Lymphadenopathy after the Anti-COVID-19 Vaccine: Multiparametric Ultrasound Findings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. US Protocol
2.3. Statistical Analysis
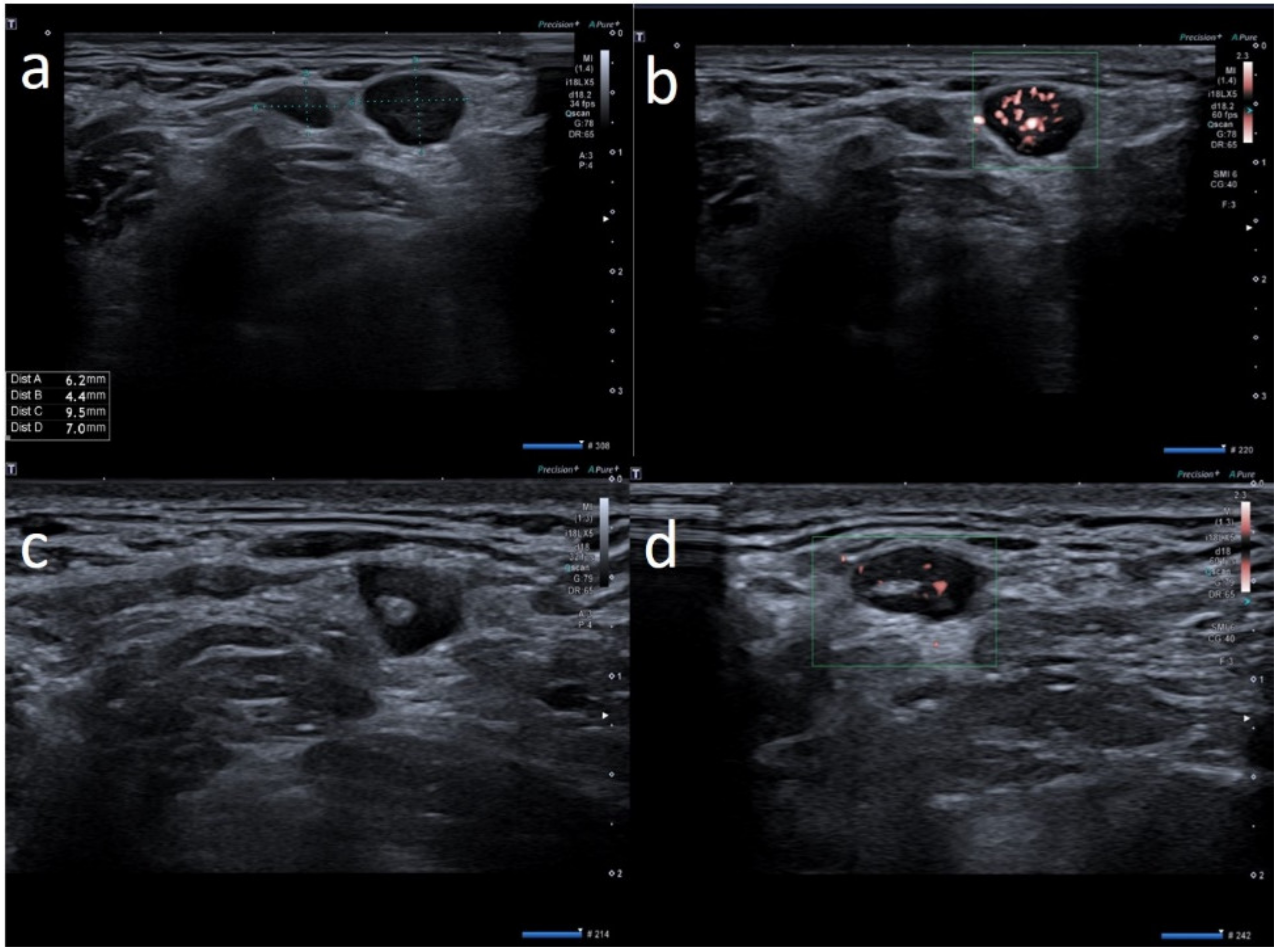
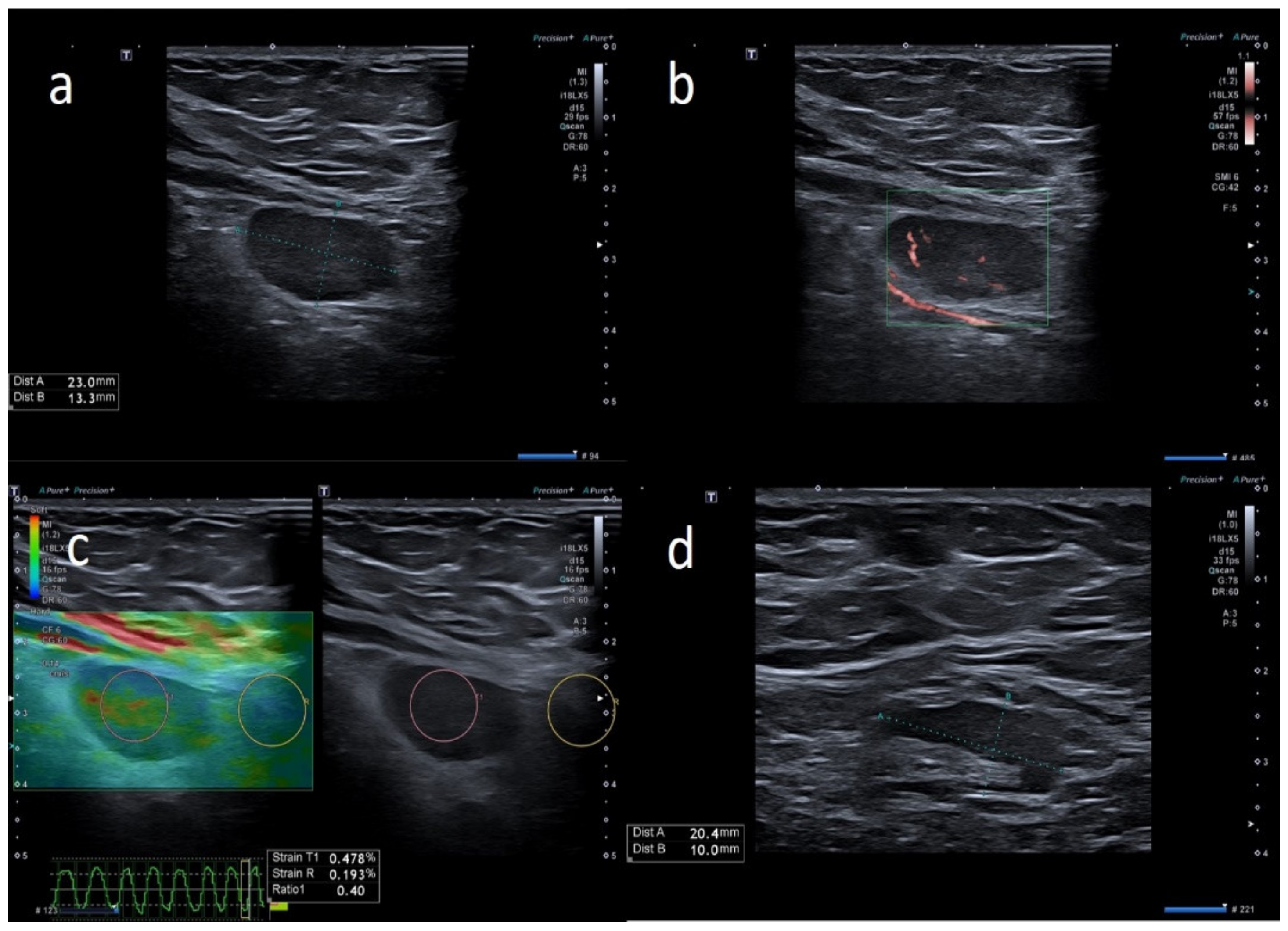
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Fernández-Prada, M.; Rivero-Calle, I.; Calvache-González, A.; Martinón-Torres, F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Eurosurveillance 2021, 26, 2100193. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef]
- Mehta, N.; Sales, R.M.; Babagbemi, K.; Levy, A.D.; McGrath, A.L.; Drotman, M.; Dodelzon, K. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin. Imaging 2021, 75, 12–15. [Google Scholar] [CrossRef]
- Özütemiz, C.; Krystosek, L.A.; Church, A.L.; Chauhan, A.; Ellermann, J.M.; Domingo-Musibay, E.; Steinberger, D. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 2021, 300, E296–E300. [Google Scholar] [CrossRef]
- Edmonds, C.E.; Zuckerman, S.P.; Conant, E.F. Management of Unilateral Axillary Lymphadenopathy Detected on Breast MRI in the Era of Coronavirus Disease (COVID-19) Vaccination. Am. J. Roentgenol. 2021. [Google Scholar] [CrossRef]
- Lehman, C.D.; D’Alessandro, H.A.; Mendoza, D.P.; Succi, M.D.; Kambadakone, A.; Lamb, L.R. Unilateral Lymphadenopathy Post COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties. J. Am. College Radiol. 2021, 18, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.S.; Perez-Johnston, R.; Chikarmane, S.A.; Chen, M.M.; El Homsi, M.; Feigin, K.N.; Gallagher, K.M.; Hanna, E.Y.; Hicks, M.; Ilica, A.T.; et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology 2021, 210436. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.; Galdiero, R.; Picone, C.; Izzo, F.; D’Aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 Covid-19 Vaccine: Preliminary Ultrasound Findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Sofia, S.; Boccatonda, A.; Montanari, M.; Spampinato, M.; D’Ardes, D.; Cocco, G.; Accogli, E.; Cipollone, F.; Schiavone, C. Thoracic ultrasound and SARS-COVID-19: A pictorial essay. J. Ultrasound 2020, 23, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Boccatonda, A.; Cocco, G.; Ianniello, E.; Montanari, M.; D’Ardes, D.; Borghi, C.; Giostra, F.; Copetti, R.; Schiavone, C. One year of SARS-CoV-2 and lung ultrasound: What has been learned and future perspectives. J. Ultrasound 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Boccatonda, A.; Ianniello, E.; D’Ardes, D.; Cocco, G.; Giostra, F.; Borghi, C.; Schiavone, C. Can Lung Ultrasound be Used to Screen for Pulmonary Embolism in Patients with SARS-CoV-2 Pneumonia? Eur. J. Case Rep. Intern. Med. 2020, 7, 001748. [Google Scholar] [CrossRef]
- Wagner, J.M.; Alleman, A.M. Ultrasonography of Cervical Lymph Nodes. Radiol. Clin. N. Am. 2019, 57, 485–500. [Google Scholar] [CrossRef]
