Evolutionary Analysis of Cystatins of Early-Emerging Metazoans Reveals a Novel Subtype in Parasitic Cnidarians
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Mining
2.2. Verification of Selected Cystatin Sequences
2.3. Phylogenetic Analyses
2.4. Comparison of the Protein Architecture
3. Results
3.1. Cystatin Gene Repertoire and Diversity in Early-Emerging Metazoans
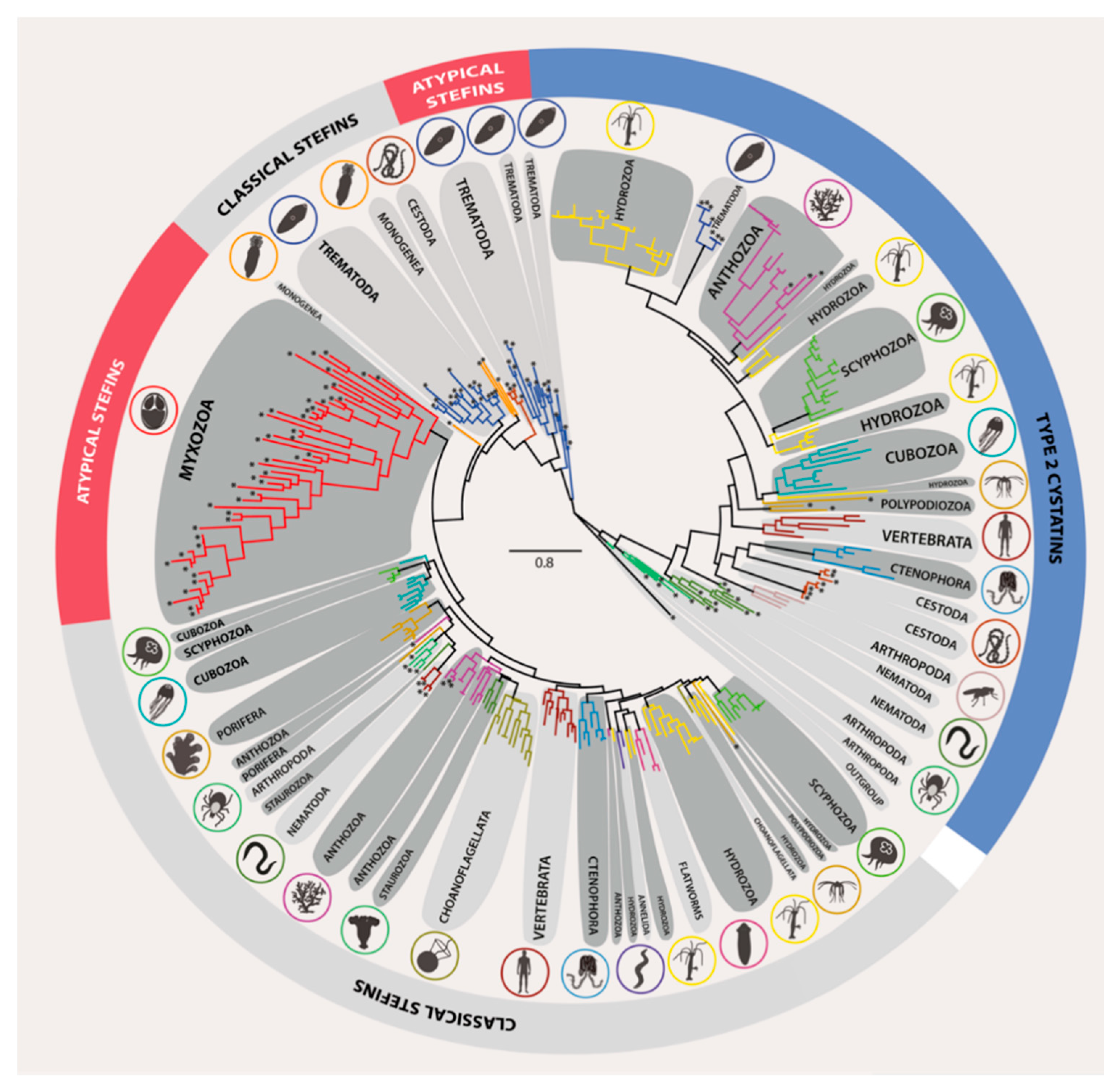
3.2. Phylogeny of Metazoan Cystatins did not Mirror Animal Phylogeny and Was Highly Diverse within Individual Groups
3.3. Structural Modifications of Cystatin Homologues in Basal Metazoans
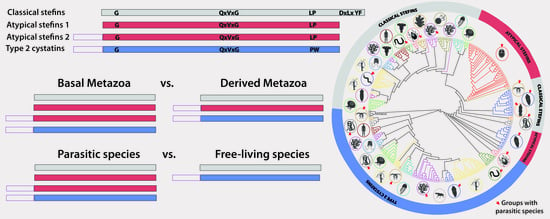
3.4. Structural and Evolutionary Comparison of Cystatins of Parasitic vs. Free-Living Groups of Basal Metazoans: The Special Case of Myxozoa
4. Discussion
4.1. A Diverse Repertoire of Cystatins in Early-Emerging Metazoans
4.2. Atypical Stefins: A Unique Type of Cystatins in Myxozoans and Some Trematodes
4.3. Obstacles to Reconstruction of Cystatin Phylogenies
4.4. Hypothetical Evolution of Metazoan Cystatins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancela, M.; Corvo, I.; da Silva, E.; Teichmann, A.; Roche, L.; Diaz, A.; Tort, J.F.; Ferreira, H.B.; Zaha, A. Functional characterization of single-domain cystatin-like cysteine proteinase inhibitors expressed by the trematode Fasciola hepatica. Parasitology 2017, 144, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Buša, M.; Matoušková, Z.; Řezáčová, P.; Bartošová-Sojková, P.; Štefanič, S.; Mareš, M. Evolutionary upgrade of stefins for secretion in parasites. In Proceedings of the 11th General Meeting of the International Proteolysis Society “Interfaces in Proteolysis”, Mariánské Lázně, Czech Republic, 29 September–4 October 2019. [Google Scholar]
- Siricoon, S.; Grams, S.V.; Grams, R. Efficient inhibition of cathepsin B by a secreted type 1 cystatin of Fasciola gigantica. Mol. Biochem. Parasit. 2012, 186, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Tarasuk, M.; Grams, S.V.; Viyanant, V.; Grams, R. Type I cystatin (stefin) is a major component of Fasciola gigantica excretion/secretion product. Mol. Biochem. Parasit. 2009, 167, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, M.; Alvarez-Fernandez, M.; Nathanson, C. Cystatins. Biochem. Soc. Symp. 2003, 70, 179–199. [Google Scholar]
- Turk, V.; Stoka, V.; Turk, D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008, 13, 5406–5420. [Google Scholar] [CrossRef] [Green Version]
- Turk, V.; Turk, D.; Dolenc, I.; Stoka, V. Characteristics, structure, and biological role of stefins (type-1 cystatins) of human, other mammals, and parasite origin. Acta Chim. Slov. 2019, 66, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Ranasinghe, S.; McManus, D. Protease inhibitors of parasitic flukes: Emerging roles in parasite survival and immune defence. Trends Parasitol. 2017, 33, 400–413. [Google Scholar] [CrossRef]
