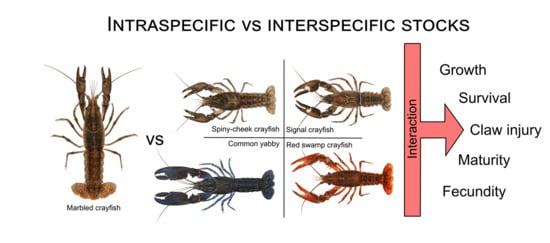
Survival, Growth, and Reproduction: Comparison of Marbled Crayfish with Four Prominent Crayfish Invaders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Experimental Animals and Selection of Juveniles
2.2. Experimental Design
2.3. Culturing Conditions and Feeding
2.4. Statistical Analyses
3. Results
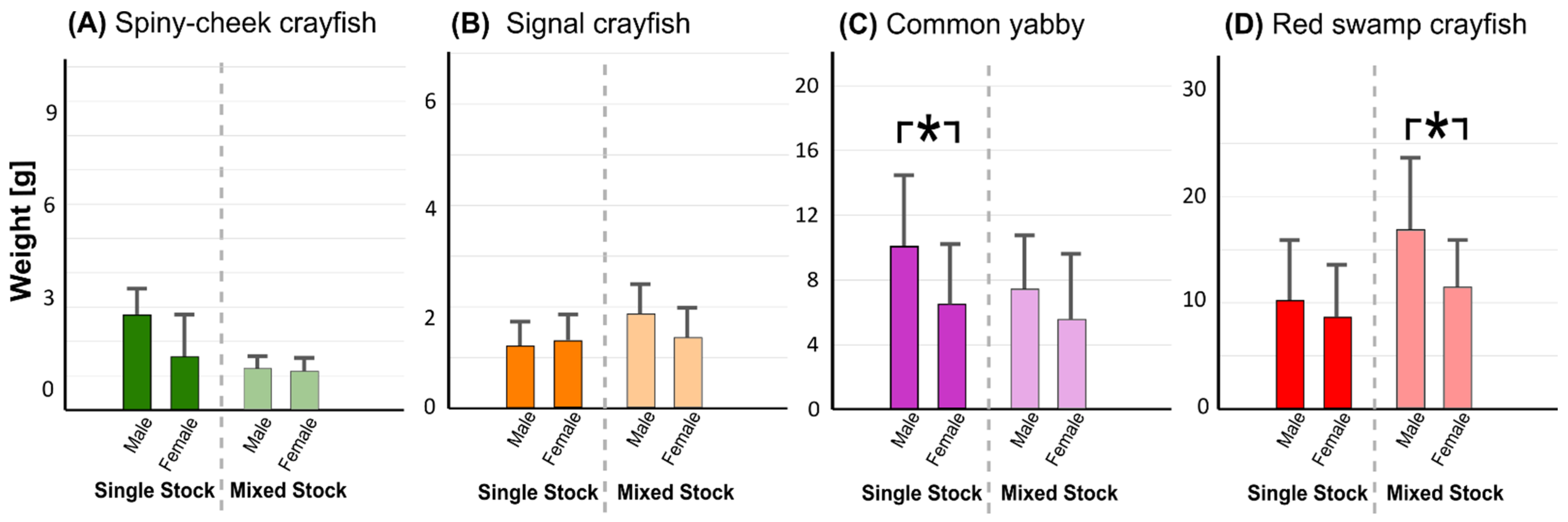
3.1. Growth Analysis
3.2. Survival Rate
3.3. Missing and/or Regenerating Claws
3.4. Speed of Maturation and Fecundity Rates
4. Discussion
4.1. Intra- and Interspecific Growth and Survival Rates in Single and Mixed Stocks
4.2. Incidence of Missing and/or Regenerating Claws
4.3. Speed of Maturation and Fecundity Rates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyšek, P.; Bacher, S.; Chytrý, M.; Jarošík, V.; Wild, J.; Celesti-Grapow, L.; Gassó, N.; Kenis, M.; Lambdon, P.W.; Nentwig, W.; et al. Contrasting patterns in the invasions of European terrestrial and freshwater habitats by alien plants, insects and vertebrates. Glob. Ecol. Biogeogr. 2010, 19, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Hanafiah, M.M.; Leuven, R.S.E.W.; Sommerwerk, N.; Tockner, K.; Huijbregts, M.A.J. Including the introduction of exotic species in life cycle impact assessment: The case of inland shipping. Environ. Sci. Technol. 2013, 47, 13934–13940. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [Green Version]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T. Accelerating invasion rate in a highly invaded estuary. Science 1998, 279, 555–558. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Blackburn, T.M.; Carlton, J.T.; Dick, J.T.; Hulme, P.E.; Iacarella, J.C.; Jeschke, J.M.; Liebhold, A.M.; Lockwood, J.L.; MacIsaac, H.J. Invasion science: A horizon scan of emerging challenges and opportunities. Trends Ecol. Evol. 2017, 32, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Patoka, J.; Prabowo, R.E.; Petrtýl, M.; Reynolds, J.D.; Kuříková, P.; Zámečníková-Wanma, B.P.; Kalous, L. Marine hitchhikers: A preliminary study on invertebrates unintentionally transported via the international pet trade. NeoBiota 2020, 61, 33. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Pyšek, P. Tens Rule. In Invasion Biology: Hypotheses and Evidence; Jeschke, J.M., Heger, T., Eds.; CAB International: Wallingford, UK, 2018; pp. 124–132. [Google Scholar]
- Allendorf, F.W.; Lundquist, L.L. Introduction: Population biology, evolution, and control of invasive species. Conserv. Biol. 2003, 17, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, R.N.; Pattison, Z.; Taylor, N.G.; Verbrugge, L.; Diagne, C.; Ahmed, D.A.; Leroy, B.; Angulo, E.; Briski, E.; Capinha, C.; et al. Global economic costs of aquatic invasive alien species. Sci. Total Environ. 2021, 775, 145238. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 1–6. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Catford, J.A.; Vesk, P.A.; Richardson, D.M.; Pysek, P. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Glob. Chang. Biol. 2012, 18, 44–62. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2020, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Sataruddin, N.S.; Heard, A.D. Over-invasion by functionally equivalent invasive species. Ecology 2014, 95, 2268–2276. [Google Scholar] [CrossRef] [Green Version]
