A Uremic Goat Model Created by Subtotal Renal Artery Embolization and Gentamicin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Enimal
2.2. Subtotal Renal Artery Embolization
2.3. Measurement of Glomerular Filtration Rate and Renal Plasma Flow
2.4. The Effect of Gentamicin on Uremic Toxins
2.5. Biochemical Monitoring
2.6. Blood Pressure
2.7. Acetazolamide and Ramipril
2.8. Complement Activity
2.9. Histopathology
2.10. Hemodialysis Experiments
2.11. Standard Peritoneal Permeability Analysis
2.12. Statistical Analysis
3. Results
3.1. Subtotal Renal Artery Embolization
3.2. Survival, General Clinical Condition and Bodyweight
3.3. Effect of Gentamicin on Plasma Urea, Creatinine, Phosphate, Potassium Plasma Concentrations
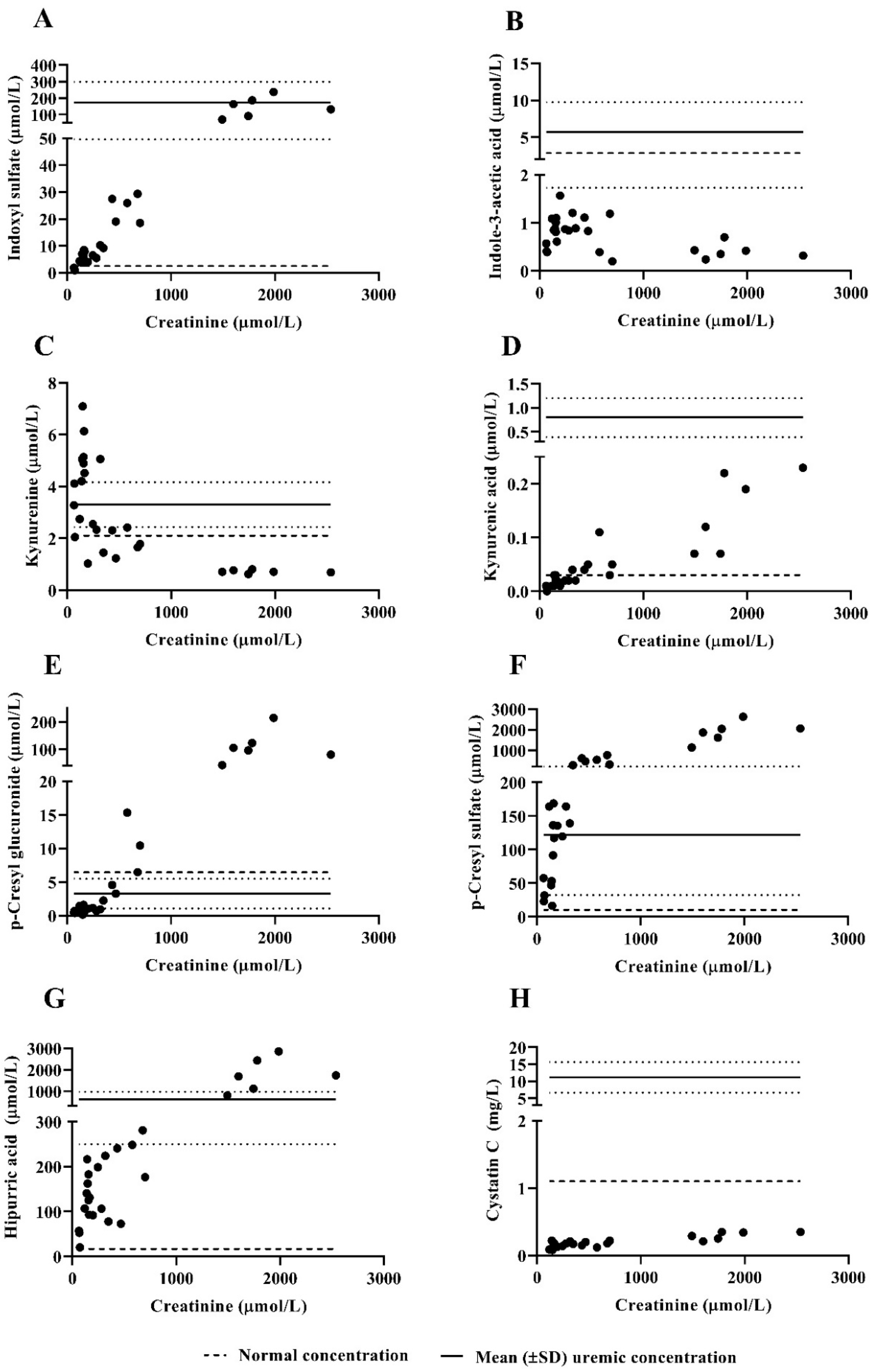
3.4. Protein-Bound Uremic Toxins and Cystatin C Plasma Concentrations
3.5. Blood Pressure
3.6. Effect of Acetazolamide and Ramipril on Plasma Bicarbonate Concentrations
3.7. Complement Activity
3.8. Histopathology
3.9. Hemodialysis Experiments
3.10. Standard Peritoneal Permeability Analysis (SPA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gelder, M.K.; Mihaila, S.M.; Jansen, J.; Wester, M.; Verhaar, M.C.; Joles, J.A.; Stamatialis, D.; Masereeuw, R.; Gerritsen, K.G.F. From portable dialysis to a bioengineered kidney. Expert Rev. Med. Devices 2018, 15, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, D.; Giasafaki, D.; Charalambopoulou, G.; van Geffen, E.; Gerritsen, K.G.F.; Steriotis, T.; Stamatialis, D. Carbon Adsorbents With Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 14914. [Google Scholar] [CrossRef]
- Pavlenko, D.; van Geffen, E.; van Steenbergen, M.J.; Glorieux, G.; Vanholder, R.; Gerritsen, K.G.; Stamatialis, D. New low-flux mixed matrix membranes that offer superior removal of protein-bound toxins from human plasma. Sci. Rep. 2016, 6, 34429. [Google Scholar] [CrossRef]
