Identification of Differential N-Glycan Compositions in the Serum and Tissue of Colon Cancer Patients by Mass Spectrometry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Patients and Controls
2.3. Samples
2.3.1. Serum
2.3.2. Tissue
2.4. Protein Extraction from Tissue Samples
2.5. N-Glycan Isolation and Derivatization
2.6. Determination of N-Glycan Profile from Blood Samples
2.7. Determination of N-Glycan Profile from Tissue Samples
2.8. Mass Spectrometry Acquisitions and Analysis
2.9. MALDI-TOF/MS
2.10. ESI-LC-MS/MS
3. Results
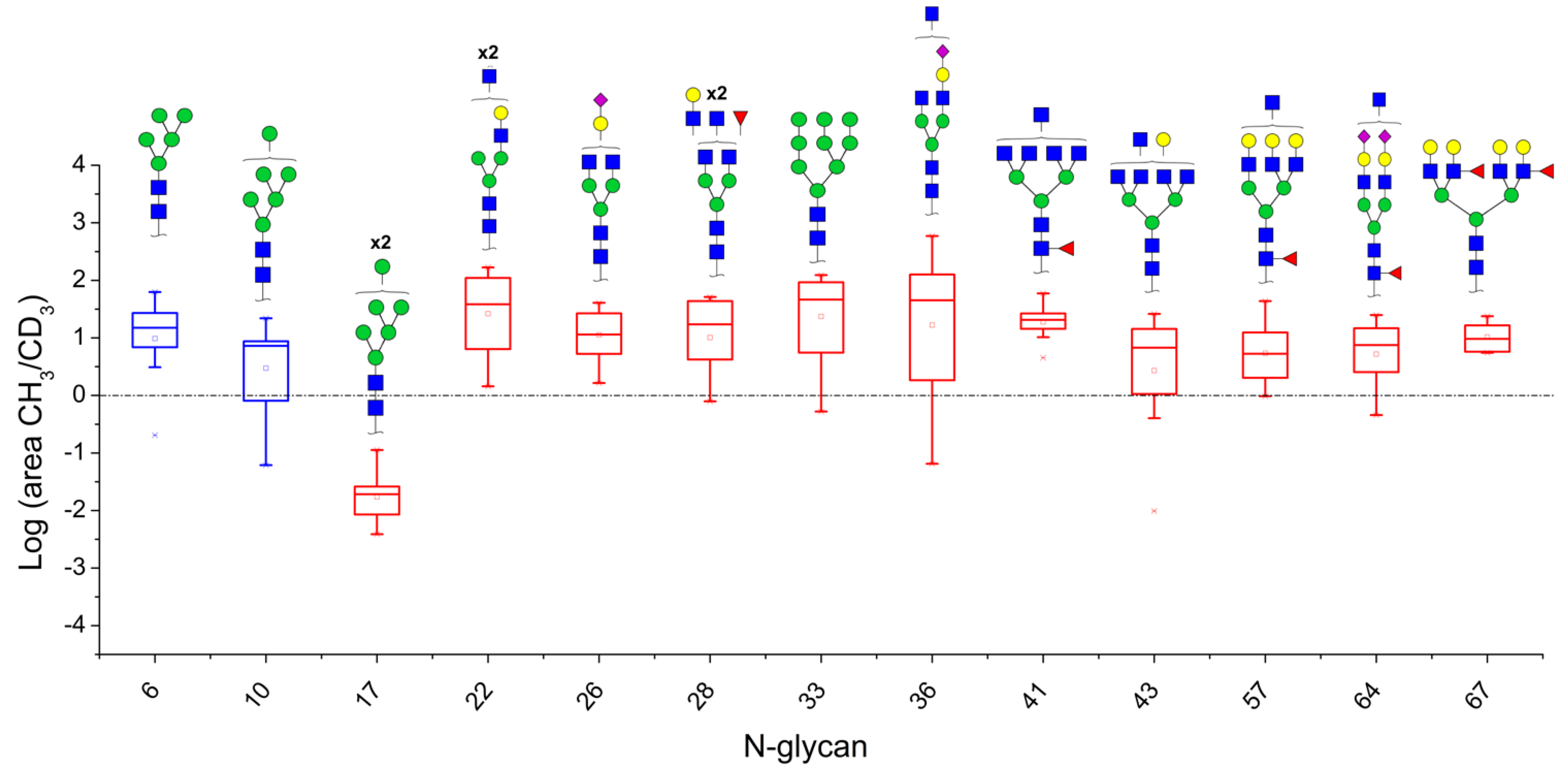
3.1. Patients with Colon Cancer Present Differential N-Glycans in Their Blood Serum in Relation to Normal Individuals
3.2. N-Glycan Profiles in CRC Tissue Are Heterogeneous, However, Some Glycan Compositions Are Consistently Altered in Patients
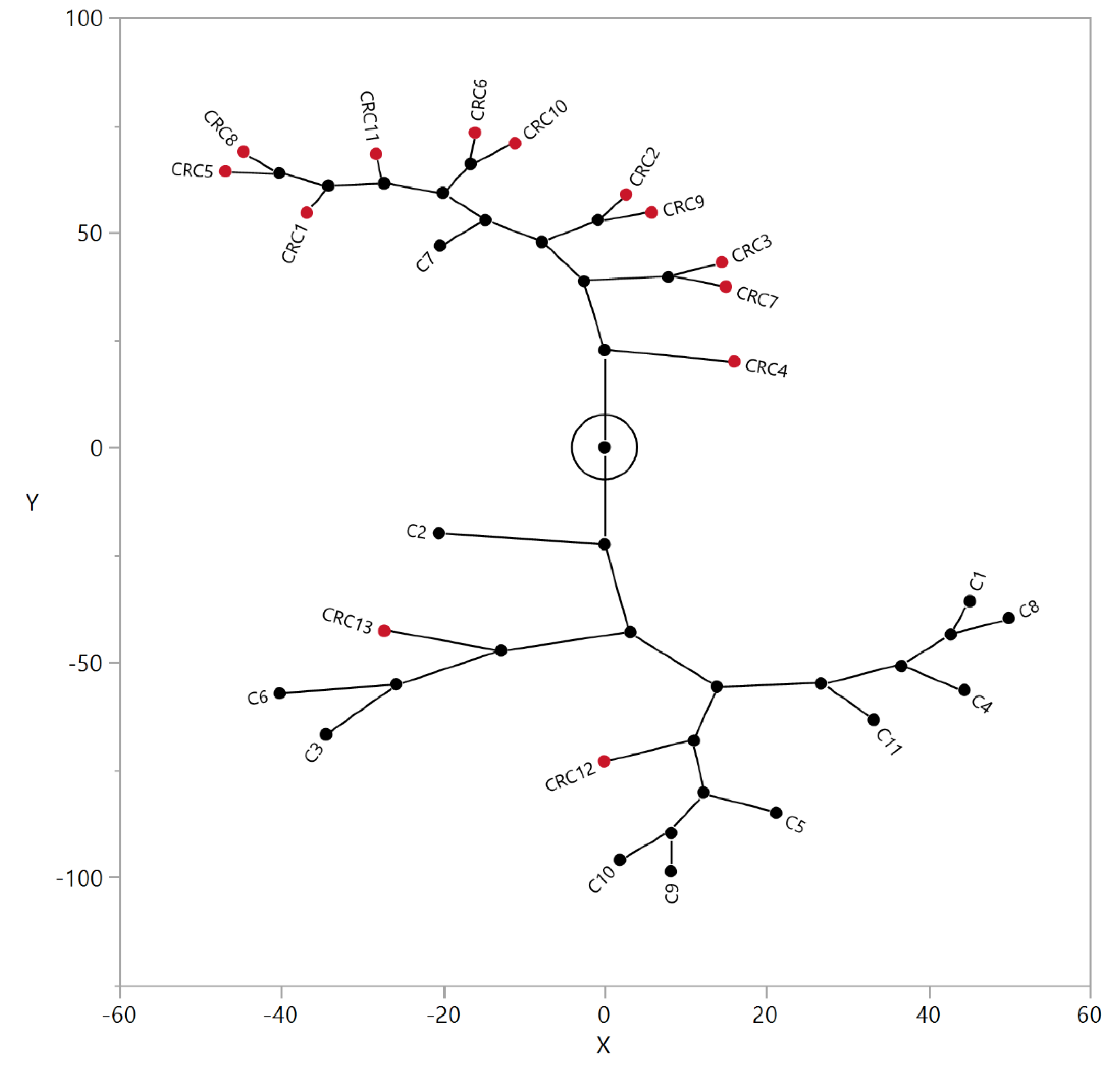
3.3. Correlation between Relative Levels of N-Glycans in Serum and Tissue of CRC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- PDQ Screening and Prevention Editorial Board. Colorectal Cancer Screening (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. NIH.gov. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65825/ (accessed on 29 March 2021).
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; García, F.A.R.; Gillman, M.W.; Harper, D.M.; Kemper, A.R.; et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 2016, 315, 2564–2575. [Google Scholar] [CrossRef]
- An, H.J.; Kronewitter, S.R.; de Leoz, M.L.A.; Lebrilla, C.B. Glycomics and disease markers. Curr. Op. Chem. Biol. 2009, 13, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäbert, K.; Cojoc, M.; Peitzsch, C.; Kurth, I.; Souchelnytskyi, S.; Dubrovska, A. Cancer biomarker discovery: Current status and future perspectives. Int. J. Rad. Biol. 2014, 90, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauc, G.; Zoldoš, V. Protein glycosylation—An evolutionary crossroad between genes and environment. Mol. BioSys. 2010, 6, 2373–2379. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Mechref, Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis 2012, 33, 1768–1777. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Zauner, G.; Huhn, C.; Bruggink, C.; Deelder, A.M.; Wuhrer, M. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010, 397, 3457–3481. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.G.; Balmaña, M.; Macedo, J.A.; Poças, J.; Fernandes, Â.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhães, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell Immun. 2018, 333, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [Green Version]