- Copp, G.H.; Fox, M.G. Can invasiveness in freshwater fishes be predicted from life-history traits? Front. Ecol. Evol. 2020, 8, 408. [Google Scholar] [CrossRef]
- Fox, M.; Vila-Gispert, A.; Copp, G. Life-history traits of introduced Iberian pumpkinseed Lepomis gibbosus relative to native populations. Can differences explain colonization success? J. Fish Biol. 2007, 71, 56–69. [Google Scholar] [CrossRef]
- Crandall, K.A.; De Grave, S. An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. J. Crustacean Biol. 2017, 37, 615–653. [Google Scholar] [CrossRef] [Green Version]
- Momot, W.T. Redefining the role of crayfish in aquatic ecosystems. Rev. Fish Sci. 1995, 3, 33–63. [Google Scholar] [CrossRef]
- Lipták, B.; Veselý, L.; Ercoli, F.; Bláha, M.; Buřič, M.; Ruokonen, T.; Kouba, A. Trophic role of marbled crayfish in a lentic freshwater ecosystem. Aquat. Invasions 2019, 14, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Lodge, D.M.; Deines, A.; Gherardi, F.; Yeo, D.C.J.; Arcella, T.; Baldridge, A.K.; Barnes, M.A.; Chadderton, W.L.; Feder, J.L.; Gantz, C.A.; et al. Global Introductions of Crayfishes: Evaluating the Impact of Species Invasions on Ecosystem Services. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 449–472. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Dieguez-Uribeondo, J.; Tricarico, E. Managing invasive crayfish: Is there a hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 11. [Google Scholar] [CrossRef] [Green Version]
- Kouba, A.; Petrusek, A.; Kozák, P. Continental-wide distribution of crayfish species in Europe: Update and maps. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 5. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, 57, 35. [Google Scholar]
- EU. Commission Implementing Regulation (EU) 2016/1141 of 13 July 2016 adopting a list of invasive alien species of Union concern pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council. Off. J. Eur. Union 2016, 189, 4–8. [Google Scholar]
- Svoboda, J.; Mrugała, A.; Kozubíková-Balcarová, E.; Petrusek, A. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. J. Fish Dis. 2017, 40, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Vorburger, C.; Ribi, G. Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshw. Biol. 1999, 42, 111–119. [Google Scholar] [CrossRef]
- Kouba, A.; Tíkal, J.; Císař, P.; Veselý, L.; Fořt, M.; Příborský, J.; Patoka, J.; Buřič, M. The significance of droughts for hyporheic dwellers: Evidence from freshwater crayfish. Sci. Rep. 2016, 6, 26569. [Google Scholar] [CrossRef] [Green Version]
- Kozák, P.; Buřič, M.; Policar, T.; Hamáčková, J.; Lepičová, A. The effect of inter-and intra-specific competition on survival and growth rate of native juvenile noble crayfish Astacus astacus and alien spiny-cheek crayfish Orconectes limosus. Hydrobiologia 2007, 590, 85–94. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.; Haffner, P. Atlas of Crayfish in Europe; Muséum National d’Histoire Naturelle: Paris, France, 2006. [Google Scholar]
- Lodge, D.M.; Taylor, C.A.; Holdich, D.M.; Skurdal, J. Nonindigenous crayfishes threaten North American freshwater biodiversity: Lessons from Europe. Fisheries 2000, 25, 7–20. [Google Scholar] [CrossRef]
