A Reliable and Standardizable Differential PCR and qPCR Methodology Assesses HER2 Gene Amplification in Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
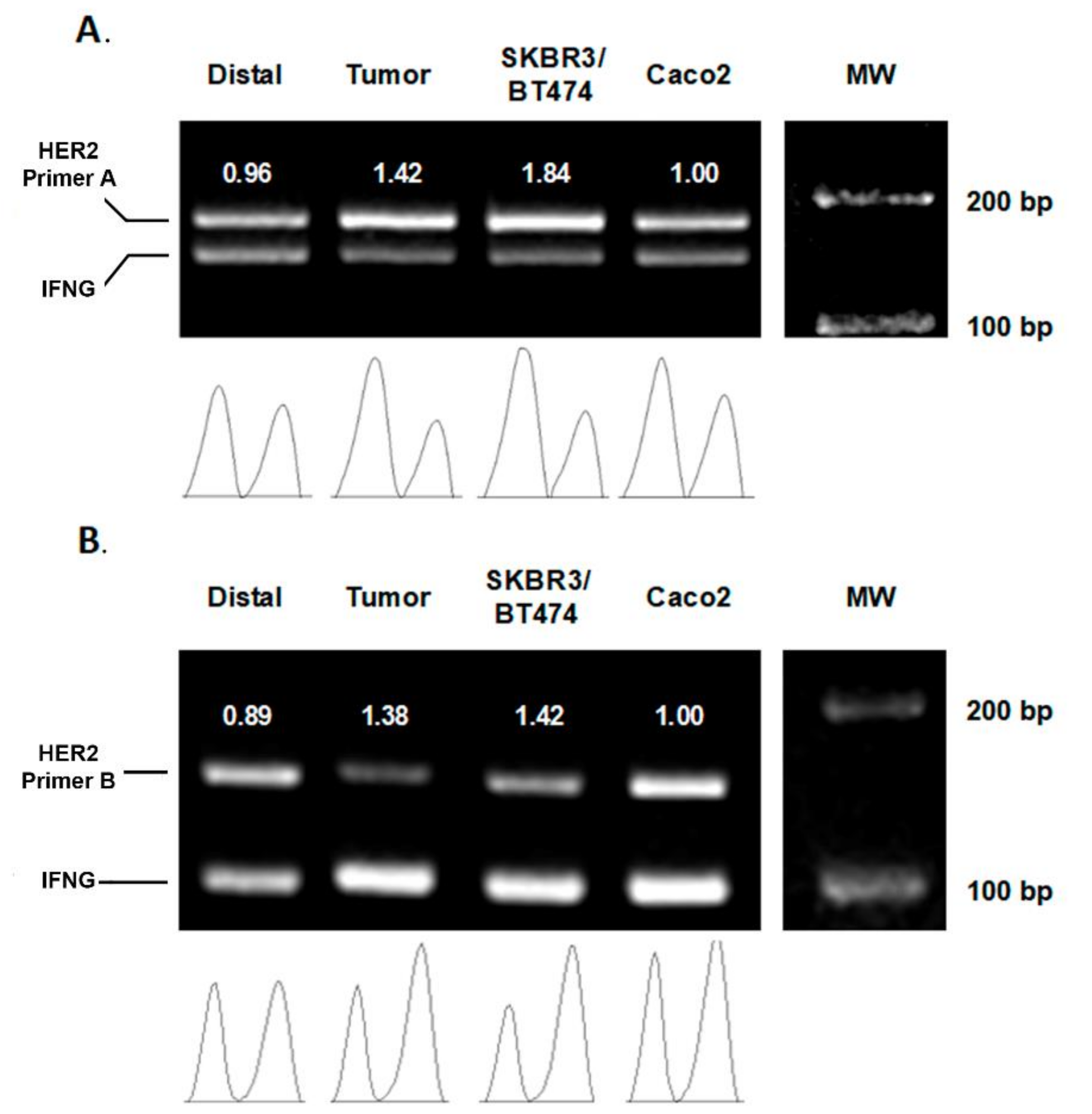
3.1. diffPCR
3.2. qPCR
3.3. Immunohistochemistry and Immunofluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Hofmann, M.; Stoss, O.; Shi, D.; Büttner, R.; Van De Vijver, M.; Kim, W.; Ochiai, A.; Rüschoff, J.; Henkel, T. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Shu, S.; Yorozu, K.; Hashizume, K.; Moriya, Y.; Fujimoto-Ouchi, K.; Harada, N. Importance of formalin fixing conditions for HER2 testing in gastric cancer: Immunohistochemical staining and fluorescence in situ hybridization. Gastric Cancer 2014, 17, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arienti, C.; Pignatta, S.; Tesei, A. Epidermal growth Factor receptor family and its role in gastric cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Merrett, N.D. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—A systematic review. Int. J. Cancer 2011, 130, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Hollmen, M.; Junttila, T.T.; Kapanen, A.I.; Tommola, S.; Soini, Y.; Helin, H.; Salo, J.; Joensuu, H.; Sihvo, E.; et al. Amplification of HER-2 in gastric carcinoma: Association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 2005, 16, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Xie, S.D.; Xu, C.Y.; Shen, J.G.; Jiang, Z.N. HER 2/neu protein expression in gastric cancer is associated with poor survival. Mol. Med. Rep. 2009, 2, 943–946. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.; Gundacker, H.; Benedetti, J.; Macdonald, J.; Baranda, J.; Levin, W.; Blanke, C.; Elatre, W.; Weng, P.; Zhou, J.; et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann. Oncol. 2013, 24, 1754–1761. [Google Scholar] [CrossRef]
- Kunz, P.L.; Mojtahed, A.; Fisher, G.A.; Ford, J.M.; Chang, D.T.; Balise, R.R.; Bangs, C.D.; Cherry, A.M.; Pai, R.K. HER2 Expression in gastric and gastroesophageal junction adenocarcinoma in a US population. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Warneke, V.S.; Behrens, H.-M.; Böger, C.; Becker, T.; Lordick, F.; Ebert, M.P.A.; Röcken, C. Her2/neu testing in gastric cancer: Evaluating the risk of sampling errors. Ann. Oncol. 2013, 24, 725–733. [Google Scholar] [CrossRef]
- Perez, E.A.; Cortés, J.; Gonzalez-Angulo, A.M.; Bartlett, J.M. HER2 testing: Current status and future directions. Cancer Treat. Rev. 2014, 40, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto-Ouchi, K.; Sekiguchi, F.; Yasuno, H.; Moriya, Y.; Mori, K.; Tanaka, Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother. Pharmacol. 2006, 59, 795–805. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Wei, X.; Tang, C.; Zhang, W. HER2-targeted therapies in gastric cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188549. [Google Scholar] [CrossRef] [PubMed]
