Targeted Dorsal Dentate Gyrus or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Irradiation Procedure
2.3. Morris Water Maze: Set-Up and Training Procedure
2.4. 5-Bromo-2’-Deoxyuridine (BrdU) Administration
2.5. Brain Sample and Tissue Section
2.6. Immunohistofluorescence
2.7. Cell Quantification
2.8. Statistical Analysis
3. Results
3.1. Postnatal Irradiation Did not Affect Learning Performances, Only Long–Term Spatial Retrieval after DDG Exposure at 1 Gy
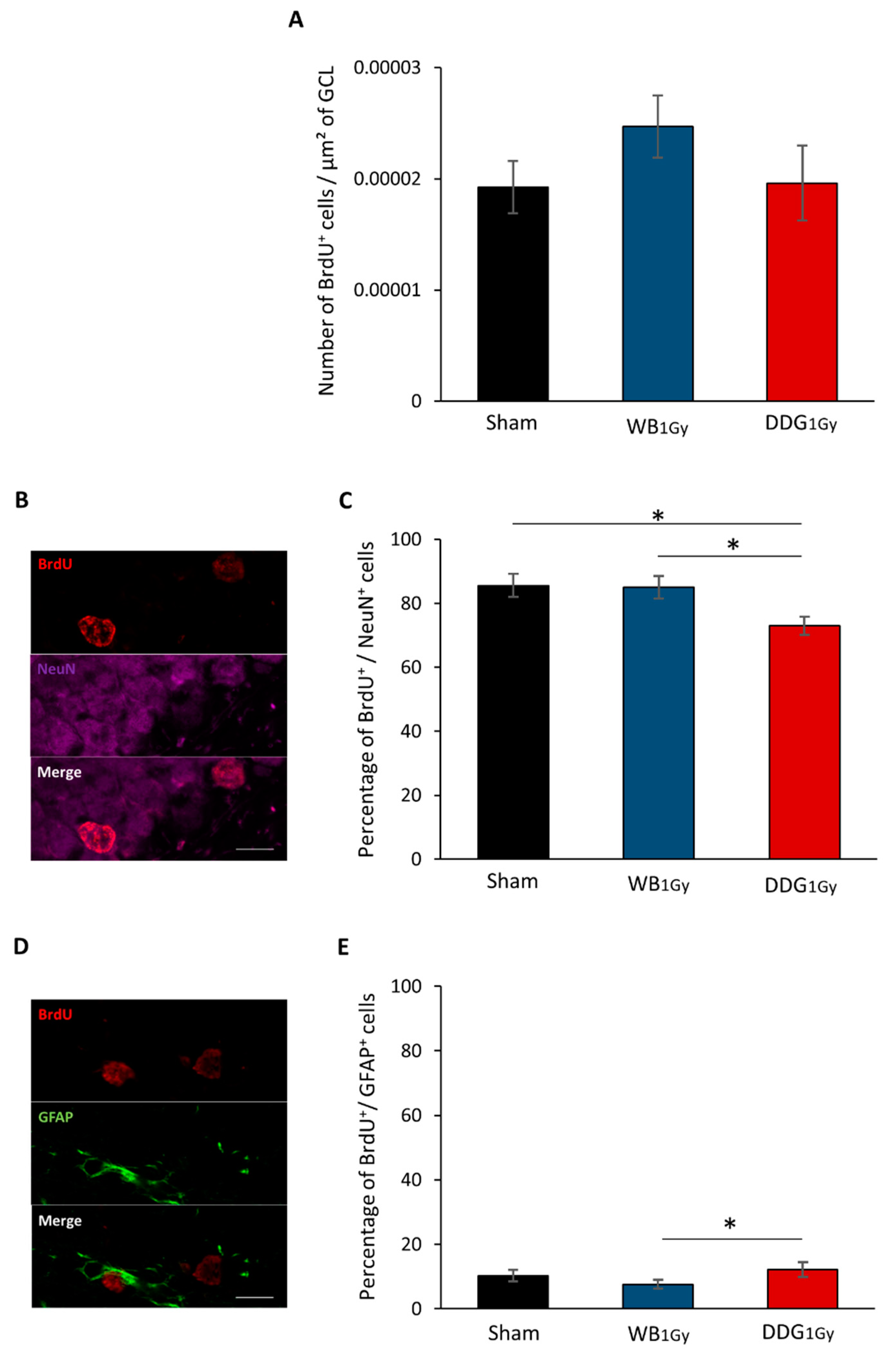
3.2. Postnatal Irradiation at 1 Gy Impacted Adult Dentate Gyrus Cell Proliferation Differently in the Two Models
3.3. Postnatal Irradiation at 1 Gy Impacted Differentiation Only after DDG Targeted Exposure
4. Discussion
4.1. Long-Term Spatial Memory Is Not Impacted in Adult Mice Whole Brain Postnatally Exposed to Ionizing Irradiation
4.2. Dose and Brain Irradiated Volume Effects on Long-Term Spatial Memory in Adult Mice Postnatally Exposed to Ionizing Radiation: A Complex Response
4.3. One-Gray Postnatal Irradiation of the DDG/Whole Brain Leads to Contrasting Disturbances of Adult Hippocampal Neurogenesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouladi, M.; Gilger, E. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J. Clin. Oncol. 2005, 23, 7152–7160. [Google Scholar] [CrossRef]
- Palmer, S.L.; Reddick, W.E. Understanding the cognitive impact on children who are treated for medulloblastoma. J. Pediatr. Psychol. 2007, 32, 1040–1049. [Google Scholar] [CrossRef]
- Gibson, E.; Monje, M. Effect of cancer therapy on neural stem cells: Implications for cognitive function. Curr. Opin. Oncol. 2012, 24, 672–678. [Google Scholar] [CrossRef]
- Pasqual, E.; Bosch de Basea, M. Neurodevelopmental effects of low dose ionizing radiation exposure: A systematic review of the epidemiological evidence. Environ. Int. 2020, 136, 105371. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Journy, N. Organ dose estimation accounting for uncertainty for pediatric and young adult ct scans in the united kingdom. Radiat. Prot. Dosim. 2018, 184, 44–53. [Google Scholar] [CrossRef]
- Salonen, E.; Nyman, H. Cognitive function following head CT in childhood: A randomized controlled follow-up trial. Acta Radiol. 2018, 59, 221–228. [Google Scholar] [CrossRef]
- Hall, P.; Adami, H.O. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ 2004, 328, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomstrand, M.; Holmberg, E. No clinically relevant effect on cognitive outcomes after low-dose radiation to the infant brain: A population-based cohort study in Sweden. Acta Oncol. 2014, 53, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.J.; Hall, E.J. Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Pearce, M.S.; Salotti, J.A. Socio-economic variation in CT scanning in Northern England, 1990–2002. BMC Health Serv. Res. 2012, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Mathews, J.D.; Forsythe, A.V. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokora, R.; Krille, L. Computed Tomography in Germany. Dtsch. Arztebl. Int. 2016, 113, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sienkiewicz, Z.J.; Saunders, R.D. Prenatal irradiation and spatial memory in mice: Investigation of critical period. Int. J. Radiat. Biol. 1992, 62, 211–219. [Google Scholar] [CrossRef]
