Spatial Movement Patterns and Local Co-Occurrence of Nutria Individuals in Association with Habitats Using Geo-Self-Organizing Map (Geo-SOM)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
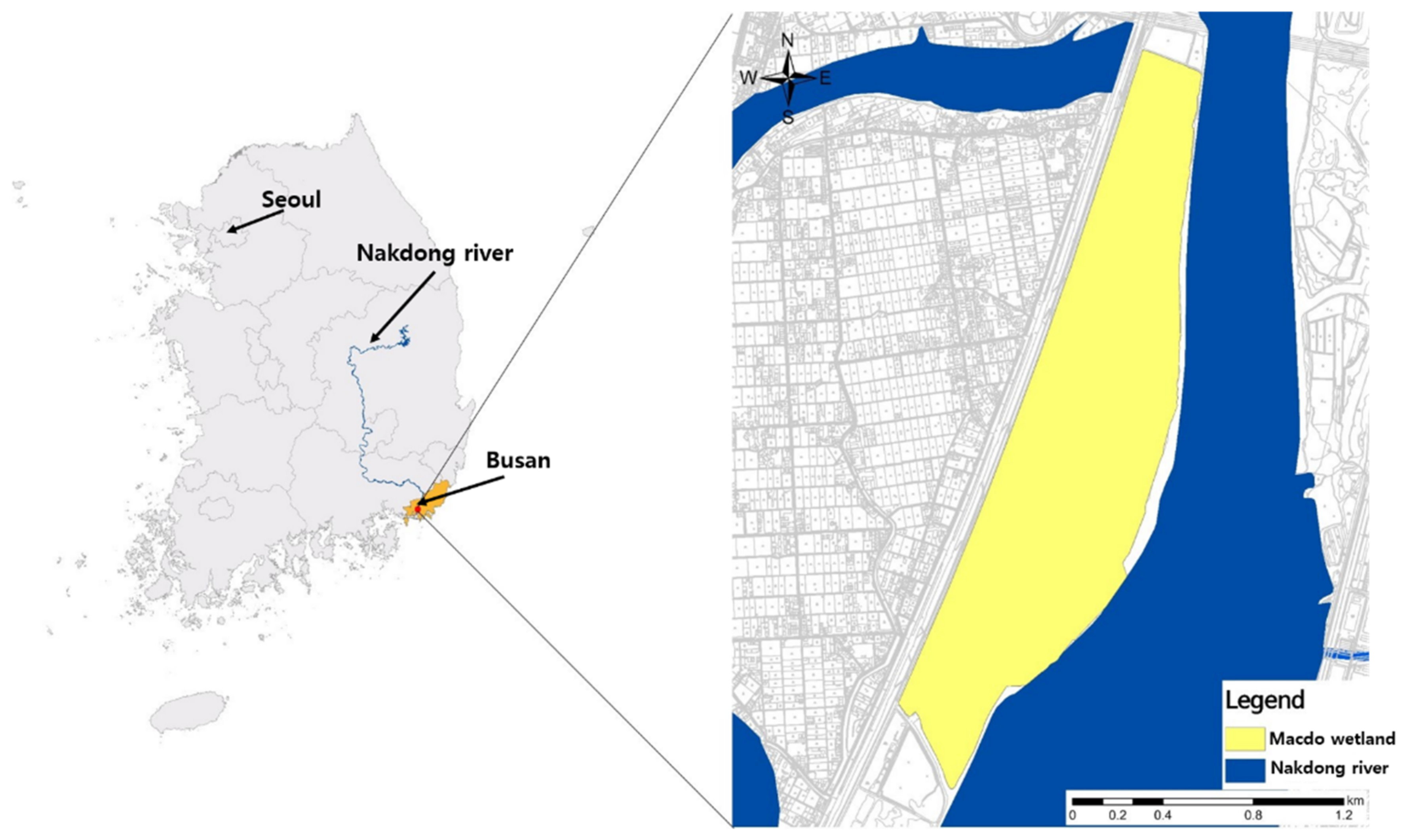
2.1. Study Area
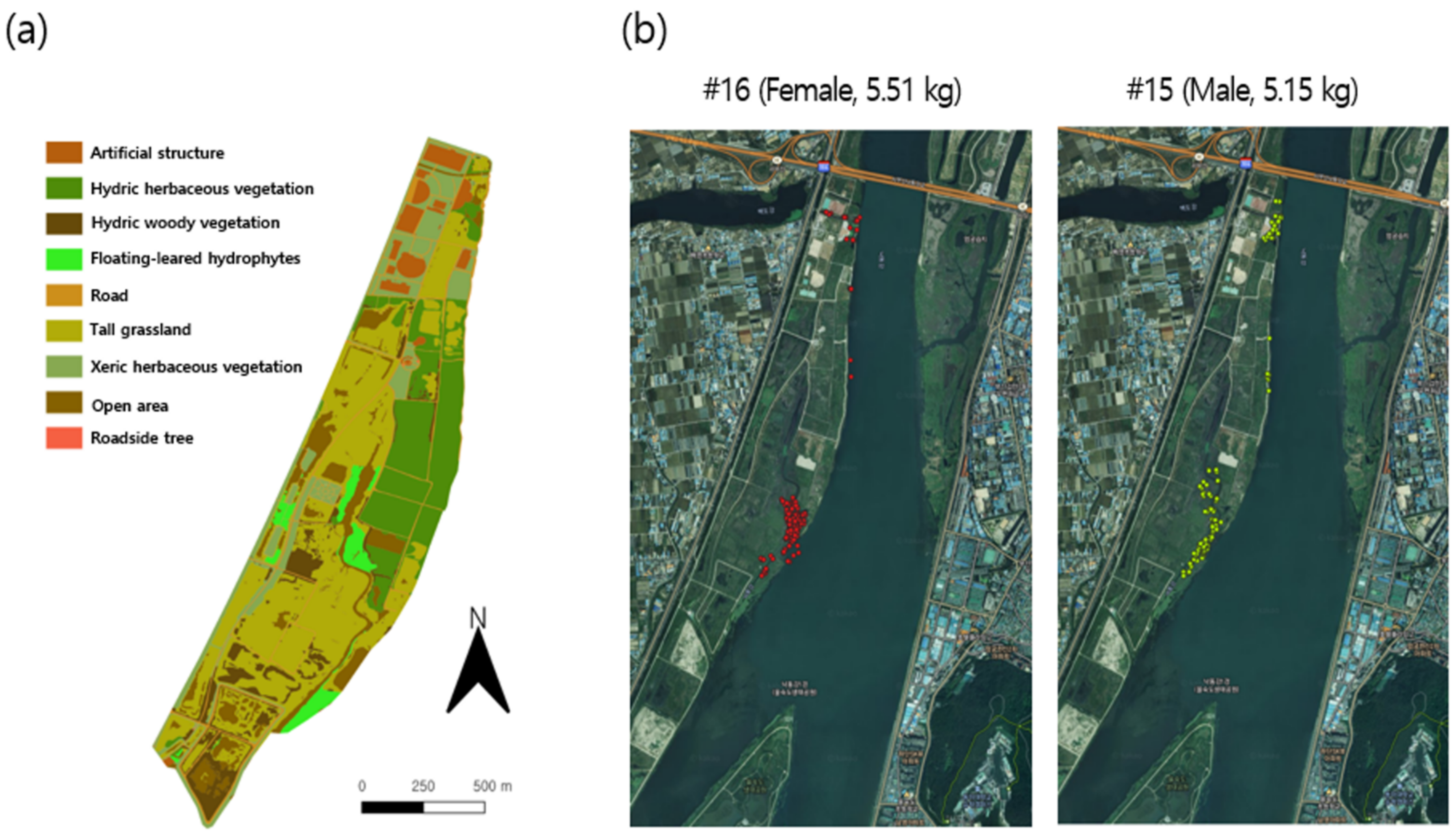
2.2. Monitoring of Individual Movement
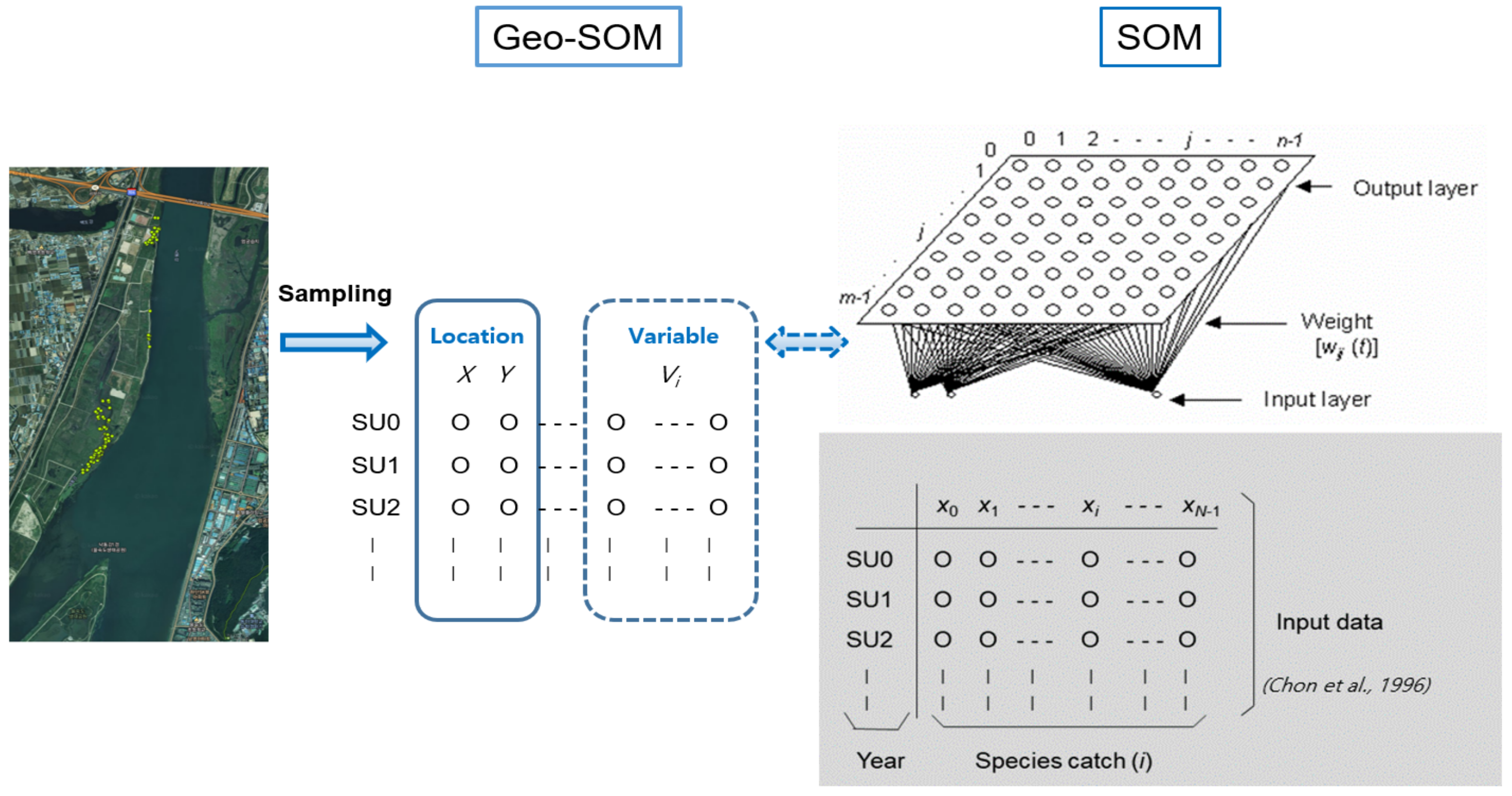
2.3. Geo-Self-Organizing Map (Geo-SOM)
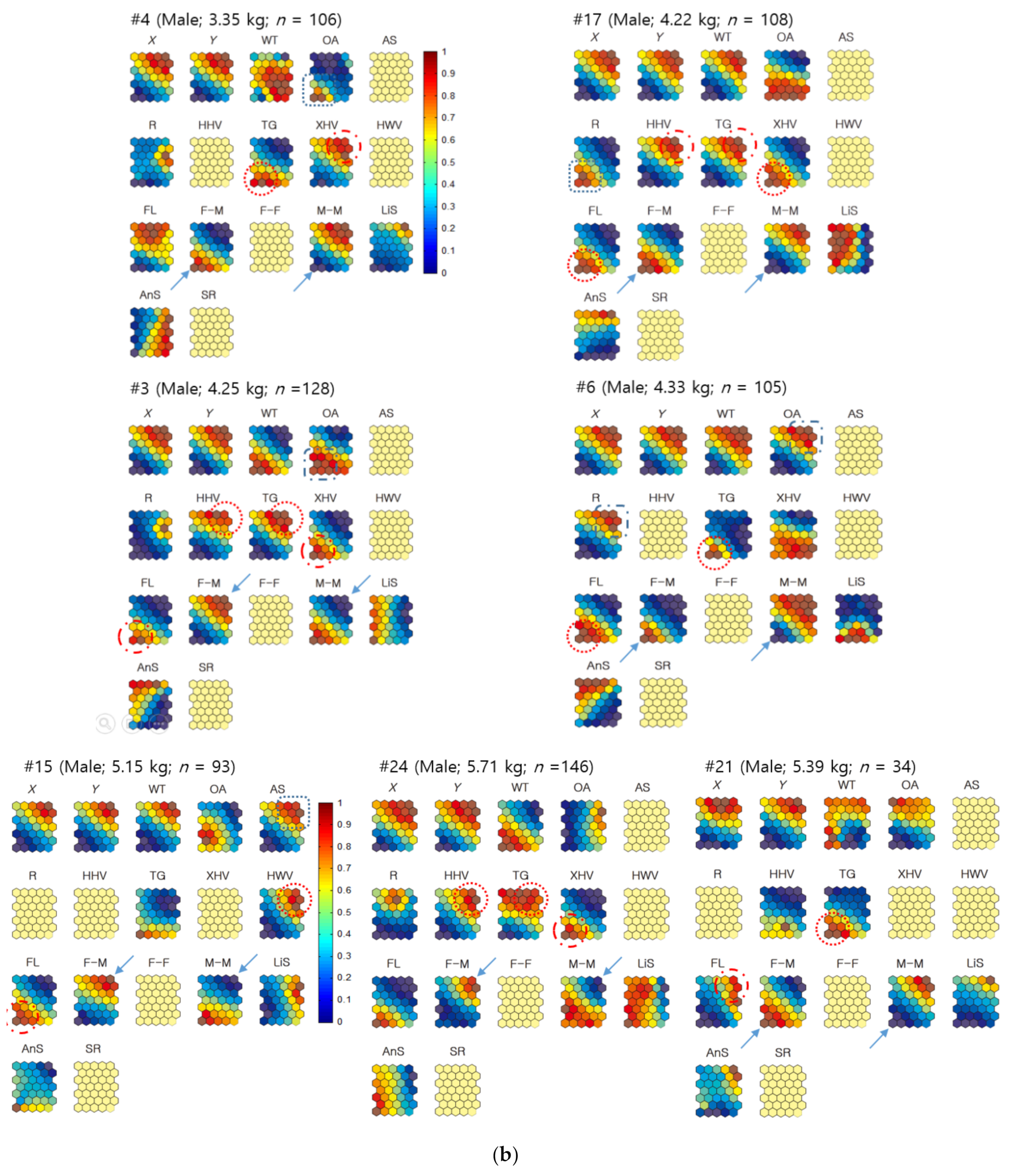
3. Results
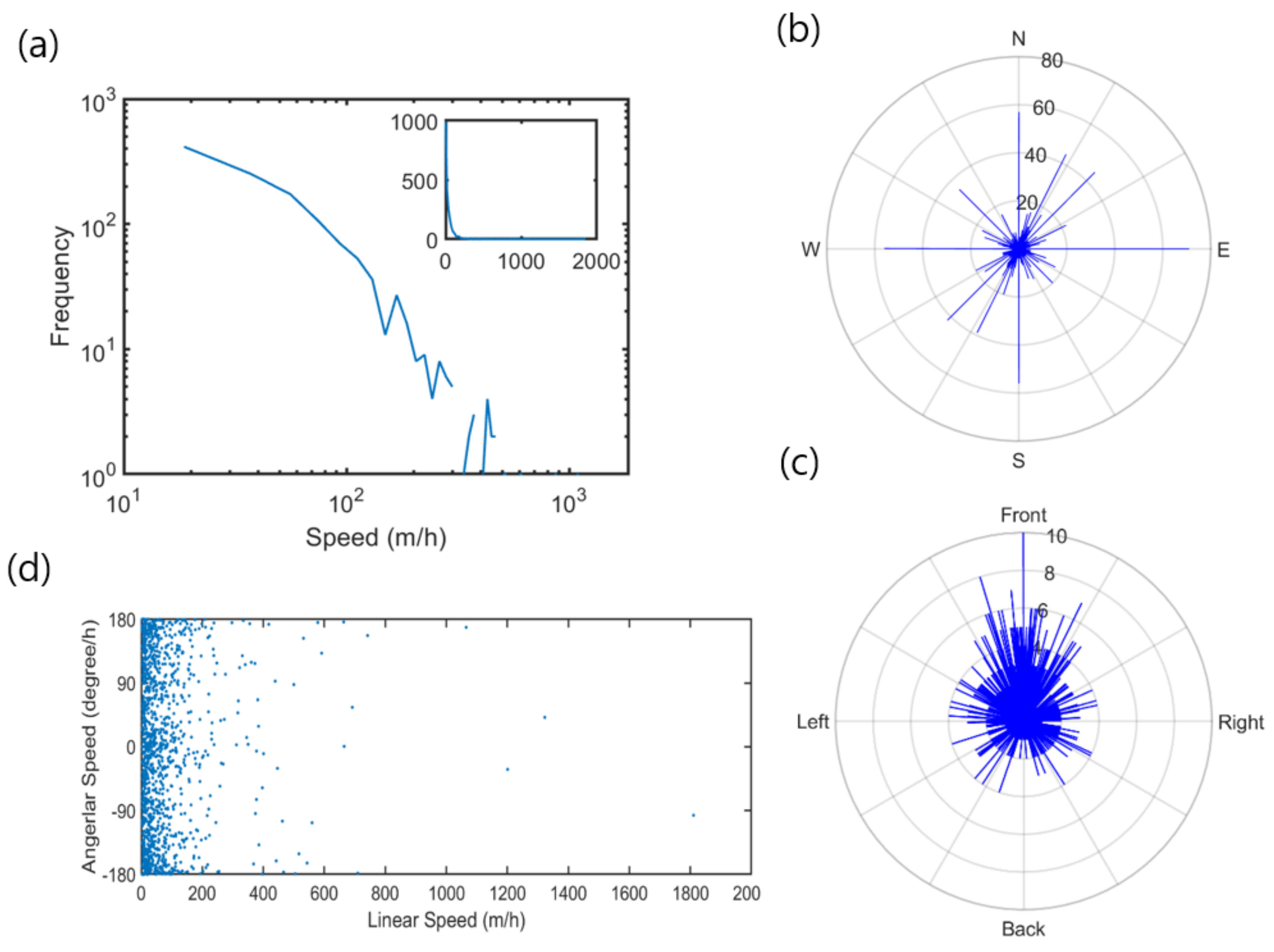
3.1. Movement Parameters
3.2. Spatial Movement Patterns
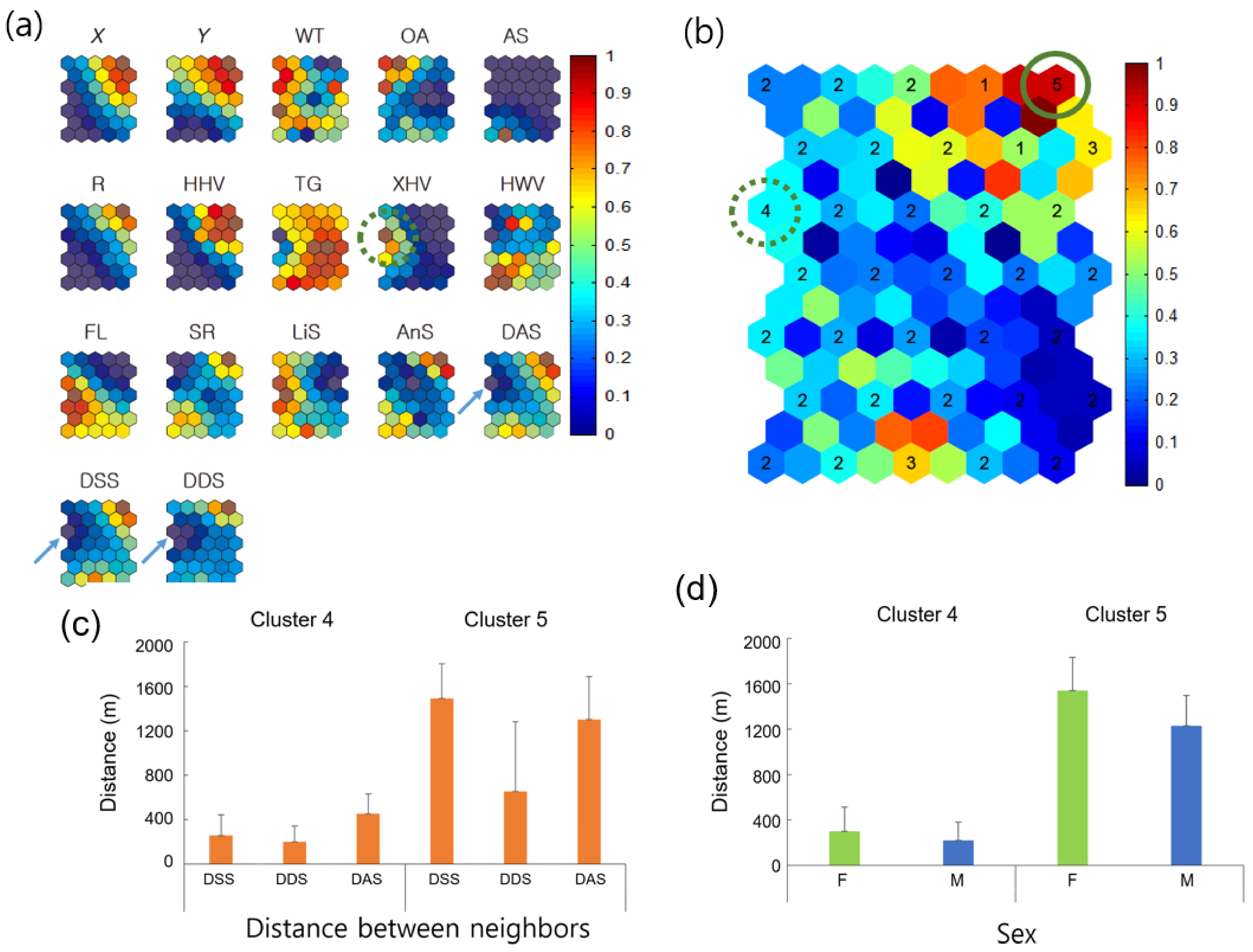
3.2.1. Nearest-Neighbor Distance According to Sex
3.2.2. Neighbor Distances Associated with Biological and Environmental Factors
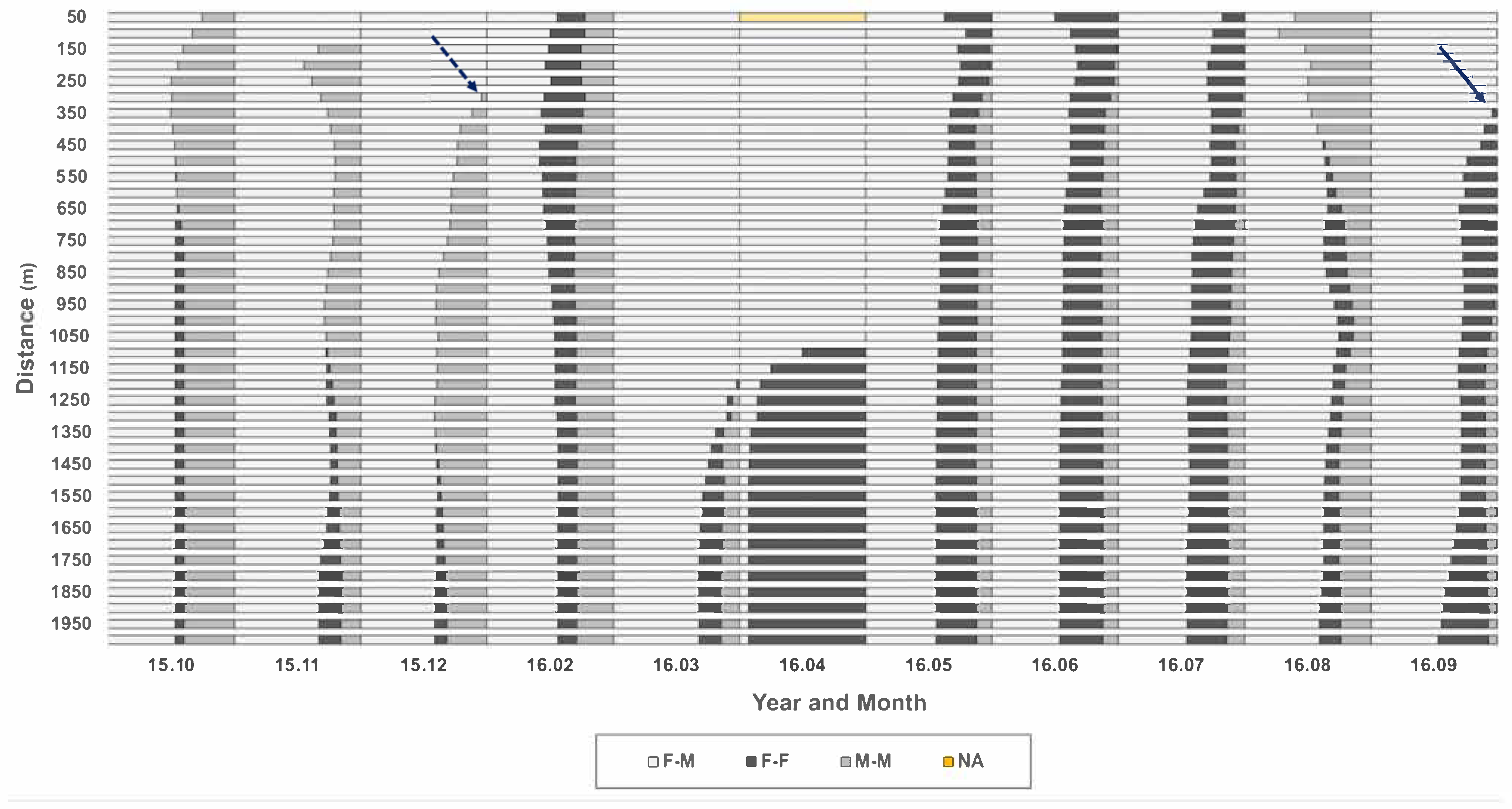
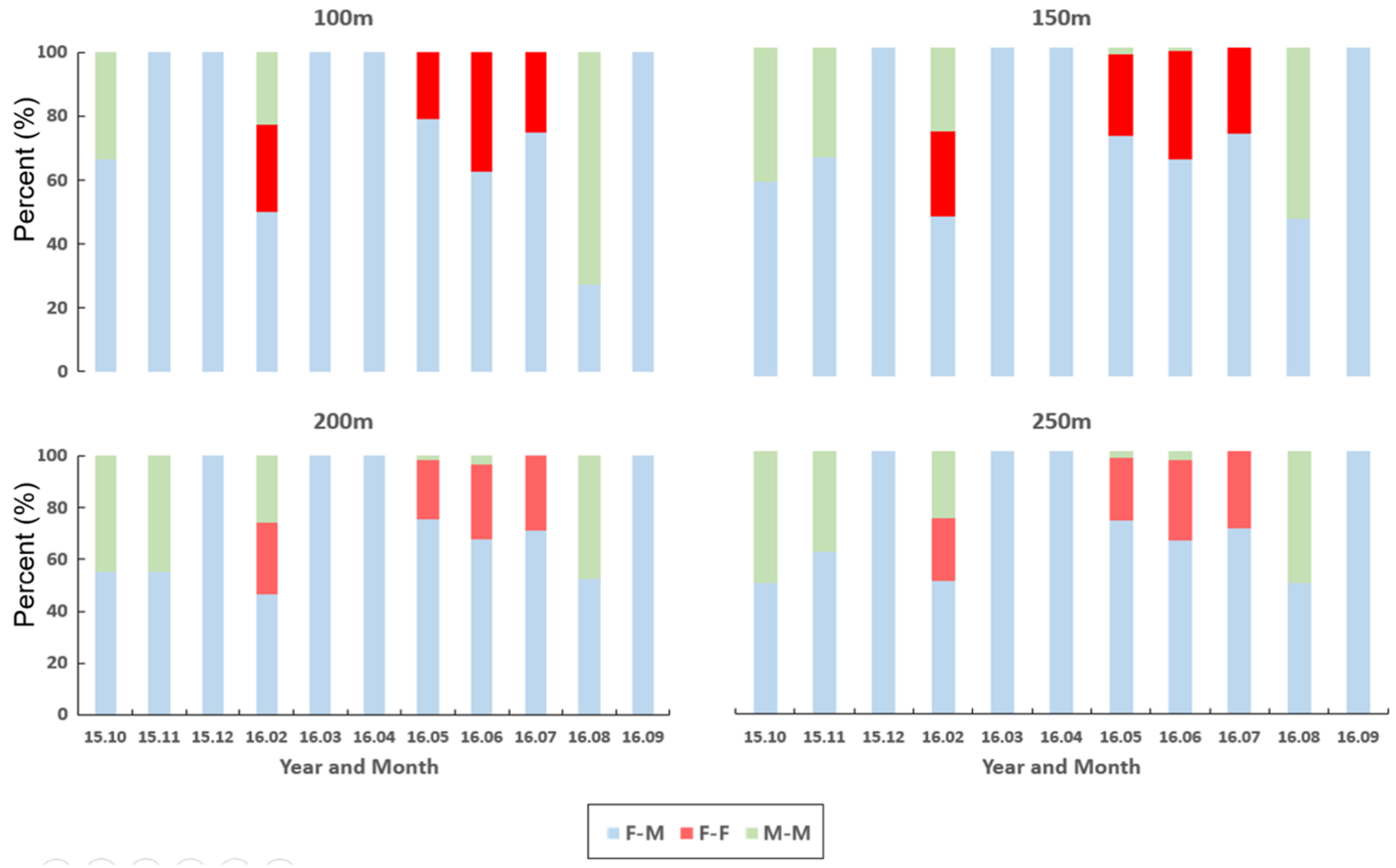
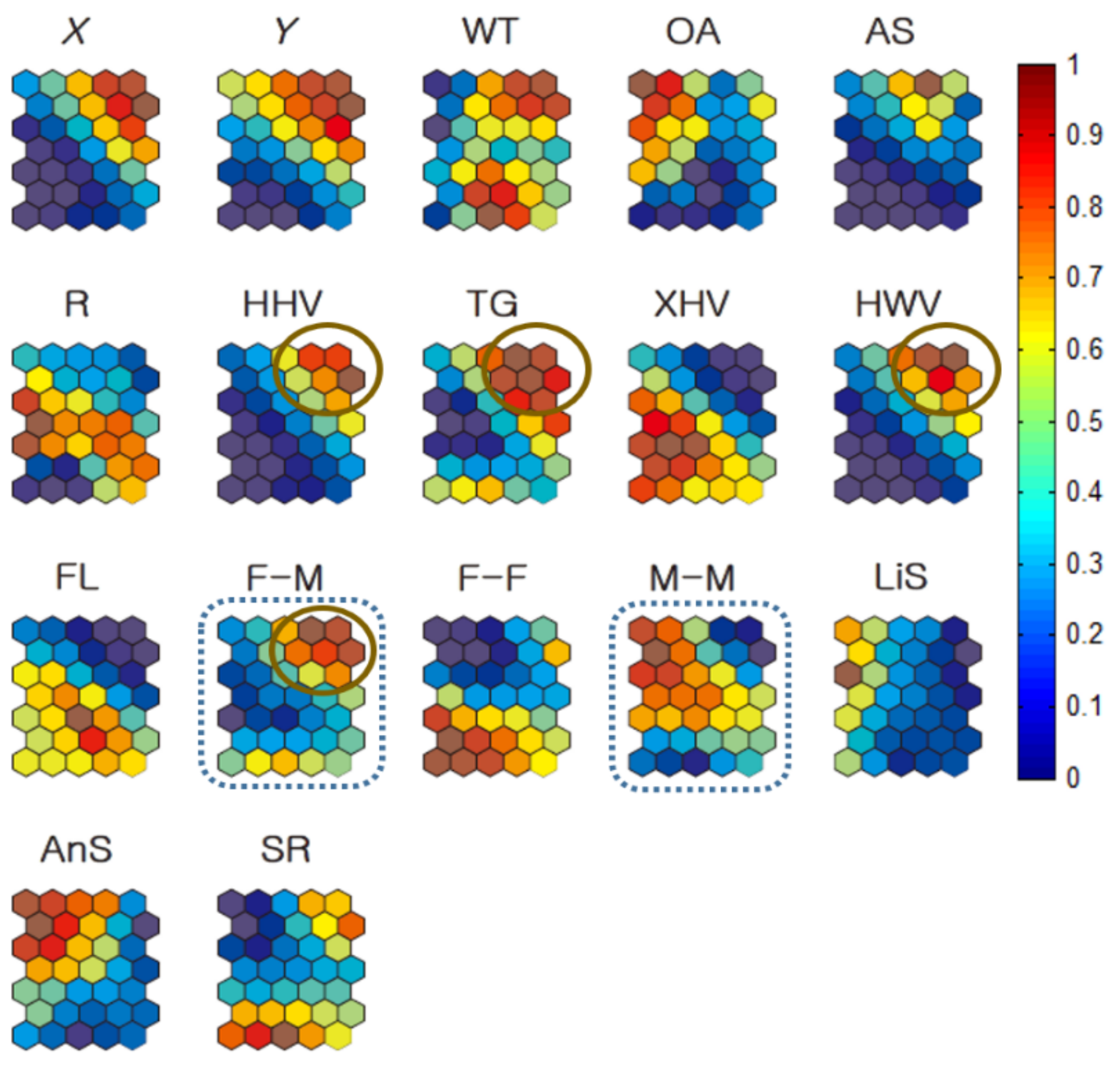
3.3. Co-Occurrence Patterns in Association with Habitat Types According to Sex
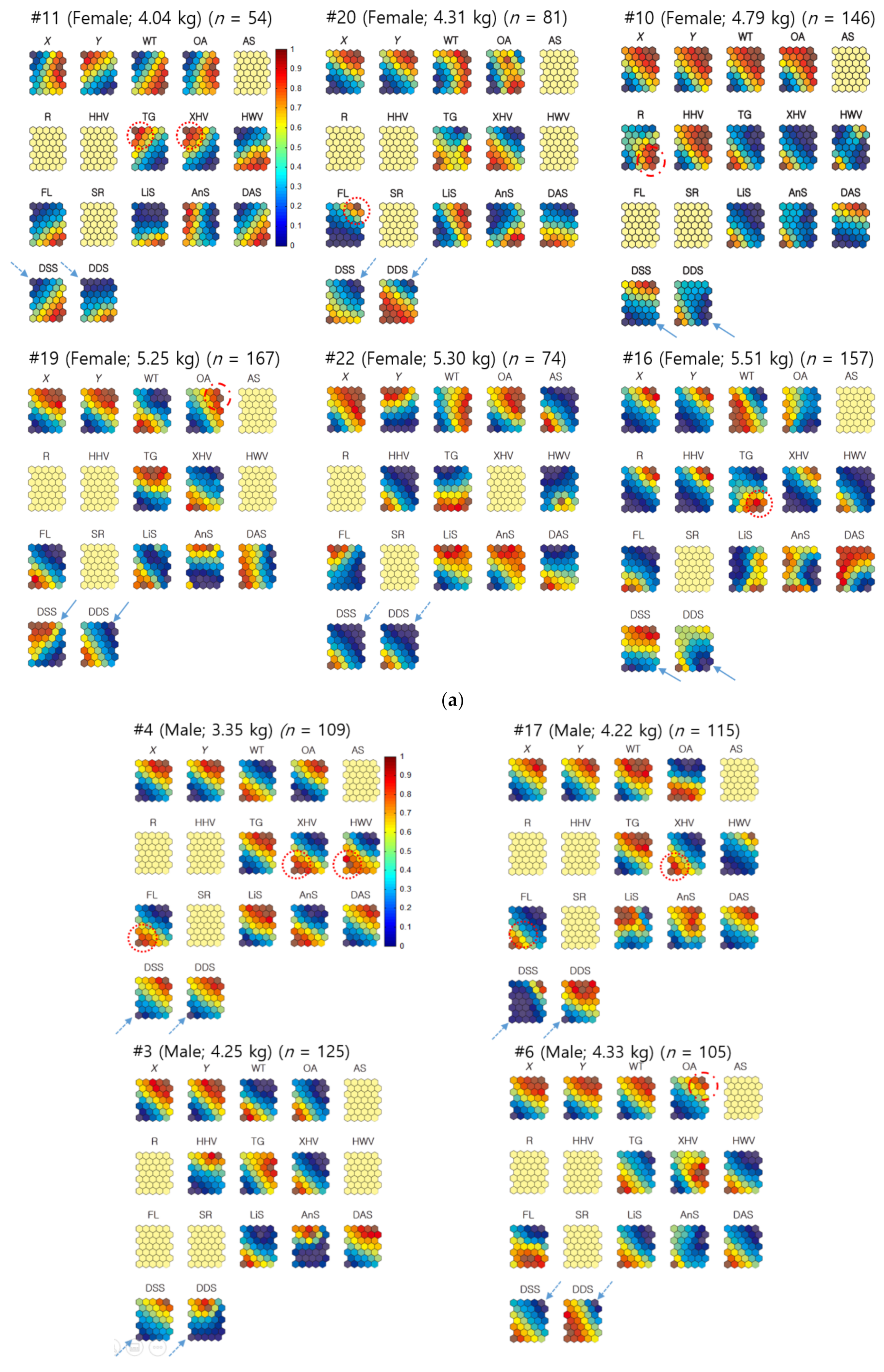
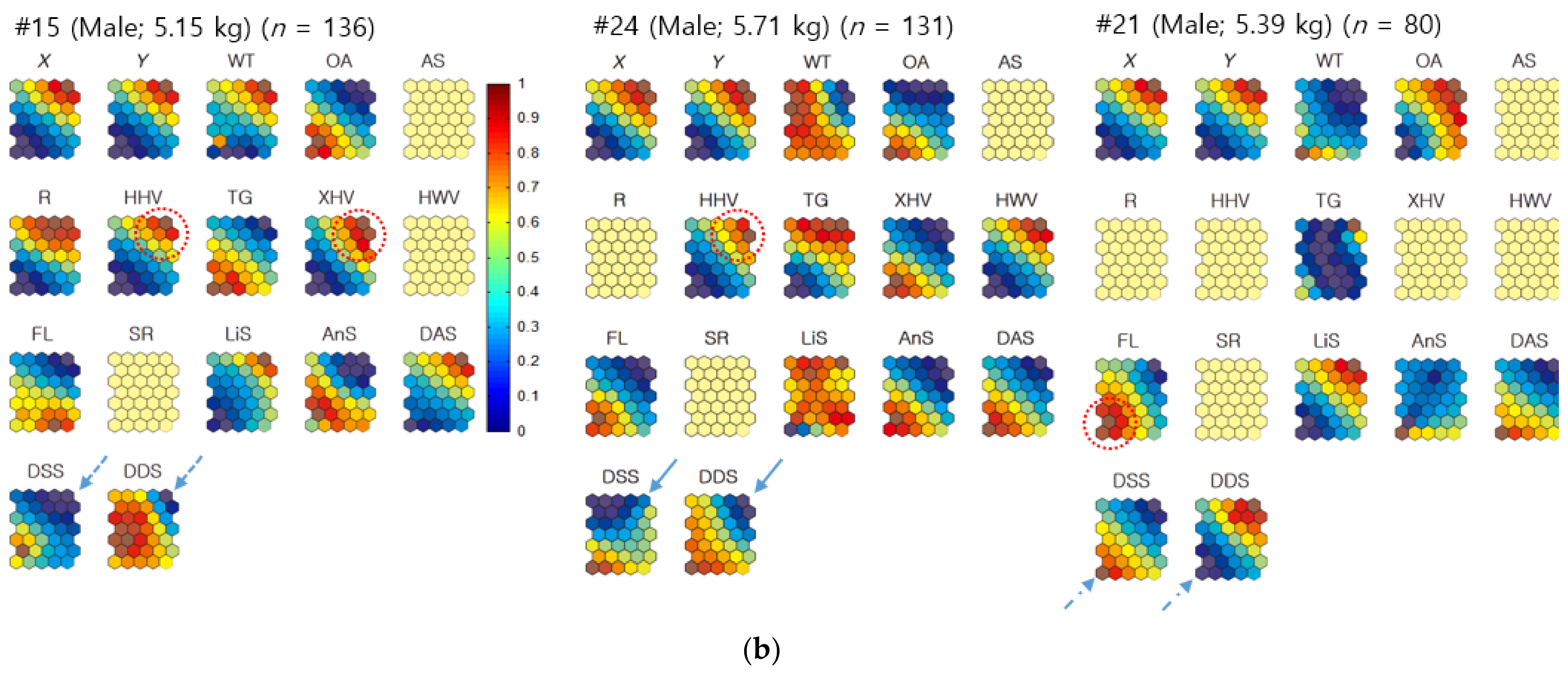
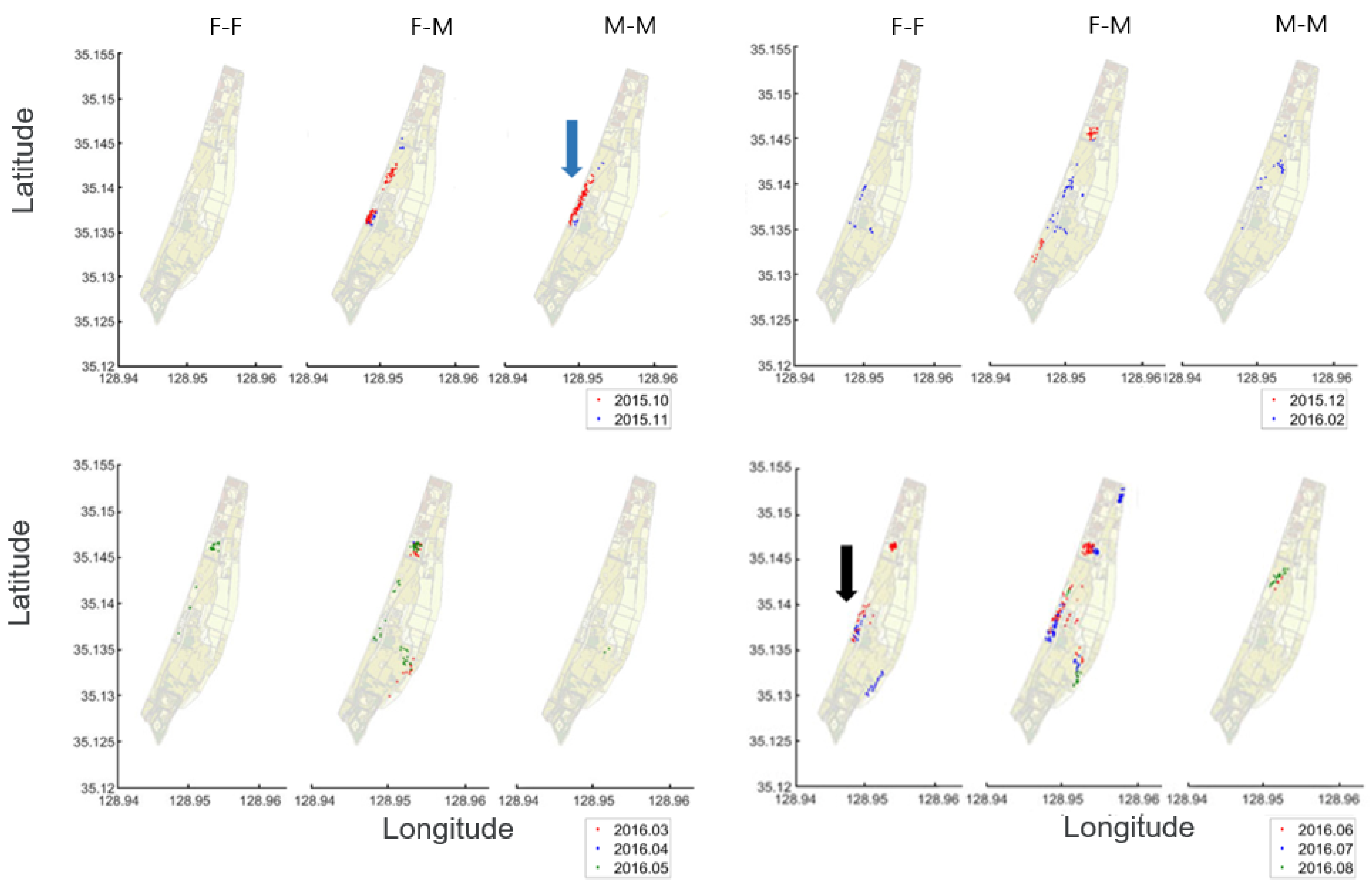
3.3.1. Individual Co-Occurrence Patterns
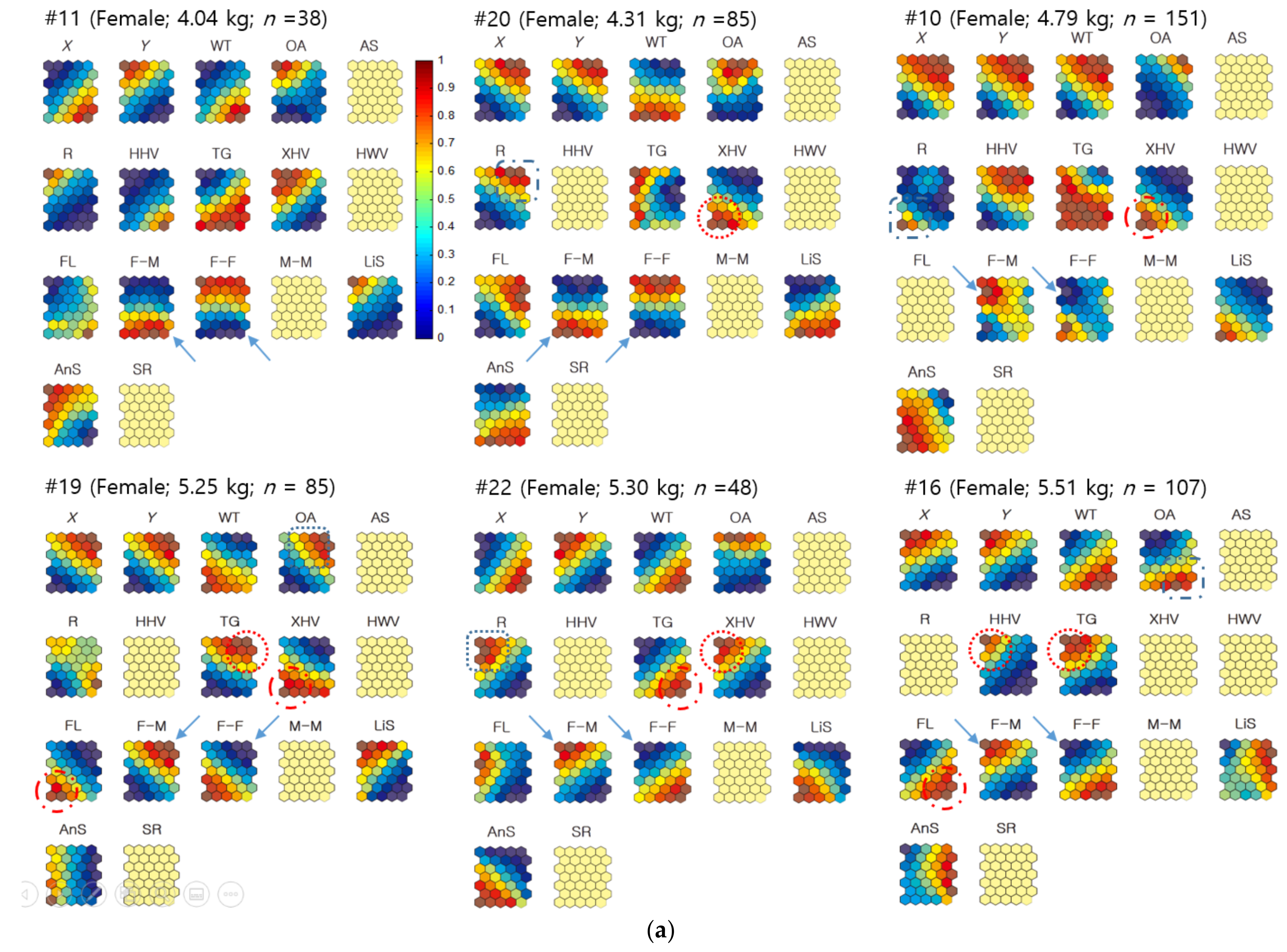
3.3.2. Co-Occurrences Associated with Biological and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
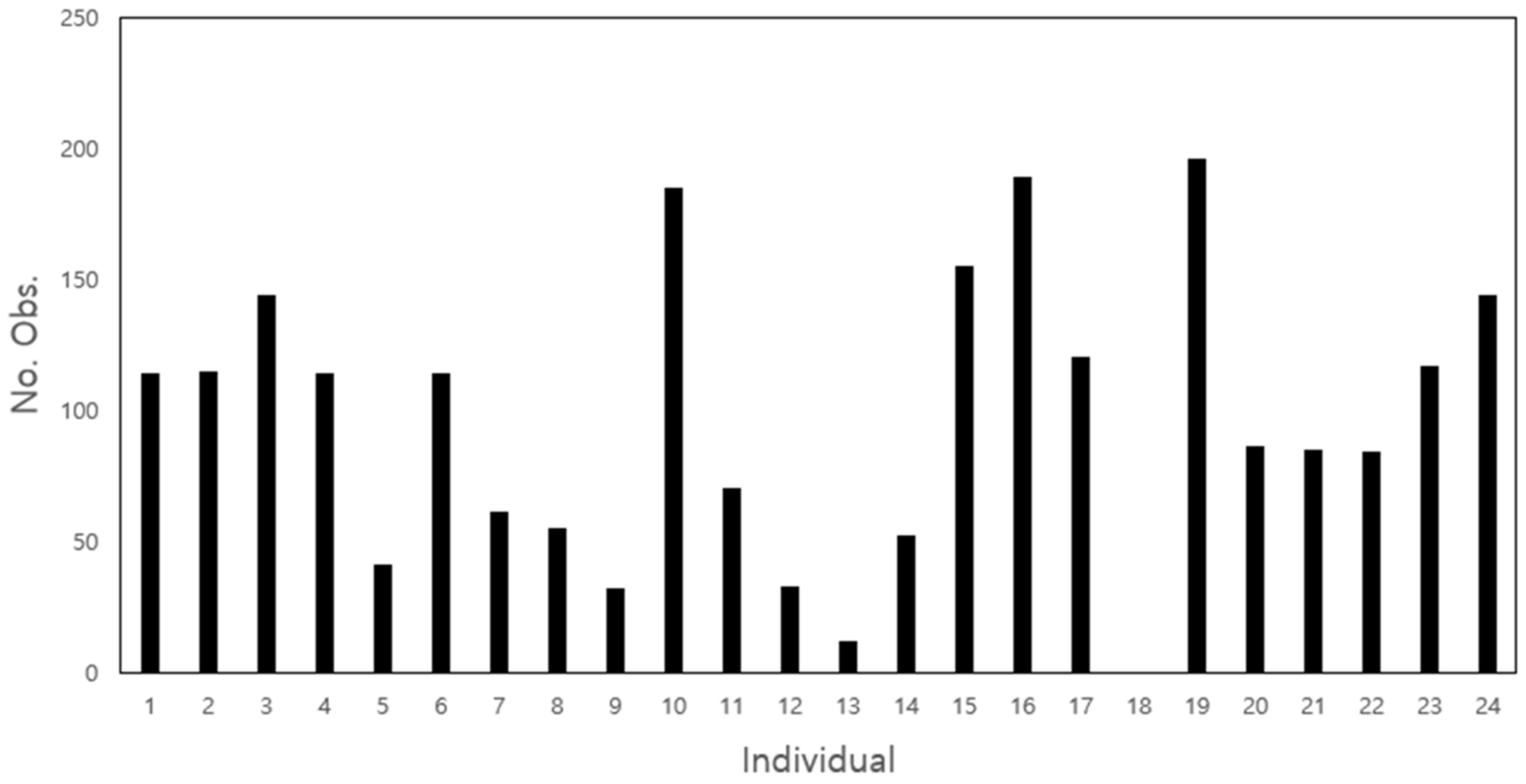
| Individual | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 | No. 12 |
| Sex | Female | Female | Male | Male | Male | Male | Male | Female | Male | Female | Female | Male |
| Weight (Kg) | 6.12 | 3.13 | 4.25 | 3.35 | 3.73 | 4.33 | 6.55 | 4.35 | 5.18 | 4.79 | 4.04 | 5.36 |
| Individual | No. 13 | No. 14 | No. 15 | No. 16 | No. 17 | No. 18 | No. 19 | No. 20 | No. 21 | No. 22 | No. 23 | No. 24 |
| Sex | Female | Female | Male | Female | Male | Male | Female | Female | Male | Female | Female | Male |
| Weight (Kg) | 3.84 | 5.27 | 5.15 | 5.51 | 4.22 | 4.74 | 5.25 | 4.31 | 5.39 | 5.30 | 5.20 | 5.71 |
Appendix B
| Indivdua | n | Sex | Range | X | Y | WT | OA | AS | R | HHV | TG | XHV | HWV | FL | SR | LiS | AnS | DAS | DSS | DDS |
| 11 | 54 | Female | Min | 128.9459 | 35.1245 | 4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 208.2 | 21.2 | 24.8 |
| Max | 128.9525 | 35.1394 | 16.2 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 928.4 | 234.8 | 1859.6 | 2227.1 | 1905.9 | |||
| 20 | 81 | Female | Min | 128.9476 | 35.1358 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 | 0 | 297.8 | 14.2 | 10 |
| Max | 128.9516 | 35.1418 | 26.9 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 286.6 | 292.2 | 1117.9 | 933 | 761.3 | |||
| 10 | 146 | Female | Min | 128.9469 | 35.1336 | 4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 36.8 | 7.9 | 8.8 |
