Effects of Chronic Hypoxia on the Immune Status of Pikeperch (Sander lucioperca Linnaeus, 1758)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
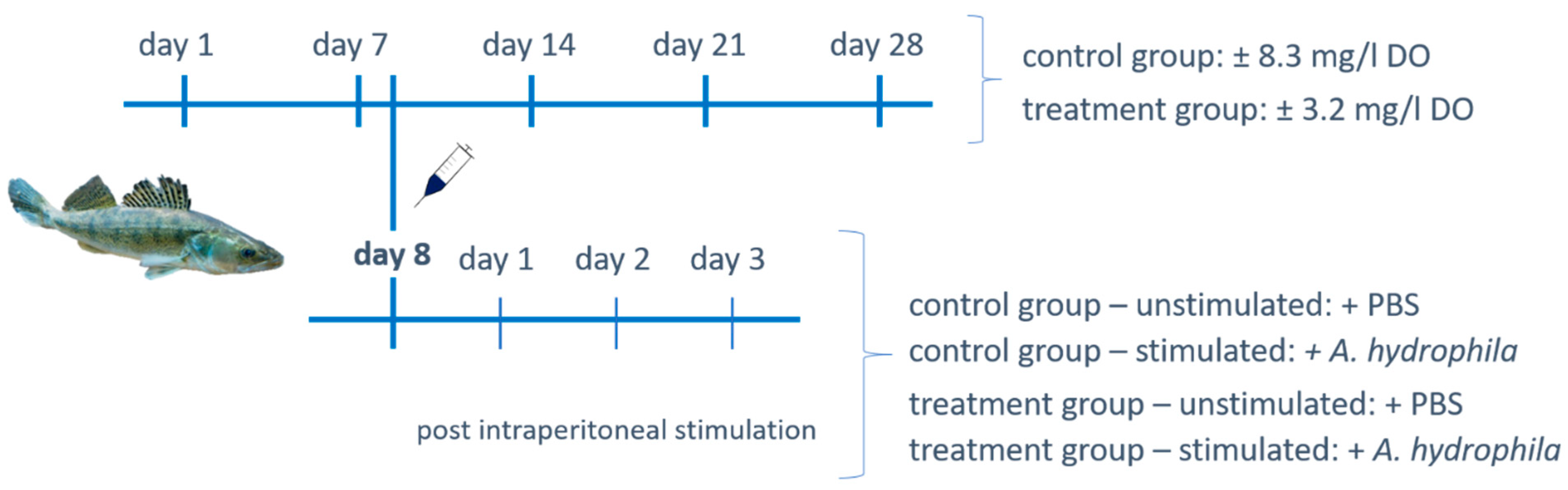
2.1. Fish Rearing and Experimental Design
2.2. Intraperitoneal Stimulation with Aeromonas hydrophila
2.3. Cell Isolation
2.4. Flow Cytometry
2.5. Health Parameters
2.6. Gene Selection and Primer Design
2.7. RNA/cDNA Preparation
2.8. Real-TIME Quantitative PCR (rt-qPCR)
2.9. Statistics
3. Results
3.1. Reduced Oxygen Saturation Induces Marginal Changes in the Process of Adaptation
3.1.1. Blood and Health Parameters of Challenged Pikeperch
3.1.2. Composition of HK and Peripheral Blood upon Low DO Exposure
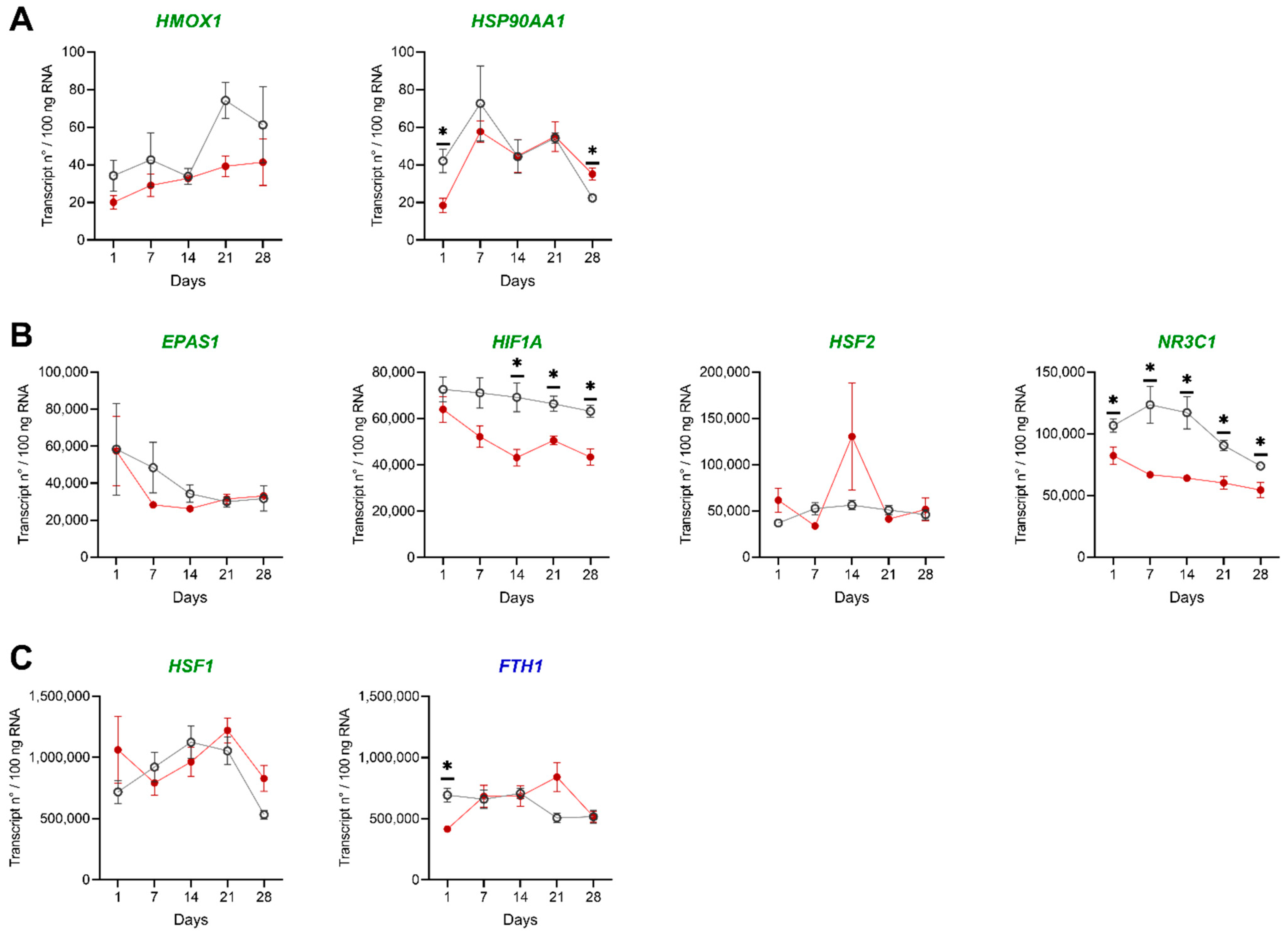
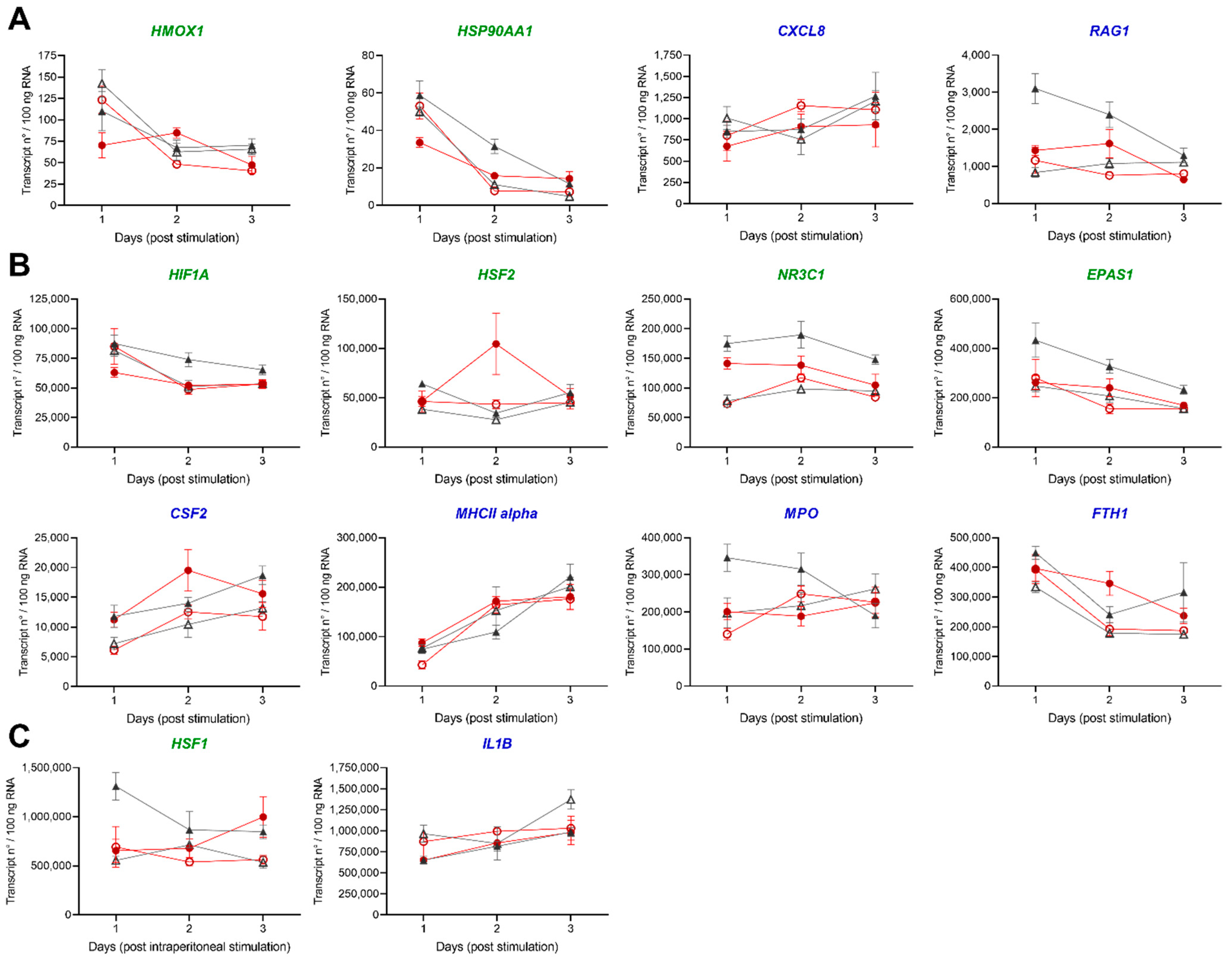
3.1.3. Gene Expression Analysis in HK and Liver of Challenged Pikeperch
3.2. Induction of Peritoneal Inflammation under Low DO Levels
3.2.1. SSI after Intraperitoneal Stimulation
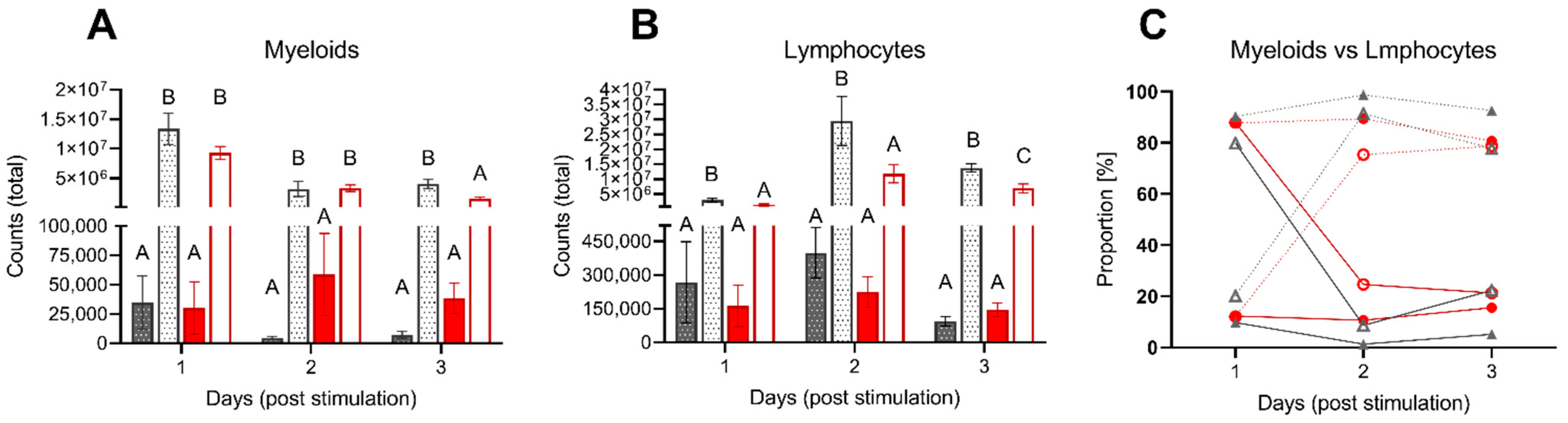
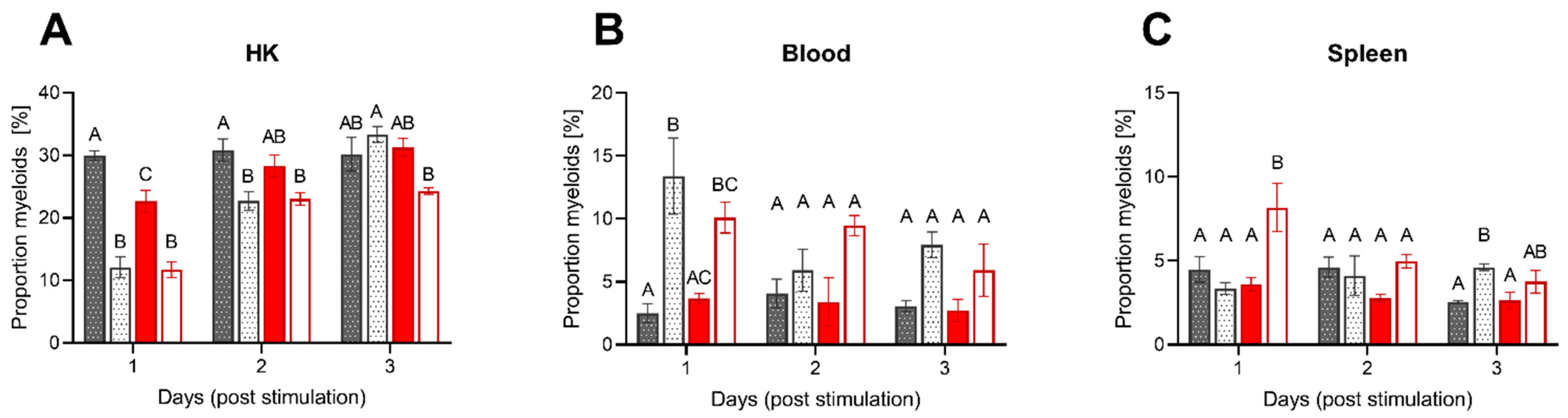
3.2.2. Leukocyte Migration upon Intraperitoneal Stimulation
3.2.3. Gene Profiling in HK and Spleen during Acute Inflammation
4. Discussion
4.1. The Hypoxic Challenge of 40% DO Does Not Induce Substantial Changes in Major Health Parameters
4.2. Effects of Low DO on Cell Distribution and Gene Expression
4.3. Acute Inflammation Is Moderately Influenced by Low DO
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef]
- Bregnballe, J. Recirculation Aquaculture; FAO and Eurofish International Organisation: Copenhagen, Denmark, 2015; ISBN 0971264600. [Google Scholar]
- Rogers, N.J.; Urbina, M.A.; Reardon, E.E.; McKenzie, D.J.; Wilson, R.W. A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (Pcrit). Conserv. Physiol. 2016, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Landman, M.J.; Van Den Heuvel, M.R.; Ling, N. Relative sensitivities of common freshwater fish and invertebrates to acute hypoxia. N. Z. J. Mar. Freshw. Res. 2005, 39, 1061–1067. [Google Scholar] [CrossRef]
- Timmons, M.B.; Summerfelt, S.T.; Vinci, B.J. Review of circular tank technology and management. Aquac. Eng. 1998, 18, 51–69. [Google Scholar] [CrossRef] [Green Version]
- Frisk, M.; Skov, P.V.; Steffensen, J.F. Thermal optimum for pikeperch (Sander lucioperca) and the use of ventilation frequency as a predictor of metabolic rate. Aquaculture 2012, 324–325, 151–157. [Google Scholar] [CrossRef]
- Ali, M.S.; Stead, S.M.; Houlihan, D.F. Effects of socking [sic] density on ammonia excretion and the growth of Nile tilapia (Oreochromis niloticus L.)-Aquatic Commons. Bangladesh J. Fish. Res. 2006, 10, 13–24. [Google Scholar]