- Guo, Q.; Dong, Z.; Zhang, L.; Ning, C.; Li, Z.; Wang, D.; Liu, C.; Zhao, M.; Tian, J. Ultrasound Features of Breast Cancer for Predicting Axillary Lymph Node Metastasis. J. Ultrasound Med. 2018, 37, 1353–1354. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.-Z.; Huang, Y.-H.; Shen, H.-I.; Liu, X.-T. Clinical Applications of Superb Microvascular Imaging in the Liver, Breast, Thyroid, Skeletal Muscle, and Carotid Plaques. J. Ultrasound Med. 2019, 38, 2811–2820. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.Y.; Yoon, R.G.; Kim, J.-H.; Hong, H.S. The Value of Microvascular Imaging for Triaging Indeterminate Cervical Lymph Nodes in Patients with Papillary Thyroid Carcinoma. Cancers 2020, 12, 2839. [Google Scholar] [CrossRef]
- Özel, D.; Özel, B.D. Evaluation of diagnostic value of conventional and color Doppler ultrasound with elastography strain ratios in differentiation between benign and malignant lymph nodes. Pol. J. Radiol. 2018, 83, 32–36. [Google Scholar] [CrossRef]
- Cui, X.-W.; Hocke, M.; Jenssen, C.; Ignee, A.; Klein, S.; Schreiber-Dietrich, D.; Dietrich, C.F. Conventional ultrasound for lymph node evaluation, update 2013. Z. Gastroenterol. 2014, 52, 212–221. [Google Scholar] [CrossRef]
- Cohen, J.; Powderly, W.G.; Opal, S.M. Infectious diseases. Elsevier Health Sci. 2017, 2, 1s45. [Google Scholar]
- Newfield, L.; Naschitz, J.E.; Yeshurun, D. BCG-induced axillary lymph-adenitis in the adult. Harefuah 1990, 119, 199–200. [Google Scholar] [PubMed]
- Studdiford, J.; Lamb, K.; Horvath, K.; Altshuler, M.; Stonehouse, A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy 2008, 28, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Net, J.M.; Mirpuri, T.M.; Plaza, M.J.; Escobar, C.A.; Whittington, E.E.; Collado-Mesa, F.; Yepes, M.M. Resident and Fellow Education Feature: US Evaluation of Axillary Lymph Nodes. Radiographics 2014, 34, 1817–1818. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Boccatonda, A.; D’Ardes, D.; Galletti, S.; Schiavone, C. Mantle cell lymphoma: From ultrasound examination to histological diagnosis. J. Ultrasound 2018, 21, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Jia, W.; Shi, J.; Yuan, C.; Zhang, Y.; Chen, M. Role of Elastography in Axillary Examination of Patients With Breast Cancer. J. Ultrasound Med. 2018, 37, 699–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Society of Breast Imaging. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination; Society of Breast Imaging: Reston, VA, USA, 2021. [Google Scholar]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Al-Quteimat, O.M.; Amer, A.M. The Impact of the COVID-19 Pandemic on Cancer Patients. Am. J. Clin. Oncol. 2020, 43, 452–455. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.T.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]