- Sethi, M.K.; Kim, H.; Park, C.K.; Baker, M.S.; Paik, Y.K.; Packer, N.H.; Hancock, W.S.; Fanayan, S.; Thaysen-Andersen, M. In-depth N-glycome profiling of paired colorectal cancer and non-tumorigenic tissues reveals cancer-, stage- and EGFR-specific protein N-glycosylation. Glycobiology 2015, 10, 1064–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balog, C.I.A.; Stavenhagen, K.; Fung, W.L.J.; Koeleman, C.A.; McDonnell, L.A.; Verhoeven, A.; Mesker, W.E.; Tollenaar, R.A.E.M.; Deelder, A.M.; Wuhrer, M. N-glycosylation of colorectal cancer tissues: A liquid chromatography and mass spectrometry-based investigation. Mol. Cell. Proteom. 2012, 11, 571–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Xie, Q.; Wang, Q.; Wang, Y.; Miao, J.; Li, L.; Zhang, T.; Cao, X.; Li, Y. Mass spectrometry analysis reveals aberrant N-glycans in colorectal cancer tissues. Glycobiology 2019, 29, 372–384. [Google Scholar] [CrossRef]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation characteristics of colorectal cancer. Adv. Cancer Res. 2015, 126, 203–256. [Google Scholar] [CrossRef]
- Muinelo-Romay, L.; Vázquez-Martín, C.; Villar-Portela, S.; Cuevas, E.; Gil-Martín, E.; Fernández-Briera, A. Expression and enzyme activity of alpha (1,6) fucosyltransferase in human colorectal cancer. Int. J. Cancer 2008, 123, 641–646. [Google Scholar] [CrossRef]
- Holm, M.; Nummela, P.; Heiskanen, A.; Satomaa, T.; Kaprio, T.; Mustonen, H.; Ristimäki, A.; Haglund, C. N-glycomic profiling of colorectal cancer according to tumor stage and location. PLoS ONE 2020, 15, e0234989. [Google Scholar] [CrossRef]
- Kaprio, T.; Satomaa, T.; Heiskanen, A.; Hokke, C.H.; Deelder, A.M.; Mustonen, H.; Hagström, J.; Carpen, O.; Saarinen, J.; Haglund, C. N-glycomic Profiling as a Tool to Separate Rectal Adenomas from Carcinomas. Mol. Cell. Proteom. 2015, 14, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Boyaval, F.; van Zeijl, R.; Dalebout, H.; Holst, S.; van Pelt, G.W.; Fariña-Sarasqueta, A.; Mesker, W.E.; Tollenaar, R.; Morreau, H.; Wuhrer, M. N-glycomic signature of stage II colorectal cancer and its association with the tumor microenvironment. Mol. Cell. Proteom. 2020. [Google Scholar] [CrossRef]
- Snyder, C.M.; Alley, W.R., Jr.; Campos, M.I.; Svoboda, M.; Goetz, J.A.; Vasseur, J.A.; Jacobson, S.C.; Novotny, M.V. Complementary glycomic analyses of sera derived from colorectal cancer patients by MALDI-TOF-MS and microchip electrophoresis. Anal. Chem. 2016, 88, 9597–9605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clerc, F.; Novokmet, M.; Dotz, V.; Reiding, K.R.; de Haan, N.; Kammeijer, G.S.M.; Dalebout, H.; Bladergroen, M.R.; Vukovic, F.; Rapp, E.; et al. Plasma N-glycan signature associated with features of inflammatory bowel disease. Gastroenterology 2018, 155, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Clerc, F.; Reiding, K.R.; Jansen, B.C.; Kammeijer, G.S.M.; Bondt, A.; Wuhrer, M. Human plasma protein N-glycosylation. Glycoconj. J. 2016, 33, 309–343. [Google Scholar] [CrossRef] [Green Version]