- Chmelař, J.; Kotál, J.; Langhansová, H.; Kotsyfakis, M. Protease inhibitors in tick saliva: The role of serpins and cystatins in tick-host-pathogen interaction. Front. Cell. Infect. Microbiol. 2017, 7, 216. [Google Scholar] [CrossRef]
- Gregory, W.F.; Maizels, R.M. Cystatins from filarial parasites: Evolution, adaptation and function in the host-parasite relationship. Int. J. Biochem. Cell Biol. 2008, 40, 1389–1398. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wu, L.Y.; Liu, X.C.; Wang, S.; Ehsan, M.; Yan, R.F.; Song, X.K.; Xu, L.X.; Li, X.R. Characterization of a secreted cystatin of the parasitic nematode Haemonchus contortus and its immune-modulatory effect on goat monocytes. Parasit. Vectors 2017, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- Kotál, J.; Stergiou, N.; Buša, M.; Chlastáková, A.; Beránková, Z.; Řezáčová, P.; Langhansová, H.; Schwarz, A.; Calvo, E.; Kopecký, J.; et al. The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin. Cell Mol. Life Sci. 2019, 76, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Valdés, J.J.; Kotsyfakis, M. The role of cystatins in tick physiology and blood feeding. Ticks Tick Borne Dis. 2012, 3, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, C.; Ziegler, T.; Figueiredo, A.S.; Rausch, S.; Hepworth, M.R.; Obsivac, N.; Sers, C.; Lang, R.; Hammerstein, P.; Lucius, R.; et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011, 7, e1001248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maizels, R.M.; Gomez-Escobar, N.; Gregory, W.F.; Murray, J.; Zang, X.X. Immune evasion genes from filarial nematodes. Int. J. Parasitol. 2001, 31, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Klotz, C.; Ziegler, T.; Daniłowicz-Luebert, E.; Hartmann, S. Cystatins of parasitic organisms. Adv. Exp. Med. Biol. 2011, 712, 208–221. [Google Scholar] [PubMed]
- Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Parasite cystatin: Immunomodulatory molecule with therapeutic activity against immune mediated disorders. Pathogens 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Kopitar-Jerala, N. The role of cystatins in cells of the immune system. FEBS Lett. 2006, 580, 6295–6301. [Google Scholar] [CrossRef] [Green Version]
- Salát, J.; Paesen, G.C.; Rezáčová, P.; Kotsyfakis, M.; Kovářová, Z.; Šanda, M.; Majtán, J.; Grunclová, L.; Horká, H.; Andersen, J.F.; et al. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem. J. 2010, 429, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Lucius, R. Modulation of host immune responses by nematode cystatins. Int. J. Parasitol. 2003, 33, 1291–1302. [Google Scholar] [CrossRef]
- Schierack, P.; Lucius, R.; Sonnenburg, B.; Schilling, K.; Hartmann, S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect. Immun. 2003, 71, 2422–2429. [Google Scholar] [CrossRef] [Green Version]
- Khaznadji, E.; Collins, P.; Dalton, J.P.; Bigot, Y.; Moire, N. A new multi-domain member of the cystatin superfamily expressed by Fasciola hepatica. Int. J. Parasitol. 2005, 35, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- McKerrow, J.; Engel, J.; Caffrey, C. Cysteine protease inhibitors as chemotherapy for parasitic infections. Bioorg. Med. Chem. 1999, 7, 639–644. [Google Scholar] [CrossRef]