- Petrosky, B.R.; Magnuson, J.J. Behavioral Responses of Northern Pike, Yellow Perch and Bluegill to Oxygen Concentrations under Simulated Winterkill Conditions. Copeia 1973, 1973, 124. [Google Scholar] [CrossRef]
- Suthers, I.M.; Gee, J.H. Role of Hypoxia in Limiting Diel Spring and Summer Distribution of Juvenile Yellow Perch (Perca flavescens) in a Prairie Marsh. Can. J. Fish. Aquat. Sci. 1986, 43, 1562–1570. [Google Scholar] [CrossRef]
- Carlson, A.R.; Blocher, J.; Herman, L.J. Growth and survival of channel catfish and yellow perch exposed to lowered constant and diurnally fluctuating dissolved oxygen concentrations. Progress. Fish-Cult. 1980, 42, 73–78. [Google Scholar] [CrossRef]
- Arend, K.K.; Beletsky, D.; Depinto, J.V.; Ludsin, S.A.; Roberts, J.J.; Rucinski, D.K.; Scavia, D.; Schwab, D.J.; Höök, T.O. Seasonal and interannual effects of hypoxia on fish habitat quality in central Lake Erie. Freshw. Biol. 2011, 56, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Brandt, S.B.; Costantini, M.; Kolesar, S.; Ludsin, S.A.; Mason, D.M.; Rae, C.M.; Zhang, H. Does hypoxia reduce habitat quality for lake erie walleye (Sander vitreus)? A bioenergetics perspective. Can. J. Fish. Aquat. Sci. 2011, 68, 857–879. [Google Scholar] [CrossRef]
- Roberts, J.J.; Brandt, S.B.; Fanslow, D.; Ludsin, S.A.; Pothoven, S.A.; Scavia, D.; Höök, T.O. Effects of hypoxia on consumption, growth, and RNA: DNA ratios of young yellow perch. Trans. Am. Fish. Soc. 2011, 140, 1574–1586. [Google Scholar] [CrossRef] [Green Version]
- Stejskal, V.; Kouřil, J.; Valentová, O.; Hamáčková, J.; Policar, T. Size-related oxygen consumption and ammonia excretion of Eurasian perch (Perca fluviatilis L.) reared in a recirculating system. Aquac. Res. 2009, 41, 135–142. [Google Scholar] [CrossRef]
- Haponski, A.E.; Stepien, C.A. Phylogenetic and biogeographical relationships of the Sander pikeperches (percidae: Perciformes): Patterns across north america and eurasia. Biol. J. Linn. Soc. 2013, 110, 156–179. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2018; The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018; ISBN 9789210472340. [Google Scholar]
- Kratochvíl, M.; Čech, M.; Vašek, M.; Kubečka, J.; Hejzlar, J.; Matěna, J.; Peterka, J.; Macháček, J.; Seďa, J. Diel vertical migrations of age 0+ percids in a shallow, well-mixed reservoir. J. Limnol. 2010, 69, 305–310. [Google Scholar] [CrossRef]
- Horký, P.; Slavík, O.; Bartoš, L.; Kolářová, J.; Randák, T. The effect of the moon phase and seasonality on the behaviour of pikeperch in the Elbe River. Folia Zool. 2006, 55, 411–417. [Google Scholar]
