The Sperm Structure and Spermatogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Scanning Electron Microscopy (SEM)
2.3. Transmission Electron Microscopy (TEM)
3. Results
3.1. Gross Morphology of the Male Reproductive System
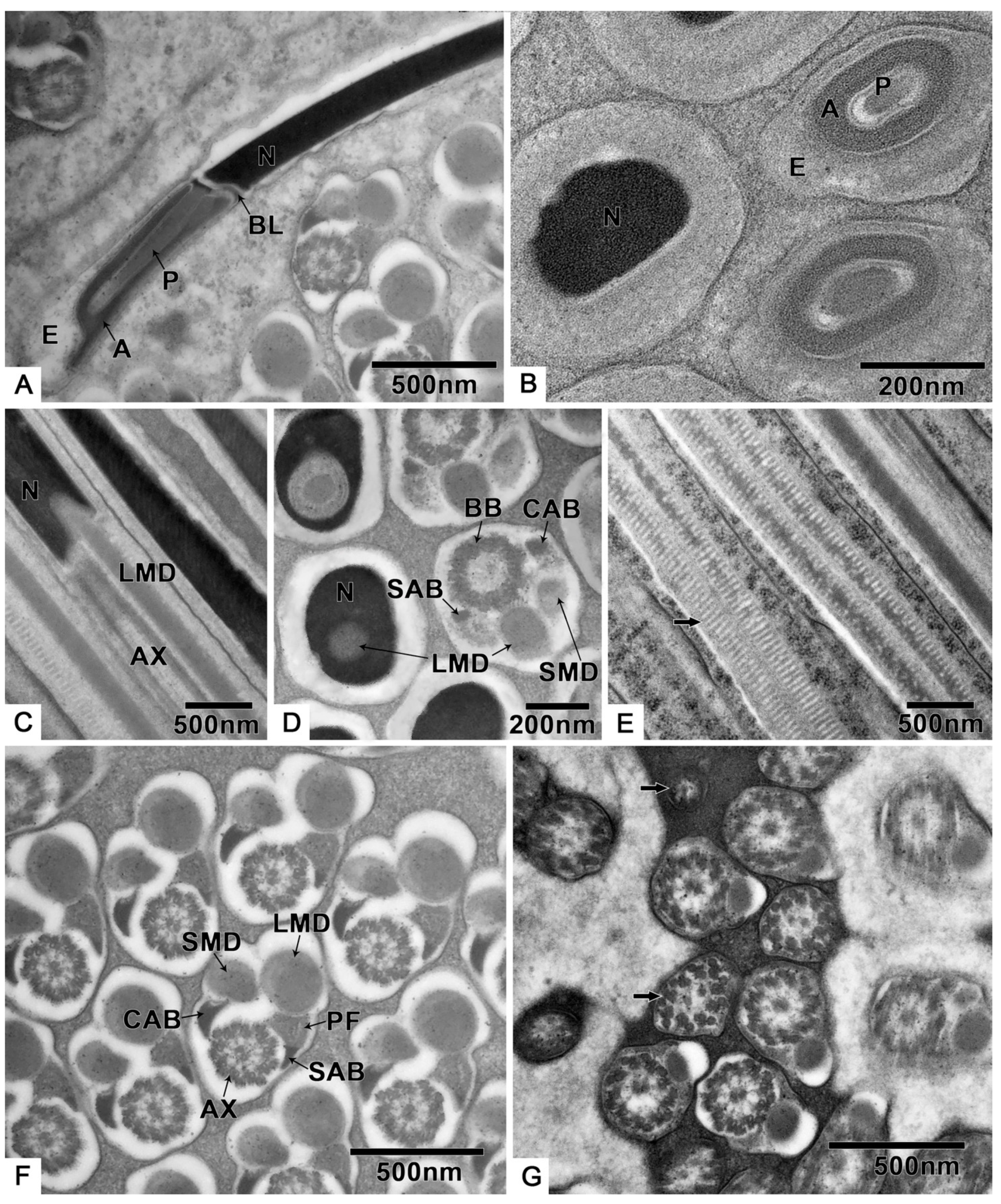
3.2. Spermatogenesis
3.2.1. From Spermatogonia to Spermatids
3.2.2. Spermiogenesis
3.3. Spermatozoa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, R.S. Weevils and plants: Phylogenetic versus ecological mediation of evolution of host plant associations in curculioninae (coleoptera: Curculionidae). Memoirs Èntomol. Soc. Can. 1993, 125, 197–232. [Google Scholar] [CrossRef]
- Gao, G. Occurrence and Host Selection Mechanism of Trypophloeus klimeschi Eggers. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2018. [Google Scholar]
- Marchetti, S.B.; Worrall, J.J.; Eager, T. Secondary insects and diseases contribute to sudden aspen decline in southwestern Colorado, USA. Can. J. For. Res. 2011, 41, 2315–2325. [Google Scholar] [CrossRef]
- Legalov, A.A. Revised checklist of weevils (Coleoptera: Curculionoidea excluding Scolytidae and Platypodidae) from Siberia and the Russian Far East. Acta Biol. Sib. 2020, 6, 437–549. [Google Scholar] [CrossRef]
- Cave, G.L.; Redlin, S.C. Importation of Chinese Penjing into the United States with Particular Reference to Ehretia microphylla; United States Department of Agriculture: Riverdale, MD, USA, 1996.