- Weiperth, A.; Bláha, M.; Szajbert, B.; Seprős, R.; Bányai, Z.; Patoka, J.; Kouba, A. Hungary: A European hotspot of non-native crayfish biodiversity. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 43. [Google Scholar] [CrossRef]
- Weiperth, A.; Gál, B.; Kuříková, P.; Bláha, M.; Kouba, A.; Patoka, J. Cambarellus patzcuarensis in Hungary: The first dwarf crayfish established outside of North America. Biologia 2017, 72, 1529–1532. [Google Scholar] [CrossRef] [Green Version]
- Szendőfi, B.; Bérces, S.; Csányi, B.; Gábris, V.; Gál, B.; Gönye, Z.; Répás, E.; Seprős, R.; Tóth, B.; Kouba, A.; et al. Egzotikus halfajok és decapodák a Barát-és Dera-patakban, valamint a torkolatuk dunai élőhelyein (Occurrence of exotic fish and crayfish species in Barát and Dera creeks and their adjacent section of the River Danube). Pisces Hung. 2018, 12, 47–51. [Google Scholar]
- Grandjean, F.; Collas, M.; Uriarte, M.; Rousset, M. First record of a marbled crayfish Procambarus virginalis (Lyko, 2017) population in France. Bioinvasions Rec. 2021, 10. in press. [Google Scholar] [CrossRef]
- Jackson, M.C.; Jones, T.; Milligan, M.; Sheath, D.; Taylor, J.; Ellis, A.; England, J.; Grey, J. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshw. Biol. 2014, 59, 1123–1135. [Google Scholar] [CrossRef] [Green Version]
- Veselý, L.; Buřič, M.; Kouba, A. Hardy exotics species in temperate zone: Can “warm water” crayfish invaders establish regardless of low temperatures? Sci. Rep. 2015, 5, 16340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veselý, L.; Ruokonen, T.J.; Weiperth, A.; Kubec, J.; Szajbert, B.; Guo, W.; Ercoli, F.; Bláha, M.; Buřič, M.; Hämäläinen, H. Trophic niches of three sympatric invasive crayfish of EU concern. Hydrobiologia 2021, 848, 727–737. [Google Scholar] [CrossRef]
- Vogt, G. Biology, Eecology, Evolution, Systematics and Utilization of the Parthenogenetic Marbled crayfish, Procambarus virginalis. In Crayfish: Evolution, Habitat and Conservation Strategies; Ribeiro, F.B., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2020; pp. 137–227. [Google Scholar]
- Kawai, T.; Kouba, A. A description of postembryonic development of Astacus astacus and Pontastacus leptodactylus. Freshw. Crayfish 2020, 25, 103–116. [Google Scholar] [CrossRef]
- Kouba, A.; Hamáčková, J.; Buřič, M.; Policar, T.; Kozak, P. Use of three forms of decapsulated Artemia cysts as food for juvenile noble crayfish (Astacus astacus). Czech J. Anim. Sci. 2011, 56, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Veselý, L.; Hrbek, V.; Kozák, P.; Buřič, M.; Sousa, R.; Kouba, A. Salinity tolerance of marbled crayfish Procambarus fallax f. virginalis. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 21. [Google Scholar] [CrossRef] [Green Version]