- Tang, B.; Liu, M.; Wang, L.; Yu, S.; Shi, H.; Boireau, P.; Cozma, V.; Wu, X.; Liu, X. Characterisation of a high-frequency gene encoding a strongly antigenic cystatin-like protein from Trichinella spiralis at its early invasion stage. Parasit. Vectors 2015, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, M.; Lim, K.; Sajid, M.; McKerrow, J.; Caffrey, C. Schistosomiasis mansoni: Novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007, 4, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Alam, M.; Paik, D.; Karmakar, K.; De, T.; Chakrahorti, T. Protease inhibitors in potential drug development for leishmaniasis. Indian J. Biochem. Biol. 2013, 50, 363–376. [Google Scholar]
- Duschak, V. Targets and patented drugs for chemotherapy of Chagas disease in the last 15 years-period. Recent Pat. Antiinfect. Drug Discov. 2016, 11, 74–173. [Google Scholar] [CrossRef]
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth immunomodulation in autoimmune disease. Front. Immunol. 2017, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Breznik, B.; Mitrovic, A.; Lah, T.T.; Kos, J. Cystatins in cancer progression: More than just cathepsin inhibitors. Biochimie 2019, 166, 233–250. [Google Scholar] [CrossRef]
- Kordiš, D.; Turk, V. Phylogenomic analysis of the cystatin superfamily in eukaryotes and prokaryotes. BMC Evol. Biol. 2009, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Diaz, I. The origin and evolution of plant cystatins and their target cysteine proteinases indicate a complex functional relationship. BMC Evol. Biol. 2008, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.M.; Dziegielewska, K.M. Friends and relations of the cystatin superfamily—New members and their evolution. Protein Sci. 1997, 6, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, N.D.; Barrett, A.J. Evolution of proteins of the cystatin superfamily. J. Mol. Evol. 1990, 30, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Verdes, A.; Simpson, D.; Holford, M. Are fireworms venomous? Evidence for the convergent evolution of toxin homologs in three species of fireworms (Annelida, Amphinomidae). Genome Biol. Evol. 2018, 10, 249–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta-Astroz, Y.; Scholte, L.L.S.; Pais, F.S.M.; Oliveira, G.; Nahum, L.A. Evolutionary analysis of the cystatin family in three Schistosoma species. Front. Genet. 2014, 5, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, A.J. Comparative analysis of cystatin superfamily in Platyhelminths. PLoS ONE 2015, 10, e0124683. [Google Scholar] [CrossRef] [Green Version]
- Ilgová, J.; Jedličková, L.; Dvořaková, H.; Benovics, M.; Mikeš, L.; Janda, L.; Vorel, J.; Roudnický, P.; Potěšil, D.; Zdráhal, Z.; et al. A novel type I cystatin of parasite origin with atypical legumain-binding domain. Sci. Rep. 2017, 7, 17526. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.W.; Xu, J.; Song, H.Y.; Liu, Y.C.; Wu, M.D.; Zhang, H.J.; Jing, B.; Lai, W.M.; Gu, X.B.; Xie, Y.; et al. Molecular characterization of a Dirofilaria immitis cysteine protease inhibitor (cystatin) and its possible role in filarial immune evasion. Genes 2019, 10, 300. [Google Scholar] [CrossRef] [Green Version]