| US Features | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Vaccine | Lymph Node Localization | Clinical Presentation | Oncological History | Nm | Size | Form | Cortical Thickening and Hilum | Sonoelasto | SMI | US Follow-Up to 2 Weeks |
| W | 25 | Pfizer | Axillary ipsilateral to vaccine injection | Three days after first dose of vaccine, axillary swelling and pain present. Also hypomobility ipsilateral arm | None | 6 | Variable: from 0.7 cm to 2.8 cm | Ovular | Prev. no hilum evidence | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 60 days |
| M | 64 | Pfizer | Supraclavicular ipsilateral to vaccine injection | Occasional autopalpation 2 weeks after second dose vaccine | None | 2 | Around 1.0 cm | Ovular | Assimetric cortical thickening with hilum evidence | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| W | 28 | Astazeneca | Supraclavicular ipsilateral to vaccine injection | Two days after first dose vaccine, supraclavicular swelling and pain present | None | 3 | Variable: from 0.6 to 1.5 cm | Ovular | No evidence hilum | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 45 days |
| W | 72 | Astazeneca | Supraclavicular ipsilateral to vaccine injection | One day after first dose vaccine, supraclavicular swelling and pain present | None | 2 | Subcentimetric size | Round | Asimmetric cortical thickening with hilum evidence | Prevalent hard pattern | Peripheral vascular signals | Normalized to 30 days |
| M | 42 | Pfizer | Axillary ipsilateral to vaccine injection | Occasionally 1 weeks after second dose during chest ct | None | 3 | Variable: from 1.5 to 2.0 cm | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| M | 39 | Astazeneca | Supraclavicular ipsilateral to vaccine injection | Four days after first dose vaccine, supraclavicular swelling and pain present | None | 1 | Subcentimetric size | Round | No evidence hilum | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 30 days |
| M | 60 | Pfizer | Supraclavicular ipsilateral to vaccine injection | Occasional autopalpation 12 days after second dose vaccine | None | 2 | Around 1.0 cm | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Central and peripheral vascular signals | Unnecessary other follow-up |
| W | 49 | Pfizer | Axillary ipsilateral to vaccine injection | Occasionally, 6 days after first dose, during breast sonography for oncological surveillance | Breast cancer 3 years ago | 4 | Variable: from 1.0 to 2.0 cm | Ovular | Asimmetric cortical thickening and poor evidence hilum | Stiffness similar to surrounding tissue | Central and peripheral vascular signals | Unnecessary other follow-up |
