechanism. Virology 2001, 286, 62–71. [Google Scholar] [CrossRef]
- Banki, K.; Hutter, E.; Gonchoroff, N.J.; Perl, A. Molecular ordering in HIV-induced apoptosis oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J. Biol. Chem. 1998, 273, 11944–11953. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative stress during HIV infection: Mechanisms and consequences. Oxidative Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F. HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef]
- Sakuma, Y.; Kodama, Y.; Eguchi, T.; Uza, N.; Tsuji, Y.; Shiokawa, M.; Maruno, T.; Kuriyama, K.; Nishikawa, Y.; Yamauchi, Y. Chemokine CXCL16 mediates acinar cell necrosis in cerulein induced acute pancreatitis in mice. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Perkins, P.; Zaninovic, V.; Sandoval, D.; Rutherford, R.; Fitzsimmons, T.; Pandol, S.J.; Poucell-Hatton, S. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology 1996, 110, 875–884. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Woods, J.; Hardin, J.; Campana, G.; Ortiz, M.; Jaeger, B.; Reichart, B.; Bonnin, J.; Currin, A.; Cosgrove, S. Early prediction of cardiac allograft vasculopathy and heart transplant failure. Am. J. Transplant. 2011, 11, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Leurquin-Sterk, G.; Schepers, K.; Delhaye, M.; Goldman, S.; Verset, L.; Matos, C. Diffuse pancreatic lesion mimicking autoimmune pancreatitis in an HIV-infected patient: Successful treatment by antiretroviral therapy. Jop. J. Pancreas 2011, 12, 477–481. [Google Scholar]
- Kaiser, A.M.; Saluja, A.K.; Sengupta, A.; Saluja, M.; Steer, M.L. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am. J. Physiol. Cell Physiol. 1995, 269, C1295–C1304. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Gukovsky, I. Which way to die: The regulation of acinar cell death in pancreatitis by mitochondria, calcium, and reactive oxygen species. Gastroenterology 2011, 140, 1876. [Google Scholar] [CrossRef]
- Bläuer, M.; Laaninen, M.; Sand, J.; Laukkarinen, J. Reciprocal stimulation of pancreatic acinar and stellate cells in a novel long-term in vitro co-culture model. Pancreatology 2016, 16, 570–577. [Google Scholar] [CrossRef]
- Apte, M.; Haber, P.; Darby, S.; Rodgers, S.; McCaughan, G.; Korsten, M.; Pirola, R.; Wilson, J. Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut 1999, 44, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Luttenberger, T.; Schmid-Kotsas, A.; Menke, A.; Siech, M.; Beger, H.; Adler, G.; Grünert, A.; Bachem, M.G. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: Implications in pathogenesis of pancreas fibrosis. Lab. Investig. 2000, 80, 47–55. [Google Scholar] [CrossRef]
- Schneider, E.; Schmid-Kotsas, A.; Zhao, J.; Weidenbach, H.; Schmid, R.M.; Menke, A.; Adler, G.; Waltenberger, J.; Grünert, A.; Bachem, M.G. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am. J. Physiol. Cell Physiol. 2001, 281, C532–C543. [Google Scholar] [CrossRef]
- Shek, F.W.-T.; Benyon, R.C.; Walker, F.M.; McCrudden, P.R.; Pender, S.L.F.; Williams, E.J.; Johnson, P.A.; Johnson, C.D.; Bateman, A.C.; Fine, D.R. Expression of transforming growth factor-β1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am. J. Pathol. 2002, 160, 1787–1798. [Google Scholar] [CrossRef]
- Phillips, P.; Wu, M.; Kumar, R.; Doherty, E.; McCarroll, J.; Park, S.; Pirola, R.C.; Wilson, J.; Apte, M. Cell migration: A novel aspect of pancreatic stellate cell biology. Gut 2003, 52, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Mews, P.; Phillips, P.; Fahmy, R.; Korsten, M.; Pirola, R.; Wilson, J.; Apte, M. Pancreatic stellate cells respond to inflammatory cytokines: Potential role in chronic pancreatitis. Gut 2002, 50, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hama, K.; Ohnishi, H.; Aoki, H.; Kita, H.; Yamamoto, H.; Osawa, H.; Sato, K.; Tamada, K.; Mashima, H.; Yasuda, H. Angiotensin II promotes the proliferation of activated pancreatic stellate cells by Smad7 induction through a protein kinase C pathway. Biochem. Biophys. Res. Commun. 2006, 340, 742–750. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Alcohol Abuse and Alcoholism Alcohol Facts and Statistics. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics (accessed on 17 November 2020).