- Policar, T.; Blecha, M.; Křišťan, J.; Mráz, J.; Velíšek, J.; Stará, A.; Stejskal, V.; Malinovskyi, O.; Svačina, P.; Samarin, A.M. Comparison of production efficiency and quality of differently cultured pikeperch (Sander lucioperca L.) juveniles as a valuable product for ongrowing culture. Aquac. Int. 2016, 24, 1607–1626. [Google Scholar] [CrossRef]
- Jarmołowicz, S.; Rożyński, M.; Kowalska, A.; Zakęś, Z. Growth in juvenile pikeperch (Sander lucioperca L.) stimulated with yeast, Saccharomyces cerevisiae, extract. Aquac. Res. 2018, 49, 614–620. [Google Scholar] [CrossRef]
- Blecha, M.; Kristan, J.; Policar, T. Adaptation of Intensively Reared Pikeperch (Sander lucioperca) Juveniles ot Pond Culture. Turkish J. Fish. Aquat. Sci. 2016, 16, 953–959. [Google Scholar] [CrossRef]
- Stejskal, V.; Matousek, J.; Drozd, B.; Blaha, M.; Policar, T.; Kouril, J. The effect of long-term hyperoxia and hypoxia on growth in pikeperch (Sander lucioperca). In Abstract Book from AQUA 2012 Conference; USB of Abstracts: Prague, Czech Republic, 2012; p. 1058. [Google Scholar]
- Baekelandt, S.; Redivo, B.; Mandiki, S.N.M.; Bournonville, T.; Houndji, A.; Bernard, B.; El Kertaoui, N.; Schmitz, M.; Fontaine, P.; Gardeur, J.N.; et al. Multifactorial analyses revealed optimal aquaculture modalities improving husbandry fitness without clear effect on stress and immune status of pikeperch Sander lucioperca. Gen. Comp. Endocrinol. 2018, 258, 194–204. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Zrnčić, S.; Radosavljević, V. West Balkans Regional Aquatic Animal Disease Diagnostic Manual; FAO: Rome, Italy, 2017; ISBN 9789251096420. [Google Scholar]
- Pichavant, K.; Person-Le-Ruyet, J.; Le Bayon, N.; Sévère, A.; Le Roux, A.; Quéméner, L.; Maxime, V.; Nonnotte, G.; Boeuf, G. Effects of hypoxia on growth and metabolism of juvenile turbot. Aquaculture 2000, 188, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Korytář, T.; Jaros, J.; Verleih, M.; Rebl, A.; Kotterba, G.; Kühn, C.; Goldammer, T.; Köllner, B. Novel insights into the peritoneal inflammation of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Swirplies, F.; Wuertz, S.; Baßmann, B.; Orban, A.; Schäfer, N.; Brunner, R.M.; Hadlich, F.; Goldammer, T.; Rebl, A. Identification of molecular stress indicators in pikeperch Sander lucioperca correlating with rising water temperatures. Aquaculture 2019, 501, 260–271. [Google Scholar] [CrossRef]
