Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
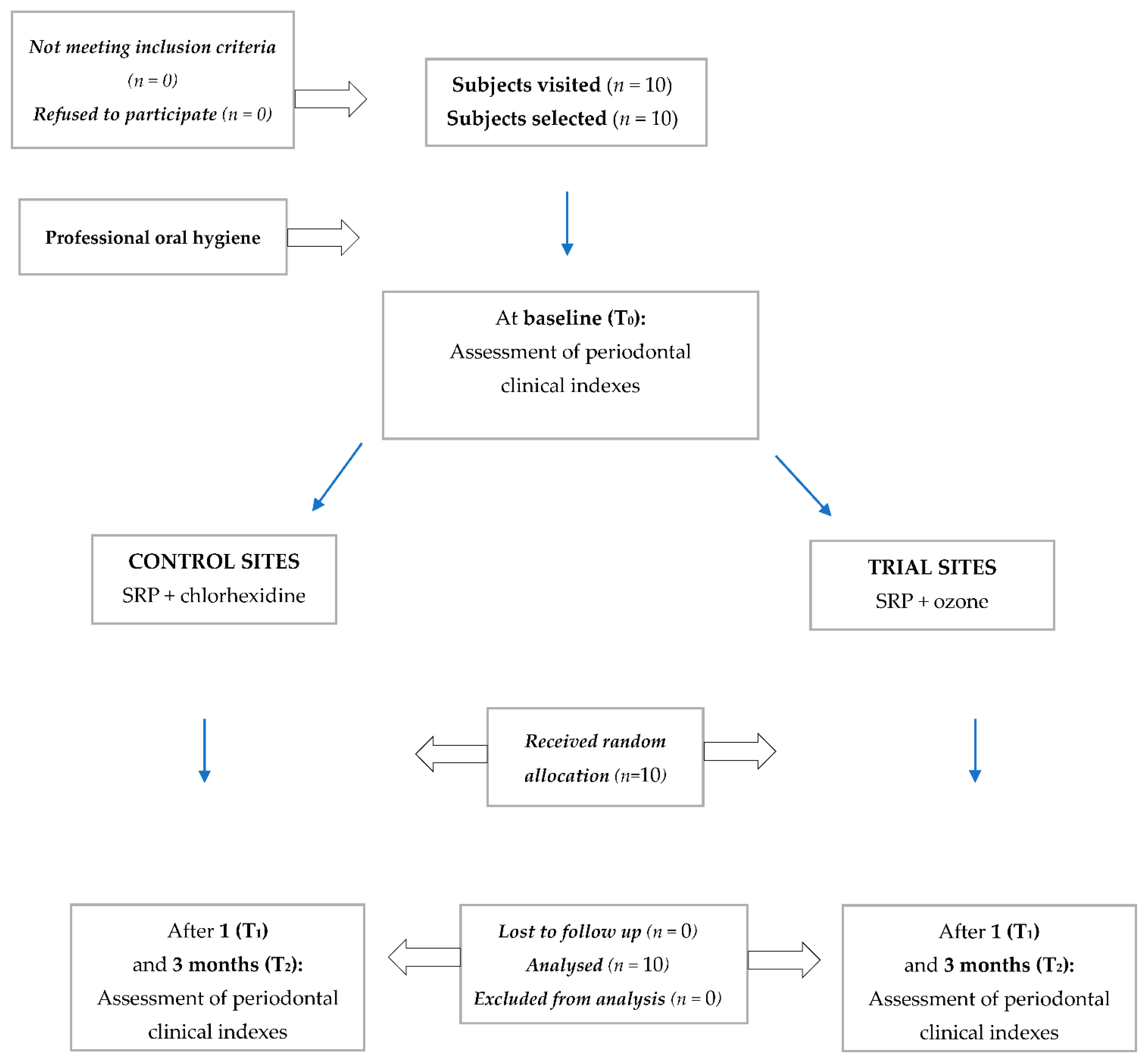
2.2. Trial Design
2.3. Participants, Eligibility Criteria, and Settings
2.4. Interventions and Outcomes
2.5. Sample Size Calculation
2.6. Randomization, Sequence Generation, Allocation Concealment and Implementation
2.7. Blinding
2.8. Statistical Methods
3. Results
3.1. Participant Flow
3.2. Recruitment
3.3. Baseline Data
3.4. Numbers Analyzed
3.5. Outcomes and Estimation
3.5.1. Probing Pocket Depth (PPD)
3.5.2. Clinical Attachment Level (CAL)
3.5.3. Gingival Index (GI)
3.5.4. Plaque Index (PI)
3.5.5. Bleeding on Probing (BoP)
3.6. Ancillary Analyses
3.7. Harms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Lezcano, I.; Nuñez, N.; Espino, M.; Gómez, M. Antibacterial activity of ozonized sunflower oil, oleozon, against Staphylococcus aureus and Staphylococcus epidermidis. Ozone Sci. Eng. 2000, 22, 207–214. [Google Scholar] [CrossRef]
- Monzillo, V.; Lallitto, F.; Russo, A.; Poggio, C.; Scribante, A.; Arciola, C.R.; Bertuccio, F.R.; Colombo, M. Ozonized Gel Against Four Candida Species: A Pilot Study and Clinical Perspectives. Materials (Basel) 2020, 13, 1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, Y.; Shrey, P.; Re, K.; Gandhi, J.; Joshi, G. Clinical utility of ozone therapy in dental and oral medicine. Med. Gas. Res. 2019, 9, 163–167. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; D’Ercole, S.; Pilloni, A.; D’Archivio, D.; Lisanti, L.; Graziani, F.; Femminella, B.; Sammartino, G.; Perillo, L.; Tetè, S.; et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a xhantan-based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J. Periodontol. 2009, 80, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Sutthiboonyapan, P.; Wang, H.L.; Charatkulangkun, O. Flowcharts for Easy Periodontal Diagnosis Based on the 2018 New Periodontal Classification. Clin. Adv. Periodontics 2020, 10, 155–160. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal diseases in pregnancy. 1. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B., Jr.; Taba, M., Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: A preliminary randomized controlled clinical study. J. Periodontol. 2007, 78, 965–973. [Google Scholar] [CrossRef]