- Santamaria, M.E.; Hernandez-Crespo, P.; Ortego, F.; Grbic, V.; Grbic, M.; Diaz, I.; Martinez, M. Cysteine peptidases and their inhibitors in Tetranychus urticae: A comparative genomic approach. BMC Genom. 2012, 13, 307. [Google Scholar] [CrossRef] [Green Version]
- Rangel, C.K.; Parizi, L.F.; Sabadin, G.A.; Costa, E.P.; Romeiro, N.C.; Isezaki, M.; Githaka, N.W.; Seixas, A.; Logullo, C.; Konnai, S.; et al. Molecular and structural characterization of novel cystatins from the taiga tick Ixodes persulcatus. Ticks Tick Borne Dis. 2017, 8, 432–441. [Google Scholar] [CrossRef]
- Ibelli, A.M.G.; Hermance, M.M.; Kim, T.K.; Gonzalez, C.L.; Mulenga, A. Bioinformatics and expression analyses of the Ixodes scapularis tick cystatin family. Exp. Appl. Acarol. 2013, 60, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.; Martinez, M. Insights into the molecular evolution of peptidase inhibitors in arthropods. PLoS ONE 2017, 12, e0187643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, S.B.; Kuris, A.M. Independent origins of parasitism in Animalia. Biol. Lett. 2016, 12, 20160324. [Google Scholar] [CrossRef] [PubMed]
- Dnyansagar, R.; Zimmermann, B.; Moran, Y.; Praher, D.; Sundberg, P.; Moller, L.; Technau, U. Dispersal and speciation: The cross Atlantic relationship of two parasitic cnidarians. Mol. Phylogenet. Evol. 2018, 126, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Stefanik, D.J.; Lubinski, T.J.; Granger, B.R.; Byrd, A.L.; Reitzel, A.M.; DeFilippo, L.; Lorenc, A.; Finnerty, J.R. Production of a reference transcriptome and transcriptomic database (EdwardsiellaBase) for the lined sea anemone, Edwardsiella lineata, a parasitic cnidarian. BMC Genom. 2014, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Shpirer, E.; Chang, E.S.; Diamant, A.; Rubinstein, N.; Cartwright, P.; Huchon, D. Diversity and evolution of myxozoan minicollagens and nematogalectins. BMC Evol. Biol. 2014, 14, 205. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.S.; Neuhof, M.; Rubinstein, N.D.; Diamant, A.; Philippe, H.; Huchon, D.; Cartwright, P. Genomic insights into the evolutionary origin of Myxozoa within Cnidaria. Proc. Natl. Acad. Sci. USA 2015, 112, 14912–14917. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Abd-Elfattah, A.; El-Matbouli, M. Identification of differentially expressed genes of brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) in response to Tetracapsuloides bryosalmonae (Myxozoa). Parasitol. Res. 2015, 114, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Guri, E.; Philippe, H.; Okamura, B.; Holland, P.W.H. Buddenbrockia is a cnidarian worm. Science 2007, 317, 116–118. [Google Scholar] [CrossRef]