- Schäfer, N.; Kaya, Y.; Rebl, H.; Stüeken, M.; Rebl, A.; Nguinkal, J.A.; Franz, G.P.; Brunner, R.M.; Goldammer, T.; Grunow, B.; et al. Insights into early ontogenesis: Characterization of stress and development key genes of pikeperch (Sander lucioperca) in vivo and in vitro. Fish Physiol. Biochem. 2021, 47, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Nguinkal, J.A.; Brunner, R.M.; Verleih, M.; Rebl, A.; de los Ríos-Pérez, L.; Schäfer, N.; Hadlich, F.; Stüeken, M.; Wittenburg, D.; Goldammer, T. The First Highly Contiguous Genome Assembly of Pikeperch (Sander lucioperca), an Emerging Aquaculture Species in Europe. Genes 2019, 10, 708. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Porchas, M.; Martínez-Córdova, L.R.; Ramos-Enriquez, R. Cortisol and glucose: Reliable indicators of fish stress? Panam. J. Aquat. Sci. 2009, 4, 158–178. [Google Scholar]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.M.; Moon, T.W. Acute Handling Stress Alters Hepatic Glycogen Metabolism in Food-Deprived Rainbow Trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 1992, 49, 2260–2266. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleost: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Aerts, J.; Metz, J.R.; Ampe, B.; Decostere, A.; Flik, G.; De Saeger, S. Scales tell a story on the stress history of fish. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culbert, B.M.; Gilmour, K.M. Rapid recovery of the cortisol response following social subordination in rainbow trout. Physiol. Behav. 2016, 164, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I.M. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef]
- Schram, E.; Roques, J.A.C.; Van Kuijk, T.; Abbink, W.; Van de Heul, J.; De Vries, P.; Bierman, S.; Van de Vis, H.; Flik, G. The impact of elevated water ammonia and nitrate concentrations on physiology, growth and feed intake of pikeperch (Sander lucioperca). Aquaculture 2014, 420–421, 95–104. [Google Scholar] [CrossRef]
- Siwicki, A.; Zakes, Z.; Trapkowska, S.; Kowalska, A.; Kazun, K.; Glabski, E. Selected hematological and biochemical parameters of pikeperch Sander lucioperca (L.) from intensive culture. Achives Polish Fish. 2003, 11, 17–22. [Google Scholar]
- Douxfils, J.; Deprez, M.; Mandiki, S.N.M.; Milla, S.; Henrotte, E.; Mathieu, C.; Silvestre, F.; Vandecan, M.; Rougeot, C.; Mélard, C.; et al. Physiological and proteomic responses to single and repeated hypoxia in juvenile Eurasian perch under domestication—Clues to physiological acclimation and humoral immune modulations. Fish Shellfish Immunol. 2012, 33, 1112–1122. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Pottinger, T.G.; Sneddon, L.U. The effects of acute and chronic hypoxia on cortisol, glucose and lactate concentrations in different populations of three-spined stickleback. Fish Physiol. Biochem. 