| Max | 128.9555 | 35.1468 | 26.9 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 472.6 | 420.3 | 2102.1 | 2227.1 | 1819.3 | |||
| 19 | 167 | Female | Min | 128.9479 | 35.1357 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 148.6 | 14.2 | 11.5 |
| Max | 128.9522 | 35.1432 | 30.9 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 378.9 | 430.1 | 1487.3 | 1843.2 | 847 | |||
| 22 | 74 | Female | Min | 128.9436 | 35.1275 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 0.1 | 47.5 | 23.6 | 90.7 |
| Max | 128.9524 | 35.139 | 26.9 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 315.8 | 289.3 | 2217.6 | 2102.1 | 2088.2 | |||
| 16 | 157 | Female | Min | 128.9497 | 35.1296 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 47.5 | 47.5 | 8.8 |
| Max | 128.9581 | 35.1526 | 30.9 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 168.4 | 355.4 | 1434.7 | 2092.5 | 964.4 | |||
| 4 | 109 | Male | Min | 128.9475 | 35.135 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0.4 | 0 | 207.6 | 8.8 | 15 |
| Max | 128.9523 | 35.1432 | 20.8 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | −1 | 713.9 | 523.3 | 1537.8 | 2868.4 | 1207.6 | |||
| 17 | 115 | Male | Min | 128.9477 | 35.1358 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0.2 | 0 | 105.2 | 27.4 | 11.5 |
| Max | 128.9542 | 35.1461 | 30.9 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | −1 | 245.4 | 383 | 1157.8 | 1223.9 | 1091.7 | |||
| 3 | 125 | Male | Min | 128.938 | 35.1171 | 4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0.2 | 0 | 52.7 | 16.2 | 8.8 |
| Max | 128.9545 | 35.1467 | 20.8 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | −1 | 461.2 | 659.9 | 2594.9 | 2868.4 | 1637.7 | |||
| 6 | 105 | Male | Min | 128.9458 | 35.1316 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0.3 | 0 | 193 | 8.8 | 37.1 |
| Max | 128.9528 | 35.1447 | 20.8 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | −1 | 443.4 | 277.4 | 1140.8 | 1065.2 | 1377 | |||
| 15 | 136 | Male | Min | 128.9497 | 35.1296 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0.3 | 0 | 93.2 | 127.9 | 3.9 |
| Max | 128.9582 | 35.153 | 30.9 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | −1 | 225.6 | 321.1 | 1548.8 | 1404.5 | 1208.4 | |||
| 24 | 131 | Male | Min | 128.9476 | 35.1358 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0 | 0 | 50.5 | 27.4 | 3.9 |
| Max | 128.9545 | 35.1467 | 30.9 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | −1 | 199.6 | 365.4 | 1200.6 | 1316.5 | 1200.6 | |||
