Romantic Love and Sleep Variations: Potential Proximate Mechanisms and Evolutionary Functions
Abstract
Simple Summary
Abstract
1. Introduction
2. Biological Perspectives on Romantic Love and Sleep
2.1. Romantic Love
2.2. Sleep
2.2.1. Definition, Characteristics, and Measurement
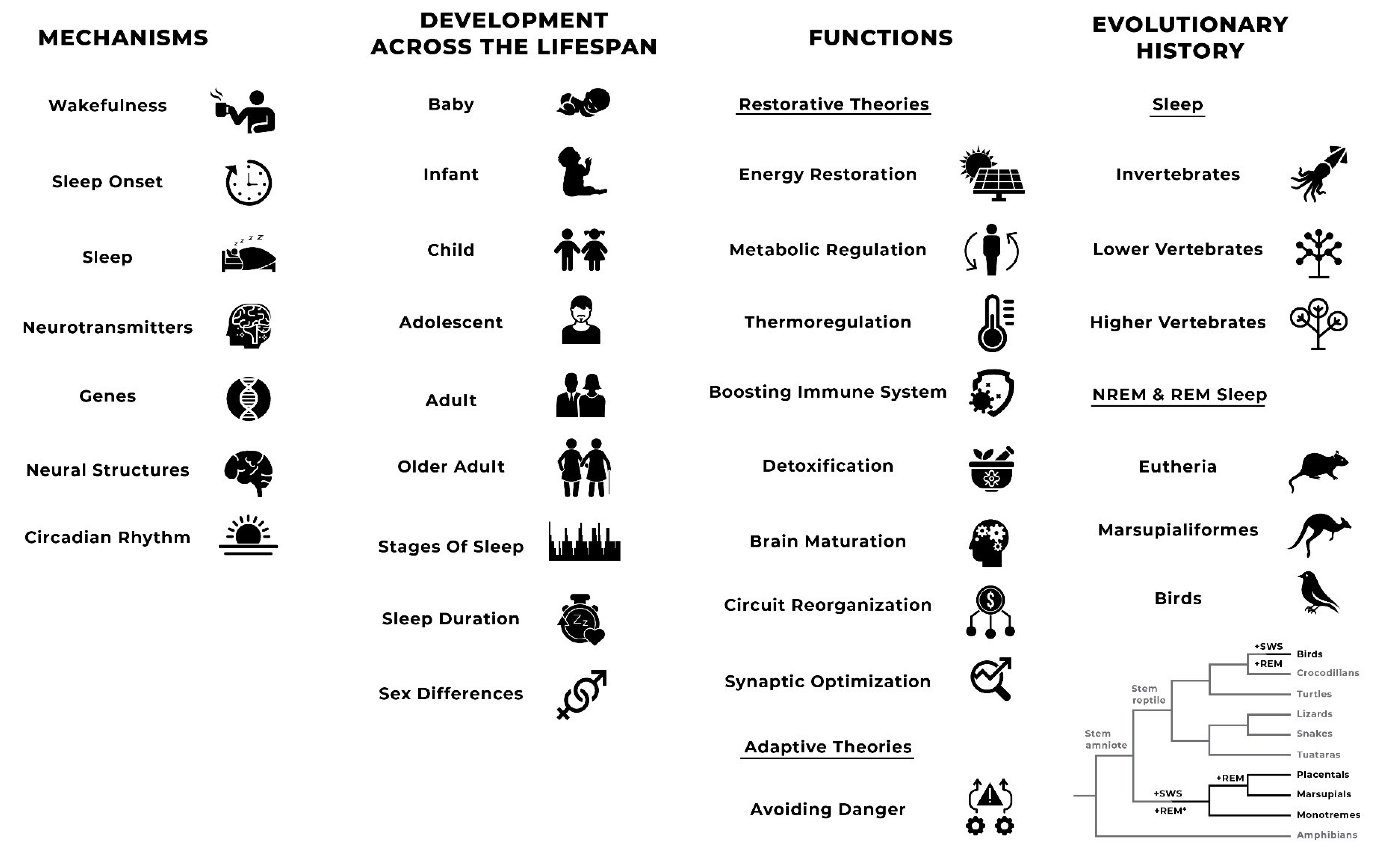
2.2.2. Mechanisms
2.2.3. Development across the Lifespan
2.2.4. Functions
2.2.5. Evolutionary History
3. Romantic Love and Sleep Variations
Psychopathological Symptoms Associated with Sleep Variations
4. Potential Mechanisms Explaining Sleep Variations in People Experiencing Romantic Love
Potential Mechanisms Explaining the Relationship between Symptoms of Psychopathology and Sleep Variations
5. Potential Functions of Sleep Variations in People Experiencing Romantic Love
Potential Functions of Sleep Variations Associated with Symptoms of Psychopathology
