Color of Pan Trap Influences Sampling of Bees in Livestock Pasture Ecosystem
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Study Site: History and Preparation
2.3. Pan Traps and Sampling
2.4. Light Reflectance Analysis of Pan Traps
2.5. Data Analyses
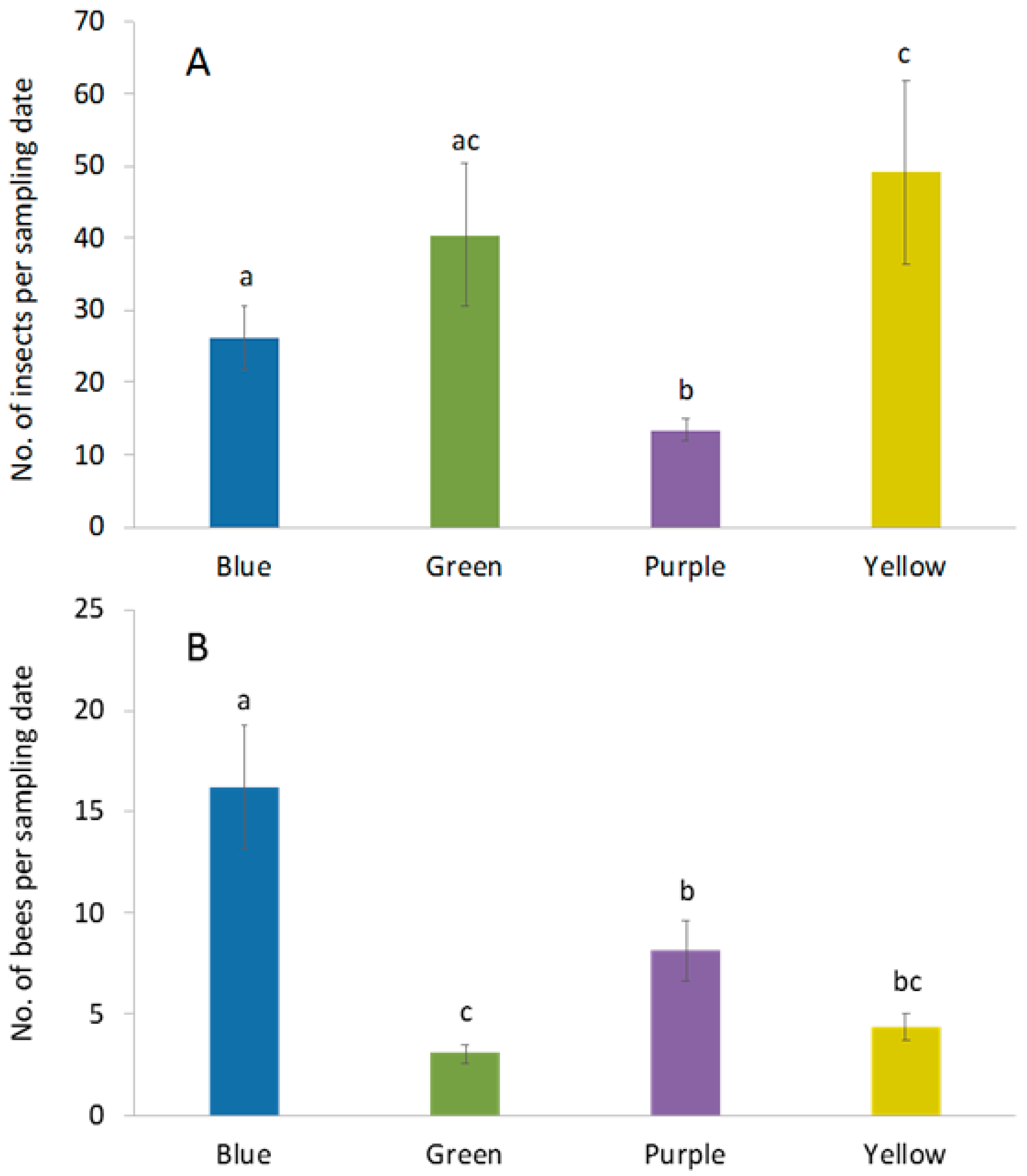
3. Results
3.1. Abundance and Diversity
3.2. Light Reflectance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanbergen, A.J.; Initiative, t.I.P. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Le Féon, V.; Schermann-Legionnet, A.; Delettre, Y.; Aviron, S.; Billeter, R.; Bugter, R.; Hendrickx, F.; Burel, F. Intensification of agriculture, landscape composition and wild bee communities: A large scale study in four European countries. Agric. Ecosyst. Environ. 2010, 137, 143–150. [Google Scholar] [CrossRef]
- Kells, A.R.; Holland, J.M.; Goulson, D. The value of uncropped field margins for foraging bumblebees. J. Insect Conserv. 2001, 5, 283–291. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.S.; Griswold, T.; Messinger, O.J. Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: Are pan traps sufficient? J. Kans. Entomol. Soc. 2008, 81, 288–300. [Google Scholar] [CrossRef]
- Gezon, Z.J.; Wyman, E.S.; Ascher, J.S.; Inouye, D.W.; Irwin, R.E. The effect of repeated, lethal sampling on wild bee abundance and diversity. Methods Ecol. Evol. 2015, 6, 1044–1054. [Google Scholar] [CrossRef] [Green Version]
- Leong, J.M.; Thorp, R.W. Colour-coded sampling: The pan trap colour preferences of oligolectic and nonoligolectic bees associated with a vernal pool plant. Ecol. Entomol. 1999, 24, 329–335. [Google Scholar] [CrossRef]
- Campbell, J.W.; Hanula, J. Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Cane, J.H.; Minckley, R.L.; Kervin, L.J. Sampling bees (Hymenoptera: Apiformes) for pollinator community studies: Pitfalls of pan-trapping. J. Kans. Entomol. Soc. 2000, 73, 225–231. [Google Scholar]
- Roulston, T.a.H.; Smith, S.A.; Brewster, A.L. A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J. Kans. Entomol. Soc. 2007, 80, 179–181. [Google Scholar] [CrossRef]
- Vrdoljak, S.M.; Samways, M.J. Optimising coloured pan traps to survey flower visiting insects. J. Insect Conserv. 2012, 16, 345–354. [Google Scholar] [CrossRef]
- Joshi, N.K.; Leslie, T.; Rajotte, E.G.; Kammerer, M.A.; Otieno, M.; Biddinger, D.J. Comparative trapping efficiency to characterize bee abundance, diversity, and community composition in apple orchards. Ann. Entomol. Soc. Am. 2015, 108, 785–799. [Google Scholar] [CrossRef]
