Alterations in Mycelial Morphology and Flow Cytometry Assessment of Membrane Integrity of Ganoderma boninense Stressed by Phenolic Compounds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, Culture Conditions and Treatments
2.2. Screening of Phenolic Compounds on Growth of G. boninense
Poison Food Technique
- C: radial growth of mycelia in the control plate, and
- T: radial growth of mycelia in treatment plate.
2.3. Mechanism(s) Involved in the Suppression of G. boninense
2.3.1. Electrolyte and Sugar Leakage
Electrolyte Leakage Measurement
Sugar Leakage Measurement
2.3.2. Membrane Depolarization Assay
2.3.3. Membrane Permeability Assay
2.3.4. Ergosterol Determination
2.3.5. Alteration in Structural Morphology
Scanning Electron Microscopy (SEM)
High-Resolution Transmission Electron Microscopy (HR-TEM)
2.3.6. Statistical Analysis
3. Results
3.1. Screening of Phenolic Compounds on the Growth of G. boninense
3.1.1. Poison Food Technique
Lethal Concentration (LC50 and LC90)
3.2. Mechanism(s) Involved in Suppression of G. boninense
3.2.1. Electrolyte and Sugar Leakage
3.2.2. Membrane Depolarization Assay
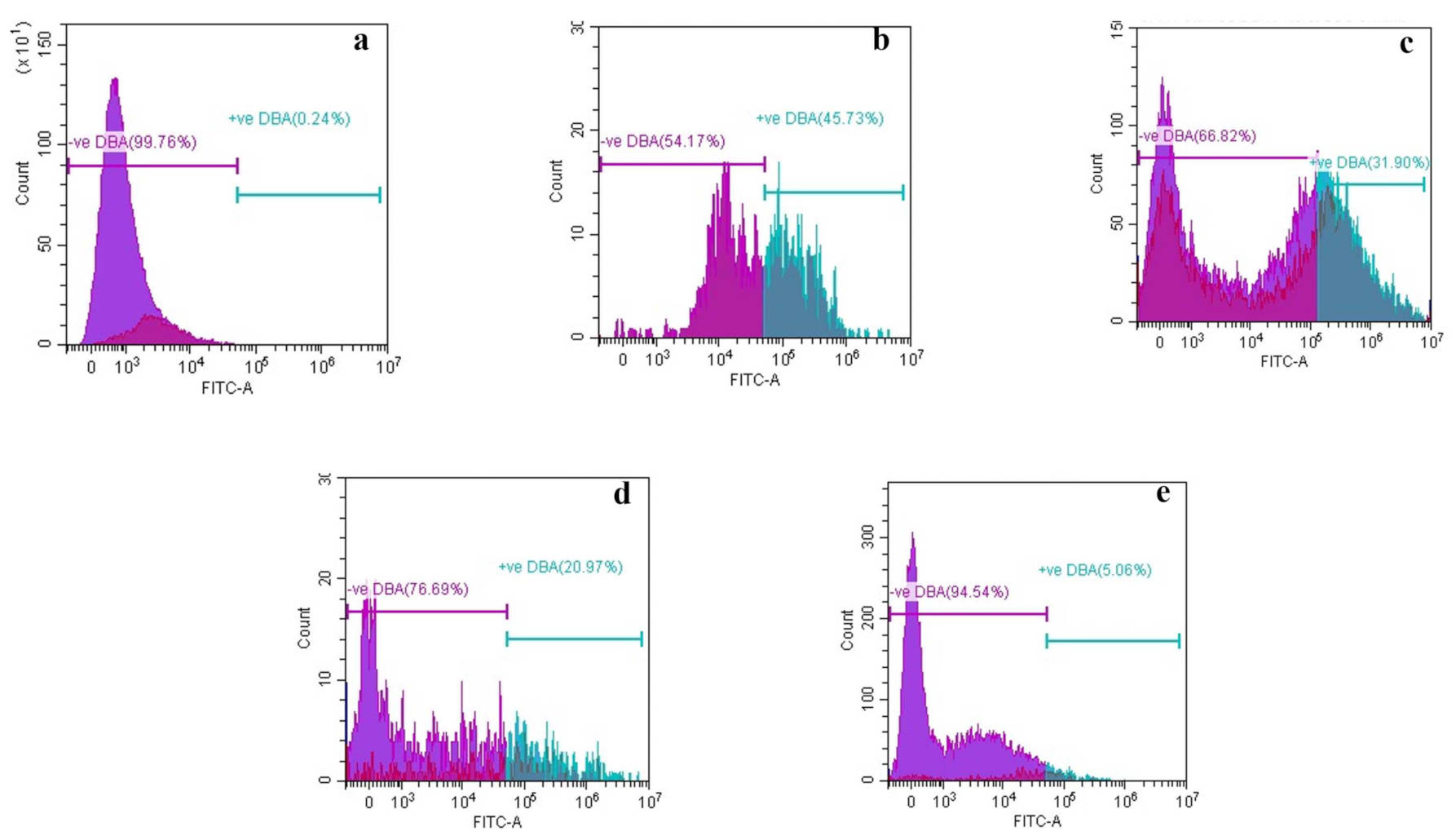
3.2.3. Membrane Permeability Assay
3.2.4. Ergosterol Determination
3.2.5. Alteration in Structural Morphology
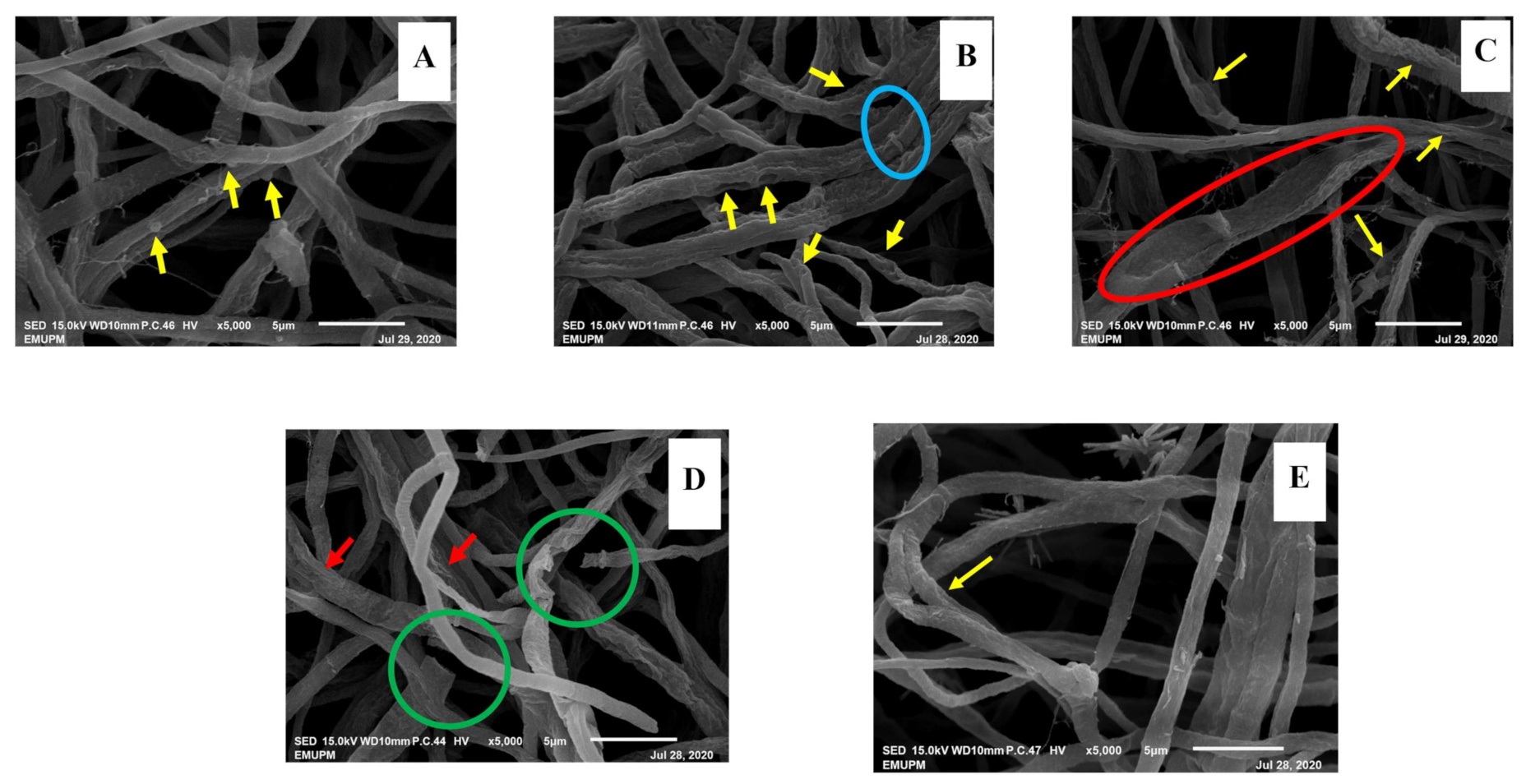
Scanning Electron Microscopy (SEM)
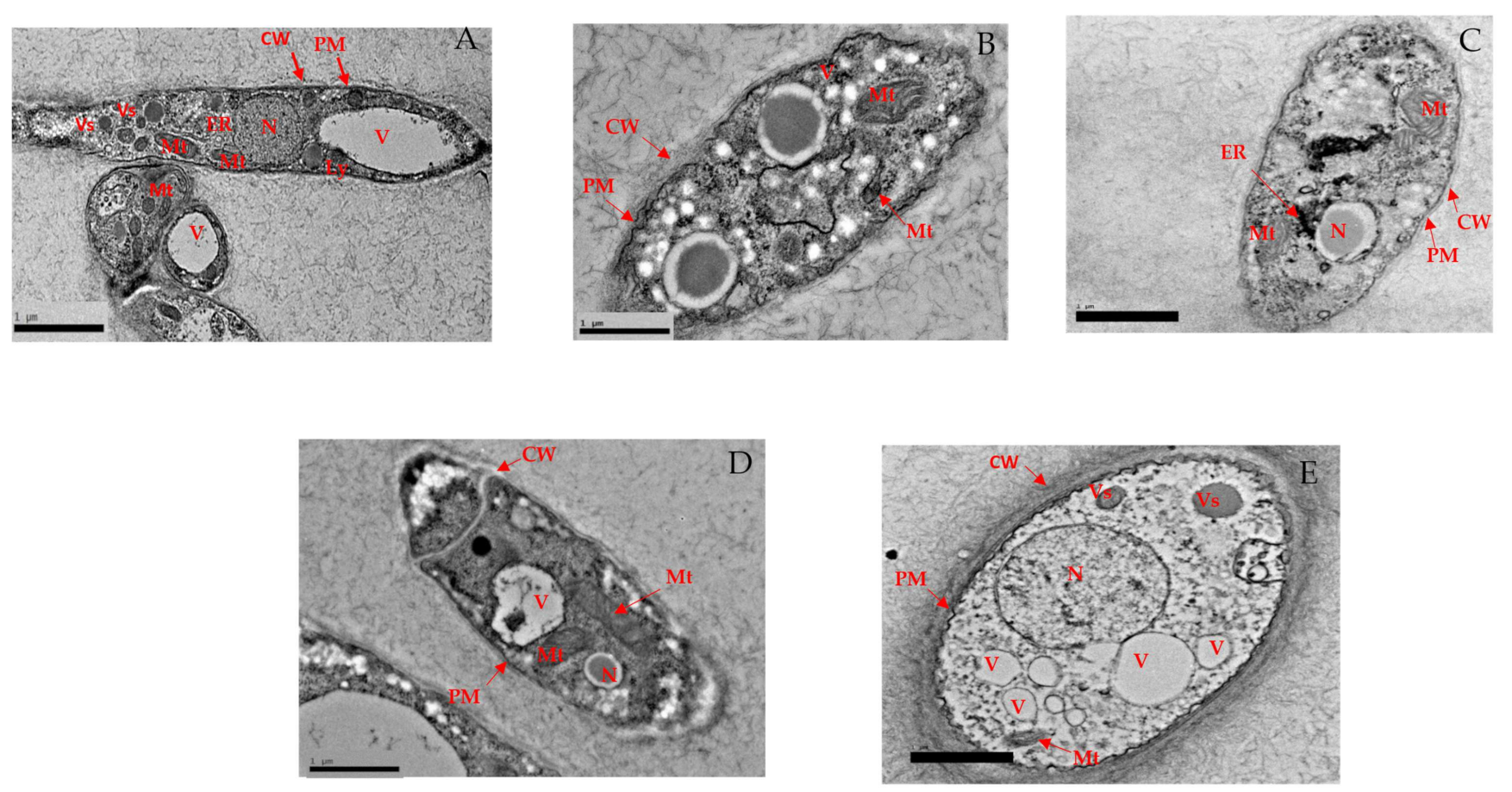
High-Resolution Transmission Electron Microscopy (HR-TEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malaysian Palm Oil Industry—MPOC. 2020. Available online: http://mpoc.org.my/malaysian-palm-oil-industry/ (accessed on 2 January 2020).