| M | 41 | Moderna | Supraclavicular ipsilateral to vaccine injection | Two days after first dose vaccine, supraclavicular swelling and pain present | None | 3 | Subcentimetric size | Ovular | No evidence hilum | Stiffness similar to surrounding tissue | Central and peripheral vascular signals | Normalized to 30 days |
| W | 54 | Pfizer | Axillary ipsilateral to vaccine injection | Occasionally, 14 days after second dose, during breast sonography for surveillance | None | 3 | Variable: from 1.0 to 2.0 cm | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| W | 74 | Astazeneca | Supraclavicular ipsilateral to vaccine injection | Ten days after first dose vaccine | None | 2 | Subcentimetric size | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| M | 35 | Pfizer | Supraclavicular ipsilateral to vaccine injection | Day after the first dose vaccine, supraclavicular swelling and pain present | None | 1 | Around 1.5 cm | Round | No evidence hilum | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 60 days |
| W | 52 | Pfizer | Axillary ipsilateral to vaccine injection | Occasionally during breast sonography for oncological surveillance | None | 3 | Variable: from 1.0 to 2.5 cm. | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| W | 26 | Astazeneca | Supraclavicular ipsilateral to vaccine injection | 2 days after the first dose vaccine, axillary swelling and pain present | None | 5 | Subcentimetric size | Ovular | No hilum evidence | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 45 days |
| W | 53 | Pfizer | Axillary ipsilateral to vaccine injection | Occasionally, 16 days after first dose, during breast sonography for oncological surveillance | Breast cancer 2 years ago | 3 | Variable: from 1.0 to 2.0 cm | Ovular | Asimmetric cortical thickening with hilum evidence | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| M | 62 | Pfizer | Axillary ipsilateral to vaccine injection | Occasionally, 2 weeks after first dose, during chest ct to monitor small polmonary nodules | None | 3 | Variable: from 1.5 to 2.0 cm. | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| M | 57 | Pfizer | Axillary ipsilateral to vaccine injection | Occasional autopalpation 2 weeks after second dose vaccine | Kidney cancer 4 years ago | 2 | Around 1.0 cm | Ovular | Asimmetric cortical thickening with hilum evidence | Stiffness similar to surrounding tissue | Normal | Normalized to 30 days |
| W | 69 | Astazeneca | Supraclavicular ipsilateral to vaccine injection | Three days after first dose vaccine | None | 3 | Subcentimetric size | Round | Asimmetric cortical thickening with hilum evidence | Stiffness similar to surrounding tissue | Central and peripheral vascular signals | Unnecessary other follow-up |
