Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Reactive Oxygen Species Related to Male Infertility
2.1. Endogenous Sources of ROS
2.1.1. Age
2.1.2. Diseases
Obesity
Diabetes
Cancer
Varicocele
2.2. Exogenous Sources of ROS
2.2.1. Infections
2.2.2. Pollution
2.2.3. Radiation
2.3. Measuring ROS
3. Reproductive Consequences of ROS and Oxidative Stress
3.1. Sperm Cells
3.2. In-Vitro Fertilization (IVF) / Intracytoplasmic Sperm Injection (ICSI) Outcomes
4. Antioxidants
4.1. Physiological Enzymatic Factors
4.1.1. Superoxide Dismutase (SOD)
4.1.2. Catalase (CAT)
4.1.3. Glutathione Peroxidase (GPX)
4.2. Non-Enzymatic Factors
4.2.1. Q-10 Coenzyme (CoQ, CoQ10)
4.2.2. Carnitines
4.2.3. Lycopene
4.3. Micronutrients
4.3.1. Vitamins
Vitamin C
Vitamin E
Vitamin B9 (Folic Acid)
4.3.2. Minerals
Zinc
Selenium
4.4. Others
4.4.1. N-Acetyl-Cysteine (NAC)
4.4.2. Melatonin
4.4.3. Alpha-Lipoic-Acid (ALA)
4.4.4. ω-3 Fatty Acids
| Antioxidant & Doses Relating to Male Fertility | Article | Specie | Level of Evidence | Dose & Duration | Main Conclusions | Gaps in the Evidence |
|---|---|---|---|---|---|---|
| CoQ10 [112] RDD: N/A RSD: 200–300 mg MDD: 12 mg/kg | [135] | Human | Review and Meta-analysis | N/A | CoQ10 supplementation improved sperm motility and concentration. | RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [139] | Human | Review and Meta-analysis | N/A | CoQ10 is positively associated with sperm motility. | ||
| [136] | Human | RCT | 200 mg/day for 24 weeks | CoQ10 supplementation improved sperm motility. | ||
| [138] | Human | RCT | 200 mg/day for 12 weeks | CoQ10 supplementation improved TAC concentrations and decreased MDA levels. | ||
| [139] | Human | Clinical trial (no control group) | 300 mg/day fro 26 weeks | CoQ10 supplementation improved sperm concentration and motility. | ||
| Carnitines [112] RDD: N/A RSD: 3000 mg MDD: 3000 mg | [146] | Human | RCT | 25 mg/day for 3 months | Carnitines supplementation improved sperm count and motility. | RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [147] | Human | Observational | N/A | Higher seminal carnitines are positively associated with higher sperm counts, motility and morphology. | ||
| [148] | Human | Observational | N/A | Higher seminal carnitines are positively associated with higher sperm count and motility. | ||
| Lycopene [251] RDD: Unknown RSD 4-20 mg MDD: Unknown | [153] | Human | Clinical trial (no control group) | 10 mg/twice a day for 3 months | Lycopene supplementation increased seminal Omega3. | RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [158] | Human | RCT | 25 mg/day for 12 weeks | Lycopene supplementation improved sperm count, concentration, motility; and higher TAC. | ||
| [159] | Human | RCT | 10 mg/twice a day for 12 weeks | Lycopene supplementation decreases seminal oxidative stress. | ||
| Vitamin C [112] RDD: 90 mg RSD:200–1000 mg MDD: 2000 mg | [163] | Human | Review | N/A | Vitamin C is linked to decrease in agglutination and DNA damage parameters. | Higher grade evidence, such as a meta-analysis, RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [166] | Human | RCT | 1.0 g/day for 60 days | Vitamin C supplementation improved semen agglutination and increased viability. | ||
| [167] | Human | RCT | 1000 mg of vitamin C were given every other day for 6 months | Vitamin C supplementation improved sperm concentration and motility. | ||
| [168] | Human | Observational | N/A | Vitamin C intake levels is positively associated with higher fertilization rates | ||
| Vitamin E [112] RDD: 15 mg RSD: 300–600 mg MDD: 1000 mg | [172] | Human and others | Review | N/A | Vitamin E in humans plays a crucial role in the modulation of telomerase activity. | Higher grade evidence, such as a meta-analysis, RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [173] | Albino Wistar Rats | RCT | 100 mg/kg /day | Vitamin E supplementation improved sperm motility in nicotine exposed, stress induced rats and rats exposed to both nicotine and stress. | ||
| [252] | Albino Wistar Rats | RCT | 500 mg/kg, 3 times a week for 2 weeks | Histological damage to the testes caused by aluminum was diminished by vitamin E supplementation. | ||
| [174] | Human | Clinical trial (no control group) | 200mg/day for 3 months | Vitamin E supplementation decreased MDA levels and increased fertilization rates. | ||
| [9] | Human | RCT | 600 mg/d for 3 months | Vitamin E supplementation improved sperm cells morphology in-vitro, during the zona binding assay. | ||
| [10] | Human | RCT | 100mg/3 times a day for 6 months or until pregnancy | Vitamin E supplementation decrease MDA levels and improved sperm motility. | ||
| Vitamin B9 [251] RDD: 400 mcg RSD: 400 mcg MDD: 1000 mcg | [182] | Human | RCT | 5mg/day for 26 weeks | Vitamin B9 and zinc supplementation improved sperm count. | RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [183] | Human | Systematic Review and Meta-analysis | N/A | Vitamin B9 is positively associated with higher sperm concentration in infertile men. | ||
| [8] | Human | Systematic Review and Meta-analysis | N/A | Vitamin B9 is positively associated with sperm morphology. | ||
| Zinc [112] RDD: 11 mg RSD: 30–40 mg MDD: 40 mg | [8] | Human | Systematic Review and Meta-analysis | N/A | Zinc supplementation was positively associated with improvements in sperm chromatin integrity index, sperm concentration, motility, membrane integrity, fertilizing capacity, conception, and pregnancy. | Comparative studies determining the best dosage-effect in zinc supplementation. |
| [93] | Human | Systematic Review | N/A | Zinc concentration is significantly higher in fertile men. | ||
| [196] | Human | RCT | 250 mg/twice a day for 3 months | Zinc supplementation improved sperm count, motility, fertilizing and reduction in the incidence of antisperm antibodies. | ||
| [193] | Human | RCT | 220 mg/day for 16 weeks | Zinc supplementation improved sperm chromatin integrity. | ||
| [188] | Human | Systematic Review and Meta-analysis | N/A | Higher mean seminal Zinc levels are found in fertile men. Zinc supplementation is positively associated with semen volume, sperm motility and the percentage of normal sperm morphology. | ||
| [195] | Human | Review | N/A | Zinc is positively associated with lower ROS production in smokers. | ||
| [194] | Human | Observational Study | N/A | Higher seminal Zinc is positively associated with sperm count and morphology. | ||
| Selenium [112] RDD: 55 mcg RSDl: 100 mcg MDD: 400 mcg | [199] | Human | RCT | 200 μg /day for 3 months | Selenium supplementation improved TAC and sperm motility. | Higher grade evidence, such as a meta-analysis, RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [202] | Human | RCT | 200 μg /day for 26 weeks | Selenium supplementation improved sperm concentration, motility, and morphology. | ||
| [201] | Human | RCT | 100 mg/day for 3 months | Selenium supplementation improved sperm count and motility. | ||
| [206] | Human | Observational | N/A | Higher seminal selenium values are positively associated with sperm count and motility. | ||
| [204] | Human | Observational | N/A | Higher selenium intake is positively associated with sperm motility. | ||
| [205] | Human | Observational | N/A | Seminal selenium is positively associated with sperm concentration and total sperm count. | ||
| [207] | Human | Observational | N/A | Seminal selenium is positively associated with pregnancy and live birth. | ||
| NAC [112] RDD: N/A RSD: 600 mg MDD: N/A | [8] | Human | Systematic Review and Meta-analysis | 600 mg/day for 6 months | NAC supplementation improved semen volume, sperm count and concentration, sperm motility, and morphology. | RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [210] | Albino Wistar Rat | RCT | Single dose of 20 mg/kg NAC intravenous | NAC administration improved MDA levels in a postreperfusion testicular injury. | ||
| [212] | Human | Observational | N/A | NAC incubation reduces the apoptotic rate by 68% compared to controls with no NAC. | ||
| [213] | Human | RCT | 600 mg/day for 3 months | NAC supplementation improved sperm volume, motility, and viscosity, as well as TAC. | ||
| [214] | Human | RCT | 600 mg/day for 3 months | NAC supplementation improved sperm morphology, DNA fragmentation and protamine deficiency. TAC significantly increased and MDA levels decreased under this supplementation. | ||
| [215] | Human | Observational | N/A | NAC incubation of sperm cells is positively associated with a decrease in ROS production. | ||
| [216] | Human | RCT | 600 mg/day for 3 months | NAC supplementation affects NRF2 expression and therefore decrease in ROS. | ||
| [217] | Human & Albino Wistar Rat | Systematic Review | N/A | NAC supplementation improved DNA fragmentation indices and ROS production. | ||
| [219] | Goat (Capra hircus) | Observational | N/A | Sperm NAC incubation resulted in positively associated with a decrease of testicular cell apoptosis. | ||
| Melatonin RDD: Unknown RSD: Unknown MDD: Unknown | [221] | Human | Observational | N/A | Sperm melatonin incubation is positively associated with less DNA damage, and MDA levels; and higher sperm viability. | RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes, and studies without involving alterations in the circadian rhythm. |
| [223] | Human | Observational | N/A | Mean seminal plasma melatonin levels are higher in fertile men, with higher sperm motility than infertile individuals. | ||
| [224] | Human | Observational | N/A | Lower melatonin serum and seminal levels are present in men with oligoasthenoteratozoospermia compared to controls. Melatonin is positively associated with sperm motility. | ||
| [228] | Human | Observational | N/A | Sperm melatonin incubation is positively associated with higher sperm cell viability. | ||
| [227] | Human | Observational | N/A | Sperm melatonin incubation is positively associated with sperm motility and less static cells. | ||
