Phosphorus-Induced Adaptation Mechanisms of Rye Grown on Post-Flotation Copper Tailings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Properties of the Sediment
2.2. Plant Material and Treatment
2.3. Gas Exchange and Chlorophyll Fluorescence
2.4. Purification of Plasma Membrane
2.5. PM H+-ATPase Hydrolytic Activity and H+ Transport
2.6. Determination of ATP
2.7. Determination of Organic Acids and P in Root Exudates
2.8. Elemental Analyses
2.9. Statistical Analyses
3. Results and Discussion
3.1. The High Cu Content Is Not the Most Critical Factor Limiting Revegetation of the Post-Flotation Tailings; It Is Low Bioavailability of P
3.2. The Activity of the Mechanisms Involved in P Mobilisation in Rye Is Reversibly Correlated with P Availability in the Post-Flotation Tailings and Requires Initial P Supplementation
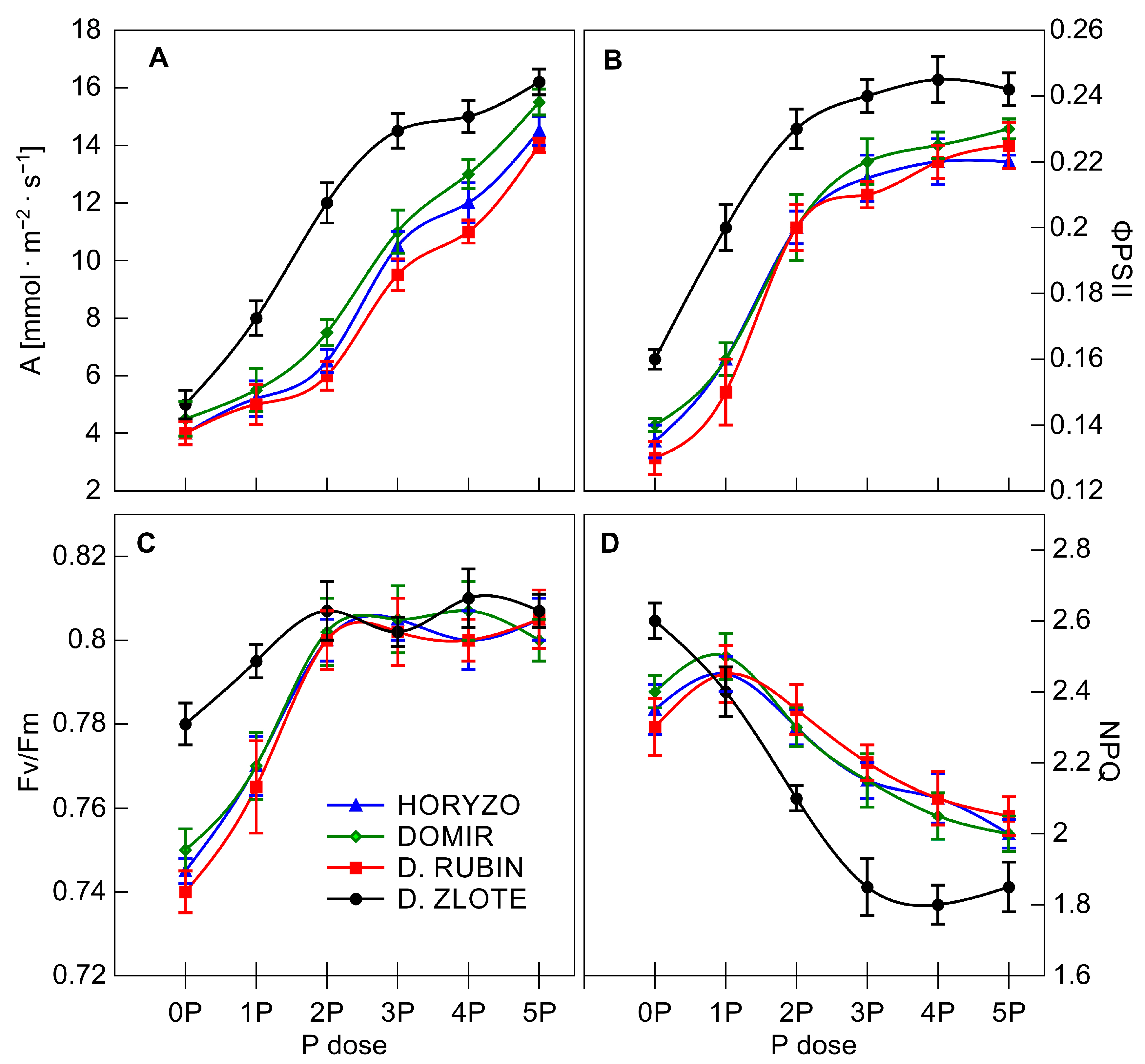
3.3. The Photosynthetic Performance Is Essential in Determining the Capacity of Rye for P Mobilisation
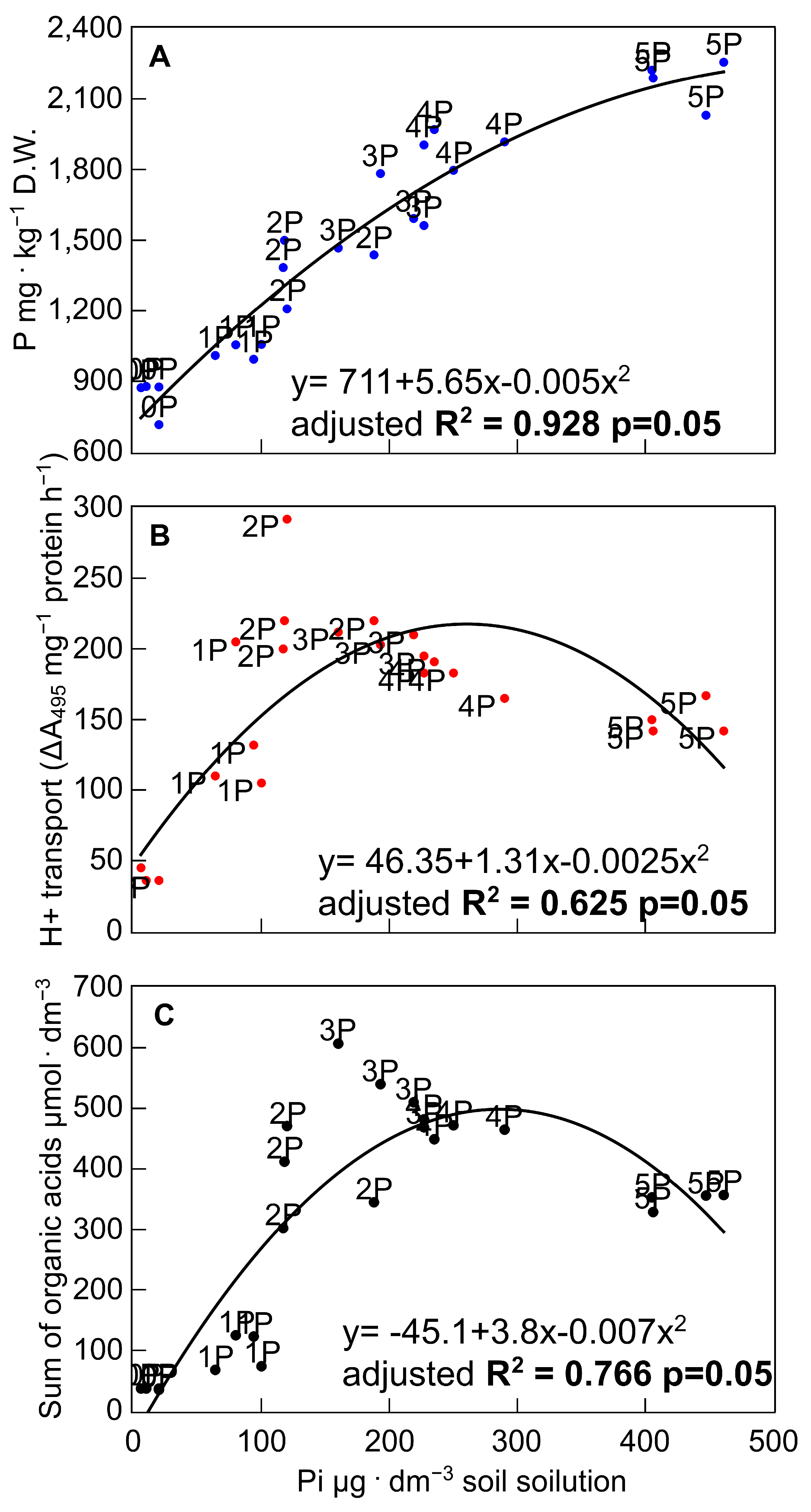
3.4. Effectiveness of the P-Mobilising Mechanisms in the Context of P Rendered Available in the Soil Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sverdrup, H.U.; Olafsdottir, H.A.; Ragnarsdottir, K.V. On the long-term sustainability of copper, zinc and lead supply, using a system dynamics model. Res. Cons. Rec. X 2019, 4, 1–21. [Google Scholar] [CrossRef]
- Younger, P.L.; Wolkersdorfer, C. Mining Impacts on the Fresh Water Environment: Technical and Managerial Guidelines for Catchment Scale Management. Mine Water Environ. 2004, 23, 2–80. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Mackline, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, L. Toward a New Paradigm for Tailings Phytostabilization—Nature of the Substrates, Amendment Options, and Anthropogenic Pedogenesis. Crit. Rev. Environ. Sci. Technol. 2014, 45, 813–839. [Google Scholar] [CrossRef]