- Dotz, V.; Wuhrer, M. N-glycome signatures in human plasma: Associations with physiology and major diseases. FEBS Lett. 2019, 593, 2966–2976. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ruan, C.; Wang, H.; Hu, Z.; Fang, M.; Gu, X.; Ji, J.; Zhao, J.; Gao, C. Identification and assessment of new biomarkers for colorectal cancer with serum N-glycan profiling. Cancer 2011, 118, 639–650. [Google Scholar] [CrossRef]
- Doherty, M.; Theodoratou, E.; Walsh, I.; Adamczyk, B.; Stöckmann, H.; Agakov, F.; Timofeeva, M.; Trbojević-Akmačić, I.; Vučković, F.; Duffy, F.; et al. Plasma N-glycans in colorectal cancer risk. Sci. Rep. 2018, 8, 8655–8666. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Morelle, W.; Michalski, J.C. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007, 2, 1585–1602. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.A.; Fontes, N.D.C.; Santos, S.C.L.; Lefeber, D.J.; Bloch, C.; Brum, J.M.; Brand, G.D. Relative quantification of plasma N-glycans in type II congenital disorder of glycosylation patients by mass spectrometry. Clin. Chim. Acta. 2019, 492, 102–113. [Google Scholar] [CrossRef]
- Song, T.; Aldredge, D.; Lebrilla, C.B. A Method for In-Depth Structural Annotation of Human Serum Glycans That Yields Biological Variations. Anal. Chem. 2015, 87, 7754–7762. [Google Scholar] [CrossRef] [Green Version]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- Kronewitter, S.R.; An, H.J.; de Leoz, M.L.; Lebrilla, C.B.; Miyamoto, S.; Leiserowitz, G.S. The development of retrosynthetic glycan libraries to profile and classify the human serum N-linked glycome. Proteomics 2009, 9, 2986–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldredge, D.; An, H.J.; Tang, N.; Waddell, K.; Lebrilla, C.B. Annotation of a Serum N-Glycan Library for Rapid Identification of Structures. J. Proteome Res. 2012, 11, 1958–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Z.; Ye, C.; Huang, Y.; Jiang, H.; Ye, Y.; Wang, S.; Zhou, Y.; Shen, Z.; Qiu, X. Human colorectal cancer cells frequently express IgG and display unique Ig repertoire. World J. Gastrointest. Oncol. 2019, 11, 195–207. [Google Scholar] [CrossRef]