| Alpha lipoic acid [232] RDD: Unknow RSD: 600 mg MDD: Unknown | [232] | Human | RCT | 600 mg/day for 80 days | ALA improved sperm motility and progressive motility, and less DNA damage. | Higher grade evidence, such as a meta-analysis, RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [234] | Human, rats and boars | Systematic Review | NA | ALA incubation in boars is associated with higher sperm motility, less DNA damage. ALA supplementation in humans is associated with a higher TAC. ALA supplementation in diabetic rats caused increased sperm concentration and motility compared to not supplemented diabetic rats. | ||
| [235] | Human | RCT | 600 mg/day for 12 weeks | ALA supplementation improved sperm count and concentration, higher TAC and lower MDA. | ||
| [236] | Human | Observational | N/A | Sperm incubation with 0.2 mM of ALA increased sperm viability and decreased DNA damage. | ||
| [237] | Human | Observational | N/A | Sperm incubation with 0.2 and 0.5 mM of ALA improved the motility, viability and morphology of frozen-thawed specimens. | ||
| Omega3 [251] RDD: Unknown RSD: 200 mg DHA MDD: Unknown | [243] | Human | Systematic Review | N/A | Omega-3 has a positive effect on semen quality markers in semen of infertile men. | Higher grade evidence, such as a meta-analysis, RCTs with larger sample size, DNA fragmentation consequences, and ART outcomes |
| [246] | Human | RCT | 1.8 g/day for 32 weeks | Omega3 supplements improved sperm concentration, motility and normal morphology. | ||
| [248] | Human | Observational | N/A | Omega3 (fish oil) supplements are positively associated with higher semen volume, total sperm count, testis size. | ||
| [249] | Human | Systematic Review and Meta-analysis | NA | Omega3 supplements improved sperm concentration and sperm motility. | ||
| [250] | Human | RCT | 500 mg/ 3 times a day for 10 weeks | Omega3 supplements improved TAC concentrations and reduced DNA fragmentation |
5. Antioxidant Paradox
6. Future Directions: Foods and Dietary Patterns in Male Infertility
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AHEI | Alternative Healthy Eating Index |
| ALA | Alpha-lipoic-acid |
| AMED | Alternate Mediterranean Diet Score |
| ART | Assisted Reproduction Techniques |
| CAT | Catalase |
| CoQ | Coenzyme Q/CoQ10 |
| DASH | Dietary Approaches to Stop Hypertension |
| ETC | Electron Transfer Chain |
| GPX | Gluthathione Peroxidase |
| H2O2 | Hydrogen Peroxide |
| HEI | Healthy Eating Index |
| ICSI | Intracytoplasmic Spersm Injection |
| IUI | Intrauterine insemination |
| IVF | In-vitro fertilization |
| MDA | Malondialdehyde |
| miRNA | microRNA |
| NAC | N-Acetyl-Cysteine |
| O2- | Superoxide |
| OFA | Omega Fatty Acids |
| OH | Hydroxyl radical |
| RCT | Randomized Control Trial |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutases |
| TAC | Total Antioxidant Capacity |
| TOS | Total Oxidation Status |
References
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Mehra, B.L.; Skandhan, K.P.; Prasad, B.S.; Pawankumar, G.; Singh, G.; Jaya, V. Male Infertility Rate: A Retrospective Study. Urol. J. 2018, 85, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology Guidelines on Male Infertility: The 2012 Update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J. The Rôle of Oxygen in the Metabolism and Motility of Human Spermatozoa. Am. J. Physiol. Leg. Content 1943, 138, 512–518. [Google Scholar] [CrossRef]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for Male Subfertility. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Kessopoulou, E.; Powers, H.J.; Sharma, K.K.; Pearson, M.J.; Russell, J.M.; Cooke, I.D.; Barratt, C.L.R. A Double-Blind Randomized Placebo Cross-over Controlled Trial Using the Antioxidant Vitamin E to Treat Reactive Oxygen Species Associated Male Infertility*†. Fertil. Steril. 1995, 64, 825–831. [Google Scholar] [CrossRef]
- Suleiman, S.A.; Ali, M.E.; Zaki, Z.M.; el-Malik, E.M.; Nasr, M.A. Lipid Peroxidation and Human Sperm Motility: Protective Role of Vitamin E. J. Androl. 1996, 17, 530–537. [Google Scholar] [PubMed]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary Patterns, Foods and Nutrients in Male Fertility Parameters and Fecundability: A Systematic Review of Observational Studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Rafie, N.; Mansourian, M.; Miraghajani, M.; Hajianfar, H. Dietary Patterns and Semen Quality: A Systematic Review and Meta-Analysis of Observational Studies. Andrology 2018, 6, 20–28. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Role of Reactive Oxygen Species in Male Infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free Radicals: Their Beneficial and Detrimental Effects on Sperm Function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar] [PubMed]
- Alahmar, A. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4. [Google Scholar] [CrossRef]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive Oxygen Species (ROS) in Human Semen: Determination of a Reference Range. J. Assist. Reprod. Genet. 2015, 32, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Florence, T.M. The Production of Hydroxyl Radical from Hydrogen Peroxide. J. Inorg. Biochem. 1984, 22, 221–230. [Google Scholar] [CrossRef]
- McKee, T.; McKee, J.R.; González de Buitrago, J.M. Metabolismo aerobio II: Transporte electrónico y fosforilación oxidativa. In Bioquímica: La base Molecular de la Vida; McGraw-Hill Interamericana: Madrid, Spain, 2005; Volume Chapter 10, ISBN 978-84-486-0524-7. [Google Scholar]
- Young, I.S. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Camello-Almaraz, C.; Gomez-Pinilla, P.J.; Pozo, M.J.; Camello, P.J. Mitochondrial Reactive Oxygen Species and Ca Signaling. Am. J. Physiol. Cell Physiol. 2006, 291, C1082–C1088. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory Mitochondrial Efficiency and DNA Oxidation in Human Sperm after In Vitro Myo-Inositol Treatment. JCM 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.J. Mother’s Age and Daughter’s Fecundity. An Epidemiological Analysis of Late 19th to Early 20th Century Family Reconstitutions. Int. J. Epidemiol. 2002, 31, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.B.; Kelsey, T.W. Human Ovarian Reserve from Conception to the Menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B. The Association of Age and Semen Quality in Healthy Men. Hum. Reprod. 2003, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent Age-Dependent Declines in Human Semen Quality: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)Ever after: Aging in the Context of Oxidative Stress, Proteostasis Loss and Cellular Senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Eskenazi, B.; Young, S.; Arnheim, N.; Tiemann-Boege, I.; Jabs, E.W.; Glaser, R.L.; Pearson, F.S.; Evenson, D. Advancing Age Has Differential Effects on DNA Damage, Chromatin Integrity, Gene Mutations, and Aneuploidies in Sperm. Proc. Natl. Acad. Sci. USA 2006, 103, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, F.; Dai, S.; Zhang, N.; Zhao, W.; Bai, R.; Sun, Y. Sperm Telomere Length Is Positively Associated with the Quality of Early Embryonic Development. Hum. Reprod. 2015, 30, 1876–1881. [Google Scholar] [CrossRef]
- Rocca, M.S.; Speltra, E.; Menegazzo, M.; Garolla, A.; Foresta, C.; Ferlin, A. Sperm Telomere Length as a Parameter of Sperm Quality in Normozoospermic Men. Hum. Reprod. 2016, 31, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Sabanegh, E.; Kim, T.; Agarwal, A. Free Radical Theory of Aging: Implications in Male Infertility. Urology 2010, 75, 14–19. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Obesity and Overweight. Available online: https://www.webcitation.org/71yhwREPC (accessed on 20 February 2021).
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, Male Infertility, and the Sperm Epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Guo, L.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-Related DNA Methylation at Imprinted Genes in Human Sperm: Results from the TIEGER Study. Clin. Epigenet. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Maghsoumi-Norouzabad, L.; James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Becerra-Tomás, N.; Javid, A.Z.; Abed, R.; Torres, P.J.; et al. Male Adiposity, Sperm Parameters and Reproductive Hormones: An Updated Systematic Review and Collaborative Meta-analysis. Obes. Rev. 2020, 22, e13082. [Google Scholar] [CrossRef]
- Kort, H.I. Impact of Body Mass Index Values on Sperm Quantity and Quality. J. Androl. 2006, 27, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Rybar, R.; Kopecka, V.; Prinosilova, P.; Markova, P.; Rubes, J. Male Obesity and Age in Relationship to Semen Parameters and Sperm Chromatin Integrity: Male Obesity, Age and Sperm Quality. Andrologia 2011, 43, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Bakos, H.W.; Tremellen, K. Impact of Body Mass Index on Seminal Oxidative Stress: Seminal Oxidative Stress and Body Mass Index. Andrologia 2011, 43, 121–128. [Google Scholar] [CrossRef]
- Setayesh, T.; Nersesyan, A.; Mišík, M.; Ferk, F.; Langie, S.; Andrade, V.M.; Haslberger, A.; Knasmüller, S. Impact of Obesity and Overweight on DNA Stability: Few Facts and Many Hypotheses. Mutat. Res. Rev. Mutat. Res. 2018, 777, 64–91. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.J.; Clifton, P.M.; Noakes, M.; Fenech, M. Weight Loss in Obese Men Is Associated with Increased Telomere Length and Decreased Abasic Sites in Rectal Mucosa. Rejuvenation Res. 2009, 12, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.; Caple, F.; Spiers, A.; Burtle, B.; Daly, A.K.; Williams, E.A.; Hesketh, J.E.; Mathers, J.C. Inter-Individual Variation in Nucleotide Excision Repair in Young Adults: Effects of Age, Adiposity, Micronutrient Supplementation and Genotype. BJN 2009, 101, 1316. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Diabetes. Available online: https://www.webcitation.org/70RGtswSF (accessed on 20 February 2021).