- Mason, L.; Prior, T.; Mudd, G.; Giurcoa, D. Availability, addiction and alternatives: Three criteria for assessing the impact of peak minerals on society. J. Clean. Prod. 2011, 19, 958–966. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Recycling of copper tailings as an additivein cement mortars. Constr. Build. Mater. 2012, 37, 723–727. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Mine Wastes, Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Asensio, V.; Vega, F.A.; Singh, B.R.; Covelo, E.F. Effects of tree vegetation and waste amendments on the fractionation of Cr, Cu, Ni, Pb and Zn in polluted mine soils. Sci. Total Environ. 2013, 443, 446–453. [Google Scholar] [CrossRef]
- Tshibangu, M.I.; Nsahlai, V.I.; Kiatoko, M.H.; Hornick, J.L. Heavy Metals Concentration in Adenodolichos rhomboideus (O. Hoffm.) Harms. Forage Growing on Mining Tailings in South East of Democratic Republic of Congo: Influence of Washing, pH and Soil Concentrations. Int. J. Curr. Res. Biosci. Plant Biol. 2014, 1, 16–27. [Google Scholar]
- Baycu, G.; Tolunay, D.; Ozden, H.; Csatari, I.; Karadag, S.; Agba, T.; Rognes, S.E. An abandoned copper mining site in cyprus and assessment of metal concentrations in plants and soil. Int. J. Phytoremediat. 2015, 17, 622–631. [Google Scholar] [CrossRef]
- Carrasco, L.; Caravaca, F.; Azcón, R.; Roldán, A. Soil acidity determines the effectiveness of an organic amendment and a native bacterium for increasing soil stabilisation in semiarid mine tailings. Chemosphere 2009, 74, 239–244. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Soil Reclamation of Abandoned Mine Land by Revegetation. Int. J. Soil Sediment Water 2010, 3, 1–20. [Google Scholar]
- Lilić, J.; Cupać, S.; Lalević, B.; Andrić, V.; Gajić-Kvaščev, M. Pedological characteristics of open-pit Cu wastes and postflotation tailings (Bor, Serbia). J. Soil Sci. Plant Nutr. 2014, 14, 161–175. [Google Scholar]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tang, X.; Zhu, Y.; Christie, P. Metal concentrations and mycorrhizal status of plants colonizing copper mine tailings; potential for revegetation. Sci. China 2005, 48 (Suppl. 1), 156–164. [Google Scholar] [CrossRef]
- Chen, B.D.; Zhu, Y.G.; Duan, J.; Xiao, X.Y.; Smith, S.E. Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ. Pollut. 2007, 147, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N.; Ahmed, M.J.; Dhal, N.K. Effects of iron ore tailings on growth and physiological activities of Tagetes patula L. J. Soils Sed. 2013, 14, 721–730. [Google Scholar] [CrossRef]