- Akram, Z.; Shafqat, S.S.; Aati, S.; Kujan, O.; Fawzy, A. Clinical efficacy of probiotics in the treatment of gingivitis: A systematic review and meta-analysis. Aust. Dent. J. 2020, 65, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.K.; Cappetta, E.G.; Pavaskar, R. Effectiveness of the adjunctive use of ozone and chlorhexidine in patients with chronic periodontitis. BDJ Open 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Habashneh, R.; Alsalman, W.; Khader, Y. Ozone as an adjunct to conventional nonsurgical therapy in chronic periodontitis: A randomized controlled clinical trial. J. Periodontal. Res. 2015, 50, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Microbes, inflammation, scaling and root planing, and the periodontal condition. J. Dent. Hyg. 2008, 82 (Suppl. 3), 4–9. [Google Scholar] [PubMed]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas. Res. 2017, 7, 212–219. [Google Scholar]
- Zanardi, I.; Borrelli, E.; Valacchi, G.; Travagli, V.; Bocci, V. Ozone: A multifaceted molecule with unexpected therapeutic activity. Curr. Med. Chem. 2016, 23, 304–314. [Google Scholar] [CrossRef]
- Pchepiorka, R.; Moreira, M.S.; Lascane, N.A.D.S.; Catalani, L.H.; Allegrini, S., Jr.; de Lima, N.B.; Gonçalves, E.F. Effect of ozone therapy on wound healing in the buccal mucosa of rats. Arch. Oral Biol. 2020, 119, 104889. [Google Scholar] [CrossRef]
- Kaur, A.; Bhavikatti, S.K.; Das, S.S.; Khanna, S.; Jain, M.; Kaur, A. Efficacy of Ozonised Water and 0.2% Chlorhexidine Gluconate in the Management of Chronic Periodontitis when Used as an Irrigant in Conjugation with Phase I Therapy. J. Contemp. Dent. Pract. 2019, 20, 318–323. [Google Scholar]
- Kshitish, D.; Laxman, V.K. The use of ozonated water and 0.2% chlorhexidine in the treatment of periodontitis patients: A clinical and microbiologic study. Indian J. Dent. Res. 2010, 21, 341–348. [Google Scholar] [CrossRef]
- Moraschini, V.; Kischinhevsky, I.C.C.; Calasans-Maia, M.D.; Shibli, J.A.; Sartoretto, S.C.; Figueredo, C.M.; Granjeiro, J.M. Ineffectiveness of ozone therapy in nonsurgical periodontal treatment: A systematic review and metaanalysis of randomized clinical trials. Clin. Oral Investig. 2020, 24, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Scribante, A. Ozone therapy in dentistry: From traditional applications towards innovative ones. A review of the literature. IOP Conf. Ser. Earth Environ. Sci. 2021, 707, 012001. [Google Scholar] [CrossRef]
- Colombo, M.; Ceci, M.; Felisa, E.; Poggio, C.; Pietrocola, G. Cytotoxicity evaluation of a new ozonized olive oil. Eur. J. Dent. 2018, 12, 585–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papapanou, P.N. Periodontal diseases: Epidemiology. Ann. Periodontol. 1996, 1, 1–36. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Küçük, F.; Yıldırım, S.; Çetiner, S. Cytotoxicity assessment of different doses of ozonated water on dental pulp cells. BMC Oral Health 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Dental plaque as a biofilm and a microbial community—Implications for treatment. J. Oral Biosci. 2015, 57, 185–191. [Google Scholar] [CrossRef]
