The Zebrafish Xenograft Models for Investigating Cancer and Cancer Therapeutics
Abstract
:Simple Summary
Abstract
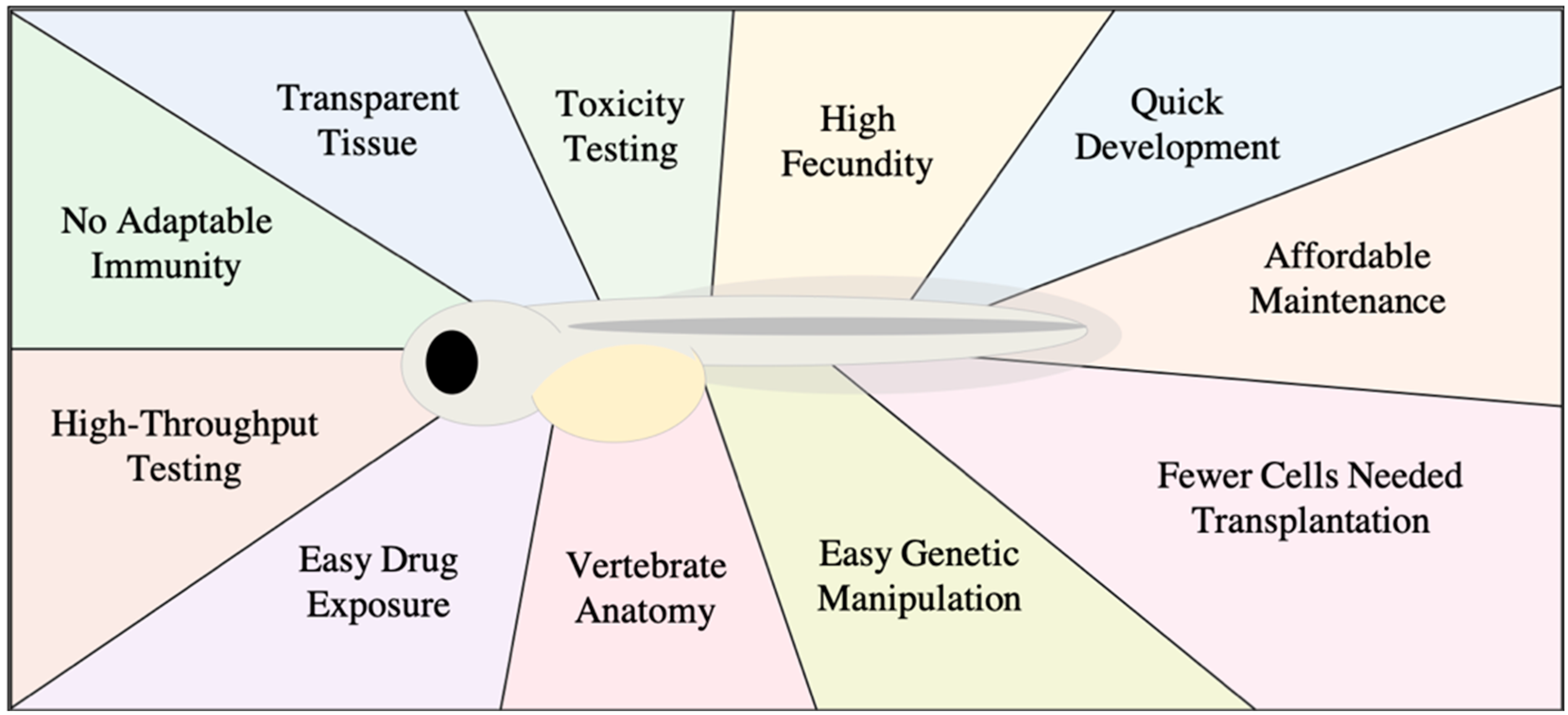
1. Zebrafish as Model for Studying Human Cancer
2. Embryo-Larval Zebrafish Xenografts
3. Zebrafish Xenografts for Cancer Drug Screening
3.1. Adult Zebrafish Xenograft Models
3.2. New Extended Utility of the Zebrafish Xenograft Model
3.3. Limitations of the Zebrafish Xenograft Model for Cancer Drug Discovery
3.4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; van Cruchten, S.; Zon, L.I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- He, S.; Jing, C.-B.; Look, A.T. Zebrafish models of leukemia. Methods Cell Biol. 2017, 138, 563–592. [Google Scholar]
- Guerra, J.; Tobia, C.; Presta, P.; Barbieri, A. Zebrafish embryo as an experimental model to study tumor angiogenesis. Tumor Vasc. 2020, 129–145. [Google Scholar] [CrossRef]
- Summerton, J.; Weller, D. Morpholino antisense oligomers: Design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A primer for morpholino use in zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Stainier, D.; Raz, E.; Lawson, N.D.; Ekker, S.C.; Burdine, R.D.; Eisen, J.S.; Ingham, P.W.; Schulte-Merker, S.; Yelon, D.; Weinstein, B.M.; et al. Guidelines for morpholino use in zebrafish. PLoS Genet. 2017, 13, e1007000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.M.J.; Seftor, E.A.; Bonde, G.; Cornell, R.A.; Hendrix, M.J.C. The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 1560–1570. [Google Scholar] [CrossRef]
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corkery, D.P.; Dellaire, G.; Berman, J.N. Leukaemia xenotransplantation in zebrafish--chemotherapy response assay in vivo. Br. J. Haematol. 2011, 153, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Pruvot, B.; Jacquel, A.; Droin, N.; Auberger, P.; Bouscary, D.; Tamburini, J.; Muller, M.; Fontenay, M.; Chluba, J.; Solary, E. Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica 2011, 96, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Stoletov, K.; Klemke, R. Catch of the day: Zebrafish as a human cancer model. Oncogene 2008, 27, 4509–4520. [Google Scholar] [CrossRef] [Green Version]
- Cabezas-Sainz, P.; Guerra-Varela, J.; Carreira, M.J.; Mariscal, J.; Roel, M.; Rubiolo, J.A.; Sciara, A.A.; Abal, M.; Botana, L.M.; López, R.; et al. Improving zebrafish embryo xenotransplantation conditions by increasing incubation temperature and establishing a proliferation index with ZFtool. BMC Cancer 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; La Du, J.; Tanguay, R.L.; Greenwood, J.A. Calpain 2 is required for the invasion of glioblastoma cells in the zebrafish brain microenvironment. J. Neurosci. Res. 2012, 90, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Hen, G.; Nicenboim, J.; Mayseless, O.; Asaf, L.; Shin, M.; Busolin, G.; Hofi, R.; Almog, G.; Tiso, N.; Lawson, N.D.; et al. Venous-derived angioblasts generate organ-specific vessels during zebrafish embryonic development. Development 2015, 142, 4266–4278. [Google Scholar] [CrossRef] [Green Version]
- Clark, B.S.; Winter, M.; Cohen, A.R.; Link, B.A. Generation of Rab-based transgenic lines for in vivo studies of endosome biology in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2011, 240, 2452–2465. [Google Scholar] [CrossRef] [Green Version]