6. Are Sleep Variations in People Experiencing Romantic Love Adaptations or By-Products?
7. Limitations of Existing Research and Areas for Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Acknowledgments
Conflicts of Interest
References
- Tinbergen, N. On aims and methods of Ethology. Z. Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Mayr, E. Cause and effect in biology—Kinds of causes, predictability, and teleology are viewed by a practicing biologist. Science 1961, 134, 1501–1506. [Google Scholar] [CrossRef]
- Bateson, P.; Laland, K.N. Tinbergen’s four questions: An appreciation and an update. Trends Ecol. Evol. 2013, 28, 712–718. [Google Scholar] [CrossRef]
- Zietsch, B.P.; Sidari, M.J.; Murphy, S.C.; Sherlock, J.M.; Lee, A.J. For the good of evolutionary psychology, let’s reunite proximate and ultimate explanations. Evol. Hum. Behav. 2020, 42, 76–78. [Google Scholar] [CrossRef]
- Satake, A. Flowering Time as a Model Trait to Bridge Proximate and Evolutionary Questions. In Mathematical Modelling in Plant Biology; Morris, R., Ed.; Springer Nature: Cham, Switzerland, 2018; pp. 171–194. [Google Scholar]
- Hughes, D.P.; Araújo, J.P.; Loreto, R.G.; Quevillon, L.; de Bekker, C.; Evans, H.C. From So Simple a Beginning: The Evolution of Behavioral Manipulation by Fungi. Adv. Genet. 2016, 94, 437–469. [Google Scholar] [CrossRef] [PubMed]
- Mellor, E.; Brilot, B.; Collins, S. Abnormal repetitive behaviours in captive birds: A Tinbergian review. Appl. Anim. Behav. Sci. 2018, 198, 109–120. [Google Scholar] [CrossRef]
- Zeifman, D.M. An ethological analysis of human infant crying: Answering Tinbergen’s four questions. Dev. Psychobiol. 2001, 39, 265–285. [Google Scholar] [CrossRef]
- Stephen, I.D.; Burke, D.; Sulikowski, D. Tinbergen’s “four questions” provides a formal framework for a more complete understanding of prosocial biases in favour of attractive people. Behav. Brain Sci. 2017, 40, 38–39. [Google Scholar] [CrossRef]
- Luoto, S.; Krams, I.; Rantala, M.J. A Life History Approach to the Female Sexual Orientation Spectrum: Evolution, Development, Causal Mechanisms, and Health. Arch. Sex Behav. 2019, 48, 1273–1308. [Google Scholar] [CrossRef]
- Bode, A.; Kushnick, G. Proximate and Ultimate Perspectives on Romantic Love. Front. Psychol. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Aron, A.; Dutton, D.G.; Aron, E.N.; Iverson, A. Experiences of falling in love. J. Soc. Pers. Relatsh. 1989, 6, 243–257. [Google Scholar] [CrossRef]
- Pines, A.M. The Role of Gender and Culture in Romantic Attraction. Eur. Psychol. 2001, 6, 96–102. [Google Scholar] [CrossRef]
- Riela, S.; Rodriguez, G.; Aron, A.; Xu, X.M.; Acevedo, B.P. Experiences of falling in love: Investigating culture, ethnicity, gender, and speed. J. Soc. Pers. Relatsh. 2010, 27, 473–493. [Google Scholar] [CrossRef]
- Buss, D.M.; Abbott, M.; Angleitner, A.; Asherian, A.; Biaggio, A.; Blanco-Villasenor, A.; Bruchon-Schweitzer, M.; Ch’u, H.-Y.; Czapinski, J.; Deraad, B.; et al. Internaitonal preferences in selecting mates—A study of 37 cultures. J. Cross-Cult. Psychol. 1990, 21, 5–47. [Google Scholar] [CrossRef]
- Fisher, H.E. Lust, attraction, and attachment in mammalian reproduction. Hum. Nat.-Interdiscip. Biosoc. Perspect. 1998, 9, 23–52. [Google Scholar] [CrossRef]