- Zablotska, I.B.; Gray, R.H.; Serwadda, D.; Nalugoda, F.; Kigozi, G.; Sewankambo, N.; Lutalo, T.; Mangen, F.W.; Wawer, M. Alcohol use before sex and HIV acquisition: A longitudinal study in Rakai, Uganda. Aids 2006, 20, 1191–1196. [Google Scholar] [CrossRef]
- Vagenas, P.; Ludford, K.T.; Gonzales, P.; Peinado, J.; Cabezas, C.; Gonzales, F.; Lama, J.R.; Sanchez, J.; Altice, F.L.; for the Peruvian HIV Sentinel Surveillance Working Group. Being unaware of being HIV-infected is associated with alcohol use disorders and high-risk sexual behaviors among men who have sex with men in Peru. AIDS Behav. 2014, 18, 120–127. [Google Scholar] [CrossRef]
- Braithwaite, R.S.; Bryant, K.J. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res. Health 2010, 33, 280. [Google Scholar]
- Sansone, R.A.; Sansone, L.A. Alcohol/Substance misuse and treatment nonadherence: Fatal attraction. Psychiatry (Edgmont) 2008, 5, 43. [Google Scholar]
- Santos, G.-M.; Emenyonu, N.I.; Bajunirwe, F.; Mocello, A.R.; Martin, J.N.; Vittinghoff, E.; Bangsberg, D.R.; Hahn, J.A. Self-reported alcohol abstinence associated with ART initiation among HIV-infected persons in rural Uganda. Drug Alcohol Depend. 2014, 134, 151–157. [Google Scholar] [CrossRef]
- Lifson, A.R.; Demissie, W.; Tadesse, A.; Ketema, K.; May, R.; Yakob, B.; Metekia, M.; Slater, L.; Shenie, T. Barriers to retention in care as perceived by persons living with HIV in rural Ethiopia: Focus group results and recommended strategies. J. Int. Assoc. Provid. Aids Care 2013, 12, 32–38. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Amaral, C.M.; White, D.; Swetsze, C.; Kalichman, M.O.; Cherry, C.; Eaton, L. Alcohol and adherence to antiretroviral medications: Interactive toxicity beliefs among people living with HIV. J. Assoc. Nurses Aids Care 2012, 23, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kader, R.; Seedat, S.; Govender, R.; Koch, J.; Parry, C. Hazardous and harmful use of alcohol and/or other drugs and health status among South African patients attending HIV clinics. AIDS Behav. 2014, 18, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, F.; Lions, C.; Winnock, M.; Salmon, D.; Durant, J.; Spire, B.; Mora, M.; Loko, M.A.; Dabis, F.; Dominguez, S. Self-reported alcohol abuse in HIV–HCV co-infected patients: A better predictor of HIV virological rebound than physician’s perceptions (HEPAVIH ARNS CO 13 cohort). Addiction 2013, 108, 1250–1258. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Grebler, T.; Amaral, C.M.; McNerney, M.; White, D.; Kalichman, M.O.; Cherry, C.; Eaton, L. Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. Int. J. Behav. Med. 2014, 21, 811–820. [Google Scholar] [CrossRef]
- Skeer, M.R.; Mimiaga, M.J.; Mayer, K.H.; O’Cleirigh, C.; Covahey, C.; Safren, S.A. Patterns of substance use among a large urban cohort of HIV-infected men who have sex with men in primary care. AIDS Behav. 2012, 16, 676–689. [Google Scholar] [CrossRef]
- Talamini, G.; Bassi, C.; Falconi, M.; Sartori, N.; Salvia, R.; Rigo, L.; Castagnini, A.; Di Francesco, V.; Frulloni, L.; Bovo, P. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig. Dis. Sci. 1999, 44, 1303–1311. [Google Scholar] [CrossRef]
- Lin, Y. Research Committee on Intractable Pancreatic Diseases. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: Findings from a case-control study in Japan. Am. J. Gastroenterol. 2001, 96, 2622–2627. [Google Scholar] [CrossRef]
- Blomgren, K.B.; Sundström, A.; Steineck, G.; Genell, S.; Sjöstedt, S.; Wiholm, B.-E. A Swedish case-control network for studies of drug-induced morbidity–acute pancreatitis. Eur. J. Clin. Pharmacol. 2002, 58, 275–283. [Google Scholar] [CrossRef]
- Kristiansen, L.; Grønbæk, M.; Becker, U.; Tolstrup, J.S. Risk of pancreatitis according to alcohol drinking habits: A population-based cohort study. Am. J. Epidemiol. 2008, 168, 932–937. [Google Scholar] [CrossRef]