- Huth, K.C.; Jakob, F.M.; Saugel, B.; Cappello, C.; Paschos, E.; Hollweck, R.; Hickel, R.; Brand, K. Effect of ozone on oral cells compared with established antimicrobials. Eur. J. Oral Sci. 2006, 114, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Smojver, I.; Vuletić, M.; Gerbl, D.; Budimir, A.; Sušić, M.; Gabrić, D. Evaluation of Antimicrobial Efficacy and Permeability of Various Sealing Materials at the Implant-Abutment Interface-A Pilot In Vitro Study. Materials (Basel.) 2021, 14, 385. [Google Scholar] [CrossRef]
- Oliver, J.C.; Bredarioli, P.A.P.; Leandro, F.D.; Ferreira, C.B.R.J.; Veiga, S.M.O.M.; Dias, A.L.T. Ozone against Pseudomonas aeruginosa biofilms in contact lenses storage cases. Rev. Inst. Med. Trop Sao Paulo 2019, 61, e23. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. [Google Scholar] [CrossRef]
- Tonon, C.C.; Panariello, B.H.D.; Spolidorio, D.M.P.; Gossweiler, A.G.; Duarte, S. Anti-biofilm effect of ozonized physiological saline solution on peri-implant-related biofilm. J. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yücesoy, T.; Seker, E.D.; Cenkcı, E.; Yay, A.; Alkan, A. Histologic and Biomechanical Evaluation of Osseointegrated Miniscrew Im-plants Treated with Ozone Therapy and Photobiomodulation at Different Loading Times. Int. J. Oral Maxillofac. Implant. 2019, 34, 1337–1345. [Google Scholar] [CrossRef]
- Sunarso Toita, R.; Tsuru, K.; Ishikawa, K. A superhydrophilic titanium implant functionalized by ozone gas modulates bone marrow cell and macrophage responses. J. Mater. Sci. Mater. Med. 2016, 27, 127. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nagano, K.; Hamaoka, K.; Kato, D.; Kawai, T.; Murakami, H.; Hasegawa, Y. Ozone Water Bactericidal and Cleaning Effects on Oral Diseases-related Planktonic and Bacterial Biofilms. J. Hard Tissue Biol. 2021, 30, 27–31. [Google Scholar] [CrossRef]
- Nardi, G.M.; Fais, S.; Casu, C.; Mazur, M.; Di Giorgio, R.; Grassi, R.; Grassi, F.R.; Orrù, G. Mouthwash Based on Ozonated Olive Oil in Caries Prevention: A Preliminary In-Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 9106. [Google Scholar] [CrossRef] [PubMed]
- Glória, J.C.R.; Douglas-de-Oliveira, D.W.; Silva, L.D.A.E.; Falci, S.G.M.; Dos Santos, C.R.R. Influence of ozonized water on pain, oedema, and trismus during impacted third molar surgery: A randomized, triple blind clinical trial. BMC Oral Health 2020, 20, 41. [Google Scholar] [CrossRef]