- Diamond, L.M. Emerging perspectives on distinctions between romantic love and sexual desire. Curr. Dir. Psychol. Sci. 2004, 13, 116–119. [Google Scholar] [CrossRef]
- Emanuele, E.; Brondino, N.; Pesent, S.; Re, S.; Geroldi, D. Genetic loading on human loving styles. Neuroendocrinol. Lett. 2007, 28, 815–821. [Google Scholar]
- Acevedo, B.P.; Poulin, M.J.; Collins, N.L.; Brown, L.L. After the Honeymoon: Neural and Genetic Correlates of Romantic Love in Newlywed Marriages. Front. Psychol. 2020, 11, 634. [Google Scholar] [CrossRef]
- Xu, X.M.; Weng, X.C.; Aron, A. The mesolimbic dopamine pathway and romantic love. In Brain Mapping: An Encyclopedic Reference; Toga, A.W., Mesulam, M.M., Kastner, S., Eds.; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Diamond, L.M.; Dickenson, J.A. The neuroimaging of love and desire: Review and future directions. Clin. Neuropsychiatry J. Treat. Eval. 2012, 9, 39–46. [Google Scholar]
- Cacioppo, S.; Bianchi-Demicheli, F.; Frum, C.; Pfaus, J.G.; Lewis, J.W. The Common Neural Bases Between Sexual Desire and Love: A Multilevel Kernel Density fMRI Analysis. J. Sex. Med. 2012, 9, 1048–1054. [Google Scholar] [CrossRef]
- Cacioppo, S.; Bianchi-Demicheli, F.; Hatfield, E.; Rapson, R.L. Social Neuroscience of Love. Clin. Neuropsychiatry 2012, 9, 3–13. [Google Scholar]
- Marazziti, D.; Akiskal, H.S.; Rossi, A.; Cassano, G.B. Alteration of the platelet serotonin transporter in romantic love. Psychol. Med. 1999, 29, 741–745. [Google Scholar] [CrossRef]
- Marazziti, D.; Canale, D. Hormonal changes when falling in love. Psychoneuroendocrinology 2004, 29, 931–936. [Google Scholar] [CrossRef]
- Emanuele, E.; Politi, P.; Bianchi, M.; Minoretti, P.; Bertona, M.; Geroldi, D. Raised plasma nerve growth factor levels associated with early-stage romantic love. Psychoneuroendocrinology 2006, 31, 288–294. [Google Scholar] [CrossRef]
- Langeslag, S.J.E.; van der Veen, F.M.; Fekkes, D. Blood Levels of Serotonin Are Differentially Affected by Romantic Love in Men and Women. J. Psychophysiol. 2012, 26, 92–98. [Google Scholar] [CrossRef]
- Weisman, O.; Schneiderman, I.; Zagoory-Sharon, O.; Feldman, R. Early Stage Romantic Love is Associated with Reduced Daily Cortisol Production. Adapt. Hum. Behav. Physiol. 2015, 1, 41–53. [Google Scholar] [CrossRef]
- Marazziti, D.; Baroni, S.; Giannaccini, G.; Piccinni, A.; Mucci, F.; Catena-Dell’Osso, M.; Rutigliano, G.; Massimetti, G.; Dell’Osso, L. Decreased lymphocyte dopamine transporter in romantic lovers. CNS Spectr. 2017, 22, 290–294. [Google Scholar] [CrossRef]
- Sorokowski, P.; Żelaźniewicz, A.; Nowak, J.; Groyecka, A.; Kaleta, M.; Lech, W.; Samorek, S.; Stachowska, K.; Bocian, K.; Pulcer, A.; et al. Romantic Love and Reproductive Hormones in Women. Int. J. Environ. Res. Public Health 2019, 16, 4224. [Google Scholar] [CrossRef]
- Renner, J.; Stanulla, M.; Walther, A.; Schindler, L. CortiLove: A pilot study on hair steroids in the context of being in love and separation. Compr. Psychoneuroendocrinol. 2021, 100061. [Google Scholar] [CrossRef]
- Hatfield, E.; Schmitz, E.; Cornelius, J.; Rapson, R.L. Passionate Love: How Early Does it Begin? J. Psychol. Hum. Sex. 1988, 1, 35–51. [Google Scholar] [CrossRef]