- Foong, S.Z.; Goh, C.K.; Supramaniam, C.V.; Ng, D.K. Input–output optimisation model for sustainable oil palm plantation development. Sustain. Prod. Consum. 2018, 17, 31–46. [Google Scholar] [CrossRef]
- Ritchie, H.; Palm Oil. Our World in Data. 2021. Available online: https://ourworldindata.org/palm-oil (accessed on 25 May 2021).
- Ebarcelos, E.; Rios, S.E.A.; Cunha, R.N.V.; Elopes, R.; Motoike, S.Y.; Ebabiychuk, E.; Eskirycz, A.; Kushnir, S. Oil palm natural diversity and the potential for yield improvement. Front. Plant Sci. 2015, 6, 190. [Google Scholar] [CrossRef]
- Dislich, C.; Keyel, A.C.; Saecker, J.; Kisel, Y.; Meyer, K.M.; Auliya, M.; Barnes, A.C.; Corre, M.D.; Darras, K.; Faust, H. A review of the ecosystem functions in oil palm plantations, using forests as a reference system. Biol. Rev. 2017, 92, 1539–1569. [Google Scholar] [CrossRef]
- Wahid, M.B.; Abdullah, S.N.A.; Henson, I.E. Oil Palm—Achievements and Potential. “New directions for a diverse planet”. Proceedings of the 4th International Crop Science Congress. Plant Prod. Sci. 2004, 8, 288–297. [Google Scholar] [CrossRef]
- Ahmad, K. The Oil Palm Industry in Malaysia: Thriving With Transformative Technologies. J. Oil Palm Res. 2008, 29, 431–439. [Google Scholar]
- Ahmad Parveez, G.K. Oil Palm Economic Performance in Malaysia and R&D Progress in 2020. J. Oil Palm Res. 2021, 33, 2. [Google Scholar]
- Statista. Global Production Volume Palm Oil, 2012–2020 | Statista. 2020. Available online: https://www.statista.com/statistics/613471/palm-oil-production-volume-worldwide/ (accessed on 30 April 2020).
- Rezania, S.; Oryani, B.; Cho, J.; Sabbagh, F.; Rupani, P.; Talaiekhozani, A.; Rahimi, N.; Lotfi Ghahroud, M. Technical Aspects of Biofuel Production from Different Sources in Malaysia—A Review. Processes 2020, 8, 993. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Surendran, A.; Paterson, R.; Ali, A.; Ahmad, K. Current strategies and perspectives in detection and control of basal stem rot of oil palm. Saudi J. Biol. Sci. 2021, 28, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Corley, R.H.V.; Tinker, P. The Oil Palm, 5th ed.; Wiley Black-Well: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Woittiez, L.; van Wijk, M.; Slingerland, M.; van Noordwijk, M.; Giller, K. Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 2017, 83, 57–77. [Google Scholar] [CrossRef]
- Midot, F.; Lau, S.Y.L.; Wong, W.C.; Tung, H.J.; Yap, M.L.; Lo, M.L.; Jee, M.S.; Dom, S.P.; Melling, L. Genetic Diversity and Demographic History of Ganoderma boninense in Oil Palm Plantations of Sarawak, Malaysia Inferred from ITS Regions. Microorganisms 2019, 7, 464. [Google Scholar] [CrossRef] [Green Version]
- Olaniyi, O.; Szulczyk, K. Estimating the economic damage and treatment cost of basal stem rot striking the Malaysian oil palms. For. Policy Econ. 2020, 116, 102163. [Google Scholar] [CrossRef]
- Seman, I.B. R&D on biology, detection and management of Ganoderma disease in oil palm. In Workshop on Basal Stem Rot (BSR) of Oil Palm; Universiti Putra Malaysia: Selangor, Malaysia, 2018; Volume 12. [Google Scholar]
- Chong, K.; Atong, M.; Rossall, S. The role of syringic acid in the interaction between oil palm and Ganoderma boninense, the causal agent of basal stem rot. Plant Pathol. 2012, 61, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D. Using modern plant breeding to improve the nutritional and technological qualities of oil crops. OCL 2014, 21, D607. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, I.; Woźniak, M.; Kwaśniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical characterization of wood treated with a formulation based on PRO, caffeine and organosilanes. Eur. J. Wood Wood Prod. 2018, 76, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Phin Chong, K.; Yu, G. Selected Biomarkers from Oil Palm-Ganoderma Infected Tissues for Detection of Basal Stem Rot Disease. WMSU Res. J. 2010, 37, 1–13. Available online: https://www.researchgate.net/profile/Khim-Chong/publication/330011060_Selected_Biomarkers_from_Oil_Palm-Ganoderma_Infected_Tissues_for_Detection_of_Basal_Stem_Rot_Disease/links/5c29ee60a6fdccfc70732b61/Selected-Biomarkers-from-Oil-Palm-Ganoderma-Infected-Tissues-for-Detection-of-Basal-Stem-Rot-Disease.pdf (accessed on 20 May 2021).