- Zamora, Z.; González, R.; Guanche, D.; Merino, N.; Menéndez, S.; Hernández, F.; Alonso, Y.; Schulz, S. Ozonized sunflower oil reduces oxidative damage induced by indomethacin in rat gastric mucosa. Inflamm. Res. 2008, 57, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Lee, S.B.; Ko, S.C. Anti-inflammatory effect of ozonated krill (Euphausia superba) oil in lipopolysaccharide-stimulated RAW 264. 7 macrophages. Fish. Aquat. Sci. 2018, 21, 15. [Google Scholar] [CrossRef]
- Dev Kumar, G.; Ravishankar, S. Ozonized water with plant antimicrobials: An effective method to inactivate Salmonella enterica on iceberg lettuce in the produce wash water. Environ. Res. 2019, 171, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Jhunkeaw, C.; Khongcharoen, N.; Rungrueng, N.; Sangpo, P.; Panphut, W.; Thapinta, A.; Senapin, S.; St-Hilaire, S.; Thanh Dong, H. Ozone nanobubble treatment effectively reduced pathogenic Gram positive and negative bacteria in freshwater and safe for tilapia. Aquaculture 2021, 534, 736286. [Google Scholar] [CrossRef]

| Product | Description | Ingredients | Manufacturer |
|---|---|---|---|
| GeliO3 | Ozonized gel | Bio-ozonized olive oil (20 mEq O2/Kg), Hydrated Silica, Arnica | Bioemmei Srl, 36100 Vicenza, Italy |
| Curasept Parodontal gel 1% Ads | Chlorhexidine gel | Sorbitol, Aqua, Hydrated Silica, Glycerin, Xylitol, PEG-40 Hydrogenated Castor Oil, Cocamidopropyl Betaine, Aroma, Cellulose Gum, Chlorhexidine, Digluconate, Ascorbic Acid, Sodium Metabisulfite, Sodium Saccharin, Sodium Methylparaben, Sodium Citrate, CI 42090 | Curasept SPA, 21047 Saronno, Varese, Italy |
| Clinical Indexes | Control Sites (Mean ± SD) | Trial Sites (Mean ± SD) |
|---|---|---|
| PPD (mm) | 5.94 ± 0.89 | 6.21 ± 0.92 |
| CAL (mm) | 6.13 ± 0.81 | 6.00 ± 0.83 |
| GI (0–3) | 1.67 ± 0.39 | 1.67 ± 0.56 |
| PI (%) | 0.86 ± 0.16 | 0.85 ± 0.18 |
| BOP (%) | Cs: 0.33 ± 0.13 | 0.43 ± 0.27 |
| Clinical Index | Treatment | Time | Mean | SD | Min | Median | Max | Intragroup Differences * | Intergroup Differences * |
|---|---|---|---|---|---|---|---|---|---|
| PPD (mm) | SRP + Ozone | T0 | 6.21 | 0.92 | 5.25 | 6.27 | 8.40 | T0-T1 p < 0.05 | T0-T0 > 0.05 T1-T1 > 0.05 T2-T2 > 0.05 |
| T1 | 4.66 | 0.74 | 3.83 | 4.48 | 5.50 | T0-T2 p < 0.05 | |||
| T2 | 4.20 | 0.48 | 3.50 | 4.15 | 5.20 | T1-T2 p > 0.05 | |||
| SRP + Chlorhexidine | T0 | 5.94 | 0.89 | 5.00 | 5.89 | 7.50 | T0-T1 p < 0.05 | ||
| T1 | 4.42 | 0.76 | 3.37 | 4.35 | 5.78 | T0-T2 p < 0.05 | |||
| T2 | 3.95 | 0.52 | 3.25 | 3.90 | 4.95 | T1-T2 p > 0.05 | |||