- Wang, A.Y.; Nguyen, H.T. Passionate love and anxiety—A cross-generational study. J. Soc. Psychol. 1995, 135, 459–470. [Google Scholar] [CrossRef]
- Fisher, H.E.; Aron, A.; Brown, L.L. Romantic love: A mammalian brain system for mate choice. Philos. Trans. R. Soc. B-Biol. Sci. 2006, 361, 2173–2186. [Google Scholar] [CrossRef]
- Meston, C.M.; Buss, D.M. Why humans have sex. Arch. Sex Behav. 2007, 36, 477–507. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.J.O.; Simpson, J.A.; Campbell, L.; Overall, N.C. Pair-Bonding, Romantic Love, and Evolution: The Curious Case of Homo sapiens. Perspect. Psychol. Sci. 2015, 10, 20–36. [Google Scholar] [CrossRef]
- Siegel, J.M. Do all animals sleep? Trends Neurosci. 2008, 31, 208–213. [Google Scholar] [CrossRef]
- Miyazaki, S.; Liu, C.Y.; Hayashi, Y. Sleep in vertebrate and invertebrate animals, and insights into the function and evolution of sleep. Neurosci. Res. 2017, 118, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chokroverty, S. Overview of normal sleep. In Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects; Chokroverty, S., Sudhansu, S.M., Eds.; Springer: New York, NY, USA, 2017. [Google Scholar]
- American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events—Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2007. [Google Scholar]
- Martoni, M.; Biagi, M. Sleep self-report measures: A literature review. Epidemiol. Psychiatr. Sci. 2007, 16, 316–329. [Google Scholar] [CrossRef]
- Caffo, B.; Swihart, B.; Laffan, A.; Crainiceanu, C.; Punjabi, N. An overview of observational sleep research with application to sleep stage transitioning. Chance (N.Y.) 2009, 22, 10–15. [Google Scholar] [CrossRef]
- Van de Water, A.T.; Holmes, A.; Hurley, D.A. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—A systematic review. J. Sleep Res. 2011, 20 1Pt 2, 183–200. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Bajoghli, H.; Joshaghani, N.; Gerber, M.; Mohammadi, M.R.; Holsboer-Trachsler, E.; Brand, S. In Iranian female and male adolescents, romantic love is related to hypomania and low depressive symptoms, but also to higher state anxiety. Int. J. Psychiatry Clin. 2013, 17, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Bajoghli, H.; Joshaghani, N.; Mohammadi, M.R.; Holsboer-Trachsler, E.; Brand, S. In female adolescents, romantic love is related to hypomanic-like stages and increased physical activity, but not to sleep or depressive symptoms. Int. J. Psychiatry Clin. 2011, 15, 164–170. [Google Scholar] [CrossRef]
- Bajoghli, H.; Keshavarzi, Z.; Mohammadi, M.-R.; Schmidt, N.B.; Norton, P.J.; Holsboer-Trachsler, E.; Brand, S. “I love you more than I can stand!”—Romantic love, symptoms of depression and anxiety, and sleep complaints are related among young adults. Int. J. Psychiatry Clin. 2014, 18, 169–174. [Google Scholar] [CrossRef]
- Brand, S.; Foell, S.; Bajoghli, H.; Keshavarzi, Z.; Kalak, N.; Gerber, M.; Schmidt, N.B.; Norton, P.J.; Holsboer-Trachsler, E. “Tell me, how bright your hypomania is, and I tell you, if you are happily in love!”—Among young adults in love, bright side hypomania is related to reduced depression and anxiety, and better sleep quality. Int. J. Psychiatry Clin. 2015, 19, 24–31. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2020. [Google Scholar]