- Chaignon, V.; Hinsinger, P. A Biotest for Evaluating Copper Bioavailability to Plants in a Contaminated Soil. J. Environ. Qual. 2003, 32, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Spiak, Z.; Gediga, K. Assessment of the applicability of some mineral wastes for revitalization of a postflotation dumping site. Przem. Chem. 2012a, 91, 990–995. [Google Scholar]
- Tordoff, G.M.; Baker, A.J.M.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Spiak, Z.; Gediga, K. Applicability of selected plant species for the occupance of land degraded by copper industry. Przem. Chem. 2012b, 91, 996–999. [Google Scholar]
- Spiak, Z.; Gediga, K. Usefulness of selected mineral wastes for reclamation of copper industry dumping site. Environ. Prot. Eng. 2009, 35, 79–88. [Google Scholar]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological Potential of Plants for Phytoremediation and Ecorestoration of Fly Ash Deposits and Mine Wastes. Front. Environ. Sci. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.; Rufaut, C.G.; Smith, C.; Mains, D.; Craw, D. Rapid plant-cover establishment on gold mine tailings in southern New Zealand: Glasshouse screening trials. Int. J. Phytoremediat. 2005, 7, 307–322. [Google Scholar] [CrossRef]
- Mains, D.; Craw, D.; Rufaut, C.G.; Smith, C.M. Phytostabilization of gold mine tailings, New Zealand. Part 1, Plant establishment in alkaline saline substrate. Int. J. Phytoremediat. 2006, 8, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, R.H.J. Rye: Genetics, Breeding, and Cultivation; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Targońska, M.; Bolibok-Brągoszewska, H.; Rakoczy-Trojanowska, M. Assessment of Genetic Diversity in Secale cereale Based on SSR Markers. Plant. Mol. Biol. Rep. 2015, 34, 37–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mench, M.; Lepp, N.; Bert, V.; Schwitzguébel, J.-P.; Gawronski, S.W.; Schröder, P.; Vangronsveld, J. Successes and limitations of phytotechnologies at field scale: Outcomes, assessment and outlook from COST Action 859. J. Soils Sed. 2010, 10, 1039–1070. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediat. 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Kidd, P.; Mench, M.; Álvarez-López, V.; Bert, V.; Dimitriou, I.; Friesl-Hanl, W.; Puschenreiter, M. Agronomic practices for improving gentle remediation of trace element-contaminated soils. Int. J. Phytoremed. 2015, 17, 1005–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtschabel, P. Das pflanzenverfügbare Magnesium des Boden und seine Bestimmung. Zeitschift Pflanz. Düngung Bodenkd. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Egner, H.; Riehm, H. Die Doppellactatmethode, zit; Methodenbuch, I., von Thun, R., Hermann, R., Knickmann, E., Eds.; Neumann-Verlag: Berlin, Germany, 1955. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langeluddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis thaliana and the halophyte Thellungiella halophila. Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, A.; Fuglsang, A.T. Purification of plant plasma membranes by two-phase partitioning and measurement of H+ pumping. Met. Mol. Biol. 2012, 913, 217–223. [Google Scholar]
- Johansson, F.; Olbe, M.; Sommarin, M.; Larsson, C. Brij 58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J. 1995, 7, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Gallahger, S.R.; Leonard, R.T. Effect of vanadate, molybdate and azide on membrane associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982, 70, 1335–1340. [Google Scholar] [CrossRef] [Green Version]
- Sze, H. H+-translocating ATPase. Advances using membrane vesicles. Ann. Rev. Plant Physiol. 1985, 36, 175–208. [Google Scholar] [CrossRef]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatases. Meth. Enzymol. 1966, 8, 115–118. [Google Scholar]
- Kłobus, G.; Buczek, J. The role of plasma membrane oxidoreductase activity in proton transport. J. Plant Physiol. 1995, 146, 103–107. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Am. Proc. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Kasowska, D.; Gediga, K.; Spiak, Z. Heavy metal and nutrient uptake in plants colonizing post-flotation copper tailings. Environ. Sci. Pollut. Res. Int. 2018, 25, 824–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Bradl, H.B. Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier Academic: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Copper. In Principles of Plant Nutrition; Springer: Dordrecht, The Netherlands, 2001; pp. 599–611. [Google Scholar]
- Hock, B.; Elstner, F.E. Plant Toxicology; Marcel Dekker: New York, NY, USA, 2005. [Google Scholar]
- Jones, J.B. Plant Nutrition and Soil Fertility Manual; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ponizovsky, A.A.; Allen, H.E.; Ackerman, A.J. Copper activity in soil solutions of calcareous soils. Environ. Pollut. 2007, 145, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elbana, T.A.; Selim, H.M. Copper Mobility in Acidic and Alkaline Soils: Miscible Displacement Experiments. Soil Sci. Soc. Am. J. 2011, 75, 2101–2110. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Reed, S.C.; Yang, X.; Thornton, P.E. Incorporating phosphorus cycling into global modeling efforts: A worthwhile, tractable endeavor. New Phytol. 2015, 208, 324–329. [Google Scholar] [CrossRef]
- Tiessen, H. Phosphorus in the global environment. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–8. [Google Scholar]
- Lis, J.; Pasieczna, A.; Mojski, J.E.; Przeniosło, S.; Sylwestrzak, H.; Strzelecki, R.; Wołkowicz, S. Geochemical Atlas of Poland; Polish Geological Institute: Warsaw, Poland, 2012. [Google Scholar]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Durism, M.; Gilucis, A.; Gregorauskiene, V.; Halamic, J.; et al. Geochemical Atlas of Europe. Part 1—Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005. [Google Scholar]
- Kęsik, K.; Jadczyszyn, T.; Lipiński, W.; Jurga, B. Adaptation of the Mehlich 3 procedure for routine determination of phosphorus, potassium and magnesium in soil. Przem. Chem. 2015, 1, 147–150. [Google Scholar]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Campbell, C.R. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Update January 2013. Available online: www.ncagr.gov/agronomi/saaesd/scsb394.pdf (accessed on 17 May 2020).