- Norden, C.; Young, S.; Link, B.A.; Harris, W.A. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell 2009, 138, 1195–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distel, M.; Hocking, J.C.; Volkmann, K.; Köster, R.W. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 2010, 191, 875–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kang, K.H.; Kim, C.-H.; Choi, S.-Y. Real-time imaging of mitochondria in transgenic zebrafish expressing mitochondrially targeted GFP. Biotechniques 2008, 45, 331–334. [Google Scholar] [PubMed]
- Progatzky, F.; Dallman, M.J.; Celso, C. Lo From seeing to believing: Labelling strategies for in vivo cell-tracking experiments. Interface Focus 2013, 3, 20130001. [Google Scholar] [CrossRef] [Green Version]
- Veinotte, C.J.; Dellaire, G.; Berman, J.N. Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis. Model. Mech. 2014, 7, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Ordoñez, A.; Seoane, S.; Cabezas, P.; Eiro, N.; Sendon-Lago, J.; Macia, M.; Garcia-Caballero, T.; Gonzalez, L.O.; Sanchez, L.; Vizoso, F.; et al. Breast cancer metastasis to liver and lung is facilitated by Pit-1-CXCL12-CXCR4 axis. Oncogene 2018, 37, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Almstedt, E.; Elgendy, R.; Hekmati, N.; Rosén, E.; Wärn, C.; Olsen, T.K.; Dyberg, C.; Doroszko, M.; Larsson, I.; Sundström, A.; et al. Integrative discovery of treatments for high-risk neuroblastoma. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- He, X.; Yin, X.; Wu, J.; Wickström, S.L.; Duo, Y.; Du, Q.; Qin, S.; Yao, S.; Jing, X.; Hosaka, K.; et al. Visualization of human T lymphocyte-mediated eradication of cancer cells in vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 22910–22919. [Google Scholar] [CrossRef] [PubMed]
- Bentley, V.L.; Veinotte, C.J.; Corkery, D.P.; Pinder, J.B.; LeBlanc, M.A.; Bedard, K.; Weng, A.P.; Berman, J.N.; Dellaire, G. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 70–76. [Google Scholar] [CrossRef]
- Xu, W.; Foster, B.A.; Richards, M.; Bondioli, K.R.; Shah, G.; Green, C.C. Characterization of prostate cancer cell progression in zebrafish xenograft model. Int. J. Oncol. 2018, 52, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Latifi, A.; Abubaker, K.; Castrechini, N.; Ward, A.C.; Liongue, C.; Dobill, F.; Kumar, J.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J. Cell. Biochem. 2011, 112, 2850–2864. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Cui, W.; Gu, A.; Xu, C.; Yu, S.C.; Li, T.T.; Cui, Y.H.; Zhang, X.; Bian, X.W. A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS ONE 2013, 8, e61801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.; Xie, X.; Walker, S.; White, D.T.; Mumm, J.S.; Cowell, J.K. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013, 13, 453. [Google Scholar] [CrossRef] [Green Version]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Nicoli, S.; Ribatti, D.; Cotelli, F.; Presta, M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007, 67, 2927–2931. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis. Model. Mech. 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Cross, L.M.; Cook, M.A.; Lin, S.; Chen, J.-N.; Rubinstein, A.L. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 911–912. [Google Scholar] [CrossRef]
- Nicoli, S.; Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007, 2, 2918–2923. [Google Scholar] [CrossRef]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Lüthi, U.; et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef]