- Haber, P.; Wilson, J.; Apte, M.; Korsten, M.; Pirola, R. Chronic ethanol consumption increases the fragility of rat pancreatic zymogen granules. Gut 1994, 35, 1474–1478. [Google Scholar] [CrossRef][Green Version]
- Gorelick, F.S. Alcohol and zymogen activation in the pancreatic acinar cell. Pancreas 2003, 27, 305–310. [Google Scholar] [CrossRef]
- Norton, I. P4502E1 is present in rat pancreas and is induced by chronic ethanol administration. Gastroenterology 1996, 110, A1280. [Google Scholar] [CrossRef]
- Foster, J.R.; Idle, J.R.; Hardwick, J.P.; Bars, R.; Scott, P.; Braganza, J.M. Induction of drug-metabolizing enzymes in human pancreatic cancer and chronic pancreatitis. J. Pathol. 1993, 169, 457–463. [Google Scholar] [CrossRef]
- Vonlaufen, A.; Wilson, J.S.; Pirola, R.C.; Apte, M.V. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res. Health 2007, 30, 48. [Google Scholar] [PubMed]
- Yokoyama, A.; Mizukami, T.; Matsui, T.; Yokoyama, T.; Kimura, M.; Matsushita, S.; Higuchi, S.; Maruyama, K. Genetic Polymorphisms of Alcohol Dehydrogenase-1 B and Aldehyde Dehydrogenase-2 and Liver Cirrhosis, Chronic Calcific Pancreatitis, Diabetes Mellitus, and Hypertension Among J apanese Alcoholic Men. Alcohol. Clin. Exp. Res. 2013, 37, 1391–1401. [Google Scholar] [CrossRef]
- Chiang, C.P.; Wu, C.W.; Lee, S.P.; Chung, C.C.; Wang, C.W.; Lee, S.L.; Nieh, S.; Yin, S.J. Expression pattern, ethanol-metabolizing activities, and cellular localization of alcohol and aldehyde dehydrogenases in human pancreas: Implications for pathogenesis of alcohol-induced pancreatic injury. Alcohol. Clin. Exp. Res. 2009, 33, 1059–1068. [Google Scholar] [CrossRef]
- Altomare, E.; Grattagliano, I.; Vendemiale, G.; Palmieri, V.; Palasciano, G. Acute ethanol administration induces oxidative changes in rat pancreatic tissue. Gut 1996, 38, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, T.; Yamada, S.; Hirayama, C. Nonoxidative metabolism of ethanol in the pancreas; implication in alcoholic pancreatic damage. Biochem. Pharmacol. 1990, 39, 241–245. [Google Scholar] [CrossRef]
- Laposata, E.A.; Lange, L.G. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science 1986, 231, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.S.; Apte, M.V.; Moran, C.; Applegate, T.L.; Pirola, R.C.; Korsten, M.A.; McCaughan, G.W.; Wilson, J.S. Non-oxidative metabolism of ethanol by rat pancreatic acini. Pancreatology 2004, 4, 82–89. [Google Scholar] [CrossRef]
- Klochkov, A.; Kudaravalli, P.; Sun, Y. Alcoholic pancreatitis. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kruger, B.; Albrecht, E.; Lerch, M.M. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am. J. Pathol 2000, 157, 43–50. [Google Scholar] [CrossRef]
- Gorelick, F.S.; Thrower, E. The acinar cell and early pancreatitis responses. Clin. Gastroenterol. Hepatol. 2009, 7, S10–S14. [Google Scholar] [CrossRef]
- Clemens, D.L.; Schneider, K.J.; Arkfeld, C.K.; Grode, J.R.; Wells, M.A.; Singh, S. Alcoholic pancreatitis: New insights into the pathogenesis and treatment. World J. Gastrointest. Pathophysiol. 2016, 7, 48. [Google Scholar] [CrossRef]
- Gerasimenko, J.V.; Gryshchenko, O.; Ferdek, P.E.; Stapleton, E.; Hébert, T.O.; Bychkova, S.; Peng, S.; Begg, M.; Gerasimenko, O.V.; Petersen, O.H. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl. Acad. Sci. USA 2013, 110, 13186–13191. [Google Scholar] [CrossRef]
- Criddle, D.N.; Murphy, J.; Fistetto, G.; Barrow, S.; Tepikin, A.V.; Neoptolemos, J.P.; Sutton, R.; Petersen, O.H. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology 2006, 130, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Wilson, J.S.; Korsten, M.A. Alcohol-related pancreatic damage: Mechanisms and treatment. Alcohol Health Res. World 1997, 21, 13. [Google Scholar]