- Alama-Bermejo, G.; Meyer, E.; Atkinson, S.D.; Holzer, A.S.; Wiśniewska, M.M.; Kolísko, M.; Bartholomew, J.L. Transcriptome-wide comparisons and virulence gene polymorphisms of host-associated genotypes of the cnidarian parasite Ceratonova shasta in salmonids. Genome Biol. Evol. 2020, 12, 1258–1276. [Google Scholar] [CrossRef]
- Nesnidal, M.; Helmkampf, M.; Bruchhaus, I.; El-Matbouli, M.; Hausdorf, B. Agent of whirling disease meets orphan worm: Phylogenomic analyses firmly place Myxozoa in Cnidaria. PLoS ONE 2013, 8, e54576. [Google Scholar] [CrossRef] [Green Version]
- Foox, J.; Ringuette, M.; Desser, S.S.; Siddall, M.E. In silico hybridization enables transcriptomic illumination of the nature and evolution of Myxozoa. BMC Genom. 2015, 16, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.L.; Xiong, J.; Zhou, Z.G.; Huo, F.M.; Miao, W.; Ran, C.; Liu, Y.C.; Zhang, J.Y.; Feng, J.M.; Wang, M.; et al. The genome of the myxosporean Thelohanellus kitauei shows adaptations to nutrient acquisition within its fish host. Genome Biol. Evol. 2014, 6, 3182–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartigan, A.; Kosakyan, A.; Pecková, H.; Eszterbauer, E.; Holzer, A.S. Transcriptome of Sphaerospora molnari (Cnidaria, Myxosporea) blood stages provides proteolytic arsenal as potential therapeutic targets against sphaerosporosis in common carp. BMC Genom. 2020, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.M.; Lindner, A.; Raikova, E.V.; Collins, A.G.; Cartwright, P. Phylogenetic placement of the enigmatic parasite, Polypodium hydriforme, within the Phylum Cnidaria. BMC Evol. Biol. 2008, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, B.; Gruhl, A. Myxozoan affinities and route to endoparasitism. In Myxozoan Evolution, Ecology and Development; Okamura, B., Gruhl, A., Bartholomew, J.L., Eds.; Springer: Cham, Switzerland, 2015; pp. 23–44. [Google Scholar]
- Fiala, I.; Bartošová-Sojková, P.; Okamura, B.; Hartikainen, H. Adaptive radiation and evolution within the Myxozoa. In Myxozoan Evolution, Ecology and Development; Okamura, B., Gruhl, A., Bartholomew, J.L., Eds.; Springer: Cham, Switzerland, 2015; pp. 69–84. [Google Scholar]
- Feng, J.M.; Xiong, J.; Zhang, J.Y.; Yang, Y.L.; Yao, B.; Zhou, Z.G.; Miao, W. New phylogenomic and comparative analyses provide corroborating evidence that Myxozoa is Cnidaria. Mol. Phylogenet. Evol. 2014, 81, 10–18. [Google Scholar] [CrossRef]
- Stubbs, M.; Laber, B.; Bode, W.; Huber, R.; Jerala, R.; Lenarcic, B.; Turk, V. The refined 2.4A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: A novel type of proteinase inhibitor interaction. EMBO J. 1990, 9, 1939–1947. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Alan, J.; Thomas, P.D.; Huang, X.D.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceroni, A.; Passerini, A.; Vullo, A.; Frasconi, P. DISULFIND: A disulfide bonding state and cysteine connectivity prediction server. Nucleic Acids Res. 2006, 34, W177–W181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.F.; Satoh, N. A likely ancient genome duplication in the speciose reef-building coral genus, Acropora. Iscience 2019, 13, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamm, K.; Osigus, H.J.; Stadler, P.F.; DeSalle, R.; Schierwater, B. Trichoplax genomes reveal profound admixture and suggest stable wild populations without bisexual reproduction. Sci. Rep. 2018, 8, 11168. [Google Scholar] [CrossRef] [PubMed]