| 21 | 80 | Male | Min | 128.9508 | 35.1338 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | 0.6 | 0.1 | 223.6 | 108.5 | 90.7 |
| Max | 128.9537 | 35.1431 | 26.9 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | −1 | 174.8 | 266.6 | 1084.7 | 1126.4 | 828.6 |
Appendix C
| Individual | n | Sex | Range | X | Y | WT | OA | AS | R | HHV | TG | XHV | HWV | FL | F-M | F-F | M-M | LiS | AnS | SR |
| 11 | 38 | Female | Min | 128.95 | 35.13 | 4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 | 1.3 | 1 |
| Max | 128.95 | 35.14 | 10.7 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 928.4 | 180 | 1 | |||
| 20 | 85 | Female | Min | 128.95 | 35.14 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 | 0.2 | 1 |
| Max | 128.95 | 35.14 | 26.9 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 286.6 | 180 | 1 | |||
| 10 | 151 | Female | Min | 128.95 | 35.13 | 4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 | 0.2 | 1 |
| Max | 128.95 | 35.15 | 26.9 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 472.6 | 180 | 1 | |||
| 19 | 85 | Female | Min | 128.95 | 35.14 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 | 0.5 | 1 |
| Max | 128.95 | 35.14 | 30.9 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 378.9 | 180 | 1 | |||
| 22 | 48 | Female | Min | 128.95 | 35.13 | 24.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 | 1.1 | 1 |
| Max | 128.95 | 35.14 | 26.9 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 315.8 | 180 | 1 | |||
| 16 | 107 | Female | Min | 128.95 | 35.13 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 | 1.2 | 1 |
| Max | 128.95 | 35.14 | 30.9 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 140.1 | 180 | 1 | |||
| 4 | 106 | Male | Min | 128.95 | 35.14 | 15.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 | 3 | −1 |
| Max | 128.95 | 35.14 | 20.8 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 713.9 | 180 | −1 | |||
| 17 | 108 | Male | Min | 128.95 | 35.14 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 | 1.8 | −1 |
| Max | 128.95 | 35.15 | 30.9 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 245.4 | 180 | −1 | |||
| 3 | 128 | Male | Min | 128.95 | 35.14 | 4.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.7 | 0 | −1 |
| Max | 128.95 | 35.15 | 20.8 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 461.2 | 180 | −1 | |||
| 6 | 105 | Male | Min | 128.95 | 35.13 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.3 | 0 | −1 |
| Max | 128.95 | 35.15 | 20.8 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 191.5 | 180 | −1 | |||
| 15 | 93 | Male | Min | 128.95 | 35.13 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 | 0 | −1 |