- Broberg, C.L.; Borden, J.H.; Humble, L.M. Distribution and abundance of Cryptorhynchus lapathi on Salix spp. in British Columbia. Can. J. For. Res. 2002, 32, 561–568. [Google Scholar] [CrossRef]
- Eggers, V.O.H. Trypophloeus klimeschi nov. spec. Entomol. Bl. 1915, 25, 7–9. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Trypophloeus klimeschi Eggers—A new insect pest to Xinjiang poplar. J. Tarim Univ. 2003, 15, 9–11. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Bionomics and control of Trypophloeus klimeschi. Entomol. Knowl. 2004, 41, 36–38. [Google Scholar]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Volatile Organic Compound Analysis of Host and Non-Host Poplars for Trypophloeus klimeschi (Coleoptera: Curculionidae: Ipinae). Russ. J. Plant Physiol. 2018, 65, 916–925. [Google Scholar] [CrossRef]
- Gao, G.; Gao, J.; Hao, C.; Dai, L.; Chen, H. Biodiversity and Activity of Gut Fungal Communities across the Life History of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Int. J. Mol. Sci. 2018, 19, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Electroantennogram, behavioural responses, and field trapping of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae) to eight host volatiles. Can. Èntomol. 2019, 151, 236–250. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Wei, L.-S.; Torres, M.A.; Zhang, X.; Wu, S.-P.; Chen, H. Morphology of the Male Reproductive System and Spermiogenesis ofDendroctonus armandiTsai and Li (Coleoptera: Curculionidae: Scolytinae). J. Insect Sci. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberprieler, R.G.; Marvaldi, A.E.; Anderson, R.S. Weevils, weevils, weevils everywhere. Zootaxa 2007, 1668, 491–520. [Google Scholar] [CrossRef] [Green Version]
- Kuschel, G. A phylogenetic classification of Curculionoidea to families and subfamilies. Mem. Entomolol. Soc. Wash. 1995, 14, 5–33. [Google Scholar]
- Dallai, R. Overview on spermatogenesis and sperm structure of Hexapoda. Arthropod Struct. Dev. 2014, 43, 257–290. [Google Scholar] [CrossRef]
- Salazar, K.; Dias, G.; Boucher, S.; Lino-Neto, J.; Serrão, J.E. Morpho-anatomy of the male reproductive tract and spermatogenesis of the South American Spasalus silvarum Kuwert (Coleoptera: Passalidae). Zoomorphology 2016, 135, 487–497. [Google Scholar] [CrossRef]
- Schubert, L.F.; Krüger, S.; Moritz, G.B.; Schubert, V. Male reproductive system and spermatogenesis of Limodromus assimilis (Paykull 1790). PLoS ONE 2017, 12, e0180492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrini, A.G.; Magnano, L.; Magnano, A.R.; Scala, C.; Baccetti, B. Spermatozoa and phylogeny of Curculionoidea (Coleoptera). Int. J. Insect Morphol. Embryol. 1988, 17, 1–50. [Google Scholar] [CrossRef]
- Dallai, R.; Afzelius, B.A.; Lupetti, P.; Osella, G. Sperm structure of some Curculionoidea and their relationship with Chrysomeloidea. Boll. Mus. Regionale Sci. Nat. Torino. 1998, 1, 27–50. [Google Scholar]
- Name, K.P.; Dos Reis, G.P.; Báo, S.N. An ultrastructural study of spermiogenesis in two species of Sitophilus (Coleoptera: Curculionidae). Biocell 2007, 31, 229–236. [Google Scholar] [CrossRef]
- Dallai, R.; Gottardo, M.; Beutel, R.G. Structure and Evolution of Insect Sperm: New Interpretations in the Age of Phylogenomics. Annu. Rev. Èntomol. 2016, 61, 1–23. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Fanciulli, P.P.; Petrioli, A.; Lupetti, P. New findings on the sperm ultrastructure of Carabidae (Insecta, Coleoptera). Arthropod Struct. Dev. 2020, 54, 100912. [Google Scholar] [CrossRef] [PubMed]
- Kuschel, G.; Leschen, R.A.B.; Zimmerman, E.C. Platypodidae under scrutiny. Invertebr. Syst. 2000, 14, 771–805. [Google Scholar] [CrossRef]