- Therneau, T.M.; Grambsch, P.M. Therneau, T.M.; Grambsch, P.M. The Cox Model. In Modeling Survival Data: Extending the Cox Model; Springer: Berlin/Heidelberg, Germany, 2000; pp. 39–77. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Cucherousset, J.; Copp, G.H.; Fox, M.G.; Sterud, E.; van Kleef, H.H.; Verreycken, H.; Záhorská, E. Life-history traits and potential invasiveness of introduced pumpkinseed Lepomis gibbosus populations in northwestern Europe. Biol. Invasions 2009, 11, 2171–2180. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, J.; Przybylski, M. Life-history traits of non-native freshwater fish invaders differentiate them from natives in the Central European bioregion. Rev. Fish Biol. Fish. 2015, 25, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Pintor, L.M.; Sih, A. Differences in growth and foraging behavior of native and introduced populations of an invasive crayfish. Biol. Invasions 2009, 11, 1895–1902. [Google Scholar] [CrossRef]
- Chucholl, C.; Morawetz, K.; Gross, H. The clones are coming—strong increase in Marmorkrebs Procambarus fallax (Hagen, 1870) f. virginalis records from Europe. Aquat. Invasions 2012, 7, 511–519. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Kouba, A. Effects of three triazine metabolites and their mixture at environmentally relevant concentrations on early life stages of marbled crayfish (Procambarus fallax f. virginalis). Chemosphere 2017, 175, 440–445. [Google Scholar] [CrossRef]
- Holdich, D.M. Biology of Freshwater Crayfish; Blackwell Science Oxford: Oxford, UK, 2002. [Google Scholar]
- Lipták, B.; Mojžišová, M.; Gruľa, D.; Christophoryová, J.; Jablonski, D.; Bláha, M.; Petrusek, A.; Kouba, A. Slovak section of the Danube has its well-established breeding ground of marbled crayfish Procambarus fallax f. virginalis. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 40. [Google Scholar] [CrossRef] [Green Version]
- Tönges, S.; Masagounder, K.; Gutekunst, J.; Lohbeck, J.; Miller, A.K.; Boehl, F.; Lyko, F. Physiological properties and tailored feeds to support aquaculture of marbled crayfish in closed systems. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Andriantsoa, R.; Tönges, S.; Panteleit, J.; Theissinger, K.; Carneiro, V.C.; Rasamy, J.; Lyko, F. Ecological plasticity and commercial impact of invasive marbled crayfish populations in Madagascar. BMC Ecol. 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.P.G.; Rasamy, J.R.; Harvey, A.; Toon, A.; Oidtmann, B.; Randrianarison, M.H.; Raminosoa, N.; Ravoahangimalala, O.R. The perfect invader: A parthenogenic crayfish poses a new threat to Madagascar’s freshwater biodiversity. Biol. Invasions 2009, 11, 1475–1482. [Google Scholar] [CrossRef]
- Maiakovska, O.; Andriantsoa, R.; Tönges, S.; Legrand, C.; Gutekunst, J.; Hanna, K.; Pârvulescu, L.; Novitsky, R.; Weiperth, A.; Sciberras, A.; et al. Genome analysis of the monoclonal marbled crayfish reveals genetic separation over a short evolutionary timescale. Commun. Biol. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Hossain, M.S.; Kouba, A.; Buřič, M. Morphometry, size at maturity, and fecundity of marbled crayfish (Procambarus virginalis). Zool. Anz. 2019, 281, 68–75. [Google Scholar] [CrossRef]
- Pieplow, U. Fischereiwissenschaftliche Monographie von Cambarus affinis Say. Z. Für Fish. 1938, 36, 349–440. [Google Scholar]
- Chybowski, Ł. Morphometrics, fecundity, density, and feeding intensity of the spinycheek crayfish, Orconectes limosus (Raf.) in natural conditions. Fish. Aquat. Life 2007, 15, 175–241. [Google Scholar]