- Martin, W.G.J.T.A.; Ye, L.; Sanders, A.J.; Lane, J. Cancer Invasion and Metastasis: Molecular and Cellular Perspective; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Stoletov, K.; Montel, V.; Lester, R.D.; Gonias, S.L.; Klemke, R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 17406–17411. [Google Scholar] [CrossRef] [Green Version]
- Siekmann, A.F.; Affolter, M.; Belting, H.G. The tip cell concept 10 years after: New players tune in for a common theme. Exp. Cell Res. 2013, 319, 1255–1263. [Google Scholar] [CrossRef]
- Krueger, J.; Liu, D.; Scholz, K.; Zimmer, A.; Shi, Y.; Klein, C.; Siekmann, A.; Schulte-Merker, S.; Cudmore, M.; Ahmed, A.; et al. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 2011, 138, 2111–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siekmann, A.F.; Lawson, N.D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 2007, 445, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Blum, Y.; Belting, H.G.; Ellertsdottir, E.; Herwig, L.; Lüders, F.; Affolter, M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 2008, 316, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.D.; Ii, M.; Park, K.W.; Suli, A.; Sorensen, L.K.; Larrieu-Lahargue, F.; Urness, L.D.; Suh, W.; Asai, J.; Kock, G.A. Netrins promote developmental and therapeutic angiogenesis. Science 2006, 313, 640–644. [Google Scholar] [CrossRef] [Green Version]
- Covassin, L.D.; Villefranc, J.A.; Kacergis, M.C.; Weinstein, B.M.; Lawson, N.D. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. USA 2006, 103, 6554–6559. [Google Scholar] [CrossRef] [Green Version]
- Habeck, H.; Odenthal, J.; Walderich, B.; Maischein, H.; Schulte-Merker, S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr. Biol. 2002, 12, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Schuermann, A.; Helker, C.S.M.; Herzog, W. Angiogenesis in zebrafish. Semin. Cell Develop. Biol. 2014, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.; Astell, K.R.; Velikova, G.; Sieger, D. A Zebrafish live imaging model reveals differential responses of microglia toward glioblastoma cells in vivo. Zebrafish 2016, 13, 523–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiens, K.M.; Lee, H.L.; Shimada, H.; Metcalf, A.E.; Chao, M.Y.; Lien, C.-L. Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel formation. PLoS ONE 2010, 5, e11324. [Google Scholar] [CrossRef] [Green Version]
- Bellail, A.C.; Hunter, S.B.; Brat, D.J.; Tan, C.; Van Meir, E.G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 2004, 36, 1046–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Moens, C.B.; Appel, B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014, 141, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Panková, K.; Rösel, D.; Novotný, M.; Brábek, J. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell. Mol. Life Sci. 2010, 67, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Wehmas, L.C.; Tanguay, R.L.; Punnoose, A.; Greenwood, J.A. Developing a novel embryo-larval zebrafish xenograft assay to prioritize human glioblastoma therapeutics. Zebrafish 2016, 13, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamble, J.T.; Reed-Harris, Y.; Barton, C.L.; La Du, J.; Tanguay, R.L.; Greenwood, J.A. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem. Biophys. Res. Commun. 2018, 506, 833–839. [Google Scholar] [CrossRef]