- Eitel, M.; Francis, W.; Varoqueaux, F.; Daraspe, J.; Osigus, H.; Krebs, S.; Vargas, S.; Blum, H.; Williams, G.; Schierwater, B.; et al. Comparative genomics and the nature of placozoan species. PLoS Biol. 2018, 16, e3000032. [Google Scholar] [CrossRef]
- Srivastava, M.; Begovic, E.; Chapman, J.; Putnam, N.; Hellsten, U.; Kawashima, T.; Kuo, A.; Mitros, T.; Salamov, A.; Carpenter, M.; et al. The Trichoplax genome and the nature of placozoans. Nature 2008, 454, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Lenarcic, B.; Ritonja, A.; Strukelj, B.; Turk, B.; Turk, V. Equistatin, a new inhibitor of cysteine proteinases from Actinia equina, is structurally related to thyroglobulin type-1 domain. J. Biol. Chem. 1997, 272, 13899–13903. [Google Scholar] [CrossRef] [Green Version]
- Irving, J.A.; Pike, R.N.; Dai, W.W.; Bromme, D.; Worrall, D.M.; Silverman, G.A.; Coetzer, T.H.T.; Dennison, C.; Bottomley, S.P.; Whisstock, J.C. Evidence that serpin architecture intrinsically supports papain-like cysteine protease inhibition: Engineering alpha(1)-antitrypsin to inhibit cathepsin proteases. Biochemistry 2002, 41, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Eszterbauer, E.; Sipos, D.; Kaján, G.L.; Szegö, D.; Fiala, I.; Holzer, A.S.; Bartošová-Sojková, P. Genetic diversity of serine protease inhibitors in myxozoan (Cnidaria, Myxozoa) fish parasites. Microorganisms 2020, 8, 1502. [Google Scholar] [CrossRef] [PubMed]
- Geadkaew, A.; Kosa, N.; Siricoon, S.; Grams, S.V.; Grams, R. A 170 kDa multi-domain cystatin of Fasciola gigantica is active in the male reproductive system. Mol. Biochem. Parasit. 2014, 196, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pol, E.; Bjork, I. Importance of the second binding loop and the C-terminal end of cystatin B (stefin B) for inhibition of cysteine proteinases. Biochemistry 1999, 38, 10519–10526. [Google Scholar] [CrossRef] [PubMed]
- Jerala, R.; Kroon-Zitko, L.; Kopitar, M.; Popovic, T.; Turk, V. Deletion of the carboxy terminal part of stefin B does not have a major effect for binding to papain. Biomed. Biochim. Acta 1991, 50, 627–629. [Google Scholar] [PubMed]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasit. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Vendelova, E.; de Lima, J.C.; Lorenzatto, K.R.; Monteiro, K.M.; Mueller, T.; Veepaschit, J.; Grimm, C.; Brehm, K.; Hrčková, G.; Lutz, M.B.; et al. Proteomic analysis of excretory-secretory products of Mesocestoides corti metacestodes reveals potential suppressors of dendritic cell functions. PLoS Negl. Trop. Dis. 2016, 10, e0005061. [Google Scholar] [CrossRef] [Green Version]