- Jacquemier, J.; Spyratos, F.; Esterni, B.; Mozziconacci, M.-J.; Antoine, M.; Arnould, L.; Lizard, S.; Bertheau, P.; Lehmann-Che, J.; Fournier, C.B.; et al. SISH/CISH or qPCR as alternative techniques to FISH for determination of HER2 amplification status on breast tumors core needle biopsies: A multicenter experience based on 840 cases. BMC Cancer 2013, 13, 351. [Google Scholar] [CrossRef] [Green Version]
- Nistor, A.; Watson, P.H.; Pettigrew, N.; Tabiti, K.; Dawson, A.; Myal, Y. Real-time PCR complements immunohistochemistry in the determination of HER-2/neu status in breast cancer. BMC Clin. Pathol. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tvrdïk, D.; Stanek, L.; Skálová, H.; Dundr, P.; Velenská, Z.; Povýšil, C. Comparison of the IHC, FISH, SISH and qPCR methods for the molecular diagnosis of breast cancer. Mol. Med. Rep. 2012, 6, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Staněk, L.; Rozkoš, T.; Laco, J.; Ryška, A.; Petruželka, L.; Důra, M.; Dundr, P. Comparison of immunohistochemistry, four in situ hybridization methods and quantitative polymerase chain reaction for the molecular diagnosis of HER2 status in gastric cancer: A study of 55 cases. Mol. Med. Rep. 2014, 10, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Valerón, P.F.; Chirino, R.; Fernandez, L.; Torres, S.; Navarro, D.; Aguiar, J.; Cabrera, J.J.; Chico, B.N.D.; Diaz-Chico, J.C. Validation of a differential PCR and an ELISA procedure in studyingHER-2/neu status in breast cancer. Int. J. Cancer 1996, 65, 129–133. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Rodríguez-Juan, C.; Sala-Silveira, L.; Pérez-Blas, M.; Valeri, A.P.; Aguilera, N.; López-Santalla, M.; Fuertes, A.; Martín-Villa, J.M. Increased levels of bovine serum albumin antibodies in patients with type 1 diabetes and celiac disease-related antibodies. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 132–135. [Google Scholar] [CrossRef]
- Grabsch, H.; Sivakumar, S.; Gray, S.; Gabbert, H.E.; Müller, W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value—Conclusions from 924 cases of two independent series. Cell. Oncol. 2010, 32, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Xu, C.; Shen, J.; Jiang, Z.; Shen, J.; Wang, L. HER 2/neu protein expression in gastric cancer is associated with poor survival. Mol. Med. Rep. 2015, 12, 4794. [Google Scholar] [CrossRef] [PubMed]
- Frye, A.R.; Benz, C.C.; Liu, E. Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene 1989, 4, 1153–1157. [Google Scholar]
- Neubauer, A.; Neubauer, B.; He, M.; Effert, P.; Iglehart, D.; A Frye, R.; Liu, E. Analysis of gene amplification in archival tissue by differential polymerase chain reaction. Oncogene 1992, 7, 1019–1025. [Google Scholar]