- Haber, P.S.; Wilson, J.S.; Apte, M.V.; Pirola, R.C. Fatty acid ethyl esters increase rat pancreatic lysosomal fragility. J. Lab. Clin. Med. 1993, 121, 759–764. [Google Scholar] [PubMed]
- Talukdar, R.; Sareen, A.; Zhu, H.; Yuan, Z.; Dixit, A.; Cheema, H.; George, J.; Barlass, U.; Sah, R.; Garg, S.K. Release of cathepsin B in cytosol causes cell death in acute pancreatitis. Gastroenterology 2016, 151, 747–758.e5. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, H.; Lewin, M.B.; Wong, A.; Deveney, C.W.; Wendland, M.F.; Leimgruber, R.M.; Geokas, M.C. Irreversible inhibition by acetaldehyde of cholecystokinin-induced amylase secretion from isolated rat pancreatic acini. Biochem. Pharmacol. 1985, 34, 2859–2863. [Google Scholar] [CrossRef]
- Casini, A.; Galli, A.; Pignalosa, P.; Frulloni, L.; Grappone, C.; Milani, S.; Pederzoli, P.; Cavallini, G.; Surrenti, C. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J. Pathol. 2000, 192, 81–89. [Google Scholar] [CrossRef]
- Chadwick, S.R.; Lajoie, P. Endoplasmic reticulum stress coping mechanisms and lifespan regulation in health and diseases. Front. Cell Dev. Biol. 2019, 7, 84. [Google Scholar] [CrossRef]
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499. [Google Scholar] [CrossRef]
- Lugea, A.; Tischler, D.; Nguyen, J.; Gong, J.; Gukovsky, I.; French, S.W.; Gorelick, F.S.; Pandol, S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011, 140, 987–997.e8. [Google Scholar] [CrossRef]
- Pandol, S.J.; Gorelick, F.S.; Gerloff, A.; Lugea, A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig. Dis. 2010, 28, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lugea, A.; Tischler, D.; Nguyen, J.; Gong, J.; Gukovsky, I.; French, S.W.; Gorelick, F.S.; Pandol, S.J. Basic-liver, pancreas, and biliary tract. Gastroenterology 2011, 140, 987–U415. [Google Scholar] [CrossRef]
- Mareninova, O.A.; Hermann, K.; French, S.W.; O’Konski, M.S.; Pandol, S.J.; Webster, P.; Erickson, A.H.; Katunuma, N.; Gorelick, F.S.; Gukovsky, I. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J. Clin. Investig. 2009, 119, 3340–3355. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Hu, R.; Lugea, A.; Gukovsky, I.; Smoot, D.; Gukovskaya, A.S.; Pandol, S.J. Ethanol feeding alters death signaling in the pancreas. Pancreas 2006, 32, 351–359. [Google Scholar] [CrossRef]
- Apte, M.V.; Pirola, R.; Wilson, J.S. Molecular mechanisms of alcoholic pancreatitis. Dig. Dis. 2005, 23, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.; Wilson, J.; Korsten, M.; McCaughan, G.; Haber, P.; Pirola, R. Effects of ethanol and protein deficiency on pancreatic digestive and lysosomal enzymes. Gut 1995, 36, 287–293. [Google Scholar] [CrossRef]
- Ponnappa, B.C.; Hoek, J.B.; Waring, A.J.; Rubin, E. Effect of ethanol on amylase secretion and cellular calcium homeostasis in pancreatic acini from normal and ethanol-fed rats. Biochem. Pharmacol. 1987, 36, 69–79. [Google Scholar] [CrossRef]
- Siegmund, E.; Lüthen, F.; Kunert, J.; Weber, H. Ethanol modifies the actin cytoskeleton in rat pancreatic acinar cells–comparison with effects of CCK. Pancreatology 2004, 4, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.S.; Korsten, M.A.; Apte, M.V.; Thomas, M.C.; Haber, P.S.; Pirola, R.C. Both ethanol consumption and protein deficiency increase the fragility of pancreatic lysosomes. J. Lab. Clin. Med. 1990, 115, 749–755. [Google Scholar]
- Duko, B.; Ayalew, M.; Ayano, G. The prevalence of alcohol use disorders among people living with HIV/AIDS: A systematic review and meta-analysis. Subst. Abus. Treat. Prev. Policy 2019, 14, 52. [Google Scholar] [CrossRef]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Alcohol and the pancreas. Pancreapedia Exocrine Pancreas Knowl. Base 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Douglas, S.D.; Metzger, D.S.; Guo, C.J.; Li, Y.; O’Brien, C.P.; Song, L.; Davis-Vogal, A.; Ho, W.Z. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol. Clin. Exp. Res. 2002, 26, 1880–1886. [Google Scholar] [CrossRef]