- Dyer, A.G.; Paulk, A.C.; Reser, D.H. Colour processing in complex environments: Insights from the visual system of bees. Proc. R. Soc. B Biol. Sci. 2011, 278, 952–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, A.G.; Spaethe, J.; Prack, S. Comparative psychophysics of bumblebee and honeybee colour discrimination and object detection. J. Comp. Physiol. A 2008, 194, 617. [Google Scholar] [CrossRef] [PubMed]
- Lotto, R.B.; Chittka, L. Seeing the light: Illumination as a contextual cue to color choice behavior in bumblebees. Proc. Natl. Acad. Sci. USA 2005, 102, 3852–3856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streinzer, M.; Paulus, H.F.; Spaethe, J. Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. J. Exp. Biol. 2009, 212, 1365–1370. [Google Scholar] [CrossRef] [Green Version]
- Gollan, J.R.; Ashcroft, M.B.; Batley, M. Comparison of yellow and white pan traps in surveys of bee fauna in New South Wales, Australia (Hymenoptera: Apoidea: Anthophila). Aust. J. Entomol. 2011, 50, 174–178. [Google Scholar] [CrossRef]
- Wang, M.; Lu, X.; Ding, S.; Ren, J.; Bian, Z.; Xu, Z. Pollinator diversity in different habitats of the agricultural landscape in the middle and lower reaches of the Yellow River based on the three-color pan trap method. Acta Ecol. Sin. 2017, 37, 148–155. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Steudel, B.; Kessler, M. Sampling Hymenoptera along a precipitation gradient in tropical forests: The effectiveness of different coloured pan traps. Entomol. Exp. Appl. 2010, 137, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Moreira, E.F.; da Silva Santos, R.L.; Penna, U.L.; Angel-Coca, C.; de Oliveira, F.F.; Viana, B.F. Are pan traps colors complementary to sample community of potential pollinator insects? J. Insect Conserv. 2016, 20, 583–596. [Google Scholar] [CrossRef]
- Laubertie, E.; Wratten, S.; Sedcole, J. The role of odour and visual cues in the pan-trap catching of hoverflies (Diptera: Syrphidae). Ann. Appl. Biol. 2006, 148, 173–178. [Google Scholar] [CrossRef]
- Shrestha, M.; Garcia, J.E.; Chua, J.H.; Howard, S.R.; Tscheulin, T.; Dorin, A.; Nielsen, A.; Dyer, A.G. Fluorescent pan traps affect the capture rate of insect orders in different ways. Insects 2019, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Heneberg, P.; Bogusch, P. To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera? J. Insect Conserv. 2014, 18, 1123–1136. [Google Scholar] [CrossRef]
- Colwell, R. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 7.5 User’s Guide and Application Published. Available online: http://purl.oclc.org/estimates (accessed on 1 March 2021).
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5). Available online: www.canoco.com (accessed on 12 March 2021).
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, NY, USA, 2003. [Google Scholar]