- Marx, A.H.; Tharun, L.; Muth, J.; Dancau, A.-M.; Simon, R.; Yekebas, E.; Kaifi, J.T.; Mirlacher, M.; Brümmendorf, T.H.; Bokemeyer, C.; et al. HER-2 amplification is highly homogenous in gastric cancer. Hum. Pathol. 2009, 40, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhan, Q.-B.P.J.-B.; Li, Y.-M. Study of differential polymerase chain reaction of C-erbB-2 oncogene amplification in gastric cancer. World J. Gastroenterol. 1999, 5, 152–155. [Google Scholar] [CrossRef]


| Median | Range | |
|---|---|---|
| Age | 68 | 38–85 |
| n | (%) | |
| Sex | ||
| Male | 16 | (57.2) |
| Female | 12 | (42.9) |
| UICC 7th edition TNM Staging | ||
| Stage I | 9 | (32.1) |
| Stage II | 7 | (25.0) |
| Stage III | 4 | (14.3) |
| Stage IV | 8 | (28.6) |
| Treatment | ||
| Surgery | 28 | (100) |
| Chemotherapy * | 28 | (100) |
| Radiotherapy | 28 | (100) |
| Primers | Sequence (5′–3′) | Size (bp) | Cycles | Denaturation | Annealing | Elongation |
|---|---|---|---|---|---|---|
| HER2 | AAGCATACGT | 180 | 35 | 94 °C | 70 °C | |
| Primer A Fwd | GATGGCTGGT | 10 min 1 | 59.4 °C | 1 min | ||
| HER2 | CAATCTGCAT | 180 | 94 °C | 1 min | 70 °C | |
| Primer B Rvs | ACACCAGTTC | 1 min | 10 min 2 | |||
| HER2 | CCTCTGACGT | 98 | 32 | 94 °C | 72 °C | |
| Primer B Fwd | CCATCATCTC | 10 min 1 | 56 °C | 1.5 min | ||
| HER2 | ATCTTCTCGT | 98 | 94 °C | 1.5 min | 72 °C | |
| Primer B Rvs | GCCGTCGCTT | 1.5 min | 10 min 2 | |||
| IFNG | TCTTTTCTTTC | 150 | Shared by both diffPCR | |||
| Primer Fwd | CCGATAGGT | |||||
| IFNG | CAGGGATGCT | 150 | ||||
| Primer Rvs | CTTCGACCTC | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez, I.; Toro-Fernandez, J.F.; Vaquero-Yuste, C.; Molina-Alejandre, M.; Lasa, I.; Gomez, R.; Lopez, A.; Martin-Villa, J.M.; Gutierrez, A. A Reliable and Standardizable Differential PCR and qPCR Methodology Assesses HER2 Gene Amplification in Gastric Cancer. Biology 2021, 10, 516. https://doi.org/10.3390/biology10060516
Juarez I, Toro-Fernandez JF, Vaquero-Yuste C, Molina-Alejandre M, Lasa I, Gomez R, Lopez A, Martin-Villa JM, Gutierrez A. A Reliable and Standardizable Differential PCR and qPCR Methodology Assesses HER2 Gene Amplification in Gastric Cancer. Biology. 2021; 10(6):516. https://doi.org/10.3390/biology10060516
Chicago/Turabian StyleJuarez, Ignacio, Juan Francisco Toro-Fernandez, Christian Vaquero-Yuste, Marta Molina-Alejandre, Inmaculada Lasa, Remedios Gomez, Adela Lopez, Jose Manuel Martin-Villa, and Alberto Gutierrez. 2021. "A Reliable and Standardizable Differential PCR and qPCR Methodology Assesses HER2 Gene Amplification in Gastric Cancer" Biology 10, no. 6: 516. https://doi.org/10.3390/biology10060516
APA StyleJuarez, I., Toro-Fernandez, J. F., Vaquero-Yuste, C., Molina-Alejandre, M., Lasa, I., Gomez, R., Lopez, A., Martin-Villa, J. M., & Gutierrez, A. (2021). A Reliable and Standardizable Differential PCR and qPCR Methodology Assesses HER2 Gene Amplification in Gastric Cancer. Biology, 10(6), 516. https://doi.org/10.3390/biology10060516