- Ambade, A.; Lowe, P.; Kodys, K.; Catalano, D.; Gyongyosi, B.; Cho, Y.; Iracheta-Vellve, A.; Adejumo, A.; Saha, B.; Calenda, C. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology 2019, 69, 1105–1121. [Google Scholar] [CrossRef]
- Liu, X.; Zha, J.; Nishitani, J.; Chen, H.; Zack, J.A. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology 2003, 307, 37–44. [Google Scholar] [CrossRef][Green Version]
- Wilen, C.; Tilton, J.; Doms, R. HIV: Cell Binding and Entry; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Miyauchi, K.; Kim, Y.; Latinovic, O.; Morozov, V.; Melikyan, G.B. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 2009, 137, 433–444. [Google Scholar] [CrossRef]
- Maréchal, V.; Clavel, F.; Heard, J.M.; Schwartz, O. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 1998, 72, 2208–2212. [Google Scholar] [CrossRef]
- Cossart, P.; Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 2014, 6, a016972. [Google Scholar] [CrossRef]
- Fredericksen, B.L.; Wei, B.L.; Yao, J.; Luo, T.; Garcia, J.V. Inhibition of Endosomal/Lysosomal Degradation Increases the Infectivity of Human Immunodeficiency Virus. J. Virol. 2002, 76, 11440–11446. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; McVicker, D.L.; Zetterman, R.K.; MacDonald, R.G.; Donohue, T.M., Jr. Flow cytometric analysis of vesicular pH in rat hepatocytes after ethanol administration. Hepatology 1997, 26, 929–934. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr.; Osna, N.A. Intracellular proteolytic systems in alcohol-induced tissue injury. Alcohol Res. Health 2003, 27, 317. [Google Scholar]
- Donohue, T.M., Jr.; Osna, N.A.; Kharbanda, K.K.; Thomes, P.G. Lysosome and proteasome dysfunction in alcohol-induced liver injury. Liver Res. 2019, 3, 191–205. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Xu, Y.; Yu, X.; Xiong, T.; Du, M.; Sun, J.; Liu, L.; Tang, Y.; Yao, P. Iron-mediated lysosomal membrane permeabilization in ethanol-induced hepatic oxidative damage and apoptosis: Protective effects of quercetin. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Jajte, J.; Stetkiewicz, J.; Wronska-Nofer, T. Combined exposure to m-xylene and ethanol: Oxidative stress in the rat liver. Int. J. Occup. Med. Environ. Health 2003, 16, 345–350. [Google Scholar]
- Curry-McCoy, T.V.; Osna, N.A.; Nanji, A.A.; Donohue, T.M., Jr. Chronic ethanol consumption results in atypical liver injury in copper/zinc superoxide dismutase deficient mice. Alcohol. Clin. Exp. Res. 2010, 34, 251–261. [Google Scholar] [CrossRef]
- Doitsh, G.; Galloway, N.L.; Geng, X.; Yang, Z.; Monroe, K.M.; Zepeda, O.; Hunt, P.W.; Hatano, H.; Sowinski, S.; Muñoz-Arias, I.; et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014, 505, 509–514. [Google Scholar] [CrossRef]
- Plymale, D.R.; Tang, D.S.; Comardelle, A.M.; Fermin, C.D.; Lewis, D.E.; Garry, R.F. Both necrosis and apoptosis contribute to HIV-1-induced killing of CD4 cells. Aids 1999, 13, 1827–1839. [Google Scholar] [CrossRef]
- Bhatia, M. Apoptosis versus necrosis in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G189–G196. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “kill” into “shock and kill”: Strategies to eliminate latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Tran, T.-A.; De Herve, M.-g.D.G.; Hendel-Chavez, H.; Dembele, B.; Le Névot, E.; Abbed, K.; Pallier, C.; Goujard, C.; Gasnault, J.; Delfraissy, J.-F. Resting regulatory CD4 T cells: A site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS ONE 2008, 3, e3305. [Google Scholar] [CrossRef]
- Rukobia Fostemsavir. Available online: https://www.rukobiahcp.com/mechanism-of-action/ (accessed on 30 December 2020).