- Kozák, P.; Ďuriš, Z.; Petrusek, A.; Buřič, M.; Horká, I.; Kouba, A.; Kozubíková-Balcarová, E.; Policar, T. Crayfish Biology and Culture; University of South Bohemia in České Budějovice, Faculty of Fisheries and Protection of Waters: Vodňany, Czech Republic, 2015. [Google Scholar]
- Guan, R.-Z.; Wiles, P.R. Growth and reproduction of the introduced crayfish Pacifastacus leniusculus in a British lowland river. Fish. Res. 1999, 42, 245–259. [Google Scholar] [CrossRef]
- Buřič, M.; Haubrock, P.J.; Veselý, L.; Kozák, P.; Kouba, A. Effective investments due to seasonal morphological changes? Possible reasons and consequences of allometric growth and reproduction in adult signal crayfish (Pacifastacus leniusculus). Can. J. Zool. 2021, 99, 85–96. [Google Scholar] [CrossRef]
- Westman, K.; Savolainen, R.; Pursiainen, M. A comparative study on the growth and moulting of the noble crayfish, Astacus astacus (L.), and the signal crayfish, Pacifastacus leniusculus (Dana), in a small forest lake in southern Finland. Freshw. Crayfish 1993, 9, 451–465. [Google Scholar]
- Abrahamsson, S.A. Density, growth and reproduction in populations of Astacus astacus and Pacifastacus leniusculus in an isolated pond. Oikos 1971, 22, 373–380. [Google Scholar] [CrossRef]
- Ackefors, H.E. Freshwater crayfish farming technology in the 1990s: A European and global perspective. Fish Fish. 2000, 1, 337–359. [Google Scholar] [CrossRef]
- Wickins, J.F.; Lee, D.O.C. Crustacean Farming: Ranching and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Oficialdegui, F.J.; Sánchez, M.I.; Clavero, M. One century away from home: How the red swamp crayfish took over the world. Rev. Fish Biol. Fish. 2020, 30, 121–135. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Oficialdegui, F.J.; Zeng, Y.; Patoka, J.; Yeo, D.C.; Kouba, A. The redclaw crayfish: A prominent aquaculture species with invasive potential in tropical and subtropical biodiversity hotspots. Rev. Aquac. 2021, accepted. [Google Scholar] [CrossRef]
- Kouba, A.; Kanta, J.; Buřič, M.; Policar, T.; Kozák, P. The effect of water temperature on the number of moults and growth of juvenile noble crayfish, Astacus astacus (Linneaus). Freshw. Crayfish 2010, 17, 37–41. [Google Scholar]
- Hartnoll, R.G. Growth in Crustacea—Twenty Years on. In Advances in Decapod Crustacean Research. Developments in Hydrobiology; Paula, J.P.M., Flores, A.A.V., Fransen, C.H.J.M., Eds.; Springer: Dordrecht, The Nezerlands, 2001; Volume 154, pp. 111–122. [Google Scholar]
- Lindqvist, O.V.; Huner, J.V. Life history characteristics of crayfish: What makes some of them good colonizers? In Crayfish in Europe as Alien Species: How to Make the Best of a Bad Situation; Gheraardi, F., Holdich, D.M., Eds.; Crustacean Issues; Routledge: London, UK, 1999; Volume 11, pp. 23–30. [Google Scholar]
- Hudina, S.; Hock, K.; Žganec, K. The role of aggression in range expansion and biological invasions. Curr. Zool. 2014, 60, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Pârvulescu, L.; Stoia, D.I.; Miok, K.; Ion, M.C.; Puha, A.E.; Sterie, M.; Vereș, M.; Marcu, I.; Muntean, D.M.; Aburel, O.M. Force and boldness: Cumulative assets of a successful crayfish invader. Front. Ecol. Evol. 2021, 9, 49. [Google Scholar] [CrossRef]