| W | 37 | Pfizer | Axillary ipsilateral to vaccine injection | Three days after first dose vaccine, axillary swelling present | Melanoma 5 years ago | 5 | Variable: from 1.5 to 2.0 cm. | Ovular | Assimetric cortical thickening with hilum evidence | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 45 days |
| M | 63 | Moderna | Axillary ipsilateral to vaccine injection | Occasionally, 16 days after first dose, during mammography | None | 3 | Variable: from 1.5 to 2.0 cm. | Ovular | Simmetric cortical thickening with normal hilum | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
| F | 32 | Astrazeneca | Supraclavicular ipsilateral to vaccine injection | Day after first dose vaccine, supraclavicular swelling and pain present | None | 1 | Around 1.2 cm | Round | No evidence hilum | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 30 days |
| F | 29 | Pfizer | Supraclavicular ipsilateral to vaccine injection | Day after first dose vaccine, supraclavicular swelling and pain present | None | 2 | Subcentimetric | Round | No evidence hilum | Prevalent hard pattern | Central and peripheral vascular signals | Normalized to 30 days |
| F | 66 | Moderna | Supraclavicular ipsilateral to vaccine injection | Occasionally, 2 weeks after second dose, during shoulder rm | None | 2 | Around 1.0 cm | Ovular | Assimetric cortical thickening with hilum evidence | Stiffness similar to surrounding tissue | Central and peripheral vascular signals | Normalized to 30 days |
| M | 59 | Astrazeneca | Supraclavicular ipsilateral to vaccine injection | Occasionally, autopalpation 4 days after second dose vaccine | None | 2 | 0.7 and 1.2 cm | Ovular | Asimetric cortical thickening with hilum evidence | Stiffness similar to surrounding tissue | Normal | Unnecessary other follow-up |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, G.; Delli Pizzi, A.; Fabiani, S.; Cocco, N.; Boccatonda, A.; Frisone, A.; Scarano, A.; Schiavone, C. Lymphadenopathy after the Anti-COVID-19 Vaccine: Multiparametric Ultrasound Findings. Biology 2021, 10, 652. https://doi.org/10.3390/biology10070652
Cocco G, Delli Pizzi A, Fabiani S, Cocco N, Boccatonda A, Frisone A, Scarano A, Schiavone C. Lymphadenopathy after the Anti-COVID-19 Vaccine: Multiparametric Ultrasound Findings. Biology. 2021; 10(7):652. https://doi.org/10.3390/biology10070652
Chicago/Turabian StyleCocco, Giulio, Andrea Delli Pizzi, Stefano Fabiani, Nino Cocco, Andrea Boccatonda, Alessio Frisone, Antonio Scarano, and Cosima Schiavone. 2021. "Lymphadenopathy after the Anti-COVID-19 Vaccine: Multiparametric Ultrasound Findings" Biology 10, no. 7: 652. https://doi.org/10.3390/biology10070652
APA StyleCocco, G., Delli Pizzi, A., Fabiani, S., Cocco, N., Boccatonda, A., Frisone, A., Scarano, A., & Schiavone, C. (2021). Lymphadenopathy after the Anti-COVID-19 Vaccine: Multiparametric Ultrasound Findings. Biology, 10(7), 652. https://doi.org/10.3390/biology10070652