- Cerezke, H.F. The Morphology and Functions of the Reproductive Systems of Dendroctonus monticolae Hopk. (Coleoptera: Scolytidae). Can. Èntomol. 1964, 96, 477–500. [Google Scholar] [CrossRef]
- Rubio, G.J.D.; Bustillo, P.A.E.; Vallejo, E.L.F.; Acuña, Z.J.R.; Benavides, M.P. Alimentary canal and reproductive tract of Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae, Scolytinae). Neotrop. Èntomol. 2008, 37, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Calder, A.A. Gross morphology of the soft parts of the male and female reproductive systems of Curculionoidea (Coleoptera). J. Nat. Hist. 1990, 24, 453–505. [Google Scholar] [CrossRef]
- Aslam, N.A. An assessment of some internal characters in the higher classification of the curculionidae s.l. (coleoptera). Trans. R. Entomol. Soc. Lond. 1961, 113, 417–480. [Google Scholar] [CrossRef]
- Ning, H.; Tang, M.; Chen, H. Mapping Invasion Potential of the Pest from Central Asia, Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae), in the Shelter Forests of Northwest China. Insects 2021, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Grodner, M.L. Aberrant spermatogenesis in hybrid progeny of sub-species of the boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1975, 4, 107–114. [Google Scholar] [CrossRef]
- Goldson, S.L.; Emberson, R.M. Reproductive morphology of the Argentine stem weevil, Hyperodes bonariensis (Coleoptera: Curculionidae). N. Z. J. Zoöl. 1981, 8, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Koçakoğlu, N.Ö.; Candan, S.; Güllü, M. The histomorphological structure of the male reproductive system of maize leaf weevil Tanymecus dilaticollis Gyllenhal, 1834 (Coleoptera: Curculionidae). Microsc. Res. Tech. 2019, 82, 1345–1352. [Google Scholar] [CrossRef]
- Barker, G.M. Functional anatomy of the reproductive system ofListronotus bonariensis (Kuschel). N. Z. Èntomol. 1989, 12, 34–42. [Google Scholar] [CrossRef]
- Gassner, G.; Childress, D.; Klemetson, D.J.; Iii, G.G. Spermiogenesis in boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1975, 4, 115–125. [Google Scholar] [CrossRef]
- Gracielle, I.M.S.; Fiorillo, B.S.; Lino-Neto, J.; Báo, S.N. Morphology of the male reproductive system and spermiogenesis in Hypanthidium foveolatum (Alfken, 1930) (Hymenoptera: Apidae: Megachilinae). Micron 2009, 40, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Hua, B. RETRACTED: Sperm ultrastructure in two species of Panorpa and one Bittacus (Mecoptera). Micron 2010, 41, 622–632. [Google Scholar] [CrossRef]
- Werner, M.; Simmons, L.W. Ultrastructure of spermatozoa of Onthophagus taurus (Coleoptera, Scarabaeidae) exhibits heritable variation. Naturwissenschaften 2011, 98, 213–223. [Google Scholar] [CrossRef]
- Dias, G.; Lino-Neto, J.; Mercati, D.; Dallai, R. The sperm ultrastructure and spermiogenesis of Tribolium castaneum (Coleoptera: Tenebrionidae) with evidence of cyst degeneration. Micron 2015, 73, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jurecic, R. Sperm cell number per bundle in Gnorimus nobilis L. (Coleoptera, Scarabaeidae). Genetica 1988, 76, 27–31. [Google Scholar] [CrossRef]
- Virkki, N. Sperm bundles and phylogenesis. Cell Tissue Res. 1969, 101, 13–27. [Google Scholar] [CrossRef]
- Paoli, F.; Dallai, R.; Cristofaro, M.; Arnone, S.; Francardi, V.; Roversi, P.F. Morphology of the male reproductive system, sperm ultrastructure and γ-irradiation of the red palm weevil Rhynchophorus ferrugineus Oliv. (Coleoptera: Dryophthoridae). Tissue Cell 2014, 46, 274–285. [Google Scholar] [CrossRef]