- Gamble, J.; Tanguay, R.; Greenwood, J.A. 4D quantitative image analysis of cancer cell invasion in a brain microenvironment using imagej softwareitle. Microsc. Microanal. 2017, 23, 1182–1183. [Google Scholar] [CrossRef] [Green Version]
- Irie, N.; Kuratani, S. Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat. Commun. 2011, 2, 248. [Google Scholar] [CrossRef] [Green Version]
- Truong, L.; Tanguay, R.L. Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 2017, 1641, 325–333. [Google Scholar]
- Lenard, A.; Alghisi, E.; Daff, H.; Donzelli, M.; McGinnis, C.; Lengerke, C. Using zebrafish to model erythroid lineage toxicity and regeneration. Haematologica 2016, 101, e164-7. [Google Scholar] [CrossRef] [Green Version]
- Guengerich, F.P. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 2011, 26, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Mione, M.C.; Trede, N.S. The zebrafish as a model for cancer. Dis. Model. Mech. 2010, 3, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leet, J.K.; Lindberg, C.D.; Bassett, L.A.; Isales, G.M.; Yozzo, K.L.; Raftery, T.D.; Volz, D.C. High-content screening in zebrafish embryos identifies butafenacil as a potent inducer of anemia. PLoS ONE 2014, 9, e104190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Lin, S.; Rallo, R.; Zhao, Y.; Damoiseaux, R.; Xia, T.; Lin, S.; Nel, A.; Cohen, Y. Automated phenotype recognition for zebrafish embryo based in vivo high throughput toxicity screening of engineered nano-materials. PLoS ONE 2012, 7, e35014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehr, P.; Martin, D.C.; Koepsell, T.; Cheadle, A. Breaking the matches in a paired t-test for community interventions when the number of pairs is small. Stat. Med. 1995, 14, 1491–1504. [Google Scholar] [CrossRef] [Green Version]
- Pearce, M.C.; Gamble, J.T.; Kopparapu, P.R.; O’Donnell, E.F.; Mueller, M.J.; Jang, H.S.; Greenwood, J.A.; Satterthwait, A.C.; Tanguay, R.L.; Zhang, X.K.; et al. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget 2018, 9, 26072–26085. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.; Gamble, J.T.; Pearce, M.C.; Elson, D.J.; Tanguay, R.L.; Kolluri, S.K.; Reich, N.O. Improved in vivo targeting of BCL-2 phenotypic conversion through hollow gold nanoshell delivery. Apoptosis 2019, 24, 529–537. [Google Scholar] [CrossRef]
- Fazio, M.; Ablain, J.; Chuan, Y.; Langenau, D.M.; Zon, L.I. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat. Rev. Cancer 2020, 20, 263–273. [Google Scholar] [CrossRef]
- Costa, B.; Estrada, M.F.; Mendes, R.V.; Fior, R. Zebrafish avatars towards personalized medicine-a comparative review between avatar models. Cells 2020, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Mercatali, L.; La Manna, F.; Groenewoud, A.; Casadei, R.; Recine, F.; Miserocchi, G.; Pieri, F.; Liverani, C.; Bongiovanni, A.; Spadazzi, C.; et al. Development of a Patient-Derived Xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. Int. J. Mol. Sci. 2016, 17, 1375. [Google Scholar] [CrossRef]
- de Almeida, C.R.; Mendes, R.V.; Pezzarossa, A.; Gago, J.; Carvalho, C.; Alves, A.; Nunes, V.; Brito, M.J.; Cardoso, M.J.; Ribeiro, J.; et al. Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy. Commun. Biol. 2020, 3, 299. [Google Scholar] [CrossRef] [PubMed]