- Surendran, A.; Siddiqui, Y.; Manickam, S.; Ali, A. Role of benzoic and salicylic acids in the immunization of oil palm seedlings-challenged by Ganoderma boninense. Ind. Crops Prod. 2017, 122, 358–365. [Google Scholar] [CrossRef]
- Surendran, A.; Siddiqui, Y.; Saud, H.M.; Ali, N.S.; Manickam, S. Inhibition and kinetic studies of lignin-degrading enzymes of Ganoderma boninense by naturally occurring phenolic compounds. J. Appl. Microbiol. 2018, 125, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Surendran, A.; Siddiqui, Y.; Ahmad, K.; Fernanda, R. Deciphering the Physicochemical and Microscopical Changes in Ganoderma boninense-Infected Oil Palm Woodblocks under the Influence of Phenolic Compounds. Plants 2021, 10, 1797. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2014, 93, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Steudler, S.; Böhmer, U.; Weber, J.; Bley, T. Biomass measurement by flow cytometry during solid-state fermentation of basidiomycetes. Cytom. Part A 2014, 87, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, D.G. An antifungal mechanism of curcumin lies in membrane-targeted action withinCandida albicans. IUBMB Life 2014, 66, 780–785. [Google Scholar] [CrossRef]
- Muniroh, M.; Sariah, M.; Zainal Abidin, M.; Lima, N.; Paterson, R. Rapid detection of Ganoderma-infected oil palms by microwave ergosterol extraction with HPLC and TLC. J. Microbiol. Methods 2014, 100, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Labrada-Delgado, G.; Aragon-Pina, A.; Campos-Ramos, A.; Castro-Romero, T.; Amador-Munoz, O.; Villalobos-Pietrini, R. Chemical and morphological characterization of PM2.5 collected during MILAGRO campaign using scanning electron microscopy. Atmos. Pollut. Res. 2012, 3, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Chong, K.P.; Eldaa, P.A.; Dayou, J. Relation of Ganoderma ergosterol content to Basal Stem Rot disease severity index. Adv. Environ. Biol. 2014, 8, 14–19. [Google Scholar]
- Ramsdale, M. Programmed cell death in pathogenic fungi. Biochim. et Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S. In vivo toxicity study of Ganoderma boninense. Afr. J. Pharm. Pharmacol. 2011, 5, 1819–1823. [Google Scholar] [CrossRef]
- Koc, A.; Silici, S.; Ayangil, D.; Ferahbas, A.; Cankaya, S. Comparison of in vitro activities of antifungal drugs and ethanolic extract of PRO against Trichophyton rubrum and T. mentagrophytes by using a microdilution assay. Mycoses 2005, 48, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Zahrani, N.; Reda, M.; Asiri, A. Recent Developments of GA derivatives and The Hybrids in Medicinal Chemistry: A Review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pu, R.; Li, Y.; Wu, Z.; Li, C.; Miao, X.; Yang, W. Chemical composition of PRO from China and the United States and their antimicrobial activities against Penicillium notatum. Molecules 2019, 24, 3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pag, U.; Oedenkoven, M.; Sass, V.P.; Shai, Y.; Shamova, O.; Antcheva, N.; Tossi, A.; Sahl, H.-G. Analysis of in vitro activities and modes of action of synthetic antimicrobial peptides derived from an α-helical ‘sequence template’. J. Antimicrob. Chemother. 2008, 61, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.; de Ávila, R.; Zara, A.; Santos, A.; Ataídes, F.; Freitas, V.; Costa, C.; Valadares, M.; Silva, M. Punicalagin triggers ergosterol biosynthesis disruption and cell cycle arrest in Cryptococcus gattii and Candida albicans. Braz. J. Microbiol. 2020, 51, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of THY and CARVCARV by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2007, 31, 1039–1045. [Google Scholar] [CrossRef]