- Chade, A.R.; Williams, M.L.; Engel, J.; Guise, E.; Harvey, T.W. A translational model of chronic kidney disease in swine. Am. J. Physiol. Ren. Physiol. 2018, 315, F364–F373. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Gordon, J.D.; Fu, A.A.; Glockner, J.F.; Chade, A.R.; Mandrekar, J.; Lerman, L.; Mukhopadhyay, D. The porcine remnant kidney model of chronic renal insufficiency. J. Surg. Res. 2006, 135, 370–379. [Google Scholar] [CrossRef]
- Fartashvand, M.; Mousavi, G.; Hajisadeghi, Y. Gentamicin-induced nephrotoxicity in adult sheep. Adv. Biores. 2012, 3, 116–120. [Google Scholar]
- Eschbach, J.W.; Adamson, J.W.; Dennis, M.B. Physiologic studies in normal and uremic sheep: I. The experimental model. Kidney Int. 1980, 18, 725–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, K.A.; Westover, A.J.; Rojas-Pena, A.; Buffington, D.A.; Pino, C.J.; Smith, P.L.; Humes, H.D. Development of a wearable bioartificial kidney using the Bioartificial Renal Epithelial Cell System (BRECS). J. Tissue Eng. Regen Med. 2017, 11, 3048–3055. [Google Scholar] [CrossRef]
- Pino, C.J.; Farokhrani, A.; Lou, L.; Smith, P.L.; Johnston, K.; Buffington, D.A.; Humes, H.D. Selective cytopheretic inhibitory device with regional citrate anticoagulation and portable sorbent dialysis. Artif. Organs 2013, 37, 203–210. [Google Scholar] [CrossRef]
- Roberts, M.B.C.; Zaragosa, J.S. Pig Trial of Automated Wearable Artificial Kidneys Based on Peritoneal Dialysis. Expert Rev. Med Devices 2016, 15, 323–336. [Google Scholar]
- Wester, M.; Gerritsen, K.G.; Simonis, F.; Boer, W.H.; Hazenbrink, D.H.; Vaessen, K.R.; Verhaar, M.C.; Joles, J.A. A regenerable potassium and phosphate sorbent system to enhance dialysis efficacy and device portability: A study in awake goats. Nephrol. Dial. Transplant. 