- Buratovic, S.; Stenerlöw, B. Neonatal exposure to a moderate dose of ionizing radiation causes behavioural defects and altered levels of tau protein in mice. Neurotoxicology 2014, 45, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kempf, S.J.; Casciati, A. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol. Neurodegener. 2014, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, P.; Buratovic, S. Neonatal exposure to whole body ionizing radiation induces adult neurobehavioural defects: Critical period, dose--response effects and strain and sex comparison. Behav. Brain Res. 2016, 304, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.; Kereselidze, D. Development of whole brain versus targeted dentate gyrus irradiation model to explain low to moderate doses of exposure effects in mice. Sci. Rep. 2018, 8, 17262. [Google Scholar] [CrossRef]
- Casciati, A.; Dobos, K. Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget 2016, 7, 28040–28058. [Google Scholar] [CrossRef] [Green Version]
- Strategic Research Agenda of the Multidisciplinary European Low Dose Initiative (MELODI)—2019. Available online: http://www.melodi-online.eu/doc/MELODI%20SRA%202019%20%20post-consultation%20draft%20FINAL2.pdf (accessed on 30 December 2020).
- Strange, B.; Witter, M. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Jalali, R.; Mallick, I. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 974–979. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Jain, N. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010, 12, 1173–1186. [Google Scholar] [CrossRef]
- Blomstrand, M.; Brodin, N.P. Estimated clinical benefit of protecting, in the developing brain during radiation therapy for pediatric medulloblastoma. Neuro Oncol. 2012, 14, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Mahone, E.M. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: A prospective study. Neuro Oncol. 2013, 15, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G.; Song, H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect Biol. 2015, 7, a018812. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.T.; Schafer, S.T. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, E.; Wen, Z. Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb. Perspect. Biol. 2016, 8, a019026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberwirth, C.; Pan, Y. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016, 1644, 127–140. [Google Scholar] [CrossRef]
- Pons-Espinal, M.; Martinez de Lagran, M. Functional implications of hippocampal adult neurogenesis in intellectual disabilities. Amino Acids 2013, 45, 113–131. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 0, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, P.; Ankarberg, E.; Fredriksson, A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Res. 2000, 853, 41–48. [Google Scholar] [CrossRef]
- Trouche, S.; Bontempi, B. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc. Natl. Acad. Sci. USA 2009, 106, 5919–5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veyrac, A.; Gros, A. Zif268/egr1 gene controls the selection, maturation and functional integration of adult hippocampal newborn neurons by learning. Proc. Natl. Acad. Sci. USA 2013, 110, 7062–7067. [Google Scholar] [CrossRef] [Green Version]
- Castillon, C.; Gonzalez, L. The intellectual disability PAK3 R67C mutation impacts cognitive functions and adult hippocampal neurogenesis. Hum. Mol. Genet. 2020, 29, 1950–1968. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.; Linard, C. IL-1β, TNFα and IL-6 induction in the rat brain after partial-body irradiation: Role of vagal afferents. Int. J. Radiat. Biol. 2003, 79, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Haridas, S. Early cognitive changes due to whole body gamma-irradiation: A behavioral and diffusion tensor imaging study in mice. Exp. Neurol. 2013, 8, 248–360. [Google Scholar]