- Fořt, M.; Hossain, S.; Kouba, A.; Buřič, M.; Kozák, P. Agonistic interactions and dominance establishment in three crayfish species non-native to Europe. Limnologica 2019, 74, 73–79. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Faulkes, Z. Can the parthenogenetic marbled crayfish Marmorkrebs compete with other crayfish species in fights? J. Ethol. 2011, 29, 115–120. [Google Scholar] [CrossRef]
- Hossain, S.; Kubec, J.; Kouba, A.; Kozák, P.; Buřič, M. Still waters run deep: Marbled crayfish dominate over red swamp crayfish in agonistic interactions. Aquat. Ecol. 2019, 53, 97–107. [Google Scholar] [CrossRef]
- Kouba, A.; Buřič, M.; Policar, T.; Kozák, P. Evaluation of body appendage injuries to juvenile signal crayfish (Pacifastacus leniusculus): Relationships and consequences. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 4. [Google Scholar] [CrossRef] [Green Version]
- Buřič, M.; Kouba, A.; Kozák, P. Chelae regeneration in European alien crayfish Orconectes limosus (Rafinesque 1817). Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 4. [Google Scholar] [CrossRef] [Green Version]
- Niksirat, H.; Kouba, A.; Kozák, P. Ultrastructure of egg activation and cortical reaction in the noble crayfish Astacus astacus. Micron 2015, 68, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Kubec, J.; Veselý, L.; Hossain, M.S.; Buřič, M.; McClain, R.; Kouba, A. High air humidity is sufficient for successful egg incubation and early post-embryonic development in the marbled crayfish (Procambarus virginalis). Freshw. Biol. 2019, 64, 1603–1612. [Google Scholar] [CrossRef] [Green Version]
- Seitz, R.; Vilpoux, K.; Hopp, U.; Harzsch, S.; Maier, G. Ontogeny of the Marmorkrebs (marbled crayfish): A parthenogenetic crayfish with unknown origin and phylogenetic position. J. Exp. Zool. Part A Comp. Exp. Biol. 2005, 303A, 393–405. [Google Scholar] [CrossRef]
- Vogt, G. Suitability of the clonal marbled crayfish for biogerontological research: A review and perspective, with remarks on some further crustaceans. Biogerontology 2010, 11, 643–669. [Google Scholar] [CrossRef] [PubMed]
- Chucholl, C.; Pfeiffer, M. First evidence for an established Marmorkrebs (Decapoda, Astacida, Cambaridae) population in Southwestern Germany, in syntopic occurrence with Orconectes limosus (Rafinesque, 1817). Aquat. Invasions 2010, 5, 405–412. [Google Scholar] [CrossRef]
- Huner, J. Procambarus; Blackwell Science: Oxford, UK, 2002; pp. 541–584. [Google Scholar]
- Avault, J.W., Jr. Crawfish farming in the United States. Freshw. Crayfish 1972, 1, 239–250. [Google Scholar]
- Oluoch, A. Breeding biology of the Louisiana red swamp crayfish Procambarus clarkii Girard in Lake Naivasha, Kenya. Hydrobiologia 1990, 208, 85–92. [Google Scholar] [CrossRef]
- Buřič, M.; Kouba, A.; Kozák, P. Intra-sex dimorphism in crayfish females. Zoology 2010, 113, 301–307. [Google Scholar] [CrossRef]
- Buřič, M.; Kouba, A.; Kozák, P. Molting and growth in relation to form alternations in the male spiny-cheek crayfish Orconectes limosus. Zool. Stud. 2010, 49, 28–38. [Google Scholar]
- Stucki, T.P. Diffrences in life history of native and introduced crayfish species in Switzerland. Freshw. Crayfish 2002, 13, 463–476. [Google Scholar]