2017, 32, 951–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poesen, R.; Mutsaers, H.A.; Windey, K.; van den Broek, P.H.; Verweij, V.; Augustijns, P.; Kuypers, D.; Jansen, J.; Evenepoel, P.; Verbeke, K.; et al. The Influence of Dietary Protein Intake on Mammalian Tryptophan and Phenolic Metabolites. PLoS ONE 2015, 10, e0140820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.Y.; Shinaberger, C.S.; Regidor, D.L.; McAllister, C.J.; Kopple, J.D.; Kalantar-Zadeh, K. Association between serum bicarbonate and death in hemodialysis patients: Is it better to be acidotic or alkalotic? Clin. J. Am. Soc. Nephrol. 2006, 1, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Black, M.D.; Robert, M. Rose & Black’s Clinical Problems in Nephrology, 1st ed.; Little Brown: New York, NY, USA, 1996. [Google Scholar]
- Nonkes, L.J.P.; van Gelder, M.K.; Kemperman, H.; Abrahams, A.C.; Boereboom, F.T.J.; Ten Berg, M.J.; Gerritsen, K.G.F. Improving home haemodialysis: Stability evaluation of routine clinical chemistry analytes in blood samples of haemodialysis patients. Biochem. Med. 2019, 29, 010709. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Dodds, A.W.; Arguello, A.; Castro, N.; Sim, R.B. The complement system of the goat: Haemolytic assays and isolation of major proteins. BMC Vet. Res. 2012, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugirdas, J.T. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv. Ren. Replace. Ther. 1995, 2, 295–304. [Google Scholar] [CrossRef]
- Pannekeet, M.M.; Imholz, A.L.; Struijk, D.G.; Koomen, G.C.; Langedijk, M.J.; Schouten, N.; de Waart, R.; Hiralall, J.; Krediet, R.T. The standard peritoneal permeability analysis: A tool for the assessment of peritoneal permeability characteristics in CAPD patients. Kidney Int. 1995, 48, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Krediet, R.T.; Boeschoten, E.W.; Zuyderhoudt, F.M.; Strackee, J.; Arisz, L. Simple assessment of the efficacy of peritoneal transport in continuous ambulatory peritoneal dialysis patients. Blood Purif. 1986, 4, 194–203. [Google Scholar] [CrossRef]
- Uremic Solutes Database. Available online: https://database.uremic-toxins.org/soluteList.php (accessed on 2 February 2021).