- Woollard, S.M.; Kanmogne, G.D. Maraviroc: A review of its use in HIV infection and beyond. Drug Des. Dev. Ther. 2015, 9, 5447. [Google Scholar]
- CytoDyn CytoDyn Seeks UK Approval of Leronlimab for HIV and COVID-19. Available online: https://www.cytodyn.com/newsroom/press-releases/detail/457/cytodyn-seeks-uk-approval-of-leronlimab-for-hiv-and-covid-19 (accessed on 4 December 2020).
- Pascua-Maestro, R.; Diez-Hermano, S.; Lillo, C.; Ganfornina, M.D.; Sanchez, D. Protecting cells by protecting their vulnerable lysosomes: Identification of a new mechanism for preserving lysosomal functional integrity upon oxidative stress. PLoS Genet. 2017, 13, e1006603. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Wang, J.-M.; Ouyang, J.-M.; Kuang, L. Antioxidant activities and repair effects on oxidatively damaged HK-2 cells of tea polysaccharides with different molecular weights. Oxidative Med. Cell. Longev. 2018, 2018, 5297539. [Google Scholar] [CrossRef]
- Song, S.B.; Hwang, E.S. High Levels of ROS Impair Lysosomal Acidity and Autophagy Flux in Glucose-Deprived Fibroblasts by Activating ATM and Erk Pathways. Biomolecules 2020, 10, 761. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Wang, S.; Chen, Y.; Liu, H. Regulation of TFEB activity and its potential as a therapeutic target against kidney diseases. Cell Death Discov. 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- New-Aaron, M.; Ganesan, M.; Dagur, R.S.; Kharbanda, K.K.; Poluektova, L.Y.; Osna, N.A. Obeticholic acid attenuates human immunodeficiency virus/alcohol metabolism-induced pro-fibrotic activation in liver cells. World J. Hepatol. 2020, 12, 965–975. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, L.; Bergmann, F.; Endris, V.; Strobel, O.; Büchler, M.W.; Kroemer, G.; Hackert, T.; Fortunato, F. The bile acid receptor FXR attenuates acinar cell autophagy in chronic pancreatitis. Cell Death Discov. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.; Lee, J.; Lee, K.; Jang, K.; Heo, J.; Choi, S.; Kim, Y.; Rhee, J. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br. J. Cancer 2011, 104, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. Biofactors 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef]
- Asaumi, H.; Watanabe, S.; Taguchi, M.; Tashiro, M.; Nagashio, Y.; Nomiyama, Y.; Nakamura, H.; Otsuki, M. Green tea polyphenol (–)-epigallocatechin-3-gallate inhibits ethanol-induced activation of pancreatic stellate cells. Eur. J. Clin. Investig. 2006, 36, 113–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
New-Aaron, M.; Ganesan, M.; Dagur, R.S.; Kharbanda, K.K.; Poluektova, L.Y.; Osna, N.A. Pancreatogenic Diabetes: Triggering Effects of Alcohol and HIV. Biology 2021, 10, 108. https://doi.org/10.3390/biology10020108
New-Aaron M, Ganesan M, Dagur RS, Kharbanda KK, Poluektova LY, Osna NA. Pancreatogenic Diabetes: Triggering Effects of Alcohol and HIV. Biology. 2021; 10(2):108. https://doi.org/10.3390/biology10020108
Chicago/Turabian StyleNew-Aaron, Moses, Murali Ganesan, Raghubendra Singh Dagur, Kusum K. Kharbanda, Larisa Y. Poluektova, and Natalia A. Osna. 2021. "Pancreatogenic Diabetes: Triggering Effects of Alcohol and HIV" Biology 10, no. 2: 108. https://doi.org/10.3390/biology10020108
APA StyleNew-Aaron, M., Ganesan, M., Dagur, R. S., Kharbanda, K. K., Poluektova, L. Y., & Osna, N. A. (2021). Pancreatogenic Diabetes: Triggering Effects of Alcohol and HIV. Biology, 10(2), 108. https://doi.org/10.3390/biology10020108