- Kozák, P.; Buřič, M.; Policar, T. The fecundity, time of egg development and juvenile production in spiny-cheek crayfish (Orconectes limosus) under controlled conditions. Bull. Français Pêche Piscic. 2006, 380–381, 1171–1182. [Google Scholar] [CrossRef] [Green Version]
- Lipták, B.; Mrugała, A.; Pekárik, L.; Mutkovič, A.; Gruľa, D.; Petrusek, A.; Kouba, A. Expansion of the marbled crayfish in Slovakia: Beginning of an invasion in the Danube catchment? J. Limnol. 2016, 75, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Kirjavainen, J.; Westman, K. Development of an introduced signal crayfish (Pacifastacus leniusculus (Dana)) population in the small Lake Karisjärvi in central Finland. Freshw. Crayfish 1995, 10, 140–150. [Google Scholar]
- Kirjavainen, J.; Westman, K. Natural history and development of the introduced signal crayfish, Pacifastacus leniusculus, in a small, isolated Finnish lake, from 1968 to 1993. Aquat. Living Resour. 1999, 12, 387–401. [Google Scholar] [CrossRef]
- Savolainen, R.; Westman, K.; Pursiainen, M. Fecundity of Finnish noble crayfish, Astacus astacus L., and signal crayfish, Pacifastacus leniusculus, in various natural habitats and in culture. Freshw. Crayfish 1996, 11, 319–338. [Google Scholar]
- Beatty, S.; Morgan, D.; Gill, H. Role of life history strategy in the colonisation of Western Australian aquatic systems by the introduced crayfish Cherax destructor Clark, 1936. Hydrobiologia 2005, 549, 219–237. [Google Scholar] [CrossRef]
- Johnston, K.; Robson, B.J.; Austin, C.M. Population structure and life history characteristics of Euastacus bispinosus and Cherax destructor (Parastacidae) in the Grampians National Park, Australia. Freshw. Crayfish 2008, 16, 165–173. [Google Scholar]
- Semple, G.; Rouse, D.; McLain, K. Cherax destructor, C. tenuimanus and C. quadricarinatus (Decapoda: Parastacidae): A comparative review of biological traits relating to aquaculture potential. Freshw. Crayfish 1995, 8, 495–503. [Google Scholar]
- Austin, C. A comparison of clutch and brood size in the Red Claw, Cherax quadricarinatus (von Martens) and the Yabby, C. destructor Clark (Decapoda: Parastacidae). Aquaculture 1998, 167, 135–145. [Google Scholar] [CrossRef]
- Huner, J.V.; Barr, J.; Coleman, E.B. Red Swamp Crawfish: Biology and Exploitation; Louisiana Sea Grant College Program, Center for Wetland Resources, Louisiana State University: Barton Rouge, LA, USA, 1984. [Google Scholar]
- Gutiérrez-Yurrita, P.J.; Del Olmo, C.M. Population dynamics of juveniles of red swamp crayfish (Procambarus clarkii) under controlled conditions. Freshw. Crayfish 2004, 14, 180–189. [Google Scholar]



| Trial | Group | Week | |||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | ||
| A | MC single | 0 A,a | 0 A,a | 2 B,a | 20 C,a | 11 BC,a | 9 BC,a |
| SCC single | 4 A,a | 6 A,a | 7 A,a | 2 A,a | 5 A,a | 11 A,a | |
| MC mixed | 0 A,a | 0 A,a | 4 B,a | 8 B,a | 8 B,a | 10 B,a | |
| SCC mixed | 0 A,a | 4 B,a | 11 B,a | 5 B,a | 20 B,a | 8 B,a | |
| B | MC single | 3 B,a | 0 A,a | 16 B,a | 6 B,a | 3 B,b | 14 B,bc |
| SC single | 0 A,a | 3 B,a | 3 B,a | 3 B,a | 3 B,b | 3 B,b | |
| MC mixed | 0 A,a | 0 A,a | 0 A,a | 0 A,a | 0 A,a | 0 A,a | |
| SC mixed | 0 A,a | 0 A,a | 0 A,a | 11 B,a | 35 BC,c | 53 C,c | |
| C | MC single | 1 A,a | 1 A,a | 7 A,a | 5 A,a | 5 A,a | 4 A,a |