- Arnandis, T.; Monteiro, P.; Adams, S.D.; Bridgeman, V.L.; Rajeeve, V.; Gadaleta, E.; Marzec, J.; Chelala, C.; Malanchi, I.; Cutillas, P.R.; et al. Oxidative stress in cells with extra centrosomes drives non-cell-autonomous invasion. Dev. Cell 2018, 47, 409–424.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, G.M.; Schmidt, M.O.; Yi, C.; Hu, Z.; Haddad, B.R.; Glasgow, E.; Riegel, A.T.; Wellstein, A. Cell growth density modulates cancer cell vascular invasion via Hippo pathway activity and CXCR2 signaling. Oncogene 2015, 34, 5879–5889. [Google Scholar] [CrossRef] [Green Version]
- Tulotta, C.; Stefanescu, C.; Beletkaia, E.; Bussmann, J.; Tarbashevich, K.; Schmidt, T. Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model. Dis. Model Mech. 2016, 9, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ma, X.-L.; Wei, Y.-Q.; Wei, X.-W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta. Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef]
- van der Ent, W.; Burrello, C.; de Lange, M.J.; van der Velden, P.A.; Jochemsen, A.G.; Jager, M.J.; Snaar-Jagalska, B.E. Embryonic zebrafish: Different phenotypes after injection of human uveal melanoma cells. Ocul. Oncol. Pathol. 2015, 1, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chou, H.L.; Chen, B.H.; Chang, K.F.; Tseng, C.H.; Fong, Y.; Fu, T.F.; Chang, H.W.; Wu, C.Y.; Tsai, E.M.; et al. BPIQ, a novel synthetic quinoline derivative, inhibits growth and induces mitochondrial apoptosis of lung cancer cells in vitro and in zebrafish xenograft model. BMC Cancer 2015, 15, 962. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, S.; Ratnakumar, K.; Langdon, E.; Kansler, E.; Kim, I.; Campbell, N.R.; Perry, E.; McMahon, A.; Kaufman, C.; van Rooijen, E.; et al. A Quantitative system for studying metastasis using transparent zebrafish. Cancer Res. 2015, 75, 4272–4282. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.C.; Tang, Q.; Yordán, N.T.; Moore, F.E.; Garcia, E.G.; Lobbardi, R.; Ramakrishnan, A.; Marvin, D.L.; Anselmo, A.; Sadreyev, R.I.; et al. Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. J. Exp. Med. 2016, 213, 2575–2589. [Google Scholar] [CrossRef]
- Tang, Q.; Moore, J.C.; Ignatius, M.S.; Tenente, I.M.; Hayes, M.N.; Garcia, E.G.; Torres Yordán, N.; Bourque, C.; He, S.; Blackburn, J.S.; et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 2016, 7, 10358. [Google Scholar] [CrossRef]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell 2019, 177, 1903–1914.e14. [Google Scholar] [CrossRef] [PubMed]
- Stachura, D.L.; Svoboda, O.; Campbell, C.A.; Espín-Palazón, R.; Lau, R.P.; Zon, L.I.; Bartunek, P.; Traver, D. The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood 2013, 122, 3918–3928. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, N.; Sabroe, I.; Renshaw, S.A. A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models. F1000Research 2018, 7, 594. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.; Melong, N.; Hing Wong, W.; King, B.; Tong, S.R.; Mahajan, N.; Gaston, D.; Lund, T.; Rittenberg, D.; Dellaire, G.; et al. Humanized zebrafish enhance human hematopoietic stem cell survival and promote acute myeloid leukemia clonal diversity. Haematologica 2020, 105, 2391–2399. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Hyeon, D.Y.; Hwang, D. Single-cell multiomics: Technologies and data analysis methods. Exp. Mol. Med. 2020, 52, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- White, M.D.; Zhao, Z.W.; Plachta, N. In vivo imaging of single mammalian cells in development and disease. Trends Mol. Med. 2018, 24, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Naber, H.P.H.; Drabsch, Y.; Snaar-Jagalska, B.E.; ten Dijke, P.; van Laar, T. Snail and slug, key regulators of TGF-β-induced EMT, are sufficient for the induction of single-cell invasion. Biochem. Biophys. Res. Commun. 2013, 435, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Fior, R.; Póvoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243. [Google Scholar] [CrossRef] [Green Version]