- Rundo, J.V.; Downey, R., III. Polysomnography. Handb. Clin. Neurol. 2019, 160, 381–392. [Google Scholar] [CrossRef]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016, 3, 67–104. [Google Scholar] [CrossRef] [PubMed]
- Eban-Rothschild, A.; Appelbaum, L.; de Lecea, L. Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology 2018, 43, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodriguez, E.; Arias-Carrion, O.; Zavala-Garcia, A.; Sarro-Ramirez, A.; Huitron-Resendiz, S.; Arankowsky-Sandoval, G. Basic sleep mechanisms: An integrative review. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 38–54. [Google Scholar] [CrossRef]
- Bollinger, T.; Schibler, U. Circadian rhythms-from genes to physiology and disease. Swiss Med. Wkly. 2014, 144, w13984. [Google Scholar] [CrossRef] [PubMed]
- Kleitman, N. The nature of sleep. In The Nature of Dreaming; Wolstenholme, G.E.W., O’Connor, M., Eds.; Churchill: London, UK, 1961; pp. 349–364. [Google Scholar]
- Lesku, J.A.; Martinez-Gonzalez, D.; Rattenborg, N.C. Phylogeny and ontogeny of sleep. In The Neuroscience of Sleep; Stickgold, R., Walker, M., Eds.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- D’Ambrosio, C.; Redline, S. Sleep across the lifespan. In Impact of Sleep and Sleep Disturbances on Obesity and Cancer; Redline, S., Berger, N.A., Eds.; Springer: New York, NY, USA, 2014; pp. 1–23. [Google Scholar]
- Grigg-Damberger, M.M. Ontogeny of Sleep and Its Functions in Infancy, Childhood, and Adolescence. In Sleep Disorders in Children; Nevšímalová, S., Bruni, O., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–29. [Google Scholar]
- Grandner, M.A.; Martin, J.L.; Patel, N.P.; Jackson, N.J.; Gehrman, P.R.; Pien, G.; Perlis, M.L.; Xie, D.; Sha, D.; Weaver, T.; et al. Age and sleep disturbances among American men and women: Data from the U.S. Behavioral Risk Factor Surveillance System. Sleep 2012, 35, 395–406. [Google Scholar] [CrossRef]
- Li, J.; Gooneratne, N.S. Sleep and health in older adults. In Sleep and Health; Grandner, M.A., Ed.; Academic Press: London, UK, 2019; pp. 21–29. [Google Scholar]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef]
- Tähkämö, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Krishnan, V.; Collop, N.A. Gender differences in sleep disorders. Curr. Opin. Pulm. Med. 2006, 12, 383–389. [Google Scholar] [CrossRef]
- Bao, A.-M.; Swaab, D.F. Sex Differences in the Brain, Behavior, and Neuropsychiatric Disorders. Neuroscientist 2010, 16, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Meers, J.; Stout-Aguilar, J.; Nowakowski, S. Sex differences in sleep health. In Sleep and Health; Grandner, M.A., Ed.; Academic Press: London, UK, 2019; pp. 21–29. [Google Scholar]
- Freiberg, A.S. Why We Sleep: A Hypothesis for an Ultimate or Evolutionary Origin for Sleep and Other Physiological Rhythms. J. Circadian Rhythm 2020, 18, 2. [Google Scholar] [CrossRef]
- Vibha, M.J.; Sushil, K.J. Sleep: Evolution and Functions; Springer: Singapore, 2020. [Google Scholar]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef] [PubMed]