- Imani, M.; Talebi, A.R.; Fesahat, F.; Rahiminia, T.; Seifati, S.M.; Dehghanpour, F. Sperm Parameters, DNA Integrity, and Protamine Expression in Patients with Type II Diabetes Mellitus. J. Obstet. Gynaecol. 2020, 1–8. [Google Scholar] [CrossRef]
- Baccetti, B. Insulin-Dependent Diabetes in Men Is Associated with Hypothalamo-Pituitary Derangement and with Impairment in Semen Quality. Hum. Reprod. 2002, 17, 2673–2677. [Google Scholar] [CrossRef]
- Tavares, R.S.; Escada-Rebelo, S.; Sousa, M.I.; Silva, A.; Ramalho-Santos, J.; Amaral, S. Can Antidiabetic Drugs Improve Male Reproductive (Dys)Function Associated with Diabetes? CMC 2019, 26, 4191–4222. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, E.L.; Albanna, G.; Frolova, A.I.; Moley, K.H. Insulin Rescues Impaired Spermatogenesis via the Hypothalamic-Pituitary-Gonadal Axis in Akita Diabetic Mice and Restores Male Fertility. Diabetes 2012, 61, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Malini, T.; Rengarajan, S.; Balasubramanian, K. Impact of Experimental Diabetes and Insulin Replacement on Epididymal Secretory Products and Sperm Maturation in Albino Rats. J. Cell. Biochem. 2009, 108, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yamamoto, T.; Abé, S.I. IGF-I, IGF-II and Insulin Promote Differentiation of Spermatogonia to Primary Spermatocytes in Organ Culture of Newt Testes. Int. J. Dev. Biol. 1999, 43, 343–347. [Google Scholar] [PubMed]
- Lampiao, F.; du Plessis, S.S. Insulin and Leptin Enhance Human Sperm Motility, Acrosome Reaction and Nitric Oxide Production. Asian J. Androl. 2008, 10, 799–807. [Google Scholar] [CrossRef]
- Andy, P.; Alberti, L.; Melo, M.; Almeida, L. Relation between Diabetes Mellitus and Male Fertility. Einstein 2009, 7, 407–410. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. Ca A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Cancer. Available online: https://www.webcitation.org/72FLbCSZa (accessed on 20 February 2021).
- Dawane, J.S. Understanding Redox Homeostasis and Its Role in Cancer. JCDR 2012, 6, 1796. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.H. Superoxide and Hydrogen Peroxide in Relation to Mammalian Cell Proliferation. Free Radic. Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- del Pilar SosaIdelchik, M.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS Control of Cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef]
- Azizi, F.; Ghafouri-Fard, S. Outer Dense Fiber Proteins: Bridging between Male Infertility and Cancer. Arch. Iran. Med. 2017, 20, 320–325. [Google Scholar]
- Ribas-Maynou, J.; Yeste, M. Oxidative Stress in Male Infertility: Causes, Effects in Assisted Reproductive Techniques, and Protective Support of Antioxidants. Biology 2020, 9, 77. [Google Scholar] [CrossRef]
- Santana, V.P.; James, E.R.; Miranda-Furtado, C.L.; de Souza, M.F.; Pompeu, C.P.; Esteves, S.C.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; dos Reis, R.M. Differential DNA Methylation Pattern and Sperm Quality in Men with Varicocele. Fertil. Steril. 2020, 114, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Santana, V.P.; Miranda-Furtado, C.L.; Pedroso, D.C.C.; Eiras, M.C.; Vasconcelos, M.A.C.; Ramos, E.S.; Calado, R.T.; Ferriani, R.A.; Esteves, S.C.; dos Reis, R.M. The Relationship among Sperm Global DNA Methylation, Telomere Length, and DNA Fragmentation in Varicocele: A Cross-Sectional Study of 20 Cases. Syst. Biol. Reprod. Med. 2019, 65, 95–104. [Google Scholar] [CrossRef]
- Pastuszak, A.; Wang, R. Varicocele and Testicular Function. Asian J. Androl. 2015, 17, 659. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C. Oxidative Stress and Role of Antioxidants in Normal and Abnormal Sperm Function. Front. Biosci. 1996, 1, e78–e86. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Morriss, A.; Khairy, M.; Khalaf, Y.; Braude, P.; Coomarasamy, A.; El-Toukhy, T. A Systematic Review of the Effect of Oral Antioxidants on Male Infertility. Reprod. Biomed. Online 2010, 20, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Han, R.; Wu, H.; Han, D. Viral Threat to Male Fertility. Andrologia 2018, 50, e13140. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of Oxidative Stress, Infection and Inflammation in Male Infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef] [PubMed]
- Jeršovienė, V.; Gudlevičienė, Ž.; Rimienė, J.; Butkauskas, D. Human Papillomavirus and Infertility. Medicina 2019, 55, 377. [Google Scholar] [CrossRef] [PubMed]
- Lundy, S.D.; Vij, S.C.; Rezk, A.H.; Cohen, J.A.; Bajic, P.; Ramasamy, R. The Microbiome of the Infertile Male. Curr. Opin. Urol. 2020, 30, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, F.; Zhang, M.; Lan, L.; Qiao, Z.; Cui, Y.; An, J.; Wang, N.; Fan, Z.; Zhao, X.; et al. Association between Air Pollution and Sperm Quality: A Systematic Review and Meta-Analysis. Environ. Pollut. 2016, 208, 663–669. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Leiser, C.L.; Hanson, H.A.; Sawyer, K.; Steenblik, J.; Al-Dulaimi, R.; Madsen, T.; Gibbins, K.; Hotaling, J.M.; Ibrahim, Y.O.; VanDerslice, J.A.; et al. Acute Effects of Air Pollutants on Spontaneous Pregnancy Loss: A Case-Crossover Study. Fertil. Steril. 2019, 111, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does Air Pollution Play a Role in Infertility?: A Systematic Review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef]
- Adams, J.A.; Galloway, T.S.; Mondal, D.; Esteves, S.C.; Mathews, F. Effect of Mobile Telephones on Sperm Quality: A Systematic Review and Meta-Analysis. Environ. Int. 2014, 70, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Deepinder, F.; Sharma, R.K.; Ranga, G.; Li, J. Effect of Cell Phone Usage on Semen Analysis in Men Attending Infertility Clinic: An Observational Study. Fertil. Steril. 2008, 89, 124–128. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile Phone Radiation Induces Reactive Oxygen Species Production and DNA Damage in Human Spermatozoa In Vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A. Sperm Function Is Affected by the Electromagnetic Radiation Emitted by Mobile Phone. Afr. J. Microbiol. Res. 2011, 5, 4896–4900. [Google Scholar] [CrossRef]
- Biedka, M.; Kuźba-Kryszak, T.; Nowikiewicz, T.; Żyromska, A. Fertility Impairment in Radiotherapy. Wo 2016, 3, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Klosky, J.L.; Simmons, J.L.; Russell, K.M.; Foster, R.H.; Sabbatini, G.M.; Canavera, K.E.; Hodges, J.R.; Schover, L.R.; McDermott, M.J. Fertility as a Priority among At-Risk Adolescent Males Newly Diagnosed with Cancer and Their Parents. Support Care Cancer 2015, 23, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulos, I.; Dimou, P.; Anagnostou, I.; Zeginiadou, T. Impact of Cancer and Cancer Treatment on Male Fertility. HJ 2015. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Capaldo, A.; Stendardi, A.; Cappelli, V.; Cianci, A.; La Marca, A.; Luddi, A.; De Leo, V. Protein Modification as Oxidative Stress Marker in Follicular Fluid from Women with Polycystic Ovary Syndrome: The Effect of Inositol and Metformin. J. Assist. Reprod. Genet. 2014, 31, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Moretti, E.; Micheli, L.; Menchiari, A.; Moltoni, L.; Cerretani, D. Semen Characteristics and Malondialdehyde Levels in Men with Different Reproductive Problems. Andrology 2015, 3, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Total antioxidant capacity. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; Volume 37, pp. 219–292. ISBN 978-0-12-010337-9. [Google Scholar]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Powanda, P.; Robaire, B. Oxidative Stress and Reproductive Function in the Aging Male. Biology 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.E.; Al Hasani, S.; Rosenbaum, P.; Schmidt, W.; Fischer Hammadeh, C. Reactive Oxygen Species, Total Antioxidant Concentration of Seminal Plasma and Their Effect on Sperm Parameters and Outcome of IVF/ICSI Patients. Arch. Gynecol. Obs. 2008, 277, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.D.; Welch, G.R. Effects of Reactive Oxygen Species on Sperm Function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Diagnostic Value of Routine Semen Analysis in Clinical Andrology. Andrologia 2021, 53, e13614. [Google Scholar] [CrossRef] [PubMed]