- Menzel, R.; Backhaus, W. Colour vision in insects. Vis. Vis. Dysfunct. 1991, 6, 262–293. [Google Scholar]
- Kevan, P.; Giurfa, M.; Chittka, L. Why are there so many and so few white flowers? Trends Plant Sci. 1996, 1, 252. [Google Scholar] [CrossRef]
- Avarguès-Weber, A.; Mota, T.; Giurfa, M. New vistas on honey bee vision. Apidologie 2012, 43, 244–268. [Google Scholar] [CrossRef]
- von Frisch, K. Der Farbensinn und Formensinn der Biene. Zool. Jahrb. 1914, 35, 1–188. [Google Scholar]
- Hertz, M. New experiments on colour vision in bees. J. Exp. Biol. 1939, 16, 1–8. [Google Scholar] [CrossRef]
- Stephenson, P.L.; Griswold, T.L.; Arduser, M.S.; Dowling, A.P.; Krementz, D.G. Checklist of bees (Hymenoptera: Apoidea) from managed emergent wetlands in the lower Mississippi Alluvial Valley of Arkansas. Biodivers. Data J. 2018, 6, e24071. [Google Scholar] [CrossRef]
- Little, C.Z. Bee Communities in the Arkansas River Valley; University of Central Arkansas: Conway, AR, USA, 2013. [Google Scholar]
- Kimoto, C.; DeBano, S.J.; Thorp, R.W.; Taylor, R.V.; Schmalz, H.; DelCurto, T.; Johnson, T.; Kennedy, P.L.; Rao, S. Short-term responses of native bees to livestock and implications for managing ecosystem services in grasslands. Ecosphere 2012, 3, 1–19. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.; Longing, S.; Brown, C.; Green, P.; Barkowsky, E. Pollinator abundance in semiarid pastures as affected by forage species. Crop Sci. 2018, 58, 2665–2671. [Google Scholar] [CrossRef] [Green Version]
- Thapa-Magar, K.B.; Davis, T.S.; Kondratieff, B. Livestock grazing is associated with seasonal reduction in pollinator biodiversity and functional dispersion but cheatgrass invasion is not: Variation in bee assemblages in a multi-use shortgrass prairie. PLoS ONE 2020, 15, e0237484. [Google Scholar] [CrossRef]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuell, J.K.; Isaacs, R. Elevated pan traps to monitor bees in flowering crop canopies. Entomol. Exp. Appl. 2009, 131, 93–98. [Google Scholar] [CrossRef]





| Diversity of Bees | Pan Trap Color | |||||
|---|---|---|---|---|---|---|
| Family | Genus | Species | Blue | Green | Purple | Yellow |
| Apidae | Apis | mellifera | x | x | ||
| Bombus | griseocollis | x | x | x | ||
| Bombus | pensylvanicus | x | x | |||
| Ceratina | strenua | x | x | x | ||
| Ceratina | calcarata | x | x | |||
| Melissodes | niveus | x | x | |||
| Melissodes | veroninae | x | ||||
| Melissodes | bimaculata | x | ||||
| Melissodes | communis | x | x | |||
| Melissodes | comptoides | x | ||||
| Peponapis | timberlakei | x | x | |||
| Ptilothrix | bombiformis | x | x | x | ||
| Svastra | atripes | x | ||||