- Ihara, H.; Tsukamoto, H.; Gu, J.; Miyoshi, E.; Taniguchi, N.; Ikeda, Y. Fucosyltransferase 8. GDP-Fucose N-Glycan Core α6-Fucosyltransferase (FUT8). In Handbook of Glycosyltransferases and Related Genes; Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 581–596. ISBN 13: 978-4-431-54240-7. [Google Scholar]
- Theodoratou, E.; Thaçi, K.; Agakov, F.; Timofeeva, M.N.; Štambuk, J.; Pučić-Baković, M.; Vučković, F.; Orchard, P.; Agakova, A.; Din, F.V.N.; et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep. 2016, 6, 28098–28110. [Google Scholar] [CrossRef] [PubMed]
- Vučković, F.; Theodoratou, E.; Thaçi, K.; Timofeeva, M.; Vojta, A.; Štambuk, J.; Pučić-Baković, M.; Rudd, P.M.; Đerek, L.; Servis, D.; et al. IgG Glycome in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 3078–3086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Rudd, P.M. Acute phase glycoproteins: Bystanders or participants in carcinogenesis? Ann. N. Y. Acad. Sci. 2012, 1253, 122–132. [Google Scholar] [CrossRef]
- de Vroome, S.W.; Holst, S.; Girondo, M.R.; van der Burgt, Y.E.M.; Mesker, W.E.; Tollenaar, R.A.E.M.; Wuhrer, M. Serum N-glycome alterations in colorectal cancer associate with survival. Oncotarget 2018, 9, 30610–30623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Carethers, J.M.; Jung, B.H. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015, 149, 1177–1190.e3. [Google Scholar] [CrossRef] [Green Version]
- Hardiman, K. Update on Sporadic Colorectal Cancer Genetics. Clin. Colon Rectal Surg. 2018, 31, 147–152. [Google Scholar] [CrossRef] [PubMed]
- de Leoz, M.L.A.; Young, L.J.T.; An, H.J.; Kronewitter, S.R.; Kim, J.; Miyamoto, S.; Borowsky, A.D.; Chew, H.K.; Lebrilla, C.B. High-mannose glycans are elevated during breast cancer progression. Mol. Cell. Proteom. 2011, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, K.; Miyoshi, E.; Kameyama, M.; Ishikawa, O.; Kabuto, T.; Sasaki, Y.; Hiratsuka, M.; Ohigashi, H.; Ishiguro, S.; Ito, S.; et al. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin. Cancer Res. 2000, 6, 1772–1777. [Google Scholar] [PubMed]
- De Freitas Junior, J.C.M.; Morgado-Diaz, J.A. The Role of N-glycans in Colorectal Cancer Progression: Potential Biomarkers and Therapeutic Applications. Oncotarget 2017, 7, 19395–19413. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.; Reis, C.A.; Pinho, S.S. Cadherins Glycans in Cancer: Sweet Players in a Bitter Process. Trends Cancer 2016, 2, 519–531. [Google Scholar] [CrossRef]
- Mare, L.; Caretti, A.; Albertini, R.; Trinchera, M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013, 45, 792–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, J.N.; Saldova, R.; Hamid, U.M.A.; Rudd, P.M. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics 2008, 8, 3284–3293. [Google Scholar] [CrossRef]