- Opuwari, C.S.; Henkel, R.R. An Update on Oxidative Damage to Spermatozoa and Oocytes. Biomed. Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Allamaneni, S.S.R.; Nallella, K.P.; George, A.T.; Mascha, E. Correlation of Reactive Oxygen Species Levels with the Fertilization Rate after in Vitro Fertilization: A Qualified Meta-Analysis. Fertil. Steril. 2005, 84, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive Analysis of Sperm DNA Fragmentation by Five Different Assays: TUNEL Assay, SCSA, SCD Test and Alkaline and Neutral Comet Assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic Review of Antioxidant Types and Doses in Male Infertility: Benefits on Semen Parameters, Advanced Sperm Function, Assisted Reproduction and Live-Birth Rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Frapsauce, C.; Pionneau, C.; Bouley, J.; de Larouzière, V.; Berthaut, I.; Ravel, C.; Antoine, J.-M.; Soubrier, F.; Mandelbaum, J. Infertilité masculine chez les patients normospermiques: Analyse protéomique des spermes normaux non fécondants en fécondation in vitro classique. Gynécologie Obs. Fertil. 2009, 37, 796–802. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.I.; James, E.R.; Salas-Huetos, A. Clinical Implications of Sperm DNA Damage in IVF and ICSI: Updated Systematic Review and Meta-analysis. Biol. Rev. 2021, e12700. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Ahlering, P.; Rodriguez, H.; Levy, S.; Sutovsky, P. Sperm Chromatin Structure Correlates with Spontaneous Abortion and Multiple Pregnancy Rates in Assisted Reproduction. Reprod. Biomed. Online 2011, 22, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Atig, F.; Kerkeni, A.; Saad, A.; Ajina, M. Effects of Reduced Seminal Enzymatic Antioxidants on Sperm DNA Fragmentation and Semen Quality of Tunisian Infertile Men. J. Assist. Reprod. Genet. 2017, 34, 373–381. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, S.S.; Makker, K.; Desai, N.R.; Agarwal, A. Impact of Oxidative Stress on IVF. Expert Rev. Obstet. Gynecol. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Lampiao, F. Free Radicals Generation in an in Vitro Fertilization Setting and How to Minimize Them. WJOG 2012, 1, 29. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA Damage Caused by Oxidative Stress: Modifiable Clinical, Lifestyle and Nutritional Factors in Male Infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-C.; Lin, Y.-C.; Chang, Y.-C.; Lin, H.-J.; Tsai, Y.-R.; Kang, H.-Y. Limited Relationships between Reactive Oxygen Species Levels in Culture Media and Zygote and Embryo Development. J. Assist. Reprod. Genet. 2019, 36, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Sabanegh, E.S.; Agarwal, A. Male Infertility Testing: Reactive Oxygen Species and Antioxidant Capacity. Fertil. Steril. 2014, 102, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Kidd, S.A.; Marks, A.R.; Sloter, E.; Block, G.; Wyrobek, A.J. Antioxidant Intake Is Associated with Semen Quality in Healthy Men. Hum. Reprod. 2005, 20, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Sgrò, P.; Salacone, P.; Paoli, D.; Gilio, B.; Lombardo, F.; Santulli, M.; Agarwal, A.; Gandini, L. A Placebo-Controlled Double-Blind Randomized Trial of the Use of Combined l-Carnitine and l-Acetyl-Carnitine Treatment in Men with Asthenozoospermia. Fertil. Steril. 2004, 81, 1578–1584. [Google Scholar] [CrossRef]
- Saylam, B.; Çayan, S. Do Antioxidants Improve Serum Sex Hormones and Total Motile Sperm Count in Idiopathic Infertile Men? Turk. J. Urol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA 2020, 323, 35. [Google Scholar] [CrossRef]
- Aghajani, M.M.R.; Mahjoub, S.; Mojab, F.; Namdari, M.; Gorji, N.M.; Dashtaki, A.; Mirabi, P. Comparison of the Effect of Ceratonia Siliqua L. (Carob) Syrup and Vitamin E on Sperm Parameters, Oxidative Stress Index, and Sex Hormones in Infertile Men: A Randomized Controlled Trial. Reprod. Sci. 2021, 28, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An Overview on Role of Some Trace Elements in Human Reproductive Health, Sperm Function and Fertilization Process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and Nutritional Factors in Male (In)Fertility—Underestimated Factors. JCM 2020, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; Barbagallo, F.; Calogero, A.E.; Cannarella, R.; Crafa, A.; La Vignera, S. D-Chiro-Inositol Improves Sperm Mitochondrial Membrane Potential: In Vitro Evidence. JCM 2020, 9, 1373. [Google Scholar] [CrossRef]
- Peeker, R. Superoxide Dismutase Isoenzymes in Human Seminal Plasma and Spermatozoa. Mol. Hum. Reprod. 1997, 3, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Holguín, E.; Lledó-García, E.; Rebollo-Román, Á.; González-García, J.; Jara-Rascón, J.; Hernández-Fernández, C. Antioxidants to Improve Sperm Quality. In Male and Sperm Factors that Maximize IVF Success; Aitken, J., Mortimer, D., Kovacs, G., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 106–120. ISBN 978-1-108-76257-1. [Google Scholar]
- Younus, H. Therapeutic Potentials of Superoxide Dismutase. Int. J. Health Sci. (Qassim) 2018, 12, 88–93. [Google Scholar]
- Kakimoto, K.; Kojima, Y.; Ishii, K.; Onoue, K.; Maeda, H. The Suppressive Effect of Gelatin-Conjugated Superoxide Dismutase on Disease Development and Severity of Collagen-Induced Arthritis in Mice. Clin. Exp. Immunol. 2008, 94, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, Y.; Zhang, G.; Tang, F.; Zhang, J.; Cao, J.; Liu, C. Targeted Elimination of Intracellular Reactive Oxygen Species Using Nanoparticle-like Chitosan- Superoxide Dismutase Conjugate for Treatment of Monoiodoacetate-Induced Osteoarthritis. Int. J. Pharm. 2020, 590, 119947. [Google Scholar] [CrossRef] [PubMed]
- Mansuroğlu, B.; Derman, S.; Yaba, A.; Kızılbey, K. Protective Effect of Chemically Modified SOD on Lipid Peroxidation and Antioxidant Status in Diabetic Rats. Int. J. Biol. Macromol. 2015, 72, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-W.; Shen, C.-J.; Tung, Y.-T.; Chen, H.-L.; Chen, Y.-H.; Chang, W.-H.; Cheng, K.-C.; Yang, S.-H.; Chen, C.-M. Extracellular Superoxide Dismutase Ameliorates Streptozotocin-Induced Rat Diabetic Nephropathy via Inhibiting the ROS/ERK1/2 Signaling. Life Sci. 2015, 135, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase Activity in Human Spermatozoa and Seminal Plasma. Gamete Res. 1989, 24, 185–196. [Google Scholar] [CrossRef]
- Medan, M.S.; Absy, G.; Zeidan, A.E.; Khalil, M.H.; Khalifa, H.H.; Abdel-Salaam, A.M.; Abdel-Khalek, T.M. Survival and Fertility Rate of Cooled Dromedary Camel Spermatozoa Supplemented with Catalase Enzyme. J. Reprod. Dev. 2008, 54, 84–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yano, S.; Yano, N. Regulation of Catalase Enzyme Activity by Cell Signaling Molecules. Mol. Cell. Biochem. 2002, 240, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ruottinen, M.; Kaaronen, V.; Saimanen, I.; Kuosmanen, V.; Kärkkäinen, J.; Selander, T.; Aspinen, S.; Eskelinen, M. The Induction of Antioxidant Catalase Enzyme With Decrease of Plasma Malonidialdehyde: An Important Reactive Oxidative Species Inhibiting Mechanism. Anticancer Res. 2020, 40, 5701–5706. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Takahara, S.; Hamilton, H.B.; Neel, J.V.; Kobara, T.Y.; Ogura, Y.; Nishimura, E.T. HYPOCATALASEMIA: A NEW GENETIC CARRIER STATE*. J. Clin. Investig. 1960, 39, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.R.; Mirault, M.-E.; Moret, R.; Zbinden, I.; Cerutti, P.A. Molecular Defect in Human Acatalasia Fibroblasts. Biochem. Biophys. Res. Commun. 1988, 153, 59–66. [Google Scholar] [CrossRef]
- Kósa, Z.; Fejes, Z.; Nagy, T.; Csordás, M.; Simics, E.; Remenyik, É.; Góth, L. Catalase −262C>T Polymorphisms in Hungarian Vitiligo Patients and in Controls: Further Acatalasemia Mutations in Hungary. Mol. Biol. Rep. 2012, 39, 4787–4795. [Google Scholar] [CrossRef]