- Research IoMUCoSMa. Extent and Health Consequences of Chronic Sleep Loss and Sleep Disorders. In Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; Colten, H.R., Altevogt, B.M., Eds.; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist 2014, 20, 203–219. [Google Scholar] [CrossRef]
- McNamara, P. (Ed.) Characteristics of REM and NREM Sleep; Cambridge University Press: Cambridge, UK, 2019; pp. 60–77. [Google Scholar]
- Siegel, J.M. REM sleep: A biological and psychological paradox. Sleep Med. Rev. 2011, 15, 139–142. [Google Scholar] [CrossRef]
- Laland, K.N.; Brown, G.R. Sense and Nonsense: Evolutionary Perspectives on Human Behaviour, 2nd ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Field, J.M.; Bonsall, M.B. The evolution of sleep is inevitable in a periodic world. PLoS ONE 2018, 13, e0201615. [Google Scholar] [CrossRef]
- Siegel, J.M.; Manger, P.R.; Nienhuis, R.; Fahringer, H.M.; Pettigrew, J.D. The Echidna Tachyglossus aculeatus Combines REM and Non-REM Aspects in a Single Sleep State: Implications for the Evolution of Sleep. J. Neurosci. 1996, 16, 3500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegel, J.M.; Manger, P.R.; Nienhuis, R.; Fahringer, H.M.; Pettigrew, J.D. Monotremes and the evolution of rapid eye movement sleep. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, R.; Toda, H.; Libourel, P.-A.; Hayashi, Y.; Vogt, K.; Sakurai, T. Evolutionary Origin of Distinct NREM and REM Sleep. Front. Psychol. 2020, 11, 3599. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Luethi, M.; von Planta, A.; Hatzinger, M.; Holsboer-Trachsler, E. Romantic love, hypomania, and sleep pattern in adolescents. J. Adolesc. Health 2007, 41, 69–76. [Google Scholar] [CrossRef]
- Bajoghli, H.; Farnia, V.; Joshaghani, N.; Haghighi, M.; Jahangard, L.; Ahmadpanah, M.; Sadeghi Bahmani, D.; Holsboer-Trachsler, E.; Brand, S. “I love you forever (more or less)”-stability and change in adolescents’ romantic love status and associations with mood states. Rev. Bras. Psiquiatr. 2017, 39, 323–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuula, L.; Partonen, T.; Pesonen, A.K. Emotions relating to romantic love-further disruptors of adolescent sleep. Sleep Health 2020, 6, 159–165. [Google Scholar] [CrossRef]
- Goodman, W.K.; Price, L.H.; Rasmussen, S.A.; Mazure, C.; Fleischmann, R.L.; Hill, C.L.; Heninger, G.R.; Charney, D.S. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch. Gen. Psychiatry 1989, 46, 1006–1011. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Hatfield, E.; Brinton, C.; Cornelius, J. Passioante love and anxiety in young adolescents. Motiv. Emot. 1989, 13, 271–289. [Google Scholar] [CrossRef]
- Wittert, G. The relationship between sleep disorders and testosterone in men. Asian J. Androl. 2014, 16, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Alvarenga, T.F.; Mazaro-Costa, R.; Hachul, H.C.; Tufik, S. The association of testosterone, sleep, and sexual function in men and women. Brain Res. 2011, 1416, 80–104. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- Miyamoto, H.; Nakamaru-Ogiso, E.; Hamada, K.; Hensch, T.K. Serotonergic integration of circadian clock and ultradian sleep-wake cycles. J. Neurosci. 2012, 32, 14794–14803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Oishi, Y.; Lazarus, M. The control of sleep and wakefulness by mesolimbic dopamine systems. Neurosci. Res. 2017, 118, 66–73. [Google Scholar] [CrossRef]