| Svastra | obliqua | x | x | |||
| Colletidae | Hylaeus | rudbeckiae | x | |||
| Megachilidae | Megachile | brevis | x | |||
| Ashmeadiella | floridana | x | ||||
| Halictidae | Agapostemon | texanus | x | x | x | x |
| Agapostemon | splendens | x | x | |||
| Agapostemon | sericeus | x | x | |||
| Augochlorella | aurata | x | x | x | ||
| Augochlorella | persimilis | x | x | |||
| Augochlora | pura | x | ||||
| Halictus | rubicundus | x | x | x | ||
| Halictus | confusus | x | x | x | x | |
| Halictus | ligatus | x | x | x | ||
| Halictus | parallelus | x | x | |||
| Lasioglossum | imitatum | x | x | x | x | |
| Lasioglossum | disparile | x | x | |||
| Lasioglossum | versatum | x | x | x | ||
| Lasioglossum | coreopsis | x | x | |||
| Lasioglossum | nr versans | x | x | |||
| Lasioglossum | birkmanni | x | x | x | ||
| Lasioglossum | subviridatum | x | x | x | x | |
| Lasioglossum | foxii | x | ||||
| Lasioglossum | sopinci | x | ||||
| Lasioglossum | paraforbesii | x | x | |||
| Lasioglossum | athabascence | x | ||||
| Lasioglossum | tegulare | x | x | |||
| Lasioglossum | pectorale | x | ||||
| Lasioglossum | trigeminum | x | ||||
| Lasioglossum | callidum | x | ||||
| Lasioglossum | zephyrum | x | ||||
| Lasioglossum | hitchensi | x | ||||
| Pan Trap Color | |||||
|---|---|---|---|---|---|
| Blue | Green | Purple | Yellow | ||
| Abundance | 291 | 55 | 147 | 79 | |
| Richness (observed) | 36 | 11 | 24 | 17 | |
| Richness (extrapolated; Chao1) 1 | 45 | 22 | 28 | 22 | |
| Number of unique species | 10 | 1 | 4 | 2 | |
| Similarity Indices 2 | Blue | 1 | |||
| Green | 0.38 (0.70) | 1 | |||
| Purple | 0.63 (0.89) | 0.34 (0.75) | 1 | ||
| Yellow | 0.53 (0.87) | 0.36 (0.76) | 0.59 (0.90) | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, R.S.; Leslie, T.; Fitting, E.; Burke, J.; Loftin, K.; Joshi, N.K. Color of Pan Trap Influences Sampling of Bees in Livestock Pasture Ecosystem. Biology 2021, 10, 445. https://doi.org/10.3390/biology10050445
Acharya RS, Leslie T, Fitting E, Burke J, Loftin K, Joshi NK. Color of Pan Trap Influences Sampling of Bees in Livestock Pasture Ecosystem. Biology. 2021; 10(5):445. https://doi.org/10.3390/biology10050445
Chicago/Turabian StyleAcharya, Roshani S., Timothy Leslie, Emily Fitting, Joan Burke, Kelly Loftin, and Neelendra K. Joshi. 2021. "Color of Pan Trap Influences Sampling of Bees in Livestock Pasture Ecosystem" Biology 10, no. 5: 445. https://doi.org/10.3390/biology10050445
APA StyleAcharya, R. S., Leslie, T., Fitting, E., Burke, J., Loftin, K., & Joshi, N. K. (2021). Color of Pan Trap Influences Sampling of Bees in Livestock Pasture Ecosystem. Biology, 10(5), 445. https://doi.org/10.3390/biology10050445