- Pascoal, S.; Salzer, B.; Scheuringer, E.; Wenninger-Weinzierl, A.; Sturtzel, C.; Holter, W.; Taschner-Mandl, S.; Lehner, M.; Distel, M. A preclinical embryonic zebrafish xenograft model to investigate CAR T. Cells in vivo. Cancers 2020, 12, 567. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, Y.; Lim, S.; Hosaka, K.; Yang, Y.; Pavlova, T.; Alkasalias, T.; Hartman, J.; Jensen, L.; Xing, X.; et al. A zebrafish model discovers a novel mechanism of stromal fibroblast-mediated cancer metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4769–4779. [Google Scholar] [CrossRef] [Green Version]
- Van Wijk, R.C.; Krekels, E.H.J.; Kantae, V.; Ordas, A.; Kreling, T.; Harms, A.C.; Hankemeier, T.; Spaink, H.P.; van der Graaf, P.H. Mechanistic and quantitative understanding of pharmacokinetics in zebrafish Larvae through nanoscale blood sampling and metabolite modeling of paracetamol. J. Pharmacol. Exp. Ther. 2019, 371, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; Tebby, C.; Brochot, C.; Bois, F.Y.; Bado-Nilles, A.; Dorne, J.L.; Quignot, N.; Beaudouin, R. Generic physiologically-based toxicokinetic modelling for fish: Integration of environmental factors and species variability. Sci. Total Environ. 2019, 651, 516–531. [Google Scholar] [CrossRef]
- Villacrez, M.; Hellman, K.; Ono, T.; Sugihara, Y.; Rezeli, M.; Ek, F.; Marko-Varga, G.; Olsson, R. Evaluation of drug exposure and metabolism in locust and zebrafish brains using mass spectrometry imaging. ACS Chem. Neurosci. 2018, 9, 1994–2000. [Google Scholar] [CrossRef]
- Hill, D.; Chen, L.; Snaar-Jagalska, E.; Chaudhry, B. Embryonic zebrafish xenograft assay of human cancer metastasis. F1000Research 2018, 7, 1682. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Bailey, T.J.; Hyde, D.R.; Poss, K.D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013, 29, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsu, Y.; Baker, M.E. Progesterone activation of zebrafish mineralocorticoid receptor may influence growth of some transplanted tumors. Proc. Natl. Acad. Sci. USA 2018, 115, E2908–E2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamble, J.T.; Elson, D.J.; Greenwood, J.A.; Tanguay, R.L.; Kolluri, S.K. The Zebrafish Xenograft Models for Investigating Cancer and Cancer Therapeutics. Biology 2021, 10, 252. https://doi.org/10.3390/biology10040252
Gamble JT, Elson DJ, Greenwood JA, Tanguay RL, Kolluri SK. The Zebrafish Xenograft Models for Investigating Cancer and Cancer Therapeutics. Biology. 2021; 10(4):252. https://doi.org/10.3390/biology10040252
Chicago/Turabian StyleGamble, John T., Daniel J. Elson, Juliet A. Greenwood, Robyn L. Tanguay, and Siva K. Kolluri. 2021. "The Zebrafish Xenograft Models for Investigating Cancer and Cancer Therapeutics" Biology 10, no. 4: 252. https://doi.org/10.3390/biology10040252
APA StyleGamble, J. T., Elson, D. J., Greenwood, J. A., Tanguay, R. L., & Kolluri, S. K. (2021). The Zebrafish Xenograft Models for Investigating Cancer and Cancer Therapeutics. Biology, 10(4), 252. https://doi.org/10.3390/biology10040252