2011, 37, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douxfils, J.; Lambert, S.; Mathieu, C.; Milla, S.; Mandiki, S.N.M.; Henrotte, E.; Wang, N.; Dieu, M.; Raes, M.; Rougeot, C.; et al. Influence of domestication process on immune response to repeated emersion stressors in Eurasian perch (Perca fluviatilis, L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 173, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.L.; Hung, C.Y.; Nakano, K.; Randall, D.J. An in vivo study of common carp (Cyprinus carpio L.) liver during prolonged hypoxia. Comp. Biochem. Physiol. Part D Genomics Proteomics 2007, 2, 295–302. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Dissolved Oxygen Level and Stocking Density Effects on Growth, Feed Utilization, Physiology, and Innate Immunity of Nile Tilapia, Oreochromis niloticus. J. Appl. Aquac. 2014, 26, 340–355. [Google Scholar] [CrossRef]
- Kvamme, B.O.; Gadan, K.; Finne-Fridell, F.; Niklasson, L.; Sundh, H.; Sundell, K.; Taranger, G.L.; Evensen, Ø. Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress. Fish Shellfish Immunol. 2013, 34, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Cecchini, S.; Saroglia, M. Antibody response in sea bass (Dicentrarchus Labrax L.) in relation to water temperature and oxygenation. Aquac. Res. 2002, 33, 607–613. [Google Scholar] [CrossRef]
- Welker, T.L.; McNulty, S.T.; Klesius, P.H. Effect of sublethal hypoxia on the immune response and susceptibility of channel catfish, Ictalurus punctatus, to enteric septicemia. J. World Aquac. Soc. 2007, 38, 12–23. [Google Scholar] [CrossRef]
- Martorell Ribera, J.; Nipkow, M.; Viergutz, T.; Brunner, R.M.; Bochert, R.; Koll, R.; Goldammer, T.; Gimsa, U.; Rebl, A. Early response of salmonid head-kidney cells to stress hormones and toll-like receptor ligands. Fish Shellfish Immunol. 2020, 98, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, E.; Suo, Y.; Su, Y.; Lu, M.; Zhao, Q.; Qin, J.G.; Chen, L. Histological and transcriptomic responses of two immune organs, the spleen and head kidney, in Nile tilapia (Oreochromis niloticus) to long-term hypersaline stress. Fish Shellfish Immunol. 2018, 76, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of dissolved oxygen and fish size on Nile tilapia, Oreochromis niloticus (L.): Growth performance, whole-body composition, and innate immunity. Aquac. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Korytář, T.; Nipkow, M.; Altmann, S.; Goldammer, T.; Köllner, B.; Rebl, A. Adverse Husbandry of Maraena Whitefish Directs the Immune System to Increase Mobilization of Myeloid Cells and Proinflammatory Responses. Front. Immunol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortuño, J.; Esteban, M.A.; Meseguer, J. Effects of short-term crowding stress on the gilthead seabream (Sparus aurata L.) innate immune response. Fish Shellfish Immunol. 2001, 11, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Trottier, M.D.; Newsted, M.M.; King, L.E.; Fraker, P.J. Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc. Natl. Acad. Sci. USA 2008, 105, 2028–2033. [Google Scholar] [CrossRef] [Green Version]