- Meseguer, M.; Antonio Martinez-Conejero, J.; Muriel, L.; Pellicer, A.; Remohi, J.; Garrido, N. The Human Sperm Glutathione System: A Key Role in Male Fertility and Successful Cryopreservation. DML 2007, 1, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Makarova, N.P.; Romanov, Y.A.; Dolgushina, N.V.; Parker, M.M.; Krasnyi, A.M. Comparative Analysis of the Expression of Glutathione Peroxidase and Glutathione Reductase Genes in Human Sperm after Cryopreservation. Bull. Exp. Biol. Med. 2018, 165, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Vicente-Carrillo, A.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione Peroxidase 5 Is Expressed by the Entire Pig Male Genital Tract and Once in the Seminal Plasma Contributes to Sperm Survival and In Vivo Fertility. PLoS ONE 2016, 11, e0162958. [Google Scholar] [CrossRef]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male Fertility Is Linked to the Selenoprotein Phospholipid Hydroperoxide Glutathione Peroxidase1. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of Reactive Oxygen Species in Male Infertility: An Updated Review of Literature. Arab J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Mantle, D. Coenzyme Q10 Supplementation in Fibrosis and Aging. In Reviews on Biomarker Studies in Aging and Anti-Aging Research; Guest, P.C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Germany, 2019; Volume 1178, pp. 103–112. ISBN 978-3-030-25649-4. [Google Scholar]
- Tiseo, B.C.; Gaskins, A.J.; Hauser, R.; Chavarro, J.E.; Tanrikut, C. Coenzyme Q10 Intake From Food and Semen Parameters in a Subfertile Population. Urology 2017, 102, 100–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The Effect of Coenzyme Q 10 on Morbidity and Mortality in Chronic Heart Failure. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Žmitek, K.; Pogačnik, T.; Mervic, L.; Žmitek, J.; Pravst, I. The Effect of Dietary Intake of Coenzyme Q10 on Skin Parameters and Condition: Results of a Randomised, Placebo-Controlled, Double-Blind Study: The Effect of Dietary Intake of Coenzyme Q10 on Skin Parameters and Condition. BioFactors 2017, 43, 132–140. [Google Scholar] [CrossRef]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and Male Infertility: A Meta-Analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Balercia, G.; Buldreghini, E.; Vignini, A.; Tiano, L.; Paggi, F.; Amoroso, S.; Ricciardo-Lamonica, G.; Boscaro, M.; Lenzi, A.; Littarru, G. Coenzyme Q10 Treatment in Infertile Men with Idiopathic Asthenozoospermia: A Placebo-Controlled, Double-Blind Randomized Trial. Fertil. Steril. 2009, 91, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. The Effect of Coenzyme Q₁₀ Supplementation on Partner Pregnancy Rate in Infertile Men with Idiopathic Oligoasthenoteratozoospermia: An Open-Label Prospective Study. Int. Urol. Nephrol. 2012, 44, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nadjarzadeh, A.; Sadeghi, M.R.; Amirjannati, N.; Vafa, M.R.; Motevalian, S.A.; Gohari, M.R.; Akhondi, M.A.; Yavari, P.; Shidfar, F. Coenzyme Q10 Improves Seminal Oxidative Defense but Does Not Affect on Semen Parameters in Idiopathic Oligoasthenoteratozoospermia: A Randomized Double-Blind, Placebo Controlled Trial. J. Endocrinol. Investig. 2011, 34, e224–e228. [Google Scholar] [CrossRef]
- Vishvkarma, R.; Alahmar, A.T.; Gupta, G.; Rajender, S. Coenzyme Q10 Effect on Semen Parameters: Profound or Meagre? Andrologia 2020, 52, e13570. [Google Scholar] [CrossRef]
- Balercia, G.; Mancini, A.; Paggi, F.; Tiano, L.; Pontecorvi, A.; Boscaro, M.; Lenzi, A.; Littarru, G.P. Coenzyme Q10 and Male Infertility. J. Endocrinol. Investig. 2009, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Gvozdjáková, A.; Kucharská, J.; Dubravicky, J.; Mojto, V.; Singh, R.B. Coenzyme Q10, α-Tocopherol, and Oxidative Stress Could Be Important Metabolic Biomarkers of Male Infertility. Dis. Markers 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukutomi, N.; Hosoe, K. Safety Assessment of Coenzyme Q 10 (CoQ10). BioFactors 2008, 32, 199–208. [Google Scholar] [CrossRef]
- Micic, S.; Lalic, N.; Djordjevic, D.; Bojanic, N.; Bogavac-Stanojevic, N.; Busetto, G.M.; Virmani, A.; Agarwal, A. Double-blind, Randomised, Placebo-controlled Trial on the Effect of L-carnitine and L-acetylcarnitine on Sperm Parameters in Men with Idiopathic Oligoasthenozoospermia. Andrologia 2019, 51, e13267. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant Supplements and Semen Parameters: An Evidence Based Review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Rasmussen, J.; Nielsen, O.W.; Janzen, N.; Duno, M.; Køber, L.; Steuerwald, U.; Lund, A.M. Carnitine Levels in 26,462 Individuals from the Nationwide Screening Program for Primary Carnitine Deficiency in the Faroe Islands. J. Inherit. Metab. Dis. 2014, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Moradi, A.; Alemi, M.; Ahmadnia, H.; Abdi, H.; Ahmadi, A.; Bazargan-Hejazi, S. Safety and Efficacy of Clomiphene Citrate and L-Carnitine in Idiopathic Male Infertility: A Comparative Study. Urol. J. 2010, 7, 188–193. [Google Scholar] [PubMed]
- Haseen Ahmed, S.D.; Ahsan, S.; Iqbal, T.; Ahmed Burney, S.I. Relationship of Seminal Free L-Carnitine with Functional Spermatozoal Characteristics: Results from an Observational Study Conducted in a Tertiary Care Hospital of Karachi, Pakistan. J. Pak. Med. Assoc. 2017, 67, 280–284. [Google Scholar] [PubMed]
- Sheikh, N.; Goodarzi, M.; Bab Al-Havaejee, H.; Safari, M.; Amiri, I.; Najafi, R.; Hadeie, J. L-Carnitine Level in Seminal Plasma of Fertile and Infertile Men. J. Res. Health Sci. 2007, 7, 43–48. [Google Scholar] [PubMed]
- Li, K.; Li, W.; Huang, Y. Determination of Free L-Carnitine in Human Seminal Plasma by High Performance Liquid Chromatography with Pre-Column Ultraviolet Derivatization and Its Clinical Application in Male Infertility. Clin. Chim. Acta 2007, 378, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise? Molecules 2020, 25, 182. [Google Scholar] [CrossRef]
- Sigman, M.; Glass, S.; Campagnone, J.; Pryor, J.L. Carnitine for the Treatment of Idiopathic Asthenospermia: A Randomized, Double-Blind, Placebo-Controlled Trial. Fertil. Steril. 2006, 85, 1409–1414. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Ong, C.; Prashast, P.; Agarwal, A. Lycopene and Male Infertility. Asian J. Androl. 2014, 16, 420. [Google Scholar] [CrossRef] [PubMed]
- Filipcikova, R.; Oborna, I.; Brezinova, J.; Novotny, J.; Wojewodka, G.; De Sanctis, J.B.; Radova, L.; Hajduch, M.; Radzioch, D. Lycopene Improves the Distorted Ratio between AA/DHA in the Seminal Plasma of Infertile Males and Increases the Likelihood of Successful Pregnancy. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2015, 159, 077–082. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Erdman, J.W.; Clinton, S.K. Complex Interactions between Dietary and Genetic Factors Impact Lycopene Metabolism and Distribution. Arch. Biochem. Biophys. 2013, 539, 171–180. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e1260. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and Bone: An in Vitro Investigation and a Pilot Prospective Clinical Study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Catalano, A.; Parrone, N.; Monego, G.; Ranelletti, F.O. Lycopene Regulation of Cholesterol Synthesis and Efflux in Human Macrophages. J. Nutr. Biochem. 2011, 22, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Amani, R.; Nasr-Esfahani, M.; Tarrahi, M.J. The Effects of Lycopene Supplement on the Spermatogram and Seminal Oxidative Stress in Infertile Men: A Randomized, Double-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2019, 33, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Oborna, I.; Malickova, K.; Fingerova, H.; Brezinova, J.; Horka, P.; Novotny, J.; Bryndova, H.; Filipcikova, R.; Svobodova, M. A Randomized Controlled Trial of Lycopene Treatment on Soluble Receptor for Advanced Glycation End Products in Seminal and Blood Plasma of Normospermic Men: LYCOPENE SUPPRESSES SRAGE IN SEMINAL PLASMA. Am. J. Reprod. Immunol. 2011, 66, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, A. Micronutrients in Health and Disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.; Levine, M. Vitamin C: The Known and the Unknown and Goldilocks. Oral. Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Draper, M.H. LIND’S TREATISE ON SCURVY. A Bicentenary Volume Containing a Reprint of the First Edition of “A Treatise of the Scurvy” by James Lind, M.D., with Additional Notes. Edited by C. P. Stewart and Douglas Guthrie. Edinburgh: University Press. 1953. Pp. 440. Wi. Q. J. Exp. Physiol. Cogn. Med. Sci. 1953, 38, 201–202. [Google Scholar] [CrossRef][Green Version]