| CAL (mm) | SRP + Ozone | T0 | 6.00 | 0.83 | 5.10 | 5.89 | 7.50 | T0-T1 p < 0.05 | T0-T0 p > 0.05 T1-T1 p > 0.05 T2-T2 p < 0.05 |
| T1 | 4.42 | 0.76 | 3.37 | 4.35 | 5.78 | T0-T2 p < 0.05 | |||
| T2 | 4.32 | 0.47 | 3.45 | 4.32 | 4.94 | T1-T2 p > 0.05 | |||
| SRP + Chlorhexidine | T0 | 6.13 | 0.81 | 5.25 | 6.06 | 8.00 | T0-T1 p < 0.05 | ||
| T1 | 4.85 | 0.90 | 3.83 | 4.54 | 6.50 | T0-T2 p < 0.05 | |||
| T2 | 3.99 | 0.56 | 3.15 | 4.05 | 4.91 | T1-T2 p < 0.05 | |||
| GI (1–3) | SRP + Ozone | T0 | 1.67 | 0.56 | 0.80 | 1.54 | 2.56 | T0-T1 p < 0.05 | T0-T0 p > 0.05 T1-T1 p > 0.05 T2-T2 p < 0.05 |
| T1 | 1.01 | 0.38 | 0.50 | 0.95 | 1.60 | T0-T2 p < 0.05 | |||
| T2 | 0.91 | 0.35 | 0.37 | 0.93 | 1.45 | T1-T2 p > 0.05 | |||
| T0 | 1.67 | 0.39 | 0.87 | 1.80 | 2.14 | T0-T1 p < 0.05 | |||
| SRP + Chlorhexidine | T1 | 1.06 | 0.38 | 0.45 | 1.15 | 1.50 | T0-T2 p < 0.05 | ||
| T2 | 0.71 | 0.36 | 0.05 | 0.75 | 1.30 | T1-T2 p < 0.05 | |||
| PI (%) | T0 | 85 | 18 | 55 | 90 | 100 | T0-T1 p < 0.05 | T0-T0 p > 0.05 T1-T1 p > 0.05 T2-T2 p > 0.05 | |
| SRP + Ozone | T1 | 54 | 9 | 40 | 50 | 70 | T0-T2 p < 0.05 | ||
| T2 | 39 | 7 | 27 | 40 | 50 | T1-T2 p < 0.05 | |||
| SRP + Chlorhexidine | T0 | 86 | 16 | 60 | 90 | 100 | T0-T1 p < 0.05 | ||
| T1 | 52 | 7 | 40 | 50 | 65 | T0-T2 p < 0.05 | |||
| T2 | 36 | 8 | 25 | 34 | 50 | T1-T2 p < 0.05 | |||
| BOP (%) | T0 | 43 | 27 | 7 | 40 | 87 | T0-T1 p < 0.05 | T0-T0 p > 0.05 T1-T1 p > 0.05 T2-T2 p > 0.05 | |
| SRP + Ozone | T1 | 15 | 6 | 5 | 17 | 24 | T0-T2 p < 0.05 | ||
| T2 | 9 | 4 | 2 | 9 | 15 | T1-T2 p < 0.05 | |||
| SRP + Chlorhexidine | T0 | 33 | 13 | 18 | 31 | 50 | T0-T1 p < 0.05 | ||
| T1 | 11 | 7 | 2 | 10 | 24 | T0-T2 p < 0.05 | |||
| T2 | 9 | 6 | 2 | 7 | 17 | T1-T2 p < 0.05 |
| PPD (mm) | T0 | T2 | T0-T2 | SD |
|---|---|---|---|---|
| SRP + Ozone | 6.21 | 4.20 | 2.01 | 1.42 |
| SRP + Chlorhexidine | 5.94 | 3.95 | 1.99 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. https://doi.org/10.3390/biology10070625
Colombo M, Gallo S, Garofoli A, Poggio C, Arciola CR, Scribante A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology. 2021; 10(7):625. https://doi.org/10.3390/biology10070625
Chicago/Turabian StyleColombo, Marco, Simone Gallo, Alessandro Garofoli, Claudio Poggio, Carla Renata Arciola, and Andrea Scribante. 2021. "Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application" Biology 10, no. 7: 625. https://doi.org/10.3390/biology10070625
APA StyleColombo, M., Gallo, S., Garofoli, A., Poggio, C., Arciola, C. R., & Scribante, A. (2021). Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology, 10(7), 625. https://doi.org/10.3390/biology10070625