- de Loor, H.; Bammens, B.; Evenepoel, P.; De Preter, V.; Verbeke, K. Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin. Chem. 2005, 51, 1535–1538. [Google Scholar] [CrossRef]
- Twardowski, Z.J.; Nolph, K.D.; Khanna, R.; Prowant, B.F.; Ryan, L.P.; Moore, H.L. Peritoneal equilibration test. Perit. Dial. Bull. 1987, 7, 138–147. [Google Scholar]
- Gaertner, K. Untersuchungen ueber die Groesse des Verteilungsraumes yon Harnstoff bei Ziegen. (Studies of the size of distribution pool of urea in goats). Zeitschr. Tierphysiol. Tierernaehr. Futtermittlk. 1961, 16, 366. [Google Scholar] [CrossRef]
- Laurent, G.; Carlier, M.B.; Rollman, B.; Van Hoof, F.; Tulkens, P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: In vitro and in vivo studies with gentamicin and amikacin. Biochem. Pharmacol. 1982, 31, 3861–3870. [Google Scholar] [CrossRef]
- Laurent, G.; Kishore, B.K.; Tulkens, P.M. Aminoglycoside-induced renal phospholipidosis and nephrotoxicity. Biochem. Pharmacol. 1990, 40, 2383–2392. [Google Scholar] [CrossRef]
- Kacew, S.; Bergeron, M.G. Pathogenic factors in aminoglycoside-induced nephrotoxicity. Toxicol. Lett. 1990, 51, 241–259; discussion 237–249. [Google Scholar] [CrossRef]
- Gandhi, M.; Olson, J.L.; Meyer, T.W. Contribution of tubular injury to loss of remnant kidney function. Kidney Int. 1998, 54, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingeot-Leclercq, M.P.; Tulkens, P.M. Aminoglycosides: Nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Mandell, G.L.; Bennet, J.E.; Dolin, R. Principles and Practice of Infectious Diseases, 7th ed.; Churchill Livingstone Elsevier: London, UK, 2009. [Google Scholar]
- Evenepoel, P.; Meijers, B.K.; Bammens, B.; Viaene, L.; Claes, K.; Sprangers, B.; Naesens, M.; Hoekstra, T.; Schlieper, G.; Vanderschueren, D.; et al. Phosphorus metabolism in peritoneal dialysis- and haemodialysis-treated patients. Nephrol. Dial. Transplant. 2016, 31, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.M.; Spiegel, D.M.; Du Mond, C.; Oestreicher, N.; Winkelmayer, W.C.; Kovesdy, C.P. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol. Dial. Transplant. 2018, 33, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Hydbring, E.; Cvek, K.; Olsson, K. Telemetric registration of heart rate and blood pressure in the same unrestrained goats during pregnancy, lactation and the non-pregnant, non-lactating period. Acta Physiol. Scand. 1999, 165, 135–141. [Google Scholar] [CrossRef]