| Max | 128.96 | 35.15 | 30.9 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 225.6 | 180 | −1 | |||
| 24 | 146 | Male | Min | 128.95 | 35.14 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.7 | −1 |
| Max | 128.95 | 35.15 | 30.9 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 199.6 | 180 | −1 | |||
| 21 | 34 | Male | Min | 128.95 | 35.13 | 20.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | 0.1 | −1 |
| Max | 128.95 | 35.14 | 26.9 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 161.4 | 180 | −1 |
References
- Kim, Y.-C.; Kim, A.; Lim, J.; Kim, T.-S.; Park, S.-G.; Kim, M.; Lee, J.-H.; Lee, J.R.; Lee, D.-H. Distribution and management of nutria (Myocastor coypus) populations in South Korea. Sustainability 2019, 11, 4169. [Google Scholar] [CrossRef] [Green Version]
- Park, S.G.; Lee, D.H. An inventory of alien mammals for ecological risk assessment in South Korea. Korean J. Environ. Biol. 2020, 38, 165–178. [Google Scholar] [CrossRef]
- Cronk, Q.C.B.; Fuller, J.L. Plant Invaders: The Threat to Natural Ecosystems; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Mooney, H.A.; Hobbs, R.J. Global change and invasive species: Where do we go from here. In Invasive Species in a Changing World; Island Press: Washington, DC, USA, 2000; pp. 425–434. [Google Scholar]
- Mooney, H.A. Invasive Alien Species: A New Synthesis; SCOPE Series; Island Press: Washington, DC, USA, 2005; Volume 63. [Google Scholar]
- Rawlins, K.A.; Grififin, J.E.; Moorhead, D.J.; Bargeron, C.T.; Evans, C.W. EDDMapS: Invasive Plant Mapping Handbook; The University of Georgia, Center for Invasive Species and Ecosystem Health: Tifton, GA, USA, 2011. [Google Scholar]
- Zietsman, L. Observations on Environmental Change in South Africa; SUN Media: Stellenbosch, South Africa, 2011. [Google Scholar]
- Mills, E.L.; Leach, J.H.; Carlton, J.T.; Secor, C.L. Exotic species in the Great Lakes: A history of biotic crises and anthropogenic introductions. J. Great Lakes Res. 1993, 19, 1–54. [Google Scholar] [CrossRef]
- Rejmánek, M.; Randall, J.M. Invasive alien plants in California: 1993 summary and comparison with other areas in North America. Madrono 1994, 41, 161–177. [Google Scholar]
- Ruiz, G.M.; Carlton, J.T. Invasion vectors: A conceptual framework for management. In Invasive Species: Vectors and Management Strategies; Island Press: Washington, DC, USA, 2003; pp. 459–504. [Google Scholar]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Herron, P.M.; Martine, C.T.; Latimer, A.M.; Leicht-Young, S.A. Invasive plants and their ecological strategies: Prediction and explanation of woody plant invasion. Divers. Distrib. 2007, 13, 633–644. [Google Scholar] [CrossRef]