- Dhabhar, F.S.; Miller, A.H.; McEwen, B.S.; Spencer, R.L. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J. Immunol. 1995, 154, 5511–5527. [Google Scholar] [PubMed]
- Soldatov, A.A. The effect of hypoxia on red blood cells of flounder: A morphologic and autoradiographic study. J. Fish Biol. 1996, 48, 321–328. [Google Scholar] [CrossRef]
- Nikinmaa, M. Oxygen-dependent cellular functions—Why fishes and their aquatic environment are a prime choice of study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Troll, J.V.; Somero, G.N. Hypoxia-induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc. Natl. Acad. Sci. USA 2001, 98, 1993–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Y.; Li, W.; Wu, B.; Chen, J.; Chen, X. Transcriptome analysis reveals new insights into immune response to hypoxia challenge of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 98, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Rimoldi, S.; Corà, S.; Bernardini, G.; Gornati, R.; Saroglia, M. Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax). Aquaculture 2008, 279, 150–159. [Google Scholar] [CrossRef]
- Rimoldi, S.; Terova, G.; Ceccuzzi, P.; Marelli, S.; Antonini, M.; Saroglia, M. HIF-1α mRNA levels in Eurasian perch (Perca fluviatilis) exposed to acute and chronic hypoxia. Mol. Biol. Rep. 2012, 39, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Mohindra, V.; Tripathi, R.K.; Singh, R.K.; Lal, K.K. Molecular characterization and expression analysis of three hypoxia-inducible factor alpha subunits, HIF-1α, -2α and -3α in hypoxia-tolerant Indian catfish, Clarias batrachus [Linnaeus, 1758]. Mol. Biol. Rep. 2013, 40, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Van Der Meer, D.L.M.; Van Den Thillart, G.E.E.J.M.; Witte, F.; De Bakker, M.A.G.; Besser, J.; Richardson, M.K.; Spaink, H.P.; Leito, J.T.D.; Bagowski, C.P. Gene expression profiling of the long-term adaptive response to hypoxia in the gills of adult zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 1512–1519. [Google Scholar] [CrossRef]
- Evans, J.J.; Shoemaker, C.A.; Klesius, P.H. Effects of Sublethal Dissolved Oxygen Stress on Blood Glucose and Susceptibility to Streptococcus agalactiae in Nile Tilapia Oreochromis niloticus. J. Aquat. Anim. Health 2003, 15, 202–208. [Google Scholar] [CrossRef]