- International Organisation for Standardisation. Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood, 3rd ed.; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Use of International Standard ISO-10993. Biological valuation of Medical Devices Part 1: Evaluation and Testing; Final Guidance for Industry and Food and Drug Administration Staff, US Department of Health and Human Services, FDA, Center for Devices and Radiological Health, Office of Device Evaluation: Rockville, MD, USA, 2016.
- Moist, L.M.; Hemmelgarn, B.R.; Lok, C.E. Relationship between blood flow in central venous catheters and hemodialysis adequacy. Clin. J. Am. Soc. Nephrol. 2006, 1, 965–971. [Google Scholar] [CrossRef]
- Rumpsfeld, M.; McDonald, S.P.; Johnson, D.W. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J. Am. Soc. Nephrol. 2006, 17, 271–278. [Google Scholar] [CrossRef]
- Clerbaux, G.; Francart, J.; Wallemacq, P.; Robert, A.; Goffin, E. Evaluation of peritoneal transport properties at onset of peritoneal dialysis and longitudinal follow-up. Nephrol. Dial. Transplant. 2006, 21, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixido-Planas, J. Peritoneal function and adequacy calculations: Current programs versus PD Adequest 2.0. Perit. Dial. Int. 2002, 22, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.; Terzic, J.; Chauve, S.; Laugel, V.; Muller, A.; Haraldsson, B. Effect of peritoneal dialysis fluid composition on peritoneal area available for exchange in children. Nephrol. Dial. Transplant. 2004, 19, 925–932. [Google Scholar] [CrossRef]
- Krediet, R.T.; Zweers, M.M.; van der Wal, A.C.; Struijk, D.G. Neoangiogenesis in the peritoneal membrane. Perit. Dial. Int. 2000, 20 (Suppl. 2), S19–S25. [Google Scholar] [CrossRef]
- Wester, M.; van Gelder, M.K.; Joles, J.A.; Simonis, F.; Hazenbrink, D.H.M.; van Berkel, T.W.M.; Vaessen, K.R.D.; Boer, W.H.; Verhaar, M.C.; Gerritsen, K.G.F. Removal of urea by electro-oxidation in a miniature dialysis device: A study in awake goats. Am. J. Physiol. Ren. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