- Gosling, L. The coypu in East Anglia. Trans. Norfolk Norwich Nat. Soc. 1974, 23, 49–59. [Google Scholar]
- Linscombe, G.; Kinler, N.; Wright, V. Nutria population density and vegetative changes in brackish marsh in coastal Louisiana. In Worldwide Furbearer Conference Proceedings; Chapman, J.A., Pursley, D., Eds.; Worldwide Furbearer Conference Inc.: Frostburg, MA, USA, 1981; pp. 129–141. [Google Scholar]
- Abbas, A. Impact du ragondin (Myocastor coypus Molina) sur une culture de maïs (Zea mays L.) dans le marais Poitevin. Acta Oecologica 1988, 9, 173–189. [Google Scholar]
- Woods, C.A.; Contreras, L.; Willner-Chapman, G.; Whidden, H.P. Myocastor coypus. Mamm. Species 1992, 398, 1–8. [Google Scholar] [CrossRef]
- Nyman, J.A.; Chabreck, R.H.; Kinler, N.W. Some effects of herbivory and 30 years of weir management on emergent vegetation in brackish marsh. Wetlands 1993, 13, 165–175. [Google Scholar] [CrossRef]
- LeBlanc, D.J. Nutria—The Handbook: Prevention and Control of Wildlife Damage; Animal Damage Control: Port Allen, LA, USA, 1994; Volume 16. [Google Scholar]
- Myers, R.S.; Shaffer, G.P.; Llewellyn, D.W. Baldcypress (Taxodium distichum (L.) Rich.) restoration in southeast Louisiana: The relative effects of herbivory, flooding, competition, and macronutrients. Wetlands 1995, 15, 141–148. [Google Scholar] [CrossRef]
- Johnson, L.A.; Foote, A.L. Vertebrate herbivory in managed coastal wetlands: A manipulative experiment. Aquat. Bot. 1997, 59, 17–32. [Google Scholar] [CrossRef]
- Ford, M.A.; Grace, J.B. The interactive effects of fire and herbivory on a coastal marsh in Louisiana. Wetlands 1998, 18, 1–8. [Google Scholar] [CrossRef]
- Cocchi, R.; Riga, F. Control of a coypu Myocastor coypus population in northern Italy and management implications. Ital. J. Zool. 2008, 75, 37–42. [Google Scholar] [CrossRef]
- Baker, S. Control and eradication of invasive mammals in Great Britain. Rev. Sci. Tech. Off. Int. Epiz. 2010, 29, 311–327. [Google Scholar] [CrossRef]
- Kendrot, S.R. Restoration through eradication: Protecting Chesapeake Bay marshlands from invasive nutria (Myocastor coypus). In Proceedings of the International Conference on Island Invasives: Eradication and Management, Auckland, New Zealand, 8–12 February 2010; IUCN: Gland, Switzerland, 2011; pp. 313–319. [Google Scholar]
- Lee, D.-H.; Kil, J.H.; Yang, G. Ecological Characteristics for the Sustainable Management of NUTRIA (Myocastor coypus) in Korea; National Institute of Environmental Research: Incheon, Korea, 2012.