- Saldova, R.; Royle, L.; Radcliffe, C.M.; Hamid, U.M.A.; Evans, R.; Arnold, J.N.; Banks, R.E.; Hutson, R.; Harvey, D.J.; Antrobus, R.; et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007, 17, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Kita, Y.; Miura, Y.; Furukawa, J.I.; Nakano, M.; Shinohara, Y.; Ohno, M.; Takimoto, A.; Nishimura, S.I. Quantitative glycomics of human whole serum glycoproteins based on the standardized protocol for liberating n-glycans. Mol. Cell. Proteom. 2007, 6, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
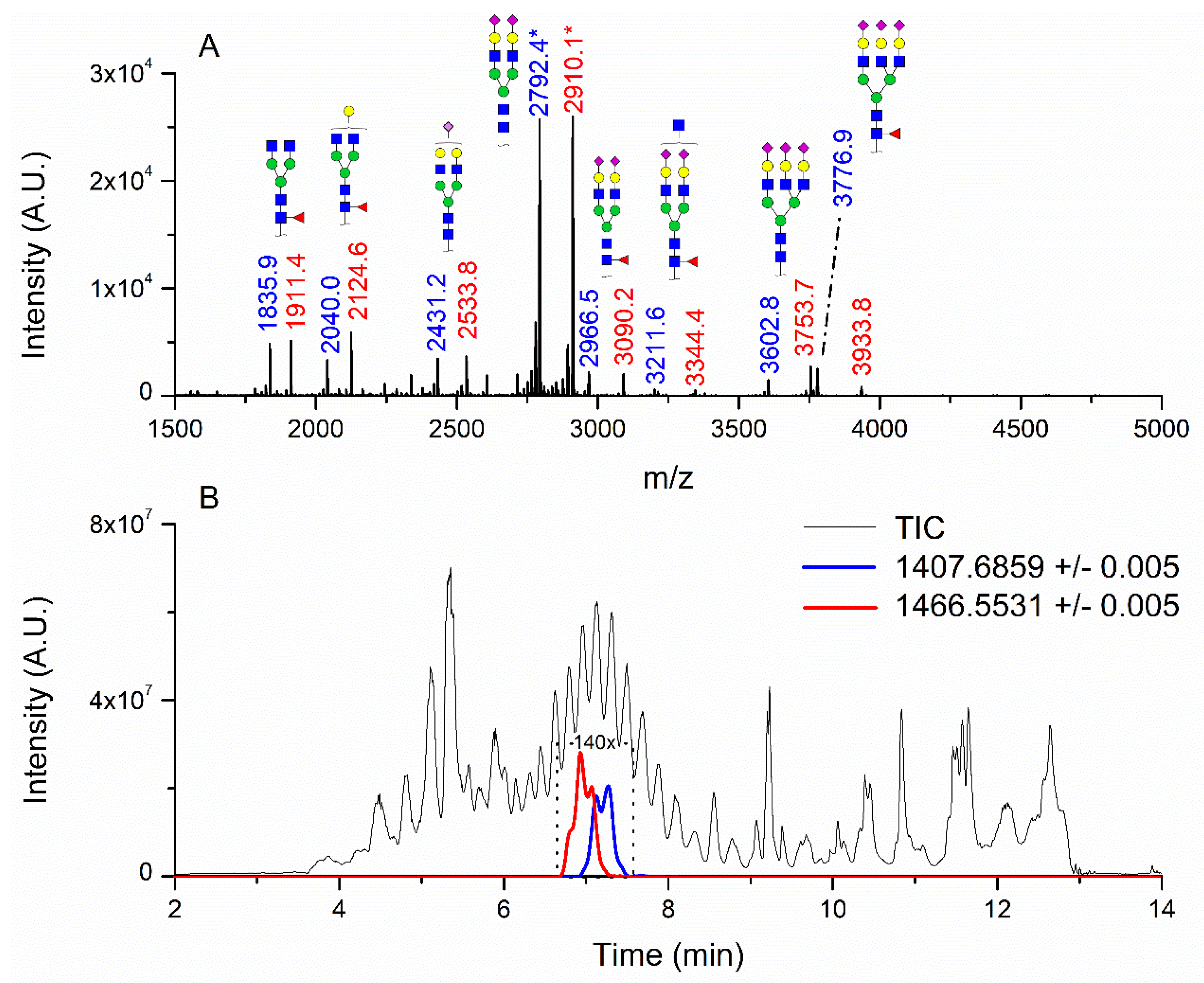
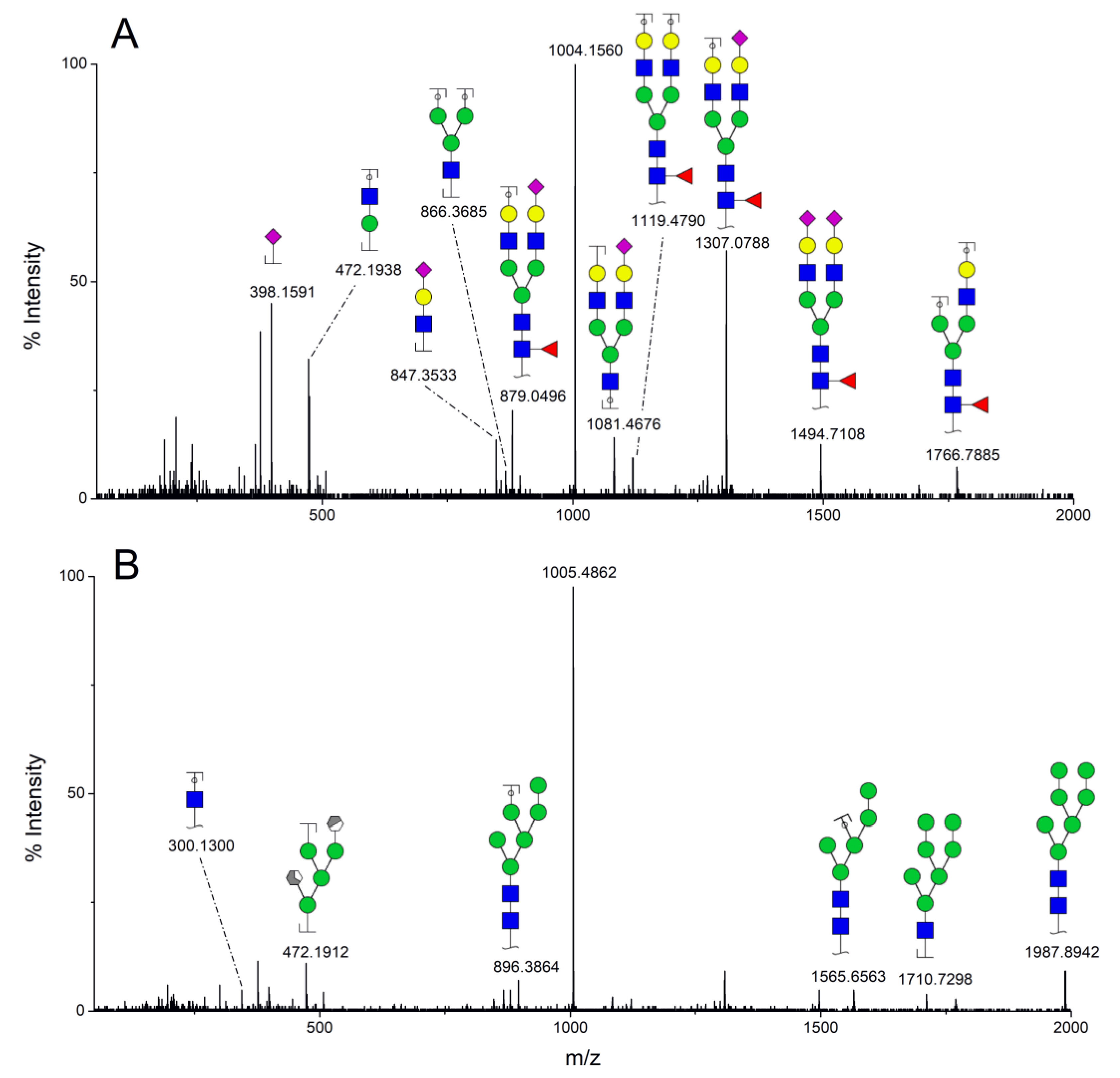
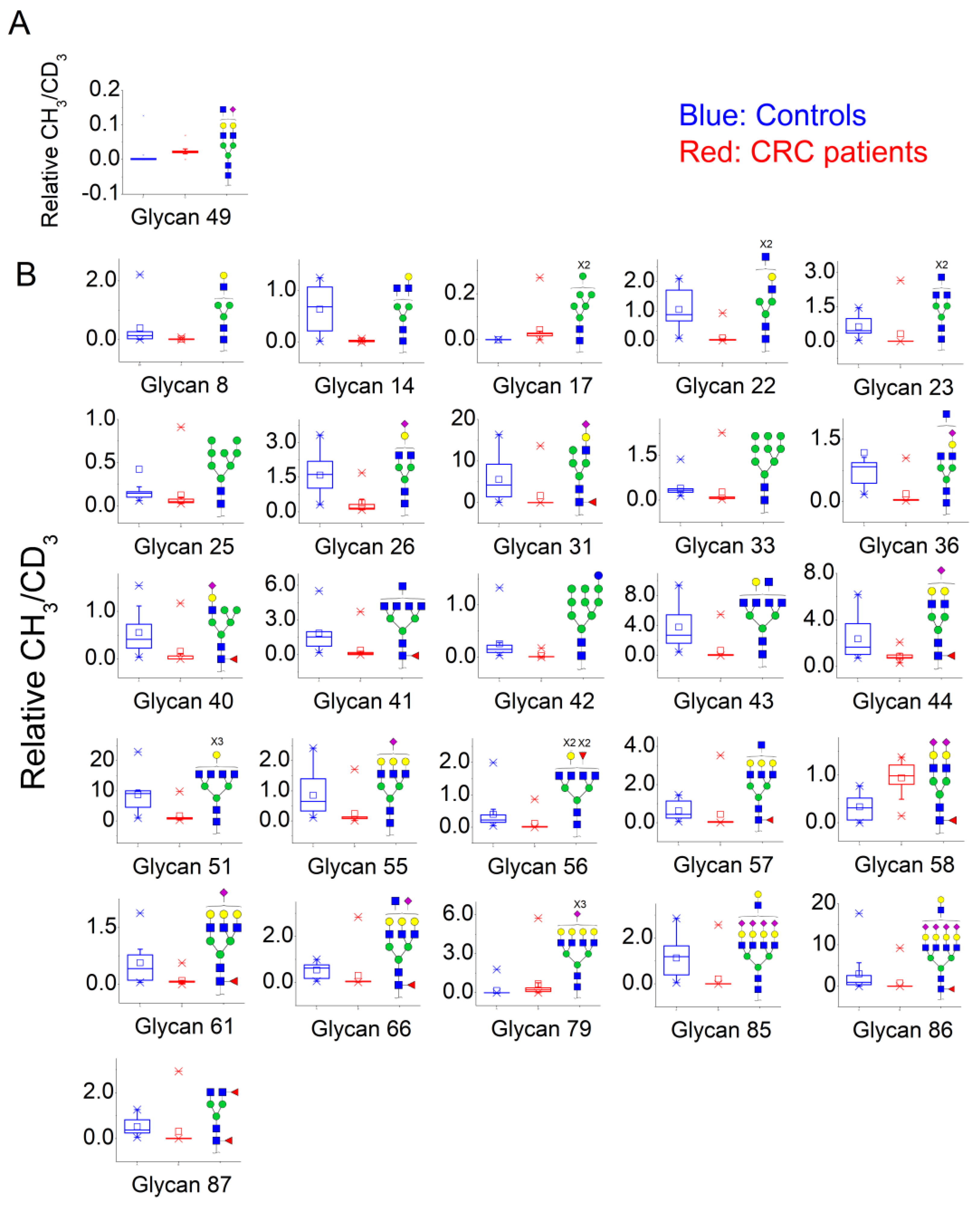
| Patient | Genre | Age (years) | Location | Stage | CEA (ng/mL) | Tumor Diameter (cm) | Mutational Nras/Kras/Braf Status |
|---|---|---|---|---|---|---|---|
| 1 | M | 37 | TRANSVERSE | II | 1.48 | 3.70 | Kras mutated |
| 2 | F | 36 | SIGMOID | II | 1.65 | 2.80 | No |
| 3 | M | 65 | CECUM | II | 1.60 | 4.30 | Kras mutated |
| 4 | M | 55 | DESCENDING | III | 5.30 | 2.60 | No |
| 5 | M | 60 | SIGMOID | II | 7.70 | 4.80 | No |
| 6 | F | 56 | SIGMOID | III | 2.10 | 3.70 | No |
| 7 | M | 67 | ASCENDING | III | 2.30 | 6.00 | Braf mutated |
| 8 | F | 64 | CECUM | III | 11.25 | 4.10 | No |
| 9 | F | 63 | CECUM | II | 6.51 | 6.20 | No |
| 10 | F | 54 | SIGMOID | III | 3.30 | 3.50 | Braf mutated |
| 11 | F | 74 | SIGMOID | III | 6.75 | 1.20 | No |
| 12 | M | 64 | CECUM | III | 5.20 | 7.20 | Kras mutated |
| 13 | F | 75 | SIGMOID | II | 1.35 | 4.60 | Kras mutated |
| Nr. | Proposed Structure, Theoretical Mass, and Composition | Mass Used for Quantitation | Adduct | Heavy Reference | Structure Confirmed by MS/MS Data a | |
|---|---|---|---|---|---|---|
| LC-MS/MS | MALDI | |||||
| 6 | 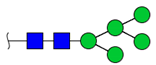 HexNac2Hex5 | 1579.7826 | [M + Na]+ | 52 | 6 | ✓ |
| 8 | 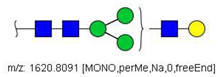 HexNAc3Hex4 | 1620.8090 | [M + Na]+ | 19 | - | * |
| 10 | 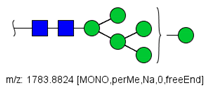 HexNAc2Hex6 | 903.4358 | [M + 2Na]2+ | 52 | 6 | ✓✓ |
| 14 | 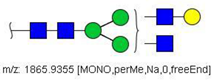 HexNAc4Hex4 | 1865.9355 | [M + Na]+ | 19 | 19 | ✓✓ |
| 17 | 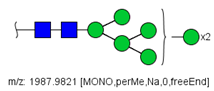 HexNAc2Hex7 | 1005.4857 * | [M + 2Na]2+ | 52 | 6 | ✓✓ |
| 22 | 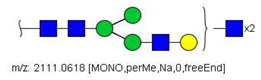 HexNAc5Hex4 | 1067.0255 | [M + 2Na]2+ | 19 | - | * |
| 23 | 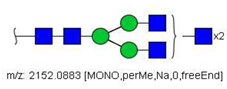 HexNAc6Hex3 | 1087.5388 * | [M + 2Na]2+ | 13 | - | ✓ |
| 25 | 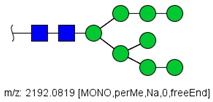 HexNAc2Hex8 | 1107.5356 | [M + 2Na]2+ | 52 | 6 | ✓✓ |