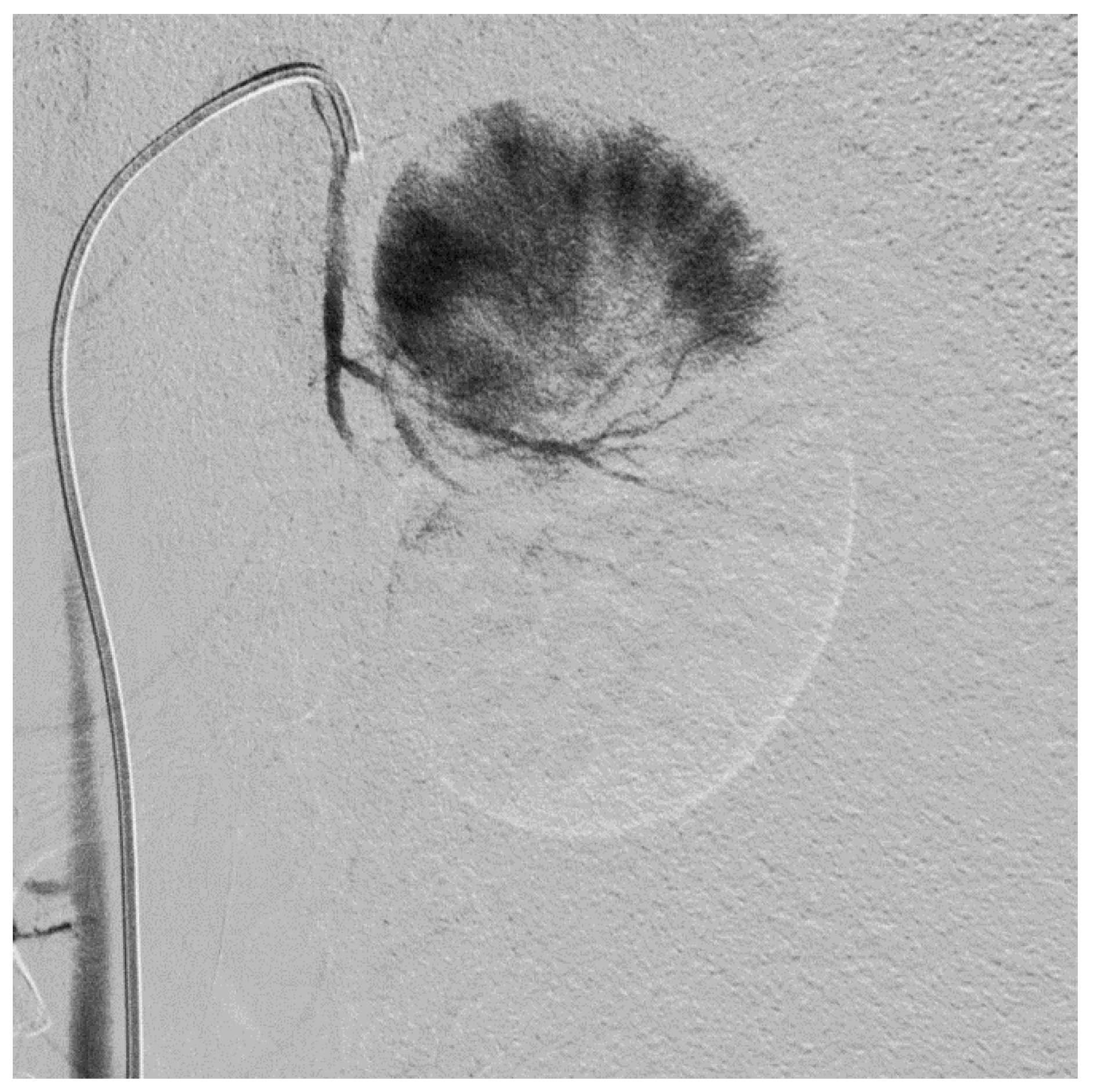



| Measurement | Pre-Embolization | Post-Embolization | AKI |
|---|---|---|---|
| Urea (mmol/L) | 5.6 ± 0.8 | 12 ± 1.4 | 29.4 ± 15.4 |
| Creatinine (µmol/L) | 65 ± 5.6 | 167 ± 13 | 841 ± 584 |
| Phosphate (mmol/L) | 1.73 ± 0.19 | 1.44 ± 0.24 | 2.18 ± 0.28 |
| Indoxyl sulfate (µmol/L) | 1.39 ± 0.50 | 69 ± 76 | |
| Indole-3-acetic acid (µmol/L) | 0.46 ± 0.10 | 0.67 ± 0.35 | |
| Kynurenine (µmol/L) | 3.15 ± 1.04 | 1.67 ± 1.18 | |
| Kynurenic acid (µmol/L) | 0.01 ± 0.01 | 0.09 ± 0.07 | |
| p-Cresyl glucuronide (µmol/L) | 0.52 ± 0.14 | 47 ± 64 | |
| p-Cresyl sulfate (µmol/L) | 37 ± 18 | 985 ± 843 | |
| Hippuric acid (µmol/L) | 43 ± 20 | 817 ± 931 | |
| Cystatin C (mg/L) | 0.13 ± 0.05 | 0.22 ± 0.08 | |
| GFR (mL/min) | 58 ± 18 | 22 ± 14 | |
| ERBF (mL/min) | 1409 ± 325 | 412 ± 210 |
| SPA | MTAC Urea (mL/min) | MTAC Creatinine (mL/min) | MTAC Phosphate (mL/min) | D/P Urea t = 4 h | D/P Creatinine t = 4 h |
|---|---|---|---|---|---|
| 1 | 3.8 | 2.3 | 3.0 | 0.4 | 0.3 |
| 2 | 6.4 | 5.1 | 6.3 | 0.4 | 0.3 |
| 3 | 7.9 | 6.0 | 4.8 | 0.6 | 0.5 |
| 4 | 7.2 | 5.5 | 5.8 | 0.7 | 0.5 |
| Mean ± SD | 6.3 ± 1.5 | 4.7 ± 1.4 | 5.0 ± 1.2 | 0.5 ± 0.1 | 0.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gelder, M.K.; de Vries, J.C.; Ahmed, S.; Monninkhof, A.S.; de Kort, G.A.P.; Vonken, E.-J.P.A.; Hazenbrink, D.H.M.; Vaessen, K.R.D.; Nguyen, T.Q.; Verhaar, M.C.; et al. A Uremic Goat Model Created by Subtotal Renal Artery Embolization and Gentamicin. Biology 2021, 10, 292. https://doi.org/10.3390/biology10040292
van Gelder MK, de Vries JC, Ahmed S, Monninkhof AS, de Kort GAP, Vonken E-JPA, Hazenbrink DHM, Vaessen KRD, Nguyen TQ, Verhaar MC, et al. A Uremic Goat Model Created by Subtotal Renal Artery Embolization and Gentamicin. Biology. 2021; 10(4):292. https://doi.org/10.3390/biology10040292
Chicago/Turabian Stylevan Gelder, Maaike K., Joost C. de Vries, Sabbir Ahmed, Anneke S. Monninkhof, Gérard A. P. de Kort, Evert-Jan P. A. Vonken, Diënty H. M. Hazenbrink, Koen R. D. Vaessen, Tri Q. Nguyen, Marianne C. Verhaar, and et al. 2021. "A Uremic Goat Model Created by Subtotal Renal Artery Embolization and Gentamicin" Biology 10, no. 4: 292. https://doi.org/10.3390/biology10040292