- Lee, D.-H.; Kil, J.H.; Kim, D.E. The study on the distribution and inhabiting status of nutria (Myocastor coypus) in Korea. Korean J. Environ. Ecol. 2013, 27, 316–326. [Google Scholar]
- Hong, S.; Do, Y.; Kim, J.Y.; Kim, D.K.; Joo, G.J. Distribution, spread and habitat preferences of nutria (Myocastor coypus) invading the lower Nakdong River, South Korea. Biol. Invasions 2015, 17, 1485–1496. [Google Scholar] [CrossRef]
- Kim, I.R.; Choi, W.; Kim, A.; Lim, J.; Lee, D.H.; Lee, J.R. Genetic diversity and population structure of nutria (Myocastor coypus) in South Korea. Animals 2019, 9, 1164. [Google Scholar] [CrossRef] [Green Version]
- Lek, S.; Guegan, J.-F. Artificial Neuronal Networks: Application to Ecology and Evolution; Springer: Berlin, Germany, 2000; p. 262. [Google Scholar]
- Recknagel, F. Ecological Informatics: Scope, Techniques and Applications, 2nd ed.; Springer: New York, NY, USA, 2006; p. 496. [Google Scholar]
- Kim, A.; Kim, Y.C.; Lee, D.H. A management plan according to the estimation of nutria (Myocastor coypus) distribution density and potential suitable habitat. Korean Sci. 2018, 27, 203–214. [Google Scholar] [CrossRef]
- Hilts, D.J.; Belitz, M.W.; Gehring, T.M.; Pangle, K.L.; Uzarski, D.G. Climate change and nutria range expansion in the Eastern United States. J. Wildl. Manag. 2019, 83, 591–598. [Google Scholar] [CrossRef]
- Kohonen, T. Self-organized formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Kohonen, T. Analysis of a Simple self-organizing process. Biol. Cybern. 1982, 44, 135–140. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps, 3rd ed.; Springer: Berlin, Germany, 2001; p. 501. [Google Scholar]
- Chon, T.-S.; Park, Y.-S.; Moon, K.-H.; Cha, E.-Y. Patternizing communities by using an artificial neural network. Ecol. Model. 1996, 90, 69–78. [Google Scholar] [CrossRef]
- Foody, G. Applications of the self-organising feature map neural network in community data analysis. Ecol. Model. 1999, 210, 97–107. [Google Scholar] [CrossRef]
- Chon, T.-S.; Park, Y.-S.; Park, J.-H. Determining temporal pattern of community dynamics by using unsupervised learning algorithms. Ecol. Model. 2000, 132, 151–166. [Google Scholar] [CrossRef]
- Lek, S.; Scardi, M.; Verdonschot, P.; Descy, J.-P.; Park, Y.-S. Modelling Community Structure in Freshwater Ecosystems; Springer: Berlin, Germany, 2005; p. 518. [Google Scholar]
- Lek, S. Uncertainty in ecological models. Ecol. Model. 2007, 207, 1. [Google Scholar] [CrossRef]
- Chon, T.-S. Self-organizing maps applied to ecological sciences. Ecol. Inform. 2011, 6, 50–61. [Google Scholar] [CrossRef]