- Di Maggio, L.S.; Tirloni, L.; Pinto, A.F.M.; Diedrich, J.K.; Yates, J.R.; Benavides, U.; Carmona, C.; Vaz, I.D.; Berasain, P. Across intra-mammalian stages of the liver fluke Fasciola hepatica: A proteomic study. Sci. Rep. 2016, 6, 32796. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.M.; Lee, K.H.; Sohn, W.M.; Na, B.K. Identification and functional characterization of CsStefin-1, a cysteine protease inhibitor of Clonorchis sinensis. Mol. Biochem. Parasit. 2011, 177, 126–134. [Google Scholar] [CrossRef]
- Cantacessi, C.; Mulvenna, J.; Young, N.D.; Kasny, M.; Horak, P.; Aziz, A.; Hofmann, A.; Loukas, A.; Gasser, R.B. A deep exploration of the transcriptome and “excretory/secretory” proteome of adult Fascioloides magna. Mol. Cell. Proteomics 2012, 11, 1340–1353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Yang, Y.; Gao, C.; Yao, Y.; Xia, R.; Hu, J.; Ran, C.; Zhang, Z.; Zhou, Z. Bioinformatics analysis and characterization of a secretory cystatin from Thelohanellus kitauei. AMB Express 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Korytář, T.; Wiegertjes, G.F.; Zusková, E.; Tomanová, A.; Lisnerová, M.; Patra, S.; Sieranski, V.; Šíma, R.; Born-Torrijos, A.; Wentzel, A.S.; et al. The kinetics of cellular and humoral immune responses of common carp to presporogonic development of the myxozoan Sphaerospora molnari. Parasit. Vectors 2019, 12, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjork, S.J.; Zhang, Y.A.; Hurst, C.N.; Alonso-Naveiro, M.E.; Alexander, J.D.; Sunyer, J.O.; Bartholomew, J.L. Defenses of susceptible and resistant Chinook salmon (Onchorhynchus tshawytscha) against the myxozoan parasite Ceratomyxa shasta. Fish Shellfish Immun. 2014, 37, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.; Strepparava, N.; Wahli, T.; Segner, H. Exploring the immune response, tolerance and resistance in proliferative kidney disease of salmonids. Dev. Comp. Immunol. 2019, 90, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, M.C.; Estensoro, I.; Calduch-Giner, J.A.; del Pozo, R.; Picard-Sánchez, A.; Perez-Sánchez, J.; Sitjà-Bobadilla, A. Hints on T cell responses in a fish-parasite model: Enteromyxum leei induces differential expression of T cell signature molecules depending on the organ and the infection status. Parasit. Vectors 2018, 11, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cordón, G.; Estensoro, I.; Benedito-Palos, L.; Calduch-Giner, J.A.; Sitjà-Bobadilla, A.; Pérez-Sanchez, J. Interleukin gene expression is strongly modulated at the local level in a fish-parasite model. Fish Shellfish Immun. 2014, 37, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Gorgoglione, B.; Wang, T.; Secombes, C.J.; Holland, J.W. Immune gene expression profiling of proliferative kidney disease in rainbow trout Oncorhynchus mykiss reveals a dominance of anti-inflammatory, antibody and Th cell-like activities. Fish Shellfish Immun. 2013, 34, 1654. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossoy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Sitjà-Bobadilla, A.; Schmidt-Posthaus, H.; Wahli, T.; Holland, J.W.; Secombes, C.J. Fish immune responses to Myxozoa. In Myxozoan Evolution, Ecology and Development; Okamura, B., Gruhl, A., Bartholomew, J.L., Eds.; Springer: Cham, Switzerland, 2015; pp. 253–280. [Google Scholar]