- Morrone, J.J.; Marvaldi, A.E. Phylogenetic systematics of weevils (Coleoptera: Curculionoidea): A reappraisal based on larval and adult morphology. Insect Syst. Evol. 2000, 31, 43–58. [Google Scholar] [CrossRef]
- Phillips, D.M. Insect sperm: Their structure and morphogenesis. J. Cell Biol. 1970, 44, 243–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatenby, J.B.; Tahmisian, T.N. Centriole adjunct, centrioles, mitochondria, and ergastoplasm in Orthopteran spermatogenesis. An electron microscope study. Cellule Rec. Trav. Originaux Cytol. Histol. Biol. Gen. 1959, 60, 105. [Google Scholar]
- Kaye, J.S. Acrosome formation in the house cricket. J. Cell Biol. 1962, 12, 411–431. [Google Scholar] [CrossRef]
- Phillips, D.M. Observations on spermiogenesis in the fungus gnat sciara coprophila. J. Cell Biol. 1966, 30, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.M. Fine structure of sciara coprophila sperm. J. Cell Biol. 1966, 30, 499–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanciulli, P.P.; Mercati, D.; Machida, R.; Dallai, R. Spermiogenesis and sperm ultrastructure of Machilontus sp (Insecta: Archaeognatha) with phylogenetic consideration. Micron 2015, 73, 47–53. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Abdelsalam, S.A.; Elmenshawy, O.M.; Abdel-Moneim, A.M. Ultrastructural Characteristics of Spermiogenesis inRhynchophorus ferrugineus (Coleoptera: Curculionidae). Fla. Èntomol. 2013, 96, 1463–1469. [Google Scholar] [CrossRef]
- Dallai, R.; Lino-Neto, J.; Dias, G.; Nere, P.H.A.; Mercati, D.; Lupetti, P. Fine structure of the ladybird spermatozoa (Insecta, Coleoptera, Coccinellidae). Arthropod Struct. Dev. 2018, 47, 286–298. [Google Scholar] [CrossRef]
- Jamieson, B.G.M.; Dallai, R.; Afzelius, B.A. Insects: Their Spermatozoa and Phylogeny; Science Publishers: Enfield, CT, USA, 1999; p. 555. [Google Scholar]
- Mojica, J.M.; Bruck, D.L. Sperm bundle coiling: Transporting long sperm bundles in Drosophila dunni dunni. J. Insect Physiol. 1996, 42, 303–307. [Google Scholar] [CrossRef]
- Moreira, J.; Zama, U.; Lino-Neto, J. Release, behavior and phylogenetic significance of spermatozoa in bundles in the seminal vesicle during sexual maturation in Aculeata (Hymenoptera). Braz. J. Morphol. Sci. 2004, 21, 185–189. [Google Scholar]
- Moreira, J.; Brito, P.; Mancini, K.; Dolder, H.; Lino-Neto, J. The descriptions of new microanatomical structures of the male reproductive system and sperm of Myschocyttarus cassununga (Hymenoptera: Vespidae). Micron 2012, 43, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Moreira, J.; Gomes, L.F.; Camargo-Mathias, M.I.; Lino-Neto, J. Sperm Bundles in the Seminal Vesicle of the Crematogaster victima (Smith) Adult Males (Hymenoptera: Formicidae). Neotrop. Èntomol. 2014, 43, 201–208. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Gao, G.; Wang, J.; Chen, H. The Sperm Structure and Spermatogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Biology 2021, 10, 583. https://doi.org/10.3390/biology10070583
Gao J, Gao G, Wang J, Chen H. The Sperm Structure and Spermatogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Biology. 2021; 10(7):583. https://doi.org/10.3390/biology10070583
Chicago/Turabian StyleGao, Jing, Guanqun Gao, Jiaxing Wang, and Hui Chen. 2021. "The Sperm Structure and Spermatogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae)" Biology 10, no. 7: 583. https://doi.org/10.3390/biology10070583
APA StyleGao, J., Gao, G., Wang, J., & Chen, H. (2021). The Sperm Structure and Spermatogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Biology, 10(7), 583. https://doi.org/10.3390/biology10070583