- Kalteh, A.M.; Hjorth, P.; Berndtsson, R. Review of the self-organizing map (SOM) approach in water resources: Analysis, modeling and application. Environ. Model. Softw. 2008, 23, 835–845. [Google Scholar] [CrossRef]
- Céréghino, R.; Park, Y.-S. Review of self-organizing map (SOM) approach in water resources: Commentary. Environ. Model. Softw. 2009, 24, 945–947. [Google Scholar] [CrossRef]
- Chon, T.-S.; Park, Y.-S.; Park, J.Y.; Choi, S.-Y.; Kim, K.-T.; Cho, E.-C. Implementation of computational methods to pattern recognition of movement behavior of Blattella germanica (Blattaria: Blattellidae) treated with Ca2+ signal inducing chemicals. Appl. Entomol. Zool. 2004, 39, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-B.; Choe, Y.; Chon, T.-S.; Kang, H.Y. Analysis of zebrafish (Danio rerio) behavior in response to bacterial infection using a self-organizing map. BMC Vet. Res. 2015, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Uehara, T.; Li, B.; Kim, B.-M.; Yoon, S.-S.; Quach, Q.K.; Kim, H.; Chon, T.-S. Inferring conflicting behavior of zebrafish (Danio rerio) in response to food and predator based on a self-organizing map (SOM) and intermittency test. Ecol. Inform. 2015, 29, 119–129. [Google Scholar] [CrossRef]
- Choi, K.-H.; Kim, J.-S.; Kim, Y.S.; Yoo, M.-A.; Chon, T.-S. Pattern detection of movement behaviors in genotype variation of Drosophila melanogaster by using self-organizing map. Ecol. Inform. 2006, 1, 219–228. [Google Scholar] [CrossRef]
- Eom, H.-J.; Liu, Y.; Kwak, G.-S.; Heo, M.; Song, K.S.; Chung, Y.D.; Chon, T.-S.; Choi, J.H. Inhalation toxicity of indoor air pollutants in Drosophila melanogaster using integrated transcriptomics and computational behavior analyses. Sci. Rep. 2017, 7, 46473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bação, F.; Lobo, V.; Painho, M. Geo-self-organizing map (Geo-SOM) for building and exploring homogeneous regions. In Proceedings of the International Conference on Geographic Information Science, Adelphi, MD, USA, 20–23 October 2004; Springer: Berlin/Heidelberg, Germany, 2004; pp. 22–37. [Google Scholar]
- Henriques, R.; Bação, F.; Lobo, V. GeoSOM Suite: A tool for spatial clustering. In Computational Science and Its Applications—ICCSA; Gervasi, O., Taniar, D., Murgante, B., Laganà, A., Mun, Y., Gavrilova, M.L., Eds.; Springer: Berlin, Germany, 2009; pp. 453–466. [Google Scholar]
- Mech, L.D.; Barber, S.M.A. Critique of Wildlife Radio-Tracking and Its Use in National Parks: A report to the U.S. National Park Service; U.S. Geological Survey, Northern Prairie Wildlife Research Center: Jamestown, ND, USA, 2002; pp. 19–20.