- Braga, R.I.; Panaitescu, A.; Bădescu, S.; Zăgrean, A.-M.; Zăgrean, L. Intranasal administration of oxytocin alters sleep architecture. Biol. Rhythm. Res. 2014, 45, 69–75. [Google Scholar] [CrossRef]
- Schneiderman, I.; Zagoory-Sharon, O.; Leckman, J.F.; Feldman, R. Oxytocin during the initial stages of romantic attachment: Relations to couples’ interactive reciprocity. Psychoneuroendocrinology 2012, 37, 1277–1285. [Google Scholar] [CrossRef]
- Schneiderman, I.; Kanat-Maymon, Y.; Zagoory-Sharon, O.; Feldman, R. Mutual influences between partners’ hormones shape conflict dialog and relationship duration at the initiation of romantic love. Soc. Neurosci. 2014, 9, 337–351. [Google Scholar] [CrossRef]
- Ulmer-Yaniv, A.; Avitsur, R.; Kanat-Maymon, Y.; Schneiderman, I.; Zagoory-Sharon, O.; Feldman, R. Affiliation, reward, and immune biomarkers coalesce to support social synchrony during periods of bond formation in humans. Brain Behav. Immun. 2016, 56, 130–139. [Google Scholar] [CrossRef]
- Kumari, M.; Badrick, E.; Ferrie, J.; Perski, A.; Marmot, M.; Chandola, T. Self-Reported Sleep Duration and Sleep Disturbance Are Independently Associated with Cortisol Secretion in the Whitehall II Study. J. Clin. Endocrinol. Metab. 2009, 94, 4801–4809. [Google Scholar] [CrossRef]
- Takahashi, S.; Krueger, J.M. Nerve growth factor enhances sleep in rabbits. Neurosci. Lett. 1999, 264, 149–152. [Google Scholar] [CrossRef]
- Yamuy, J.; Morales, F.R.; Chase, M.H. Induction of rapid eye movement sleep by the microinjection of nerve growth factor into the pontine reticular formation of the cat. Neuroscience 1995, 66, 9–13. [Google Scholar] [CrossRef]
- Andre, C.J.; Lovallo, V.; Spencer, R.M.C. The effects of bed sharing on sleep: From partners to pets. Sleep Health 2021, 7, 314–323. [Google Scholar] [CrossRef]
- Drews, H.J.; Wallot, S.; Brysch, P.; Berger-Johannsen, H.; Weinhold, S.L.; Mitkidis, P.; Baier, P.C.; Lechinger, J.; Roepstorff, A.; Göder, R. Bed-Sharing in Couples Is Associated With Increased and Stabilized REM Sleep and Sleep-Stage Synchronization. Front. Psychiatry 2020, 11, 583. [Google Scholar] [CrossRef]
- Hofer, M.K.; Chen, F.S. The Scent of a Good Night’s Sleep: Olfactory Cues of a Romantic Partner Improve Sleep Efficiency. Psychol. Sci. 2020, 31, 449–459. [Google Scholar] [CrossRef]
- Ong, A.D.; Kim, S.; Young, S.; Steptoe, A. Positive affect and sleep: A systematic review. Sleep Med. Rev. 2017, 35, 21–32. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Johnson, S.L.; Edge, M.D.; Holmes, M.K.; Carver, C.S. The behavioral activation system and mania. Annu. Rev. Clin. Psychol. 2012, 8, 243–267. [Google Scholar] [CrossRef]
- Nettle, D.; Bateson, M. The Evolutionary Origins of Mood and Its Disorders. Curr. Biol. 2012, 22, R712–R721. [Google Scholar] [CrossRef]
- Ketchesin, K.D.; Becker-Krai, l.D.; McClung, C.A. Mood-related central and peripheral clocks. Eur. J. Neurosci. 2020, 51, 326–345. [Google Scholar] [CrossRef]