- Nath, S.; Mallick, S.K.; Jha, S. An Improved Method of Genome Size Estimation by Flow Cytometry in Five Mucilaginous Species of Hyacinthaceae. Cytom. Part A 2014, 85A, 833–840. [Google Scholar] [CrossRef]
- Vanhauteghem, D.; Demeyere, K.; Callaert, N.; Boelaert, A.; Haesaert, G.; Audenaert, K.; Meyer, E. Flow Cytometry Is a Powerful Tool for Assessment of the Viability of Fungal Conidia in Metalworking Fluids. Appl. Environ. Microbiol. 2017, 83, e00938-17. [Google Scholar] [CrossRef] [Green Version]
- Cossu, A.; Le, P.; Young, G.; Nitin, N. Assessment of sanitation efficacy against Escherichia coli O157:H7 by rapid measurement of intracellular oxidative stress, membrane damage or glucose active uptake. Food Control 2017, 71, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Rao, R. Beyond ergosterol: Linking pH to antifungal mechanisms. Virulence 2010, 1, 551–554. [Google Scholar] [CrossRef] [Green Version]
- Pastinen, O.; Nyyssölä, A.; Pihlajaniemi, V.; Sipponen, M.H. Fractionation process for the protective isolation of ergosterol and trehalose from microbial biomass. Process Biochem. 2017, 58, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, T.R.; Lima, M.L.; Galuppo, M.K.; Mesquita, J.T.; Nascimento, M.A.D.; Santos, A.L.D.; Sartorelli, P.; Pimenta, D.C.; Tempone, A.G. Ergosterol isolated from the basidiomycete Pleurotus salmoneostramineus affects Trypanosoma cruzi plasma membrane and mitochondria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 30. [Google Scholar] [CrossRef] [Green Version]
- Francois, I.; Cammune, B.; Borgers, M.; Ausma, J.; Dispersyn, G. Azole: Mode of Antifungal Action and Resistance Development. Effect of Miconazole on Endogenous Reactive Oxygen Species Production in Candida Albicans. Anti-Infect. Agents Med. Chem. 2006, 5, 3–13. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2013, 3, 439. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2012.00439/full (accessed on 14 May 2021). [CrossRef] [PubMed] [Green Version]
- Hu, C.; Zhou, M.; Wang, W.; Sun, X.; Yarden, O.; Li, S. Abnormal Ergosterol Biosynthesis Activates Transcriptional Responses to Antifungal Azoles. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The fungal CYP51s: Their functions, structures, related drug resistance, and inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- An, P.; Yang, X.; Yu, J.; Qi, J.; Ren, X.; Kong, Q. A-terpineol and terpene-4-ol, the critical components of tea tree, exert antifungal activites in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control 2018, 98, 42–53. [Google Scholar] [CrossRef]
- Fernanda, R.; Siddiqui, Y.; Ganapathy, D.; Ahmad, K.; Surendran, A. Suppression of Ganoderma boninense Using Benzoic Acid: Impact on Cellular Ultrastructure and Anatomical Changes in Oil Palm Wood. Unpubl. Work. For. 2021, 12. (Accepted). [Google Scholar]