- Cuesta, A.; Esteban, M.Á.; Meseguer, J. Effects of different stressor agents on gilthead seabream natural cytotoxic activity. Fish Shellfish Immunol. 2003, 15, 433–441. [Google Scholar] [CrossRef]
- Chadzinska, M.; Leon-Kloosterziel, K.M.; Plytycz, B.; Lidy Verburg-van Kemenade, B.M. In vivo kinetics of cytokine expression during peritonitis in carp: Evidence for innate and alternative macrophage polarization. Dev. Comp. Immunol. 2008, 32, 509–518. [Google Scholar] [CrossRef]
- Castro, R.; Coll, J.; Blanco, M.D.M.; Rodriguez-Bertos, A.; Jouneau, L.; Fernández-Garayzábal, J.F.; Gibello, A. Spleen and head kidney differential gene expression patterns in trout infected with Lactococcus garvieae correlate with spleen granulomas. Vet. Res. 2019, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.J.; Chen, Q.; Rong, Y.J.; Chen, J. Mobilisation and dysfunction of haematopoietic stem/progenitor cells after Listonella anguillarum infection in ayu, Plecoglossus altivelis. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bielek, E.; Bigaj, J.; Chadzińska, M.; Płytycz, B. Depletion of head kidney neutrophils and cells with basophilic granules during peritoneal inflammation in the goldfish, Carassius auratus. Folia Biol. 1999, 47, 33–42. [Google Scholar]
- Hong, S.; Li, R.; Xu, Q.; Secombes, C.J.; Wang, T. Two Types of TNF-α Exist in Teleost Fish: Phylogeny, Expression, and Bioactivity Analysis of Type-II TNF-α3 in Rainbow Trout Oncorhynchus mykiss. J. Immunol. 2013, 191, 5959–5972. [Google Scholar] [CrossRef] [Green Version]
- Reis, M.I.R.; do Vale, A.; Pereira, P.J.B.; Azevedo, J.E.; dos Santos, N.M.S. Caspase-1 and IL-1β Processing in a Teleost Fish. PLoS ONE 2012, 7, e50450. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Secombes, C. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, metabolism and immune cell function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Melillo, G.; Varesio, L. Hypoxia: A double-edged sword of immunity. J. Mol. Med. 2011, 89, 657–665. [Google Scholar] [CrossRef]




| Gene Symbol | Official Names | Sense Primer (5′-3′) | Antisense Primer (5′-3′) | Primer Efficiency [%] | Fragment Length [bp] |
|---|---|---|---|---|---|
| Reference genes: | |||||
| EEF1A1 | Elongation factor 1 alpha | ATGGACAGACCCGTGAGCATG | TTCTTGATGTAGGTGCTCACTTC | 105 | 151 |
| RPL32 | Ribosomal protein L32 | GGCGTAAACCCAGAGGTATTGA | ACCTCGAGCTCCTTGACATTGT | 105 | 157 |
| RPS5 | Ribosomal protein S5 | GCAGGATTACATTGCTGTGAAAG | TCATCAGCTTCTTGCCATTGTTG | 101 | 161 |
| Target genes: | |||||
| Stress response | |||||
| EPAS1 | Endothelial PAS domain protein1 | AGTGCAGAGGACGCACAGATG | TCATGTTCACCTGCGTGAGCC | 100 | 139 |