- Harvey, A.G. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. Am. J. Psychiatry 2008, 165, 820–829. [Google Scholar] [CrossRef]
- Keller, M.C.; Nesse, R.M. Is low mood an adaptation? Evidence for subtypes with symptoms that match precipitants. J. Affect. Disord. 2005, 86, 27–35. [Google Scholar] [CrossRef]
- Ben Mocha, Y. Why do human and non-human species conceal mating? The cooperation maintenance hypothesis. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201330. [Google Scholar] [CrossRef]
- Wrosch, C.; Miller, G.E. Depressive Symptoms Can Be Useful: Self-Regulatory and Emotional Benefits of Dysphoric Mood in Adolescence. J. Pers. Soc. Psychol. 2009, 96, 1181–1190. [Google Scholar] [CrossRef]
- Buss, D.M. The evolution of love in humans. In The New Psychology of Love, 2nd ed.; Sternberg, R.J., Sternberg, K., Eds.; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Buss, D.M.; Haselton, M.G.; Shackelford, T.K.; Bleske, A.L.; Wakefield, J.C. Adaptations, exaptations, and spandrels. Am. Psychol. 1998, 53, 533–548. [Google Scholar] [CrossRef]
- Williams, G.C. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought; Princeton Science Library, Ed.; Princeton University Press: Woodstock, Oxfordshire, UK, 2019. [Google Scholar]
- Reeve, H.K.; Sherman, P.W. Adaptation and the goals of evolutionary research. Q. Rev. Biol. 1993, 68, 1–32. [Google Scholar] [CrossRef]
- Andrews, P.W.; Gangestad, S.W.; Matthews, D. Adaptationism—How to carry out an exaptationist program. Behav. Brain Sci. 2002, 25, 489. [Google Scholar] [CrossRef]
- Campbell, K.; Hosseini, C.; Myers, K.; Calub, N. Does Love Influence Athletic Performance? The Perspectives of Olympic Athletes. Rev. Eur. Stud. 2016, 8, 1. [Google Scholar] [CrossRef]
| Adolescents | Young Adults | Studies | |
|---|---|---|---|
| Sleep onset latency | - | Shorter | [47,48]; see also [45,46,81,82] |
| Sleep duration | Shorter | - | [81,83] *; see also [45,46,47,48,82] |
| WASO | - | Fewer | [47,48]; see also [45,46] |
| Sleep quality | - | Better | [47,48]; see also [81] and [45,46,47,82,83] |
| Restoring sleep | Increased | [47,48] |
| (Neuro) Endocrine | Neural | Social | Psychological |
|---|---|---|---|
| Testosterone | Mesolimbic pathway | Bed sharing | Mood |
| Serotonin | Joint evening activities | Attachment anxiety | |
| Dopamine | Sexual activity | Rumination | |
| Oxytocin | Stress | ||
| Cortisol | |||
| NGF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bode, A.; Kuula, L. Romantic Love and Sleep Variations: Potential Proximate Mechanisms and Evolutionary Functions. Biology 2021, 10, 923. https://doi.org/10.3390/biology10090923
Bode A, Kuula L. Romantic Love and Sleep Variations: Potential Proximate Mechanisms and Evolutionary Functions. Biology. 2021; 10(9):923. https://doi.org/10.3390/biology10090923
Chicago/Turabian StyleBode, Adam, and Liisa Kuula. 2021. "Romantic Love and Sleep Variations: Potential Proximate Mechanisms and Evolutionary Functions" Biology 10, no. 9: 923. https://doi.org/10.3390/biology10090923
APA StyleBode, A., & Kuula, L. (2021). Romantic Love and Sleep Variations: Potential Proximate Mechanisms and Evolutionary Functions. Biology, 10(9), 923. https://doi.org/10.3390/biology10090923