- Kazda, T.; Jancalek, R. Why and how to spare the hippocampus during brain radiotherapy: The developing role of hippocampal avoidance in cranial radiotherapy. Radiat. Oncol. 2014, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.D.; Hein, A.M. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running. Brain Behav. Immun. 2012, 2, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Woodbury, M.E.; Freilich, R.W. miR-155 Is Essential for Inflammation-Induced Hippocampal Neurogenic Dysfunction. J. Neurosci. 2015, 35, 9764–9781. [Google Scholar] [CrossRef] [Green Version]
- Monje, M.L.; Mizumatcho, S.; Fike, J.R.; Palmer, T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002, 8, 955–962. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Sándor, N.; Walter, F.R. Low Dose Cranial Irradiation-Induced Cerebrovascular Damage Is Reversible in Mice. PLoS ONE 2014, 9, e112397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, T.; Trouche, S. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience 2010, 171, 769–778. [Google Scholar] [CrossRef]
- Castillon, C.; Lunion, S. Selective alteration of adult hippocampal neurogenesis and impaired spatial pattern separation performance in the RSK2-deficient mouse model of Coffin-Lowry syndrome. Neurobiol. Dis. 2018, 115, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 2013, 7, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Dose (Gy) | Group | n | Distance (Block Effect) |
|---|---|---|---|
| 0 Gy | Sham | 16 | F(8,120) = 11.17 |
| p < 0.0001 | |||
| 0.25 Gy | WB | 9 | F(8,64) = 8.68 |
| p < 0.0001 | |||
| DDG | 9 | F(8,64) = 10.70 | |
| p < 0.0001 | |||
| 0.5 Gy | WB | 8 | F(8,56) = 6.79 |
| p < 0.0001 | |||
| DDG | 11 | F(8,80) = 3.30 | |
| p < 0.0001 | |||
| 1 Gy | WB | 12 | F(8,88) = 11.50 |
| p < 0.0001 | |||
| DDG | 11 | F(8,80) = 6.48 | |
| p < 0.0001 | |||
| 2 Gy | WB | 9 | F(8,64) = 4.98 |
| p < 0.0001 | |||
| DDG | 8 | F(8,56) = 5.48 | |
| p < 0.0001 |
| Models | Block | Dose | Block X Dose Interaction |
|---|---|---|---|
| WB | F(8,392) = 35.288 | F(4,49) = 0.172 | F(32,392) = 0.956 |
| p < 0.0001 | p = 0.952, ns | p = 0.809, ns | |
| DDG | F(8,400) = 29.087 | F(4,50) = 0.738 | F(32,400) = 1.114 |
| p < 0.0001 | p = 0.570, ns | p = 0.465, ns |
| Dose | Block | Model | Block X Model Interaction |
|---|---|---|---|
| 0.25 Gy | F(8,248) = 25.061 | F(2,31) = 0.107 | F(16,248) = 1.311 |
| p < 0.0001 | p = 0.899, ns | p = 0.191, ns | |
| 0.5 Gy | F(8,256) = 17.564 | F(2,32) = 0.448 | F(16,256) = 0.892 |
| p < 0.0001 | p = 0.643, ns | p = 0.579, ns | |
| 1 Gy | F(8,288) = 26.148 | F(2,36) = 0.590 | F(16,288) = 0.487 |
| p < 0.0001 | p = 0.560, ns | p = 0.953, ns | |
| 2 Gy | F(8,240) = 15.471 | F(2,30) = 0.596 | F(16,240) = 1.217 |
| p < 0.0001 | p = 0.557, ns | p = 0.3825, ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, C.; Dos Santos, M.; Kereselidze, D.; Beugnies, L.; Lestaevel, P.; Poirier, R.; Durand, C. Targeted Dorsal Dentate Gyrus or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis. Biology 2021, 10, 192. https://doi.org/10.3390/biology10030192
Serrano C, Dos Santos M, Kereselidze D, Beugnies L, Lestaevel P, Poirier R, Durand C. Targeted Dorsal Dentate Gyrus or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis. Biology. 2021; 10(3):192. https://doi.org/10.3390/biology10030192
Chicago/Turabian StyleSerrano, Céline, Morgane Dos Santos, Dimitri Kereselidze, Louison Beugnies, Philippe Lestaevel, Roseline Poirier, and Christelle Durand. 2021. "Targeted Dorsal Dentate Gyrus or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis" Biology 10, no. 3: 192. https://doi.org/10.3390/biology10030192
APA StyleSerrano, C., Dos Santos, M., Kereselidze, D., Beugnies, L., Lestaevel, P., Poirier, R., & Durand, C. (2021). Targeted Dorsal Dentate Gyrus or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis. Biology, 10(3), 192. https://doi.org/10.3390/biology10030192