| 26 | 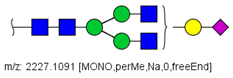 HexNAc4Hex4NeuAc1 | 1125.0492 | [M + 2Na]2+ | 26 | 35 | ✓✓ |
| 28 | 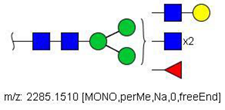 HexNAc5Hex4Fuc1 | 1154.0701 | [M + 2Na]2+ | 19 | 19 | ✓ |
| 31 | 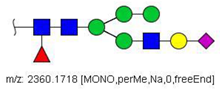 HexNAc3Hex5Fuc1NeuAc1 | 1191.5805 | [M + 2Na]2+ | 27 | - | ✓✓ |
| 33 | 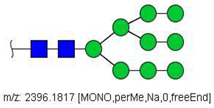 HexNAc2Hex9 | 1209.5855 | [M + 2Na]2+ | 52 | 6 | ✓✓ |
| 36 | 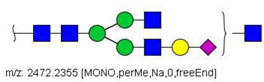 HexNAc5Hex4NeuAc1 | 1247.6123 | [M + 2Na]2+ | 52 | - | ✓✓ |
| 40 | 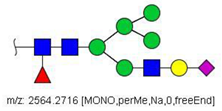 HexNAc3Hex6Fuc1NeuAc1 | 1293.6304 * | [M + 2Na]2+ | 27 | - | ✓✓ |
| 41 | 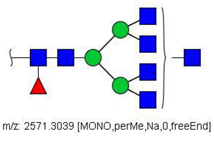 HexNAc7Hex3Fuc1 | 1297.1465 | [M + 2Na]2+ | 75 | - | * |
| 42 | 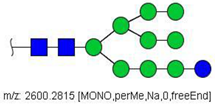 HexNAc2Hex10 | 1311.6353 | [M + 2Na]2+ | 52 | - | ✓ |
| 43 | 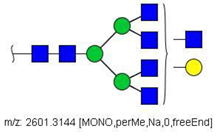 HexNAc7Hex4 | 1312.1518 | [M + 2Na]2+ | 72 | - | ✓ |
| 44 | 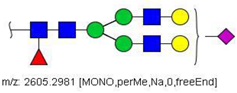 HexNAc4Hex5Fuc1NeuAc1 | 1314.1437 | [M + 2Na]2+ | 44 | 58 | ✓✓ |
| 49 | 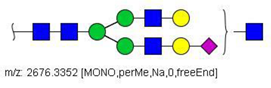 HexNAc5Hex5NeuAc1 | 907.4379 | [M + 3Na]3+ | 35 | ✓ | |
| 51 | 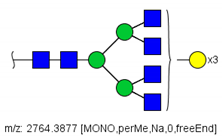 HexNAc6Hex6 | 936.7887 | [M + 3Na]3+ | 52 | 35 | ✓ |
| 52 | 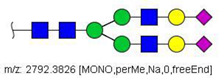 HexNAc4Hex5NeuAc2 | 1407.6859 | [M + 2Na]2+ | 52 | 52 | ✓✓ |
| 55 | 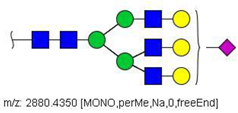 HexNAc5Hex6NeuAc1 | 975.4711 | [M + 3Na]3+ | 72 | 35 | ✓✓ |
| 56 | 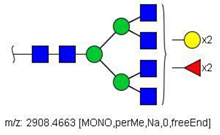 HexNAc6Hex5Fuc2 | 984.8149 | [M + 3Na]3+ | 27 | - | ✓ |
| 57 |  HexNAc6Hex6Fuc1 | 994.8184 | [M + 3Na]3+ | 27 | - | ✓ |
| 58 | 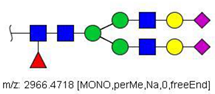 HexNAc4Hex5Fuc1NeuAc2 | 1004.1501 | [M + 3Na]3+ | 58 | 58 | ✓✓ |
| 61 |  HexNAc5Hex6Fuc1NeuAc1 | 1033.5009 * | [M + 3Na]3+ | 27 | - | ✓ |
| 64 | 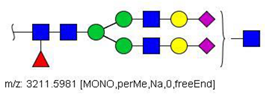 HexNAc5Hex5Fuc1NeuAc2 | 1617.2937 | [M + 2Na]2+ | 27 | 64 | ✓ |
| 66 | 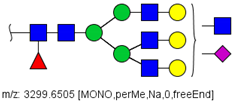 HexNAc6Hex6Fuc1NeuAc1 | 1115.2096 | [M + 3Na]3+ | 27 | - | ✓ |
| 67 | 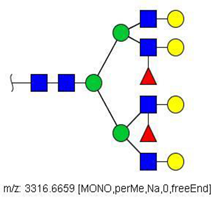 HexNAc6Hex7Fuc2 | 1669.8275 * | [M + 2Na]2+ | 27 | - | ✓ |
| 79 | 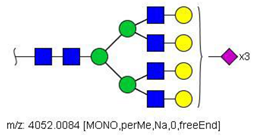 HexNAc6Hex7NeuAc3 | 1030.2440 | [M + 4Na]4+ | 80 | 52 | ✓ |
| 85 | 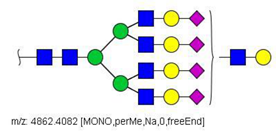 HexNAc7Hex8NeuAc4 | 1232.8439 * | [M + 4Na]4+ | 80 | - | * |
| 86 |  HexNAc7Hex8Fuc1NeuAc4 | 1276.3663 * | [M + 4Na]4+ | 80 | - | ✓ |
| 87 | 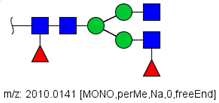 HexNAc4Hex3Fuc2 | 1016.5017 | [M + 2Na]2+ | 13 | - | ✓✓ |
 N-acetyl glucosamine (GlcNac), ● Mannose (Man), ● Galactose (Gal), ◄ Fucose (Fuc),