- Angulo, C.; Maldonado, R.; Pulgar, E.; Mancilla, H.; Córdova, A.; Villarroel, F.; Castro, M.A.; Concha, I.I. Vitamin C and Oxidative Stress in the Seminiferous Epithelium. Biol. Res. 2011, 44, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Rolf, C. Antioxidant Treatment of Patients with Asthenozoospermia or Moderate Oligoasthenozoospermia with High-Dose Vitamin C and Vitamin E: A Randomized, Placebo-Controlled, Double-Blind Study. Hum. Reprod. 1999, 14, 1028–1033. [Google Scholar] [CrossRef]
- Greco, E. Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.B.; Harris, W.A.; Powell, L.C. Relationship between Ascorbic Acid and Male Fertility. In World Review of Nutrition and Dietetics; Bourne, G.H., Ed.; S. Karger AG: Basel, Switzerland, 1990; Volume 62, pp. 1–26. ISBN 978-3-8055-4994-3. [Google Scholar]
- Rafiee, B.; Morowvat, M.H.; Rahimi-Ghalati, N. Comparing the Effectiveness of Dietary Vitamin C and Exercise Interventions on Fertility Parameters in Normal Obese Men. Urol. J. 2016, 13, 2635–2639. [Google Scholar]
- Li, M.-C.; Chiu, Y.-H.; Gaskins, A.J.; Mínguez-Alarcón, L.; Nassan, F.L.; Williams, P.L.; Petrozza, J.; Hauser, R.; Chavarro, J.E. Men’s Intake of Vitamin C and β-Carotene Is Positively Related to Fertilization Rate but Not to Live Birth Rate in Couples Undergoing Infertility Treatment. J. Nutr. 2019, 149, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Rumsey, S.C.; Daruwala, R.; Park, J.B.; Wang, Y. Criteria and Recommendations for Vitamin C Intake. JAMA 1999, 281, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and Metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Khadangi, F.; Azzi, A. Vitamin E The Next 100 Years. IUBMB Life 2018, 71, 411–415. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions: Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Bisong, S.A.; Ukoh, I.E.; Nna, V.U.; Ebong, P.E. Vitamin E Attenuates Nicotine- and Noise-Induced Reproductive Impairment in Male Albino Wistar Rats. Andrologia 2018, 50, e13050. [Google Scholar] [CrossRef] [PubMed]
- Geva, E.; Bartoov, B.; Zabludovsky, N.; Lessing, J.B.; Lerner-Geva, L.; Amit, A. The Effect of Antioxidant Treatment on Human Spermatozoa and Fertilization Rate in an in Vitro Fertilization Program. Fertil. Steril. 1996, 66, 430–434. [Google Scholar] [CrossRef]
- Waniek, S.; di Giuseppe, R.; Esatbeyoglu, T.; Plachta-Danielzik, S.; Ratjen, I.; Jacobs, G.; Nöthlings, U.; Koch, M.; Schlesinger, S.; Rimbach, G.; et al. Vitamin E (α- and γ-Tocopherol) Levels in the Community: Distribution, Clinical and Biochemical Correlates, and Association with Dietary Patterns. Nutrients 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Merrell, B.J.; McMurry, J.P. Folic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Fenech, M. Folate (Vitamin B9) and Vitamin B12 and Their Function in the Maintenance of Nuclear and Mitochondrial Genome Integrity. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2012, 733, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Leahy, L.G. Vitamin B Supplementation: What’s the Right Choice for Your Patients? J. Psychosoc. Nurs. Ment. Health Serv. 2017, 55, 7–11. [Google Scholar] [CrossRef]
- Simmons, S. Folic Acid Vitamin B9: Friend or Foe? Nursing 2013, 43, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, A.; Vojvodic, A.; Ayhan, E.; Dönmezdil, S.; Jovicevic, T.V.; Vojvodic, P.; Lotti, T.; Vestita, M. Depression, Isotretinoin, and Folic Acid: A Practical Review. Dermatol. Ther. 2019, 32, e13104. [Google Scholar] [CrossRef]
- Malouf, R.; Grimley Evans, J.; Areosa Sastre, A. Folic acid with or without vitamin B12 for cognition and dementia. In The Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2003; p. CD004514. [Google Scholar]
- Wong, W.Y.; Merkus, H.M.W.M.; Thomas, C.M.G.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: A Double-Blind, Randomized, Placebo-Controlled Trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef]
- Irani, M.; Amirian, M.; Sadeghi, R.; Lez, J.L.; Latifnejad Roudsari, R. The Effect of Folate and Folate Plus Zinc Supplementation on Endocrine Parameters and Sperm Characteristics in Sub-Fertile Men: A Systematic Review and Meta-Analysis. Urol. J. 2017, 14, 4069–4078. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D. Recent Developments in Folate Nutrition. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2018; Volume 83, pp. 195–213. ISBN 978-0-12-811803-0. [Google Scholar]
- Zhang, X.; Xu, X.; Zhong, Y.; Power, M.C.; Taylor, B.D.; Carrillo, G. Serum Folate Levels and Urinary Arsenic Methylation Profiles in the US Population: NHANES, 2003–2012. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A Necessary Ion for Mammalian Sperm Fertilization Competency. IJMS 2018, 19, 4097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc Levels in Seminal Plasma and Their Correlation with Male Infertility: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc Is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Riffo, M.; Leiva, S.; Astudillo, J. Effect of Zinc on Human Sperm Motility and the Acrosome Reaction. Int. J. Androl. 1992, 15, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ajina, T.; Sallem, A.; Haouas, Z.; Mehdi, M. Total Antioxidant Status and Lipid Peroxidation with and without in Vitro Zinc Supplementation in Infertile Men. Andrologia 2017, 49, e12703. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Bittner, J.; Weber, R.; Hüther, F.; Miska, W. Relevance of Zinc in Human Sperm Flagella and Its Relation to Motility. Fertil. Steril. 1999, 71, 1138–1143. [Google Scholar] [CrossRef]
- Raigani, M.; Yaghmaei, B.; Amirjannti, N.; Lakpour, N.; Akhondi, M.M.; Zeraati, H.; Hajihosseinal, M.; Sadeghi, M.R. The Micronutrient Supplements, Zinc Sulphate and Folic Acid, Did Not Ameliorate Sperm Functional Parameters in Oligoasthenoteratozoospermic Men. Andrologia 2014, 46, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc Levels in Seminal Plasma Are Associated with Sperm Quality in Fertile and Infertile Men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Beigi Harchegani, A.; Dahan, H.; Tahmasbpour, E.; Bakhtiari kaboutaraki, H.; Shahriary, A. Effects of Zinc Deficiency on Impaired Spermatogenesis and Male Infertility: The Role of Oxidative Stress, Inflammation and Apoptosis. Hum. Fertil. 2020, 23, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Omu, A.E.; Dashti, H.; Al-Othman, S. Treatment of Asthenozoospermia with Zinc Sulphate: Andrological, Immunological and Obstetric Outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 79, 179–184. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The Physiological Role of Zinc as an Antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, P.; Ghanghas, P.; Kaur, J.; Kaushal, N. Selenium Ameliorates Ibuprofen Induced Testicular Toxicity by Redox Regulation. Reprod. Toxicol. 2020, 96, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T.; Sengupta, P. Impact of Coenzyme Q10 and Selenium on Seminal Fluid Parameters and Antioxidant Status in Men with Idiopathic Infertility. Biol. Trace Elem. Res. 2020, 199, 1246–1252. [Google Scholar] [CrossRef]
- Schoenmakers, E.; Chatterjee, K. Human Disorders Affecting the Selenocysteine Incorporation Pathway Cause Systemic Selenoprotein Deficiency. Antioxid. Redox Signal. 2020, 33, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; MacPherson, A.; Yates, R.W.; Hussain, B.; Dixon, J. The Effect of Oral Selenium Supplementation on Human Sperm Motility. BJU Int. 1998, 82, 76–80. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Safarinejad, S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2009, 181, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Chyra-Jach, D.; Kaletka, Z.; Dobrakowski, M.; Machoń-Grecka, A.; Kasperczyk, S.; Bellanti, F.; Birkner, E.; Kasperczyk, A. Levels of Macro- and Trace Elements and Select Cytokines in the Semen of Infertile Men. Biol. Trace Elem. Res. 2020, 197, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Teixeira, M.; Daltro, A.; Torquato Filho, S.; Assis, R.; Celedonio, R.; Pires, L.; Maia, C.; Guedes, M. Unbalance of Se and Nutritional Status in Male Infertility. JBRA Assist. Reprod. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yuan, G.; Zhou, Q.; Liu, Y.; He, X.; Zhang, H.; Guo, Y.; Wen, Y.; Huang, S.; Ke, Y.; et al. The Association between Metal Exposure and Semen Quality in Chinese Males: The Mediating Effect of Androgens. Environ. Pollut. 2020, 264, 113975. [Google Scholar] [CrossRef] [PubMed]