| CY single | 0 A,a | 4 B,a | 8 BC,a | 13 BC,a | 24 CD,b | 37 D,b | |
| MC mixed | 0 A,a | 3 B,a | 2 B,a | 42 C,b | 23 BC,ab | 45 C,b | |
| CY mixed | 0 A,a | 10 B,a | 5 B,a | 14 B,ab | 16 B,ab | 22 B,ab | |
| D | MC single | 0 A,a | 3 B,a | 6 B,a | 2 B,a | 3 B,a | NA |
| RSC single | 0 A,a | 8 B,a | 23 BC,a | 32 C,b | 42 C,b | NA | |
| MC mixed | 3 A,a | 3 AB,a | 18 AB,a | 13 AB,ab | 50 B,b | NA | |
| RSC mixed | 0 A,a | 0 A,a | 13 B,a | 14 B,ab | 35 C,b | NA | |
| Trial | Group | Week | Fecundity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Absolute | Relative | ||
| A | MC single | – | – | 3 + 0 | 13 + 0 | 15 + 0 | 15 + 8 | 17 + 3 | 10 + 12 | 134 ± 66 | 5.5 ± 2.0 |
| SCC single | – | – | 1 + 0 | 2 + 0 | 3 + 0 | 3 + 0 | 3 + 0 | 3 + 0 | – | – | |
| MC mixed | – | 1 + 0 | 1 + 0 | 9 + 0 | 9 + 0 | 11 + 2 | 10 + 2 | 8 + 5 | 150 ± 65 | 6.0 ± 1.9 | |
| SCC mixed | – | – | – | – | 1 + 0 | 1 + 0 | 1 + 0 | 1 + 0 | – | – | |
| B | MC single | – | – | – | – | 5 + 0 | 11 + 1 | 16 + 2 | 12 + 6 | 220 ± 82 | 8.6 ± 2.8 |
| SC single | – | – | – | – | – | – | – | – | – | – | |
| MC mixed | – | – | – | – | 3 + 0 | 7 + 0 | 4 + 0 | 6 + 4 | 275 ± 50 | 10.0 ± 1.6 | |
| SC mixed | – | – | – | – | – | – | – | – | – | – | |
| C | MC single | 8 + 0 | 18 + 0 | 21 + 0 | 39 + 0 | 29 + 19 | 24 + 17 | 14 + 17 | 11 + 2 | 131 ± 42 | 6.1 ± 1.8 |
| CY single | – | – | – | – | 5 + 0 | 16 + 0 | 14 + 0 | 8 + 0 | – | – | |
| MC mixed | – | 1 + 0 | 6 + 0 | 8 + 0 | 8 + 0 | 7 + 5 | 5 + 2 | 2 + 2 | 126 ± 39 | 5.8 ± 1.4 | |
| CY mixed | – | – | – | – | 4 + 0 | 9 + 0 | 9 + 0 | 9 + 0 | – | – | |
| D | MC single | – | 11 + 0 | 17 + 0 | 17 + 1 | 9 + 8 | NA | NA | NA | 126 ± 32 | 6.1 ± 1.0 |
| RSC single | – | 1 + 0 | 3 + 0 | 5 + 0 | 3 + 0 | NA | NA | NA | – | – | |
| MC mixed | – | 1 + 0 | 1 + 0 | 0 + 0 | 0 + 0 | NA | NA | NA | – | – | |
| RSC mixed | – | 3 + 0 | 6 + 0 | 8 + 0 | 8 + 0 | NA | NA | NA | – | – | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouba, A.; Lipták, B.; Kubec, J.; Bláha, M.; Veselý, L.; Haubrock, P.J.; Oficialdegui, F.J.; Niksirat, H.; Patoka, J.; Buřič, M. Survival, Growth, and Reproduction: Comparison of Marbled Crayfish with Four Prominent Crayfish Invaders. Biology 2021, 10, 422. https://doi.org/10.3390/biology10050422
Kouba A, Lipták B, Kubec J, Bláha M, Veselý L, Haubrock PJ, Oficialdegui FJ, Niksirat H, Patoka J, Buřič M. Survival, Growth, and Reproduction: Comparison of Marbled Crayfish with Four Prominent Crayfish Invaders. Biology. 2021; 10(5):422. https://doi.org/10.3390/biology10050422
Chicago/Turabian StyleKouba, Antonín, Boris Lipták, Jan Kubec, Martin Bláha, Lukáš Veselý, Phillip J. Haubrock, Francisco J. Oficialdegui, Hamid Niksirat, Jiří Patoka, and Miloš Buřič. 2021. "Survival, Growth, and Reproduction: Comparison of Marbled Crayfish with Four Prominent Crayfish Invaders" Biology 10, no. 5: 422. https://doi.org/10.3390/biology10050422
APA StyleKouba, A., Lipták, B., Kubec, J., Bláha, M., Veselý, L., Haubrock, P. J., Oficialdegui, F. J., Niksirat, H., Patoka, J., & Buřič, M. (2021). Survival, Growth, and Reproduction: Comparison of Marbled Crayfish with Four Prominent Crayfish Invaders. Biology, 10(5), 422. https://doi.org/10.3390/biology10050422