N-acetyl glucosamine (GlcNac), ● Mannose (Man), ● Galactose (Gal), ◄ Fucose (Fuc),  Sialic acid (NeuAc).
Sialic acid (NeuAc).| Nr | N-Glycan Composition | Median Controls (n = 11) | Median Cancer Patients (n = 13) | Alteration in Cancer Patients | Z | Wilcoxon, 2-Sample Test, Normal Approximation, Prob>|Z| |
|---|---|---|---|---|---|---|
| MALDI-TOF/MS data | ||||||
| 49 | HexNAc5Hex5NeuAc1 | 0 | 0.022 | ↑ | −3.09 | 0.0020 |
| LC-MS/MS data | ||||||
| 8 | HexNAc3Hex4 | 0.140 | 0.012 | ↓ | 3.13 | 0.0018 |
| 14 | HexNAc4Hex4 | 0.685 | 0.023 | ↓ | 3.03 | 0.001 |
| 17 | HexNAc2Hex7 | 0.000 | 0.026 | ↑ | −3.53 | 0.0004 |
| 22 | HexNAc5Hex4 | 0.882 | 0.020 | ↓ | 3.77 | 0.0002 |
| 23 | HexNAc6Hex3 | 0.466 | 0.005 | ↓ | 2.72 | 0.0065 |
| 25 | HexNAc2Hex8 | 0.149 | 0.051 | ↓ | 2.72 | 0.0065 |
| 26 | HexNAc4Hex4NeuAc1 | 1.619 | 0.160 | ↓ | 3.30 | 0.001 |
| 31 | HeXNAc3Hex5Fuc1NeuAc1 | 4.228 | 0.021 | ↓ | 3.07 | 0.0021 |
| 33 | HexNAc2Hex9 | 0.334 | 0.072 | ↓ | 3.01 | 0.0026 |
| 36 | HexNAc5Hex4NeuAc1 | 0.838 | 0.035 | ↓ | 3.13 | 0.0018 |
| 40 | HexNAc3Hex6Fuc1NeuAc1 | 0.413 | 0.000 | ↓ | 2.97 | 0.003 |
| 41 | HexNAc7Hex3Fuc1 | 1.533 | 0.092 | ↓ | 3.25 | 0.0012 |
| 42 | HexNAc2Hex10 | 0.147 | 0.008 | ↓ | 3.42 | 0.0006 |
| 43 | HexNAc7Hex4 | 2.705 | 0.025 | ↓ | 3.36 | 0.0008 |
| 44 | HexNAc4Hex5Fuc1NeuAc1 | 1.659 | 0.772 | ↓ | 3.01 | 0.0026 |
| 51 | HexNAc6Hex6 | 9.144 | 0.842 | ↓ | 3.42 | 0.0006 |
| 55 | HexNAc5Hex6NeuAc1 | 0.650 | 0.123 | ↓ | 2.66 | 0.0077 |
| 56 | HexNAc6Hex5Fuc2 | 0.221 | 0.004 | ↓ | 3.02 | 0.0026 |
| 57 | HexNAc6Hex6Fuc1 | 0.439 | 0.030 | ↓ | 2.66 | 0.0077 |
| 58 | HexNAc4Hex5Fuc1NeuAc2 | 0.315 | 0.987 | ↑ | −3.36 | 0.0008 |
| 61 | HexNAc5Hex6Fuc1NeuAc1 | 0.414 | 0.069 | ↓ | 2.84 | 0.0045 |
| 66 | HexNAc6Hex6Fuc1NeuAc1 | 0.633 | 0.050 | ↓ | 3.07 | 0.0021 |
| 79 | HexNAc6Hex7NeuAc3 | 0.001 | 0.226 | ↑ | −3.19 | 0.0014 |
| 85 | HexNAc7Hex8NeuAc4 | 1.188 | 0.000 | ↓ | 3.44 | 0.0006 |
| 86 | HexNAc7Hex8Fuc1NeuAc4 | 0.920 | 0.019 | ↓ | 2.95 | 0.0031 |
| 87 | HexNAc4Hex3Fuc2 | 0.379 | 0.006 | ↓ | 2.95 | 0.0031 |
| Patient Nr. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nr. | N-Glycan Composition | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Median | n/Total * | Binomial Probability |
| MALDI-TOF/MS data (area ratio) | |||||||||||||||||
| 6 | HexNac2Hex5 | 29.41 | 62.5 | - | 6.88 | 15.33 | - | 7.48 | 20.8 | 0.2 | 3.09 | 14.63 | - | 27.09 | 14.98 | 9/10 | 0.0010 |
| 10 | HexNac2Hex6 | 42.63 | 7.25 | - | 8.71 | - | - | 21.94 | 4 | 0.06 | - | 7.48 | - | 23.4 | 8.10 | 7/8 | 0.0020 |
| LC-MS/MS data (area ratio) | |||||||||||||||||