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Clode, P.; Hawkins, H.J.; Laliberté, E.; Oliveira, R.S.; Reddell, P.; Shane, M.W.; Stitt, M.; Weston, P. Metabolic adaptations of the non-mycotrophic Proteaceae to soils with low phosphorus availability. In Annual Plant Reviews; Plaxton, W.C., Lambers, H., Eds.; John Wiley& Sons: Hoboken, NJ, USA, 2015; Volume 48, pp. 289–335. [Google Scholar]
- Long, M.H.; McGlathery, K.J.; Zieman, J.C.; Berg, P. The role of organic acid exudates in liberating phosphorus from seagrass-vegetated carbonate sediments. Limnol. Oceanogr. 2008, 53, 2616–2626. [Google Scholar] [CrossRef]
- Ryan, P.; Delhaize, E.; Jones, D. Function And Mechanism Of Organic Anion Exudation From Plant Roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Phosphorus starvation boosts carboxylate secretion in P-deficient genotypes of Lupinus angustifolius with contrasting root structure. Crop Pasture Sci. 2013, 64, 588–599. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Roelofs, R.F.R.; Rengel, Z.; Cawthray, G.R.; Dixon, K.W.; Lambers, H. Exudation of carboxylates in Australian Proteaceae: Chemical composition. Plant Cell Environ. 2001, 24, 891–903. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Stevens, J.; Cawthray, G.R.; Turner, S.; Grigg, A.M.; Lambers, H. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 2003, 248, 187–197. [Google Scholar] [CrossRef]
- White, P.J.; Hammond, J.P. Phosphorus nutrition of terrestrial plants. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 51–81. [Google Scholar]
- Hunter, P.J.; Teakle, G.R.; Bending, G.D. Root traits and microbial community interactions in relation to phosphorus availability and acquisition, with particular reference to Brassica. Front. Plant Sci. 2014, 5, 27. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.X.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Uhde-Stone, C. White Lupin: A Model System for Understanding Plant Adaptation to Low Phosphorus Availability. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability; Sulieman, R.S., Lam-Son, P.T., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; pp. 243–280. [Google Scholar]