- Rogers, R.L.; Hartl, D.L. Chimeric genes as a source of rapid evolution in Drosophila melanogaster. Mol. Biol. Evol. 2012, 29, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, R.L.; Bedford, T.; Hartl, D.L. Formation and longevity of chimeric and duplicate genes in Drosophila melanogaster. Genetics 2009, 181, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eszterbauer, E.; Atkinson, S.; Diamant, A.; Morris, D.J.; El-Matbouli, M.; Hartikainen, H. Myxozoan life cycles: Practical approaches and insights. In Myxozoan Evolution, Ecology and Development; Okamura, B., Gruhl, A., Bartholomew, J.L., Eds.; Springer: Cham, Switzerland, 2015; pp. 175–198. [Google Scholar]
- Holzer, A.S.; Bartošová-Sojková, P.; Born-Torrijos, A.; Lövy, A.; Hartigan, A.; Fiala, I. The joint evolution of the Myxozoa and their alternate hosts: A cnidarian recipe for success and vast biodiversity. Mol. Ecol. 2018, 27, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Melillo, D.; Marino, R.; Italiani, P.; Boraschi, D. Innate immune memory in invertebrate metazoans: A critical appraisal. Front. Immunol. 2018, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.P. Salivary (SD-type) cystatins: Over one billion years in the making-but to what purpose? Crit. Rev. Oral Biol. Med. 2002, 13, 485–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartošová-Sojková, P.; Kyslík, J.; Alama-Bermejo, G.; Hartigan, A.; Atkinson, S.D.; Bartholomew, J.L.; Picard-Sánchez, A.; Palenzuela, O.; Faber, M.N.; Holland, J.W.; et al. Evolutionary Analysis of Cystatins of Early-Emerging Metazoans Reveals a Novel Subtype in Parasitic Cnidarians. Biology 2021, 10, 110. https://doi.org/10.3390/biology10020110
Bartošová-Sojková P, Kyslík J, Alama-Bermejo G, Hartigan A, Atkinson SD, Bartholomew JL, Picard-Sánchez A, Palenzuela O, Faber MN, Holland JW, et al. Evolutionary Analysis of Cystatins of Early-Emerging Metazoans Reveals a Novel Subtype in Parasitic Cnidarians. Biology. 2021; 10(2):110. https://doi.org/10.3390/biology10020110
Chicago/Turabian StyleBartošová-Sojková, Pavla, Jiří Kyslík, Gema Alama-Bermejo, Ashlie Hartigan, Stephen D. Atkinson, Jerri L. Bartholomew, Amparo Picard-Sánchez, Oswaldo Palenzuela, Marc Nicolas Faber, Jason W. Holland, and et al. 2021. "Evolutionary Analysis of Cystatins of Early-Emerging Metazoans Reveals a Novel Subtype in Parasitic Cnidarians" Biology 10, no. 2: 110. https://doi.org/10.3390/biology10020110
APA StyleBartošová-Sojková, P., Kyslík, J., Alama-Bermejo, G., Hartigan, A., Atkinson, S. D., Bartholomew, J. L., Picard-Sánchez, A., Palenzuela, O., Faber, M. N., Holland, J. W., & Holzer, A. S. (2021). Evolutionary Analysis of Cystatins of Early-Emerging Metazoans Reveals a Novel Subtype in Parasitic Cnidarians. Biology, 10(2), 110. https://doi.org/10.3390/biology10020110