- Xu, Y.; Luo, L.; Chen, B.; Fu, Y. Recent development of chemical components in propolis. Front. Biol. China 2009, 4, 385–391. [Google Scholar] [CrossRef]
- Bivi, M.S.; Paiko, A.S.; Khairulmazmi, A.; Akhtar, M.S.; Idris, A.S. Control of Basal Stem Rot Disease in Oil Palm by Supplementation of Calcium, Copper, and Salicylic Acid. J. Plant Pathol. 2016, 32, 396. [Google Scholar] [CrossRef] [Green Version]

| Treatments (mg/mL) | PIRG (%) |
|---|---|
| Control | 0 e |
| GA 5 | 50.14 ± 3.8 c |
| GA 6 | 63.08 ± 3.8 b |
| GA 7 | 62.5 ± 3.8 b |
| GA 8 | 94 ± 3.8 a |
| THY 0.1 | 9.09 ± 3.76 e |
| THY 0.15 | 55.96 ± 3.76 c |
| THY 0.2 | 74.86 ± 3.76 b |
| THY 0.25 | 87.13 ± 3.76 a |
| PRO 2 | 35.88 ± 3.76 d |
| PRO 2.5 | 34.23 ± 3.76 d |
| PRO 3 | 32.6 ± 3.76 d |
| CARV 0.1 | 31.62 ± 3.76 d |
| CARV 0.13 | 33.2 ± 3.76 d |
| CARV 0.15 | 36 ± 3.76 d |
| Phenolic Compounds | Lethal Concentration (mg/mL) | |||
|---|---|---|---|---|
| LC50 | LC90 | Correlation (r) | R Square | |
| GA | 5.25 | 8.51 | 0.86 | 0.74 |
| THY | 0.19 | 0.22 | 0.91 | 0.84 |
| PRO | 74.13 | 91.2 | 0.99 | 0.99 |
| CARV | 0.68 | 9.33 | 0.91 | 0.83 |
| Treatments (mg/mL) | Electrolyte Leakage in G. boninense Mycelium |
|---|---|
| Control | 23.83 ± 3.7 f |
| GA-5 | 79.73 ± 3.82 ab |
| GA-6 | 79.33 ± 3.82 ab |
| GA-7 | 92.43 ± 3.82 a |
| GA-8 | 100.30 ± 3.82 a |
| THY-0.1 | 42.10 ± 3.72 d |
| THY-0.15 | 37.77 ± 3.72 e |
| THY-0.2 | 52.27 ± 3.72 c |
| THY-0.25 | 61.30 ± 3.72 b |
| PRO-2 | 43.43 ± 3.72 d |
| PRO- 2.5 | 44.8 ± 3.72 d |
| PRO-3 | 31.70 ± 3.72 e |
| PRO-3.5 | 30.27 ± 3.72 e |
| CARV-0.1 | 32.47 ± 3.72 e |
| CARV-0.13 | 22.8 ± 3.72 f |
| CARV-0.15 | 23.53 ± 3.72 f |
| Treatments (mg/mL) | Sugar Leakage of G. boninense Mycelium |
|---|---|
| Control | 0.0324 ± 0.32 c |
| GA-5 | 0.1654 ± 0.33 b |
| GA-6 | 0.0345 ± 0.33 cd |
| GA-7 | 0.1385 ± 0.33 b |
| GA-8 | 0.985 ± 0.33 a |
| THY-0.1 | 0.0923 ± 0.32 b |
| THY-0.15 | 0.1288 ± 0.32 b |
| THY-0.2 | 0.0567 ± 0.32 c |
| THY-0.25 | 0.0834 ± 0.32 b |
| PRO-2 | 0.0569 ± 0.32 c |
| PRO- 2.5 | 0.0256 ± 0.32 cd |
| PRO-3 | 0.0988 ± 0.32 b |
| PRO-3.5 | 0.2973 ± 0.32 b |
| CARV-0.1 | 0.0188 ± 0.32 d |
| CARV-0.13 | 0.0963 ± 0.32 b |
| CARV-0.15 | 0.0945 ± 0.32 b |
| Phenolic Compounds | Retention Time (min) Ergosterol | Amount (ppm) Ergosterol |
|---|---|---|
| Control | 8.337 | 122.26 |
| GA | 8.200 | 96.77 |
| THY | 8.173 | 162.76 |
| PRO | 8.253 | 158.09 |
| CARV | 8.173 | 382.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganapathy, D.; Siddiqui, Y.; Ahmad, K.; Adzmi, F.; Ling, K.L. Alterations in Mycelial Morphology and Flow Cytometry Assessment of Membrane Integrity of Ganoderma boninense Stressed by Phenolic Compounds. Biology 2021, 10, 930. https://doi.org/10.3390/biology10090930
Ganapathy D, Siddiqui Y, Ahmad K, Adzmi F, Ling KL. Alterations in Mycelial Morphology and Flow Cytometry Assessment of Membrane Integrity of Ganoderma boninense Stressed by Phenolic Compounds. Biology. 2021; 10(9):930. https://doi.org/10.3390/biology10090930
Chicago/Turabian StyleGanapathy, Daarshini, Yasmeen Siddiqui, Khairulmazmi Ahmad, Fariz Adzmi, and Kong Lih Ling. 2021. "Alterations in Mycelial Morphology and Flow Cytometry Assessment of Membrane Integrity of Ganoderma boninense Stressed by Phenolic Compounds" Biology 10, no. 9: 930. https://doi.org/10.3390/biology10090930