- Howell, T.; Moriconi, J.I.; Zhao, X.; Hegarty, J.; Fahima, T.; Santa-Maria, G.E.; Dubcovsky, J. A wheat/rye polymorphism affects seminal root length and yield across different irrigation regimes. J. Exp. Bot. 2019, 70, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P.; Herrmann, L.; Lesueur, D.; Robin, A.; Trap, J.; Waithaisong, K.; Plassard, C. Impact of roots, microorganisms and microfauna on the fate of soil phosphorus in the rhizosphere. In Annual Plant Reviews; Plaxton, W.C., Lambers, H., Eds.; John Wiley& Sons: Hoboken, NJ, USA, 2015; Volume 48, pp. 377–408. [Google Scholar]
- Yu, W.; Kan, Q.; Zhang, J.; Zeng, B.; Chen, Q. Role of the plasma membrane H+-ATPase in the regulation of organic acid exudation under aluminum toxicity and phosphorus deficiency. Plant Signal. Behav. 2016, 11, e1106660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruta, M.; Gray, W.M.; Sussman, M.R. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015, 28, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Kerkeb, L.; Venema, K.; Donaire, J.; Rodriguez-Rozales, M. Enhanced H+/ATP coupling ratio of H+-ATPase and increased 14-3-3 protein content in plasma membrane of tomato cells upon osmotic shock. Physiol. Plant 2002, 16, 37–41. [Google Scholar] [CrossRef]
- Poirier, Y.; Jung, J.-Y. Phosphate transporters. In Annual Plant Reviews; Plaxton, W.C., Lambers, H., Eds.; John Wiley&Sons: Hoboken, NJ, USA, 2015; Volume 48, pp. 125–158. [Google Scholar]
- Zhu, Y.; Yan, F.; Zörb, C.; Schubert, S. Link Between Citrate and Proton Release by Proteoid Roots of White Lupin (Lupinus albus L.) Grown Under Phosphorus-deficient Conditions? Plant Cell Physiol. 2005, 46, 892–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Col, V.; Fuchs, P.; Nietzel, T.; Elsässer, M.; Voon, C.P.; Candeo, A.; Seeliger, I.; Fricker, M.D.; Grefen, C.; Møller, I.M.; et al. ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology. eLife 2017, 6, e26770. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Hammond, J.P.; White, P.J. Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. J. Exp. Bot. 2008, 59, 93–109. [Google Scholar] [CrossRef]