- Bleau, G.; Lemarbre, J.; Faucher, G.; Roberts, K.D.; Chapdelaine, A. Semen Selenium and Human Fertility. Fertil. Steril. 1984, 42, 890–894. [Google Scholar] [CrossRef]
- Wu, S.; Wang, M.; Deng, Y.; Qiu, J.; Zhang, X.; Tan, J. Associations of Toxic and Essential Trace Elements in Serum, Follicular Fluid, and Seminal Plasma with In Vitro Fertilization Outcomes. Ecotoxicol. Environ. Saf. 2020, 204, 110965. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Alkan, Z.; Wong, K. Selenium Supplementation Does Not Affect Testicular Selenium Status or Semen Quality in North American Men. J. Androl. 2009, 30, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-Chain Selenium and Human Health: Emphasis on Intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Çay, A.; Alver, A.; Küçük, M.; Işik, O.; Eminağaoğlu, M.S.; Karahan, S.C.; Değer, O. The Effects of N-Acetylcysteine on Antioxidant Enzyme Activities in Experimental Testicular Torsion. J. Surg. Res. 2006, 131, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Mora-Esteves, C.; Shin, D. Nutrient Supplementation: Improving Male Fertility Fourfold. Semin. Reprod. Med. 2013, 31, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, K.; Hirvonen, V.; Wuokko, E.; Parvinen, M.; Dunkel, L. N-Acetyl-l-Cysteine Inhibits Apoptosis in Human Male Germ Cells in Vitro1. J. Clin. Endocrinol. Metab. 1998, 83, 2523–2531. [Google Scholar] [CrossRef]
- Ciftci, H.; Verit, A.; Savas, M.; Yeni, E.; Erel, O. Effects of N-Acetylcysteine on Semen Parameters and Oxidative/Antioxidant Status. Urology 2009, 74, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-Acetyl-Cysteine Supplementation on Sperm Quality, Chromatin Integrity and Level of Oxidative Stress in Infertile Men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Oeda, T.; Henkel, R.; Ohmori, H.; Schill, W.-B. Scavenging Effect of N-Acetyl-L-Cysteine against Reactive Oxygen Species in Human Semen: A Possible Therapeutic Modality for Male Factor Infertility? Andrologia 2009, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Parivar, K.; Hayati Roodbari, N.; Nasr-Esfahani, M.H. The Effect of N-Acetyl-Cysteine on NRF 2 Antioxidant Gene Expression in Asthenoteratozoospermia Men: A Clinical Trial Study. Int. J. Fertil. Steril. 2020, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Ghafarizadeh, A.; Malmir, M.; Naderi Noreini, S.; Faraji, T. Antioxidant Effects of N-acetylcysteine on the Male Reproductive System: A Systematic Review. Andrologia 2020, 53, e13898. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Mitra, S.; Lakhera, P.C.; Khandelwal, S. N-Acetylcysteine Effectively Mitigates Cadmium-Induced Oxidative Damage and Cell Death in Leydig Cells in Vitro. Drug Chem. Toxicol. 2016, 39, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Saraf, P.; Kumari, P.; Mittal, M.; Kumar, V. N-Acetyl-Cysteine Mediated Inhibition of Spermatogonial Cells Apoptosis against Malathion Exposure in Testicular Tissue. J. Biochem. Mol. Toxicol. 2018, 32, e22046. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological Effects in Humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Malmir, M.; Naderi Noreini, S.; Ghafarizadeh, A.; Faraji, T.; Asali, Z. Ameliorative Effect of Melatonin on Apoptosis, DNA Fragmentation, Membrane Integrity and Lipid Peroxidation of Spermatozoa in the Idiopathic Asthenoteratospermic Men: In Vitro. Andrologia 2020, 53, e13944. [Google Scholar] [CrossRef]
- Sun, T.-C.; Li, H.-Y.; Li, X.-Y.; Yu, K.; Deng, S.-L.; Tian, L. Protective Effects of Melatonin on Male Fertility Preservation and Reproductive System. Cryobiology 2020, 95, 1–8. [Google Scholar] [CrossRef]
- Awad, H.; Halawa, F.; Mostafa, T.; Atta, H. Melatonin Hormone Profile in Infertile Males. Int. J. Androl. 2006, 29, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; El‑Taieb, M.; Fares, N.; Fayed, H.; Toghan, R.; Ibrahim, H. Men with Idiopathic Oligoasthenoteratozoospermia Exhibit Lower Serum and Seminal Plasma Melatonin Levels: Comparative Effect of Night‑light Exposure with Fertile Males. Exp. Ther. Med. 2020, 20, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-L.; Ye, X.; Wang, S.; Zhang, D. Melatonin Application in Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Randomized Trials. Front. Endocrinol. 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.L.; Bhatti, S.; Abbas, S.; Kaloglu, C.; Qurat-ul-Ain Zahra, S.; Khan, Y.L.; Hassan, Z.; Turhan, N.Ö.; Aydin, H.H. Melatonin Levels and MicroRNA (MiRNA) Relative Expression Profile in the Follicular Ambient Microenvironment in Patients Undergoing in Vitro Fertilization Process. J. Assist. Reprod. Genet. 2020, 38, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High Endogenous Melatonin Concentrations Enhance Sperm Quality and Short-Term in Vitro Exposure to Melatonin Improves Aspects of Sperm Motility: Melatonin Improves Sperm Quality. J. Pineal Res. 2010, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Bejarano, I.; Ortiz, Á.; Lozano, G.M.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. Melatonin as a Potential Tool against Oxidative Damage and Apoptosis in Ejaculated Human Spermatozoa. Fertil. Steril. 2010, 94, 1915–1917. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-Lipoic Acid as a Dietary Supplement: Molecular Mechanisms and Therapeutic Potential. Biochim. Et Biophys. Acta (Bba) Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.F.; Osman, K.; Das, S.; Othman, A.M.; Majid, N.A.; Rahman, M.P.A. A Study of the Antioxidant Effect of Alpha Lipoic Acids on Sperm Quality. Clinics 2008, 63, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Molavi, N.; Tavalaee, M.; Abbasi, H.; Nasr-Esfahani, M.H. Alpha-Lipoic Acid Improves Sperm Motility in Infertile Men after Varicocelectomy: A Triple-Blind Randomized Controlled Trial. Reprod. Biomed. Online 2020, 41, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-Lipoic Acid as a Biological Antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Di Tucci, C.; Galati, G.; Mattei, G.; Bonanni, V.; Capri, O.; D’Amelio, R.; Muzii, L.; Benedetti Panici, P. The Role of Alpha Lipoic Acid in Female and Male Infertility: A Systematic Review. Gynecol. Endocrinol. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haghighian, H.K.; Haidari, F.; Mohammadi-asl, J.; Dadfar, M. Randomized, Triple-Blind, Placebo-Controlled Clinical Trial Examining the Effects of Alpha-Lipoic Acid Supplement on the Spermatogram and Seminal Oxidative Stress in Infertile Men. Fertil. Steril. 2015, 104, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Taherian, S.; Khayamabed, R.; Tavalaee, M.; Nasr-Esfahani, M.H. Alpha-lipoic Acid Minimises Reactive Oxygen Species-induced Damages during Sperm Processing. Andrologia 2019, 51, e13314. [Google Scholar] [CrossRef] [PubMed]
- Asa, E.; Ahmadi, R.; Mahmoodi, M.; Mohammadniya, A. Supplementation of Freezing Media with Alpha Lipoic Acid Preserves the Structural and Functional Characteristics of Sperm against Cryodamage in Infertile Men with Asthenoteratozoospermia. Cryobiology 2020, 96, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rago, R.; Gallo, M.; Dal Lago, A.; Licata, E.; Paciotti, G.; Amodei, M.; Meneghini, C.; Fabiani, C.; Dani, G.; Liberanome, C.; et al. Controlled, Prospective, Observational Study on the Efficiency and Tolerability of a Combination of Potential Nrf2-Inducing Antioxidants and Micronutrients as Pre-Treatment for ICSI in Dyspermic Patients with Previous Failure. Eur. Rev. Med. Pharm. Sci. 2017, 21, 1645–1652. [Google Scholar]