- White, G.C.; Garrott, R.A. Analysis of Wildlife Radio-Tracking Data; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzuge der Vegetationskunde; Springer: Wien, Austria; New York, NY, USA, 1964. [Google Scholar]
- Spencer, L.J.; Bousquin, S.G. Interim responses of floodplain wetland vegetation to phase I of the Kissimmee River restoration project: Comparisons of vegetation maps from five periods in the river’s history. Restor. Ecol. 2014, 22, 397–408. [Google Scholar] [CrossRef]
- Kim, H.G.; Hong, S.; Chon, T.-S.; Joo, G.-J. Spatial patterning of chlorophyll a and water-quality measurements for deter-mining environmental thresholds for local eutrophication in the Nakdong River basin. Environ. Pollut. 2021, 268, 115701. [Google Scholar] [CrossRef]
- Vesanto, J.; Himberg, J.; Alhoniemi, E.; Parhankangas, J.; SOM Toolbox Team. SOM Toolbox for Matlab; Helsinki University of Technology: Espoo, Finland, 2000. [Google Scholar]
- Astel, A.; Tsakovski, S.; Barbieri, P.; Simeonov, V. Comparison of self-organizing maps classification approach with cluster and principal components analysis for large environmental data sets. Water Res. 2007, 41, 4566–4578. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall International: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Aliev, F. Contribution to the study of nutria migrations (Myocastor coypus). Säugetierkd Mitt. 1968, 16, 301–303. [Google Scholar]
- Warkentin, M.J. Observations on the behavior and ecology of the nutria in Louisiana. Tulane Stud. Zool. Bot. 1968, 15, 10–17. [Google Scholar]
- Gosling, L.M. Coypu, The Handbook of British Mammals, 2nd ed.; Corbet, G.B., Southern, H.N., Eds.; Blackwell Scientific Press: Oxford, UK, 1977; pp. 256–265. [Google Scholar]
- Doncaster, C.P.; Micol, T. Annual cycle of a coypu (Myocastor coypus) population: Male and female strategies. J. Zool. 1989, 217, 227–240. [Google Scholar] [CrossRef]
- Lowery, G.H. The Mammals of Louisiana and Its Adjacent Waters; Louisiana State University Press: Baton Rouge, LA, USA, 1974; p. 565. [Google Scholar]
- Gosling, L.M. The twenty-four hour active cycle captive coypus (Myocastor coypus). J. Zool. 1979, 187, 341–367. [Google Scholar] [CrossRef]
- Gosling, L.M.; Guyon, G.E.; Wright, K.M. Diurnal activity of feral coypus (Myocastor coypus) during the cold winter of 1978–9. J. Zool. 1980, 192, 143–146. [Google Scholar] [CrossRef]
- Adams, W.H., Jr. The nutria in coastal Louisiana. Proc. La. Acad. Sci. 1956, 19, 28–41. [Google Scholar]
- Kim, P. The coypu (Myocastor coypus) in the Netherlands: Reproduction, home range and manner of seeking food. Lutra 1980, 23, 55–64. [Google Scholar]
- Chabreck, R.H.; Love, J.R.; Linscombe, G. Foods and feeding habits of nutria in brackish marsh in Louisiana. In Proceedings of the Worldwide Furbearer Conference, Frostburg, MD, USA, 3–11 August 1980. [Google Scholar]
- Nowak, R.M.; Walker, E.P. Walker’s Mammals of the World; JHU Press: Baltimore, MA, USA, 1999; Volume 1. [Google Scholar]
- Lohmeier, L. Home range, movements, and population density of nutria on a Mississippi pond. J. Miss. Acad. Sci. 1981, 26, 50–54. [Google Scholar]
- Reggiani, G.; Boitani, L.; D’Antoni, S.; Stefano, R. Biology and control of the coypu in the Mediterranean area. Suppl. Ric. Biol. Selvaggina 1993, 21, 67–100. [Google Scholar]
- Denena, M.M.C. Home Range and Movement of Nutria (Myocastor coypus) at Spring Lake in Central Texas, with Anecdotal Comments on the American Beaver (Castor canadensis) of the Same Area; Southwest Texas State University: San Marcos, TX, USA, 2002. [Google Scholar]
- Nolfo-Clements, L.E. Nutria survivorship, movement patterns, and home ranges. Southeast. Nat. 2009, 8, 399–410. [Google Scholar] [CrossRef]
- Willner, G.R.; Joseph, A.C.; Duane, P. Reproduction, physiological responses, food habits, and abundance of nutria on Maryland marshes. Wildl. Monogr. 1979, 65, 3–43. [Google Scholar]
- Gosling, L.M.; Watt, A.D.; Baker, S.J. Continuous retrospective census of the East Anglian coypu population between 1970 and 1979. J. Anim. Ecol. 1981, 50, 885–901. [Google Scholar] [CrossRef]
- Hong, S.; Chon, T.S.; Joo, G.J. Spatial distribution patterns of Eurasian Otter (Lutra Lutra) in association with environmental factors unravelled by machine learning and diffusion kernel method. J. Environ. Inform. 2021, 37, 130–141. [Google Scholar]
- Nguyen, T.V.; Liu, Y.; Jung, I.-H.; Lee, S.-H.; Chon, T.-S. Unraveling Markov processes in movement patterns of indicator species in response to chemical stressors. Mod. Phys. Lett. B 2011, 25, 1143–1149. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, S.-H.; Chon, T.-S. Analysis of behavioral changes of zebrafish (Danio rerio) in response to formaldehyde using self-organizing map and a hidden Markov model. Ecol. Model. 2011, 222, 2191–2201. [Google Scholar] [CrossRef]
| Neighbors | Plant Types | Land Cover States | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG | FL | XHV | HHV | HWV | Total | OA | R | AS | Total | ||
| Different sex | F-M | 2 | 0 | 2 | 1 | 0 | 5 | 1 | 1 | 0 | 2 |
| M-F | 5 | 2 | 1 | 2 | 1 | 11 | 1 | 1 | 1 | 3 | |
| Subtotal | 7 | 2 | 3 | 3 | 1 | 16 | 2 | 2 | 1 | 5 | |
| Same sex | F-F | 1 | 2 | 2 | 0 | 0 | 5 | 1 | 2 | 0 | 3 |
| M-M | 1 | 3 | 2 | 1 | 0 | 7 | 2 | 1 | 0 | 3 | |
| Subtotal | 2 | 5 | 4 | 1 | 0 | 12 | 3 | 3 | 0 | 6 | |
| Total | 9 | 7 | 7 | 4 | 1 | 28 | 5 | 5 | 1 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Jung, N.; Jang, Y.-H.; Lee, K.; Lim, J.; Jang, G.-S.; Lee, J.W.; Chon, T.-S. Spatial Movement Patterns and Local Co-Occurrence of Nutria Individuals in Association with Habitats Using Geo-Self-Organizing Map (Geo-SOM). Biology 2021, 10, 598. https://doi.org/10.3390/biology10070598
Lee D-H, Jung N, Jang Y-H, Lee K, Lim J, Jang G-S, Lee JW, Chon T-S. Spatial Movement Patterns and Local Co-Occurrence of Nutria Individuals in Association with Habitats Using Geo-Self-Organizing Map (Geo-SOM). Biology. 2021; 10(7):598. https://doi.org/10.3390/biology10070598
Chicago/Turabian StyleLee, Do-Hun, Nam Jung, Yong-Hyeok Jang, KyoungEun Lee, Joobaek Lim, Gab-Sue Jang, Jae Woo Lee, and Tae-Soo Chon. 2021. "Spatial Movement Patterns and Local Co-Occurrence of Nutria Individuals in Association with Habitats Using Geo-Self-Organizing Map (Geo-SOM)" Biology 10, no. 7: 598. https://doi.org/10.3390/biology10070598
APA StyleLee, D.-H., Jung, N., Jang, Y.-H., Lee, K., Lim, J., Jang, G.-S., Lee, J. W., & Chon, T.-S. (2021). Spatial Movement Patterns and Local Co-Occurrence of Nutria Individuals in Association with Habitats Using Geo-Self-Organizing Map (Geo-SOM). Biology, 10(7), 598. https://doi.org/10.3390/biology10070598