- Chu, S.; Li, H.; Zhang, X.; Yu, K.; Chao, M.; Han, S.; Zhang, D. Physiological and proteomics analyses reveal low-phosphorus stress affected the regulation of photosynthesis in soybean. Int. J. Mol. Sci. 2018, 19, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepien, P.; Johnson, G.N. Plastid terminal oxidase requires translocation to the grana stacks to act as a sink for electron transport. Proc. Natl. Acad. Sci. USA 2018, 115, 9634–9639. [Google Scholar] [CrossRef] [Green Version]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Briantain, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Golding, A.J.; Johnson, G.N. Down-regulation of linear and activation of cyclic electron transport during drought. Planta 2003, 218, 107–114. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]

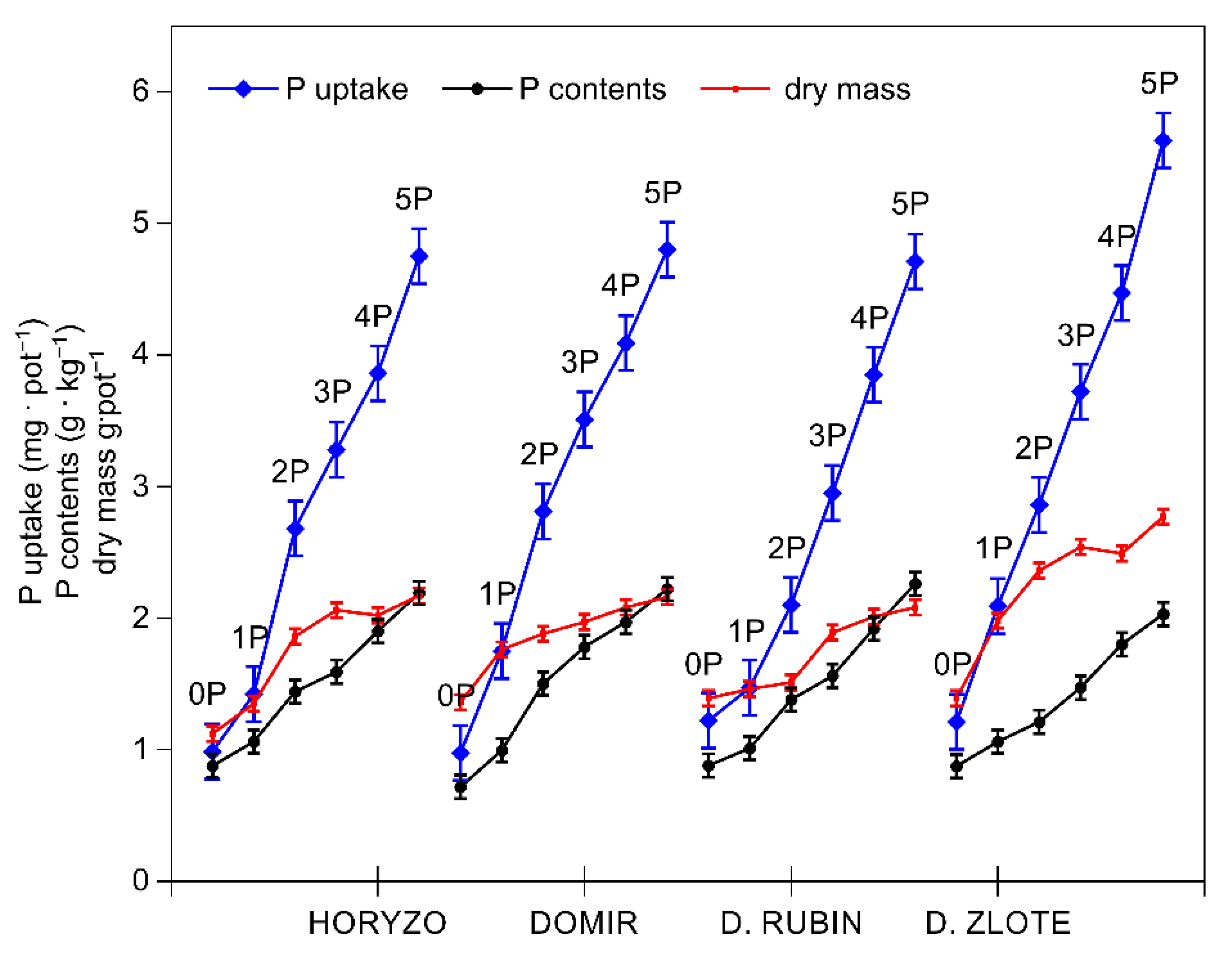
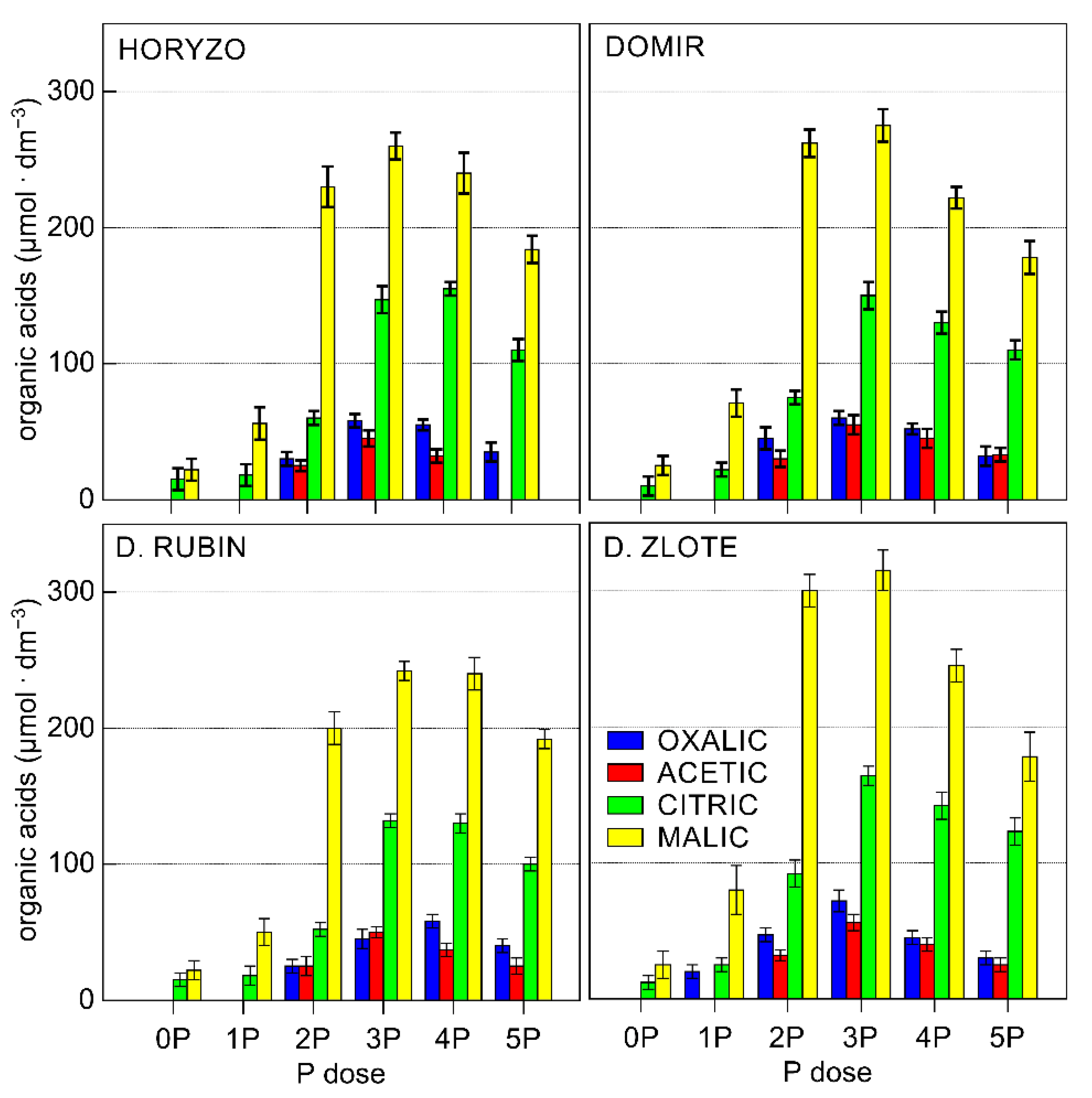
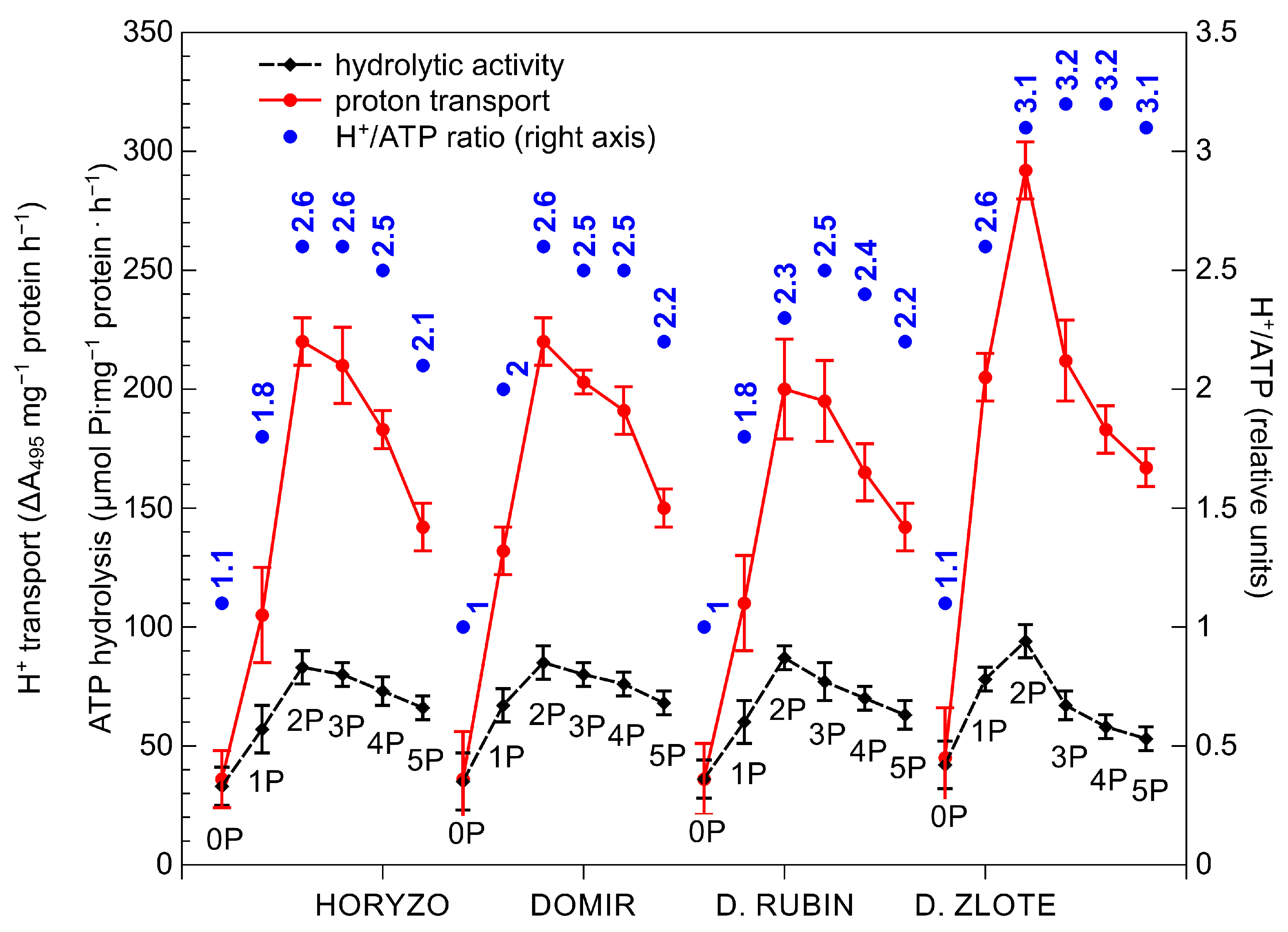
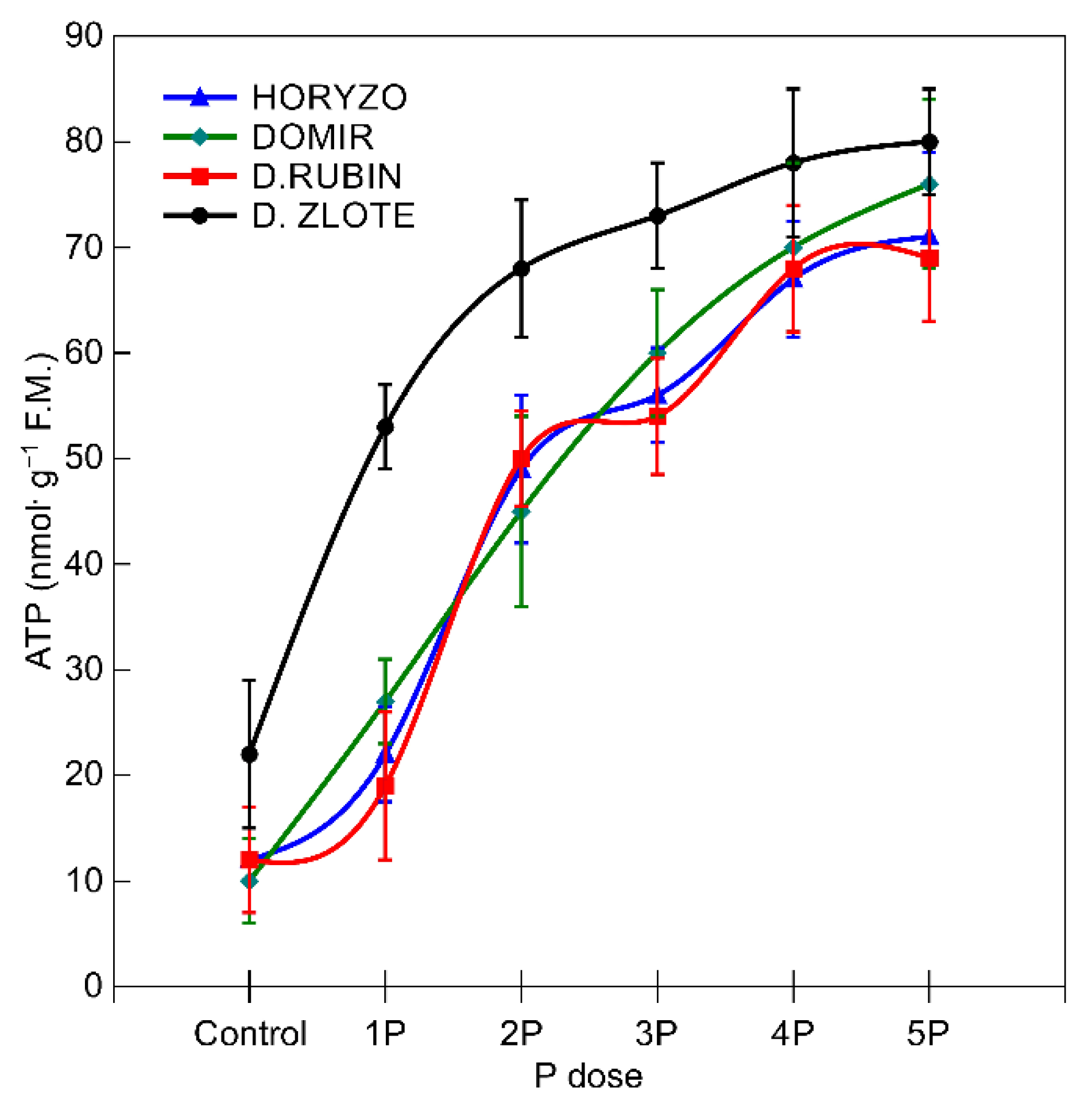

| SiO2 | Ca | Mg | K | P | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|
| % DM | mg·kg−1 (DM) | |||||||
| 54 ± 1.8 | 16.3 ± 0.5 | 2.4 ± 0.1 | 2.7 ± 0.1 | 0.08 ± 0.005 | 18,000 ± 80 | 1900 ± 9 | 70 ± 9 | 1800 ± 90 |
| Granulometric Class | |||
|---|---|---|---|
| >2.0 mm | 0.05–2.0 mm | 0.002–0.05 mm | <0.002 mm |
| 0 | 1–13 | 63–85 | 5–35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, P.; Gediga, K.; Spiak, Z. Phosphorus-Induced Adaptation Mechanisms of Rye Grown on Post-Flotation Copper Tailings. Biology 2021, 10, 818. https://doi.org/10.3390/biology10080818
Stępień P, Gediga K, Spiak Z. Phosphorus-Induced Adaptation Mechanisms of Rye Grown on Post-Flotation Copper Tailings. Biology. 2021; 10(8):818. https://doi.org/10.3390/biology10080818
Chicago/Turabian StyleStępień, Piotr, Krzysztof Gediga, and Zofia Spiak. 2021. "Phosphorus-Induced Adaptation Mechanisms of Rye Grown on Post-Flotation Copper Tailings" Biology 10, no. 8: 818. https://doi.org/10.3390/biology10080818
APA StyleStępień, P., Gediga, K., & Spiak, Z. (2021). Phosphorus-Induced Adaptation Mechanisms of Rye Grown on Post-Flotation Copper Tailings. Biology, 10(8), 818. https://doi.org/10.3390/biology10080818