| 17 | HexNAc2Hex7 | - | - | - | 0.02 | 0.03 | 0.00 | 0.03 | 0.02 | 0.01 | 0.11 | 0.01 | - | - | 0.02 | 8/8 | 0.0000 |
| 22 | HexNAc5Hex4 | 167.48 | 53.17 | 31.47 | 38.23 | 84.42 | - | 7.42 | 110.19 | 1.44 | 4.61 | 6.39 | - | 136.41 | 38.23 | 11/11 | 0.0000 |
| 26 | HexNAc4Hex4NeuAc1 | 40.70 | 31.47 | 11.41 | 10.34 | 5.26 | - | 1.64 | 24.58 | - | - | 3.43 | - | 26.69 | 11.41 | 9/9 | 0.0000 |
| 28 | HexNAc5Hex4Fuc1 | 51.49 | 17.16 | 46.90 | - | 4.20 | - | - | 43.48 | 0.79 | 1.84 | 5.27 | - | 19.55 | 17.16 | 8/9 | 0.0020 |
| 33 | HexNAc2Hex9 | 92.23 | 116.52 | 86.94 | 65.26 | 5.54 | - | 4.45 | 32.67 | 0.52 | - | 16.76 | - | 123.43 | 48.96 | 9/10 | 0.0010 |
| 36 | HexNAc5Hex4NeuAc1 | 140.70 | 113.15 | 59.98 | 142.75 | 17.23 | 0.06 | 1.98 | 35.80 | 0.78 | 1.70 | 56.29 | - | 587.41 | 46.05 | 10/12 | 0.0032 |
| 41 | HexNAc7Hex3Fuc1 | 20.10 | 26.13 | 21.01 | 10.27 | 27.09 | - | - | 20.10 | - | - | 4.52 | - | 59.21 | 20.55 | 8/8 | 0.0000 |
| 43 | HexNAc7Hex4 | 26.12 | 10.94 | 25.56 | 6.74 | - | 0.01 | 1.34 | 7.67 | 0.40 | 1.06 | 1.91 | - | 14.29 | 6.74 | 9/11 | 0.0059 |
| 57 | HexNAc6Hex6Fuc1 | - | 12.13 | 43.48 | 12.63 | 6.20 | 2.52 | 1.76 | 7.29 | 0.97 | 2.32 | 4.52 | 1.76 | 26.01 | 5.36 | 11/12 | 0.0002 |
| 64 | HexNAc5Hex5Fuc1NeuAc2 | 7.49 | 24.86 | 6.61 | 14.67 | - | 0.62 | - | - | 0.45 | 2.54 | 11.36 | - | 19.28 | 7.49 | 7/9 | 0.0195 |
| 67 | HexNAc6Hex7Fuc2 | 9.62 | 7.77 | 5.74 | 23.82 | 16.50 | - | - | 13.98 | - | - | - | - | 5.50 | 9.62 | 7/7 | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coura, M.d.M.A.; Barbosa, E.A.; Brand, G.D.; Bloch, C., Jr.; de Sousa, J.B. Identification of Differential N-Glycan Compositions in the Serum and Tissue of Colon Cancer Patients by Mass Spectrometry. Biology 2021, 10, 343. https://doi.org/10.3390/biology10040343
Coura MdMA, Barbosa EA, Brand GD, Bloch C Jr., de Sousa JB. Identification of Differential N-Glycan Compositions in the Serum and Tissue of Colon Cancer Patients by Mass Spectrometry. Biology. 2021; 10(4):343. https://doi.org/10.3390/biology10040343
Chicago/Turabian StyleCoura, Marcelo de M.A., Eder A. Barbosa, Guilherme D. Brand, Carlos Bloch, Jr., and Joao B. de Sousa. 2021. "Identification of Differential N-Glycan Compositions in the Serum and Tissue of Colon Cancer Patients by Mass Spectrometry" Biology 10, no. 4: 343. https://doi.org/10.3390/biology10040343
APA StyleCoura, M. d. M. A., Barbosa, E. A., Brand, G. D., Bloch, C., Jr., & de Sousa, J. B. (2021). Identification of Differential N-Glycan Compositions in the Serum and Tissue of Colon Cancer Patients by Mass Spectrometry. Biology, 10(4), 343. https://doi.org/10.3390/biology10040343