| HIF1A | Hypoxia inducible factor 1 subunit alpha | CCAGTCGAATCCCTTGAGAGTT | CTGTGGGGTCCTCTTAGCAAC | 97 | 156 |
| HMOX1 | Heme oxygenase 1 | GCTCGCTGTATGAGGTCTACC | TCTCTCCAGTCCTGGCCATAG | 101 | 154 |
| HSP90AA1 | Heat shock protein 90 alpha family class A member 1 | AGATACTACACCTCGGCTTCTG | TCACCAGTGATGTAGTAGATGTG | 100 | 101 |
| HSF1 | Heat shock transcription factor 1 | TGTGTCTTGTGCAGAGTGGAAC | GCTGGCCATGTTGTTGTGTTTG | 111 | 101 |
| HSF2 | Heat shock transcription factor 2 | AGCCGTCCCGCAGCTCCCT | CGGGACTCAGTTCGCACAGG | 91 | 93 |
| NR3C1 | Nuclear receptor subfamily 3 group c member 1 | CCAGTCCTGCATGGATTCACTT | AGGTCCATAGTGTTGTCACTGAA | 100 | 180 |
| Immune response | |||||
| CSF2 * | Colony stimulating factor 2 | CCAGCAGGAATACACAGAAATCT | CGCAGATAGAGACAATGATGAAG | 95 | 164 |
| CXCL8 * | Interleukin 8 | AACAGGGATGAGTCTGAGAAGC | GCTTGGAAATGAAGTCTTACATGA | 98 | 158 |
| FTH1 | Ferritin heavy chain 1 | AGAACTGGCAGACTGGGTGAC | CTGCTTTCTTTGCCCAGGGTG | 99 | 102 |
| IL1B * | Interleukin 1 beta | TCGACCTACTTGCACCCTACA | TCTGCCTCCACAACCTGAA | 101 | 137 |
| MHC II alpha * | Major histocompatibility complex II alpha | TGGACCAACCACTGACCAGAAT | CATCATCAGTCCCAGCCAATCA | 99 | 168 |
| MPO * | Myeloperoxidase | GTTTGATCGGCCGTCCTGCTA | ATTACCAGCCAAGCCATGGTCA | 98 | 152 |
| RAG1 * | Recombination activating 1 | CTCAGGCTTCAGTGTCATGATC | AACCTCTTTCTCCTCCTCGTCT | 95 | 157 |
| TNF * | Tumor necrosis factor | GTCTTTGGAACCAGGCTATTTAC | TTTATGCCTCAGGCTTGACTGG | 89 | 157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, N.; Matoušek, J.; Rebl, A.; Stejskal, V.; Brunner, R.M.; Goldammer, T.; Verleih, M.; Korytář, T. Effects of Chronic Hypoxia on the Immune Status of Pikeperch (Sander lucioperca Linnaeus, 1758). Biology 2021, 10, 649. https://doi.org/10.3390/biology10070649
Schäfer N, Matoušek J, Rebl A, Stejskal V, Brunner RM, Goldammer T, Verleih M, Korytář T. Effects of Chronic Hypoxia on the Immune Status of Pikeperch (Sander lucioperca Linnaeus, 1758). Biology. 2021; 10(7):649. https://doi.org/10.3390/biology10070649
Chicago/Turabian StyleSchäfer, Nadine, Jan Matoušek, Alexander Rebl, Vlastimil Stejskal, Ronald M. Brunner, Tom Goldammer, Marieke Verleih, and Tomáš Korytář. 2021. "Effects of Chronic Hypoxia on the Immune Status of Pikeperch (Sander lucioperca Linnaeus, 1758)" Biology 10, no. 7: 649. https://doi.org/10.3390/biology10070649
APA StyleSchäfer, N., Matoušek, J., Rebl, A., Stejskal, V., Brunner, R. M., Goldammer, T., Verleih, M., & Korytář, T. (2021). Effects of Chronic Hypoxia on the Immune Status of Pikeperch (Sander lucioperca Linnaeus, 1758). Biology, 10(7), 649. https://doi.org/10.3390/biology10070649