- Nguyen, H.; Gupta, V. Alpha-Lipoic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.J.; Mei\Ner, H.P.; Lobisch, M.; SchTte, K.; Gries, F.A. Treatment of Symptomatic Diabetic Peripheral Neuropathy with the Anti-Oxidant α-Lipoic Acid. Diabetologia 1995, 38, 1425–1433. [Google Scholar] [CrossRef]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.J.; Hasche, H.; Lobisch, M.; Schutte, K.; Kerum, G.; Malessa, R. Treatment of Symptomatic Diabetic Polyneuropathy with the Antioxidant Alpha-Lipoic Acid: A 7-Month Multicenter Randomized Controlled Trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care 1999, 22, 1296–1301. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, X.; Yang, F.; Li, J.; Yu, X.; Li, Y. Effect of Oral Alpha-Lipoic Acid (ALA) on the Treatment of Male Infertility: A Protocol for Systematic Review and Meta-Analysis. Medicine 2019, 98, e18453. [Google Scholar] [CrossRef] [PubMed]
- Falsig, A.-M.L.; Gleerup, C.S.; Knudsen, U.B. The Influence of Omega-3 Fatty Acids on Semen Quality Markers: A Systematic PRISMA Review. Andrology 2019, 7, 794–803. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Semen Profile and Enzymatic Anti-Oxidant Capacity of Seminal Plasma in Infertile Men with Idiopathic Oligoasthenoteratospermia: A Double-Blind, Placebo-Controlled, Randomised Study: Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation. Andrologia 2011, 43, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary Fat and Semen Quality among Men Attending a Fertility Clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef]
- Jensen, T.K.; Priskorn, L.; Holmboe, S.A.; Nassan, F.L.; Andersson, A.-M.; Dalgård, C.; Petersen, J.H.; Chavarro, J.E.; Jørgensen, N. Associations of Fish Oil Supplement Use with Testicular Function in Young Men. JAMA Netw. Open 2020, 3, e1919462. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Nourmohamadi, M.; Hajipour, S.; Taghizadeh, M.; Asemi, Z.; Keshavarz, S.A.; Jafarnejad, S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2019, 16, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary Supplementation with Docosahexaenoic Acid (DHA) Improves Seminal Antioxidant Status and Decreases Sperm DNA Fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Buhling, K.J.; Laakmann, E. The Effect of Micronutrient Supplements on Male Fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 199–209. [Google Scholar] [CrossRef]
- Kutlubay, R.; Oğuz, E.O.; Can, B.; Güven, M.C.; Sinik, Z.; Tuncay, Ö.L. Vitamin E Protection from Testicular Damage Caused by Intraperitoneal Aluminium. Int. J. Toxicol. 2007, 26, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ebisch, I.M.W.; Pierik, F.H.; De Jong, F.H.; Thomas, C.M.G.; Steegers-Theunissen, R.P.M. Does Folic Acid and Zinc Sulphate Intervention Affect Endocrine Parameters and Sperm Characteristics in Men? Int. J. Androl. 2006, 29, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Azizollahi, G.; Azizollahi, S.; Babaei, H.; Kianinejad, M.; Baneshi, M.R.; Nematollahi-mahani, S.N. Effects of Supplement Therapy on Sperm Parameters, Protamine Content and Acrosomal Integrity of Varicocelectomized Subjects. J. Assist. Reprod. Genet. 2013, 30, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi-Mahani, S.N.; Azizollahi, G.H.; Baneshi, M.R.; Safari, Z.; Azizollahi, S. Effect of Folic Acid and Zinc Sulphate on Endocrine Parameters and Seminal Antioxidant Level after Varicocelectomy. Andrologia 2014, 46, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Maiorino, M.; Roverato, A.; Roveri, A.; Ursini, F.; Foresta, C. Oral Carnitine Supplementation Increases Sperm Motility in Asthenozoospermic Men with Normal Sperm Phospholipid Hydroperoxide Glutathione Peroxidase Levels. Fertil. Steril. 2005, 83, 355–361. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Commercial Dietary Antioxidant Supplements Assayed for Their Antioxidant Activity by Different Methodologies. J. Agric. Food Chem. 2003, 51, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The Excessive Use of Antioxidant Therapy: A Possible Cause of Male Infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Russell, R.M. Essential Nutrients: Food or Supplements?: Where Should the Emphasis Be? JAMA 2005, 294, 351. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Tinz, J. Multivitamin/Mineral Supplements: Rationale and SafetyA Systematic Review. Nutrition 2017, 33, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Rashki Ghaleno, L.; Alizadeh, A.; Drevet, J.R.; Shahverdi, A.; Valojerdi, M.R. Oxidation of Sperm DNA and Male Infertility. Antioxidants 2021, 10, 97. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Henry-Berger, J.; Vernet, P.; Cadet, R.; Saez, F.; Drevet, J.R. Mammalian Glutathione Peroxidases Control Acquisition and Maintenance of Spermatozoa Integrity 1. J. Anim. Sci. 2010, 88, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Finley, J.W. How to Standardize the Multiplicity of Methods to Evaluate Natural Antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Vitagliano, A.; Di Nisio, A.; Foresta, C. Dietary Supplements for Male Infertility: A Critical Evaluation of Their Composition. Nutrients 2020, 12, 1472. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.-H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E. Dietary Patterns and Outcomes of Assisted Reproduction. Am. J. Obstet. Gynecol. 2019, 220, 567.e1–567.e18. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; de Vries, J.H.; Dohle, G.R.; Bonsel, G.J.; Lindemans, J.; Macklon, N.S.; van der Spek, P.J.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Associations between Dietary Patterns and Semen Quality in Men Undergoing IVF/ICSI Treatment. Hum. Reprod. 2009, 24, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Efrat, M.; Stein, A.; Pinkas, H.; Unger, R.; Birk, R. Dietary Patterns Are Positively Associated with Semen Quality. Fertil. Steril. 2018, 109, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Cutillas-Tolín, A.; Mínguez-Alarcón, L.; Mendiola, J.; López-Espín, J.J.; Jørgensen, N.; Navarrete-Muñoz, E.M.; Torres-Cantero, A.M.; Chavarro, J.E. Mediterranean and Western Dietary Patterns Are Related to Markers of Testicular Function among Healthy Men. Hum. Reprod. 2015, 30, 2945–2955. [Google Scholar] [CrossRef] [PubMed]
- Danielewicz, A.; Morze, J.; Przybyłowicz, M.; Przybyłowicz, K.E. Association of the Dietary Approaches to Stop Hypertension, Physical Activity, and Their Combination with Semen Quality: A Cross-Sectional Study. Nutrients 2019, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Jensen, T.K.; Priskorn, L.; Halldorsson, T.I.; Chavarro, J.E.; Jørgensen, N. Association of Dietary Patterns with Testicular Function in Young Danish Men. JAMA Netw. Open 2020, 3, e1921610. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Babio, N.; Carrell, D.T.; Bulló, M.; Salas-Salvadó, J. Adherence to the Mediterranean Diet Is Positively Associated with Sperm Motility: A Cross-Sectional Analysis. Sci. Rep. 2019, 9, 3389. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Chou, Y.-C.; Chao, J.C.-J.; Hsu, C.-Y.; Cha, T.-L.; Tsao, C.-W. The Association between Dietary Patterns and Semen Quality in a General Asian Population of 7282 Males. PLoS ONE 2015, 10, e0134224. [Google Scholar] [CrossRef] [PubMed]

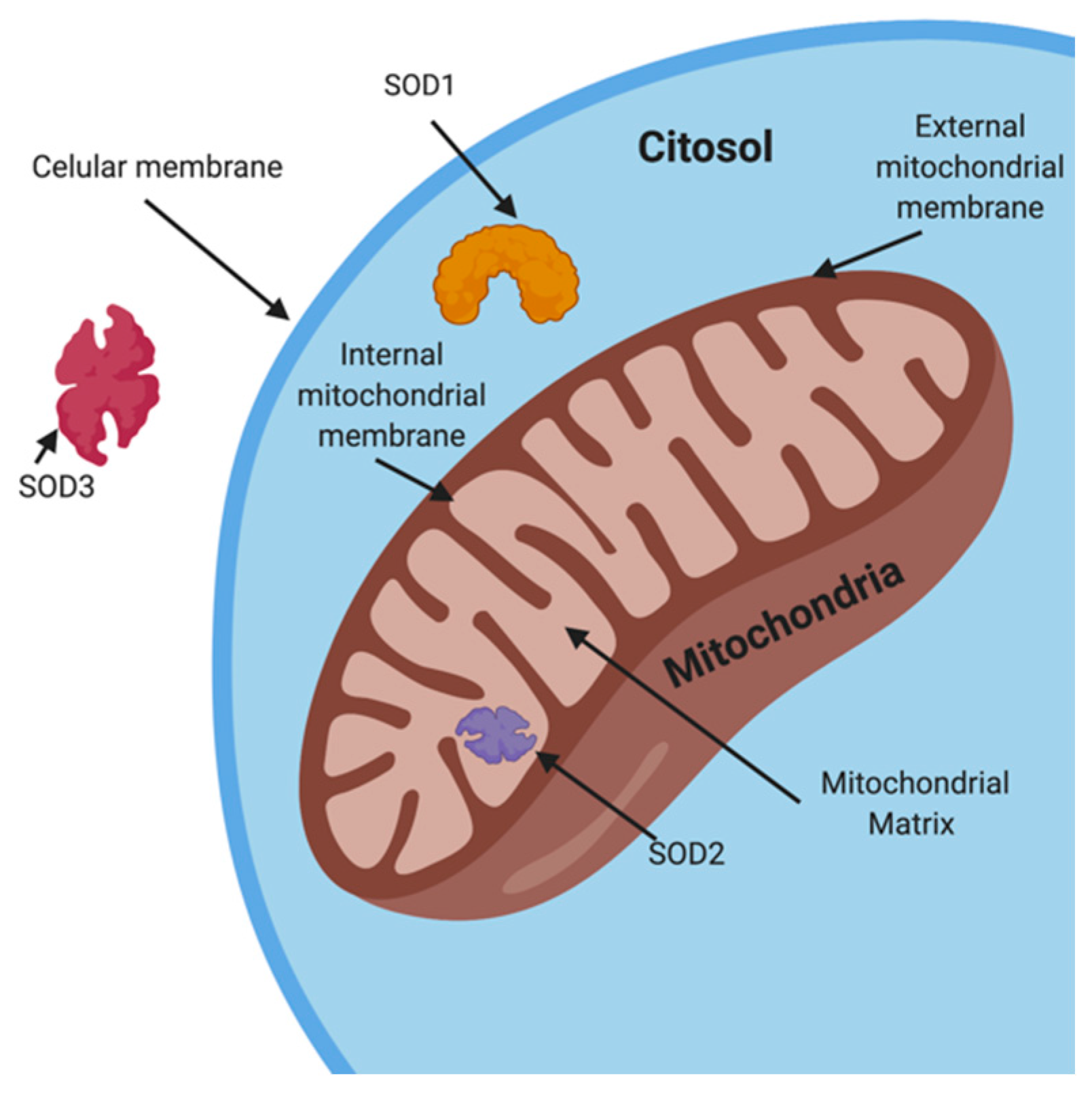
| 4.1 Physiological enzymatic factors | Superoxide Dismutase (SOD) Catalase (CAT) Glutathione Peroxidase (GPX) | |
| 4.2 Non-enzymatic factors | Q-10 coenzyme (CoQ10) Carnitines Lycopene | |
| 4.3 Micronutrients | 4.3.1 Vitamins | Vitamin C Vitamin E Vitamin B9 (Folic Acid) |
| 4.3.2 Minerals | Zinc Selenium | |
| 4.4 Others | N-acetyl-cysteine (NAC) Melatonin Alpha-lipoic acid (ALA) ω-3 fatty acid (Omega3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Arce, E.; Vizmanos, B.; Babio, N.; Márquez-Sandoval, F.; Salas-Huetos, A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology 2021, 10, 241. https://doi.org/10.3390/biology10030241
Torres-Arce E, Vizmanos B, Babio N, Márquez-Sandoval F, Salas-Huetos A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology. 2021; 10(3):241. https://doi.org/10.3390/biology10030241
Chicago/Turabian StyleTorres-Arce, Elizabeth, Barbara Vizmanos, Nancy Babio, Fabiola Márquez-Sandoval, and Albert Salas-Huetos. 2021. "Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress" Biology 10, no. 3: 241. https://doi.org/10.3390/biology10030241
APA StyleTorres-Arce, E., Vizmanos, B., Babio, N., Márquez-Sandoval, F., & Salas-Huetos, A. (2021). Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology, 10(3), 241. https://doi.org/10.3390/biology10030241










